Abstract
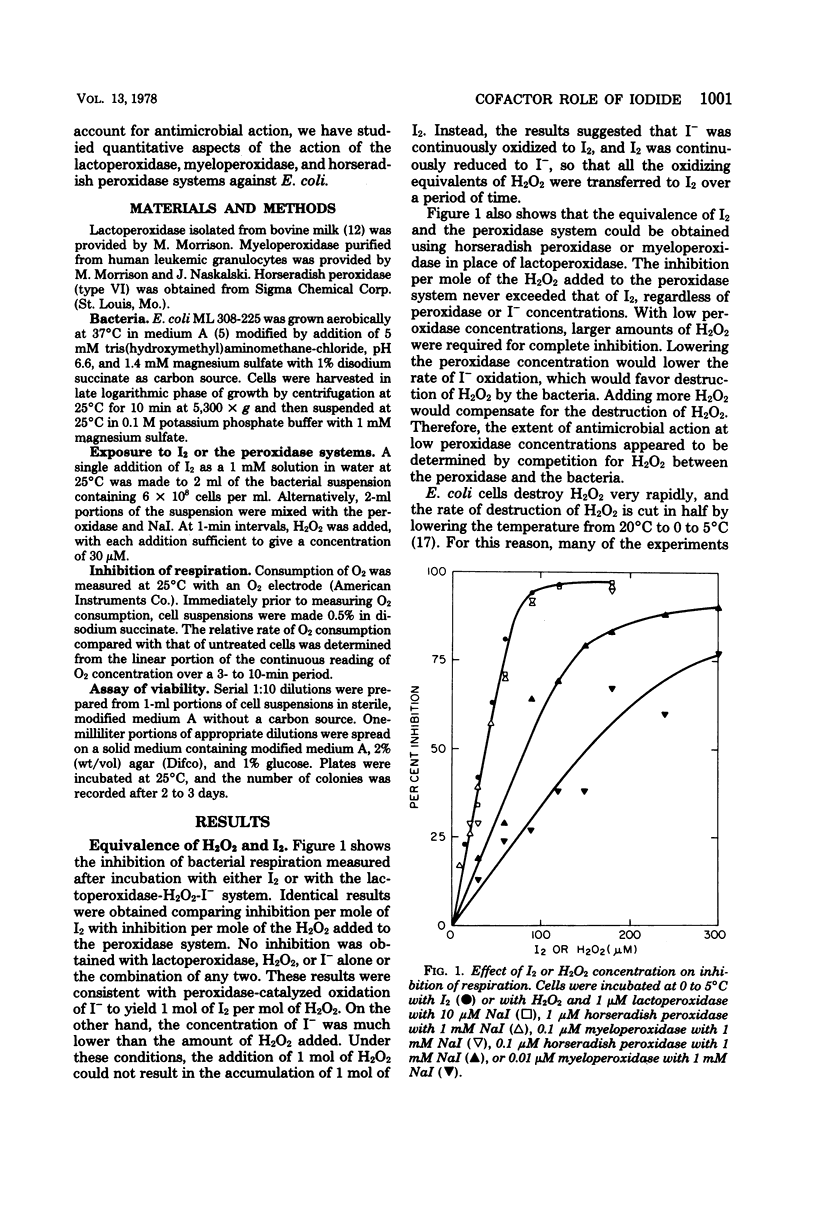
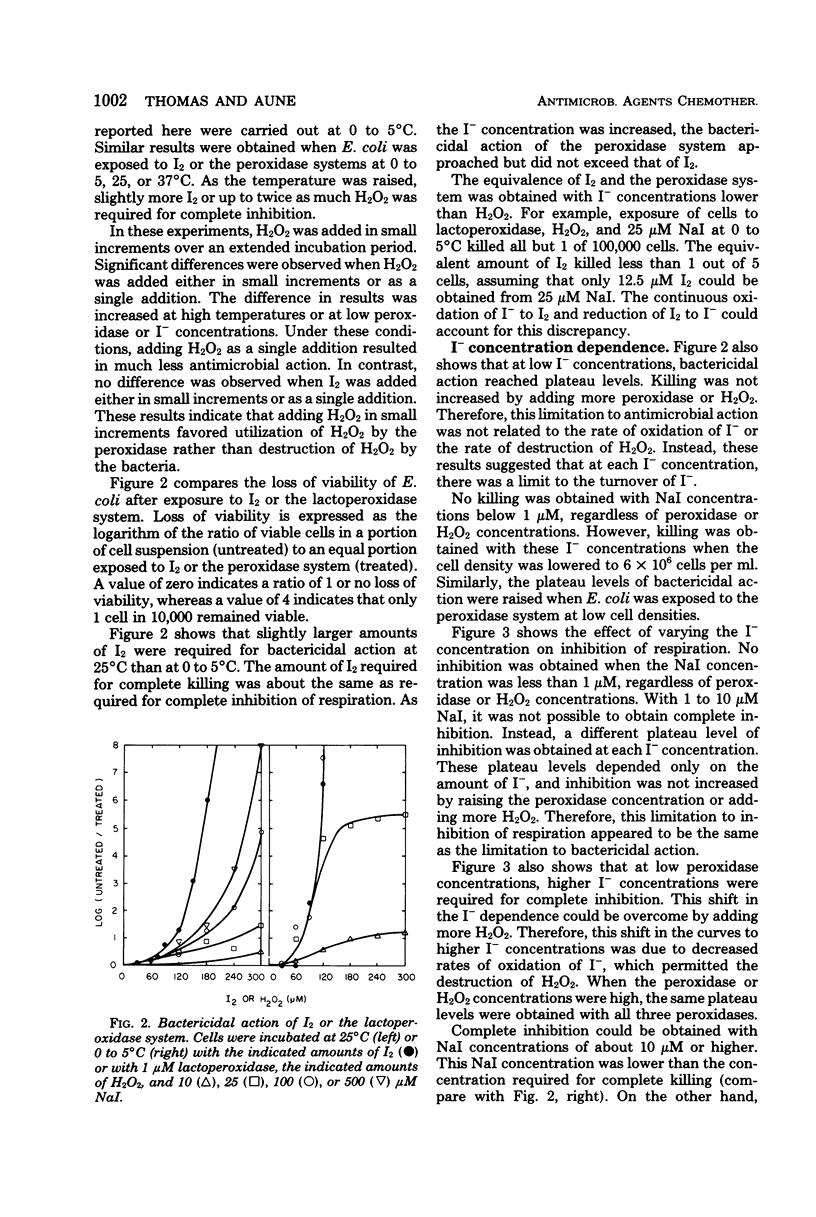
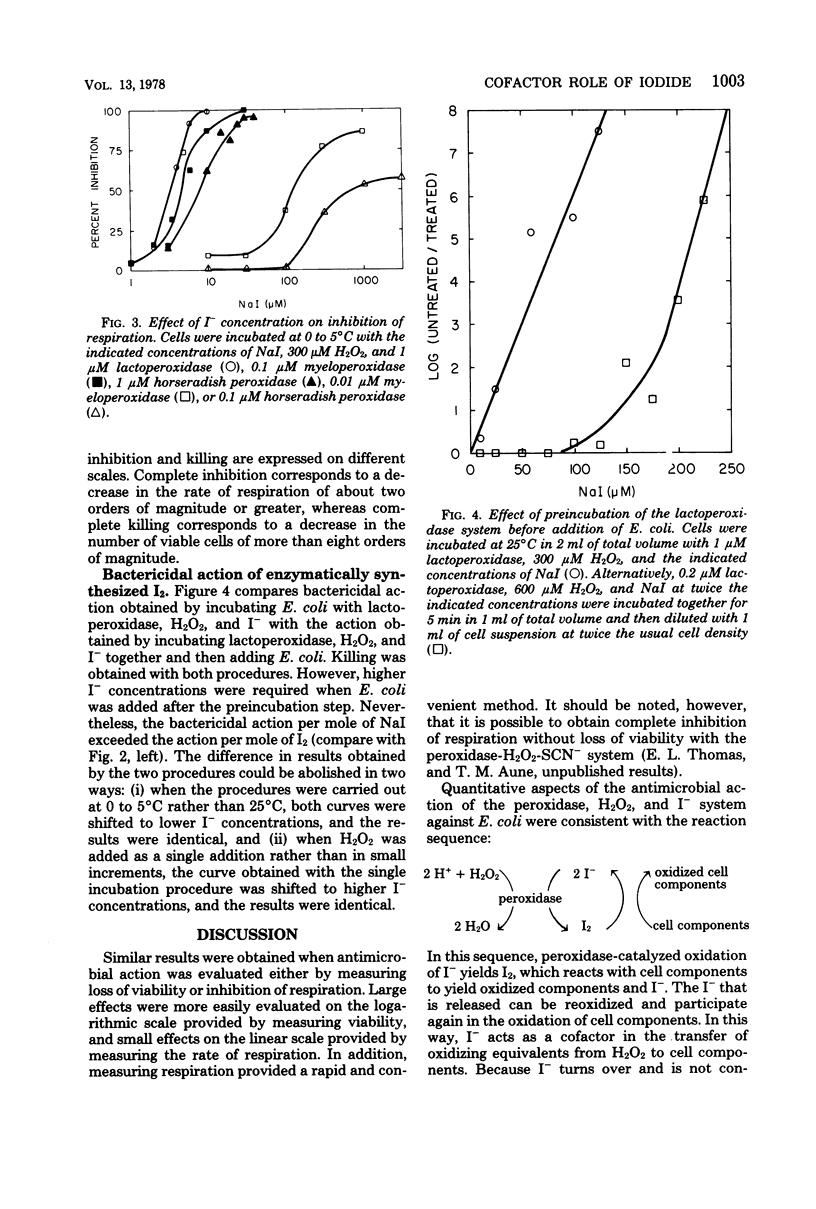
The mechanism of antimicrobial activity of the peroxidase-hydrogen peroxide (H2O2)-iodide (I−) system was investigated. Inhibition of respiration and loss of viability of Escherichia coli were used as measures of antimicrobial activity. Because the bacteria destroyed H2O2, peroxidase antimicrobial action depended on the competition for H2O2 between the bacteria and the peroxidase. Utilization of H2O2 by the peroxidase was favored by (i) increasing either the peroxidase or the I− concentration, so as to increase the rate of oxidation of I−, (ii) lowering the temperature to lower the rate of destruction of H2O2 by the bacteria, and (iii) adding H2O2 in small increments so as to avoid a large excess of H2O2 relative to I−. When utilization of H2O2 by the peroxidase system was favored, the peroxidase system and iodine (I2) were equivalent. That is, antimicrobial action per mole of H2O2 equaled that per mole of I2. Also, identical antimicrobial action was obtained either by incubating the bacteria directly with the peroxidase system or by preincubating the peroxidase system so as to form I2 and then adding the bacteria. On the other hand, peroxidase antimicrobial action could be obtained at low I− concentrations. These I− concentrations were lower than the concentration of I2 that was required for antimicrobial action. It is proposed that peroxidase-catalyzed oxidation of I− yields I2, which reacts with bacterial components to yield the oxidized components and I−. The I− that is released can be reoxidized and participate again in the oxidation of bacterial components. In this way, I− acts as a cofactor in the peroxidase-catalyzed oxidation of bacterial components.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aune T. M., Thomas E. L. Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur J Biochem. 1977 Oct 17;80(1):209–214. doi: 10.1111/j.1432-1033.1977.tb11873.x. [DOI] [PubMed] [Google Scholar]
- Aune T. M., Thomas E. L., Morrison M. Lactoperoxidase-catalyzed incorporation of thiocyanate ion into a protein substrate. Biochemistry. 1977 Oct 18;16(21):4611–4615. doi: 10.1021/bi00640a013. [DOI] [PubMed] [Google Scholar]
- Aune T. M., Thomas E. L. Oxidation of protein sulfhydryls by products of peroxidase-catalyzed oxidation of thiocyanate ion. Biochemistry. 1978 Mar 21;17(6):1005–1010. doi: 10.1021/bi00599a010. [DOI] [PubMed] [Google Scholar]
- Björck L., Rosén C., Marshall V., Reiter B. Antibacterial activity of the lactoperoxidase system in milk against pseudomonads and other gram-negative bacteria. Appl Microbiol. 1975 Aug;30(2):199–204. doi: 10.1128/am.30.2.199-204.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon C. B., Klebanoff S. J. A peroxidase-mediated, streptococcus mitis-dependent antimicrobial system in saliva. J Exp Med. 1973 Feb 1;137(2):438–450. doi: 10.1084/jem.137.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Hoogendoorn H., Piessens J. P., Scholtes W., Stoddard L. A. Hypothiocyanite ion; the inhibitor formed by the system lactoperoxidase-thiocyanate-hydrogen peroxide. I. Identification of the inhibiting compound. Caries Res. 1977;11(2):77–84. doi: 10.1159/000260252. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON M., HULTQUIST D. E. LACTOPEROXIDASE. II. ISOLATION. J Biol Chem. 1963 Aug;238:2843–2849. [PubMed] [Google Scholar]
- Morrison M., Schonbaum G. R. Peroxidase-catalyzed halogenation. Annu Rev Biochem. 1976;45:861–888. doi: 10.1146/annurev.bi.45.070176.004241. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Peroxidase-catalyzed oxidation of protein sulfhydryls mediated by iodine. Biochemistry. 1977 Aug 9;16(16):3581–3586. doi: 10.1021/bi00635a013. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Susceptibility of Escherichia coli to bactericidal action of lactoperoxidase, peroxide, and iodide or thiocyanate. Antimicrob Agents Chemother. 1978 Feb;13(2):261–265. doi: 10.1128/aac.13.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELDOW B. J. Studies on the antibacterial action of human saliva. III. Cofactor requirements of Lactobacillus bactericidin. J Immunol. 1963 Jan;90:12–16. [PubMed] [Google Scholar]


