Abstract
Half of all patients with multiple sclerosis (MS) experience cognitive impairment, for which there is no pharmacological treatment. Using magnetic resonance spectroscopy (MRS), we examined metabolic changes in the hippocampi of MS patients, compared the findings to performance on a neurocognitive test battery, and found that N-acetylaspartylglutamate (NAAG) concentration correlated with cognitive functioning. Specifically, MS patients with cognitive impairment had low hippocampal NAAG levels, whereas those with normal cognition demonstrated higher levels. We then evaluated glutamate carboxypeptidase II (GCPII) inhibitors, known to increase brain NAAG levels, on cognition in the experimental autoimmune encephalomyelitis (EAE) model of MS. Whereas GCPII inhibitor administration did not affect physical disabilities, it increased brain NAAG levels and dramatically improved learning and memory test performance compared with vehicle-treated EAE mice. These data suggest that NAAG is a unique biomarker for cognitive function in MS and that inhibition of GCPII might be a unique therapeutic strategy for recovery of cognitive function.
Keywords: neuroradiology, hippocampus, 2-(phosphonomethyl)pentanedioic acid (2-PMPA)
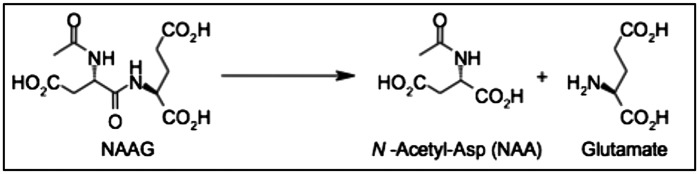
Glutamate carboxypeptidase II (GCPII), previously called N-acetylated-α-linked acidic dipeptidase (NAALADase), is a membrane-bound enzyme expressed on the surface of astrocytes that catalyzes the cleavage of N-acetylaspartylglutamate (NAAG) into N-acetylaspartate (NAA) and glutamate (Fig. 1) (1, 2). NAAG, the most abundant neuropeptide in the mammalian brain, is a selective agonist for metabotropic glutamate receptor 3 (mGluR3) (1, 2). mGluR3s are expressed on the surface of presynaptic neurons and astrocytes in the CNS. Activation of mGluR3s by NAAG reduces cAMP and cGMP levels, inhibits glutamate release, and stimulates a release of transforming growth factor beta (1, 2). A recent study reported impaired performance on spatial reference and working memory tasks in mGluR2/3 knockout mice (3), suggesting a role for group II mGluRs in cognitive function.
Fig. 1.
GCPII reaction. GCPII cleaves NAAG into NAA and glutamate.
Cognitive impairment is a frequent comorbidity of many neurological diseases, including multiple sclerosis (MS). MS affects over 2 million individuals worldwide, and ∼40–65% of all MS patients experience cognitive impairment (4). The most commonly and severely affected facets of cognition in MS patients are anterograde episodic memory, working memory, and processing speed (5–7), and these impairments strongly contribute to the high frequency of unemployment in MS patient populations (8). Global brain atrophy, candidate genetic risk factors, accelerated inflammatory processes (7), and dysregulated signaling pathways (9) have been implicated as contributing factors, but an incomplete understanding of the molecular mechanisms behind cognitive impairment in MS has prevented the development and Food and Drug Administration (FDA) approval of drug therapies.
The most widely used animal model of MS, experimental autoimmune encephalomyelitis (EAE), is a valuable tool for developing treatments for MS. Three FDA-approved disease-modifying agents were developed in EAE models before successfully moving into MS trials—glatiramer acetate, natalizumab (10), and fingolimod (11). A recent study reported memory impairment in EAE mice compared with normal controls (12). However, to the best of our knowledge, no drugs have demonstratively improved EAE-induced cognitive impairment.
A 1.5-T magnetic resonance spectroscopy (MRS) study in MS patients revealed no correlations between cognitive performance and the concentrations of metabolites commonly altered in neurodegenerative disorders, including inositol and NAA (13). However, use of a magnet at higher field strength (3 T) improves spectral resolution and sensitivity and may also be used to estimate NAAG, a neuropeptide that is significantly reduced in the brains of patients with neurodegenerative diseases (14, 15), by allowing it to be resolved from the NAA peak (16). In the present study, we found that right hippocampal NAAG/creatine (Cr) concentrations positively correlate to cognitive function in MS patients. Based on these data, we used a potent and selective small molecule inhibitor of GCPII (17) to increase brain NAAG levels in an animal model of MS with cognitive impairment (12).
Results
Right Hippocampal NAAG Correlates with Cognitive Function in MS.
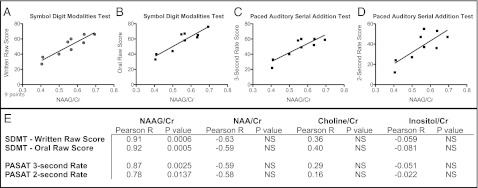
To determine if relationships exist between CNS metabolite concentrations and cognitive function in patients with MS, a neurocognitive battery and MRS were conducted in newly diagnosed relapsing–remitting MS patients at baseline and 6–12 mo after the initial clinical visit. The left and right hippocampi were evaluated for NAAG, NAA, inositol, and choline levels and normalized to Cr, a relatively constant metabolite in the brain. P values were set at 0.01 to eliminate the chance of both type I and II errors, and according to these parameters, right hippocampal NAAG/Cr significantly correlated with the subjects’ scores on 17 cognitive variables (Table 1). These striking correlations were observed in a wide range of cognitive tests, including measures of processing speed (i.e., Symbol Digit Modalities Test, Fig. 2 A and B), nonverbal learning and memory (i.e., Brief Visuospatial Memory Test, Rey-Osterrieth Complex Figure Copy Test), sustained attention (i.e., Paced Auditory Serial Addition Test, Fig. 2 C and D), and cognitive flexibility (i.e., D-KEFS Sorting Test). No correlations were discerned between cognitive performance and either right or left NAA, inositol, choline (Fig. 2E), or left hippocampal NAAG levels.
Table 1.
Cognitive test scores correlated to right hippocampal NAAG/Cr concentrations in MS patients
| P value | P value summary | Pearson r | |
| Symbol Digit Modalities Test | |||
| Written raw score | 0.0006 | *** | 0.9129 |
| Oral raw score | 0.0005 | *** | 0.9163 |
| Paced Auditory Serial Addition Test (PASAT) | |||
| 3-s rate PASAT | 0.003 | ** | 0.8664 |
| 2-s rate PASAT | 0.01 | ** | 0.7774 |
| Verbal Fluency Tests | |||
| FAS Verbal Fluency Test | 0.13 | NS | 0.549 |
| Categories | 0.005 | ** | 0.8342 |
| Judgment of Line Orientation | |||
| Line orientation | 0.06 | NS | 0.6483 |
| Rey-Osterrieth Complex Figure Copy Test | |||
| Copy | 0.03 | NS | 0.7124 |
| Immediate recall | 0.48 | NS | 0.2738 |
| Delayed recall | 0.67 | NS | 0.168 |
| Recognition total correct | 0.24 | NS | 0.4381 |
| California Verbal Learing Test II | |||
| Trial 1 recall | 0.26 | NS | 0.4188 |
| Trial 5 recall | 0.003 | ** | 0.8631 |
| Trials 1–5 total | 0.01 | ** | 0.7744 |
| Trial B | 0.02 | NS | 0.7639 |
| Short delay free recall | 0.002 | ** | 0.8843 |
| Short delay cued recall | 0.004 | ** | 0.8503 |
| Long delay free recall | 0.0008 | *** | 0.9032 |
| Long delay cued recall | 0.0008 | *** | 0.9052 |
| Recognition hits | 0.15 | NS | 0.5251 |
| Recognition false positives | 0.009 | ** | −0.8051 |
| D prime | 0.01 | ** | 0.7826 |
| C | 0.02 | NS | 0.7425 |
| Brief Visuospatial Memory Test | |||
| Trial 1 | 0.07 | NS | 0.6368 |
| Trial 2 | 0.002 | ** | 0.8837 |
| Trial 3 | 0.0008 | *** | 0.903 |
| Delayed recall | 0.001 | *** | 0.8955 |
| Hits | 0.37 | NS | 0.3653 |
| False positives | 0.15 | NS | −0.5561 |
| d-KEFS Sorting Test | |||
| Free sorting correct sorts | 0.05 | NS | 0.6616 |
| Free sorting description | 0.02 | NS | 0.7439 |
| Sort recognition description | 0.001 | *** | 0.8936 |
P value summary denoted by **P < 0.01, ***P < 0.001.
Fig. 2.
Positive correlations of cognitive task performances to right hippocampal (NAAG/Cr). Performance on the written (A) and oral (B) Symbol Digit Modalities Test positively correlated to right hippocampal (NAAG/Cr) in MS patients. (A, P < 0.001, r = 0.9129; B, P < 0.001, r = 0.9163) Performance on the 2-s (C) and 3-s (D) Paced Auditory Serial Addition Test positively correlated to right hippocampal (NAAG/Cr) in patients with multiple sclerosis (C, P < 0.01, r = 0.8644; D, P < 0.05, r = 0.7774). No differences were noted in other metabolites examined (E). n = 9 patients.
A relationship between cognitive impairment and lower levels of NAAG suggested that interventions to raise NAAG levels might enhance cognition in MS patients. To test this hypothesis, we turned to EAE and the use of a class of drugs that elevate brain NAAG levels.
2-(Phosphonomethyl)Pentanedioic Acid Administration Does Not Affect EAE Severity.
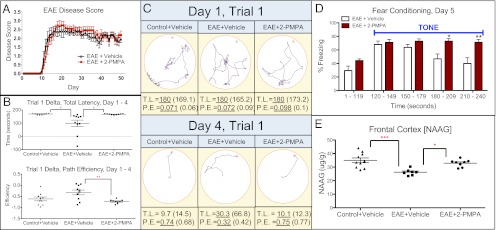
To determine the effects of GCPII inhibition in EAE, mice were administered daily injections of either 2-(phosphonomethyl)pentanedioic acid (2-PMPA) or vehicle (saline). 2-PMPA is a potent and selective GCPII inhibitor that penetrates the blood brain barrier (BBB) and raises NAAG levels (18). Mice developed signs of EAE ∼10 d postimmunization. EAE disability scores did not differ between groups for the duration of the experiment (Fig. 3A), indicating that 2-PMPA does not affect severity and progression of EAE.
Fig. 3.
Effect of 2-PMPA on disease score, cognition, and frontal cortex NAAG concentration in EAE mice. (A) Daily administration of 2-PMPA does not affect EAE severity compared with vehicle-treated mice. (B) EAE mice treated with 2-PMPA demonstrated improved Barnes maze performance as indicated by increased total latency delta (day 1 total latency − day 4 total latency) and decreased path efficiency delta (day 1 path efficiency − day 4 path efficiency) compared with controls, showing superior learning abilities. (C) Sample Barnes maze paths tracked by ANY-maze software of the first trial of day 1 or day 4 of Barnes maze testing (∼4 wk post-EAE induction). TL, total latency; PE, path efficiency. Underlined number is represented in the plot, and the number in parentheses represents the group average. (D) Daily administration of 2-PMPA increases cue-based freezing behavior, an indication of improved memory, in a fear conditioning test. (E) NAAG concentration in the frontal cortex is elevated in EAE-positive mice receiving daily injections of 2-PMPA. Significantly different at *P < 0.05, **P < 0.01, ***P < 0.001. n = 10 mice per group.
GCPII Inhibition Enhances Cognitive Performance in EAE Mice.
We tested whether GCPII inhibition could lead to improved cognition in EAE mice as assessed by Barnes maze performance. After ∼4 wk of treatment, EAE + 2-PMPA and EAE + vehicle demonstrated similar initial learning abilities, with mean primary latency (time before head poke into target box), total latency (time before target box entry), and path efficiency (total distance traveled/distance from starting point to target box) equal between groups on the first 2 d of Barnes maze testing. Trends emerged on day 3, and significant differences between EAE + 2-PMPA and EAE + vehicle mice were observed on day 4. When comparing performance between the first trial of day 4 to baseline (day 1, trial 1), EAE + 2-PMPA mice were significantly different compared with EAE + vehicle mice in primary latency (mean ± SEM: 167.4 ± 1.19 vs. 134.0 ± 10.30, respectively; P < 0.01), total latency (mean ± SEM: 167.4 ± 1.19 vs. 98.42 ± 24.66, respectively; P < 0.05), and path efficiency (mean ± SEM: −0.728 ± 0.030 vs. −0.324 ± 0.107, respectively; P < 0.01) (Fig. 3 B and C), accounting for a 25, 70, and 125% improvement in performance in EAE + 2-PMPA mice. Another research group using the Barnes maze determined that comparison of back-to-back trials on consecutive days (e.g., trial 4 of day 3 vs. trial 1 of day 4) was an indication of long-term memory in mice (19). Using this methodology, 2-PMPA enhanced long-term memory in EAE mice by significantly decreasing total latency (mean ± SEM: 13.14 ± 7.69 vs. −38.82 ± 19.04, respectively; P < 0.05) and increasing path efficiency (mean ± SEM: −0.138 ± 0.094 vs. 0.209 ± 0.098, respectively; P < 0.05) compared with EAE + vehicle mice. Maximum speed in the maze was equal between groups.
To ascertain if 2-PMPA treatment altered fear memory in EAE mice (20), fear conditioning tests were administered following completion of Barnes maze testing. Freezing levels were equal between groups on days 1–3. On day 5, however, mice treated with 2-PMPA had increased cued freezing in response to the 120-s tone, specifically in the last 60 s, indicating a stronger fear memory (Fig. 3D).
2-PMPA Treatment Is Not Anxiolytic in EAE Mice.
To confirm that 2-PMPA’s cognitive-enhancing effects were due to differences in memory and not anxiety, elevated plus maze tests were conducted 4 wk postimmunization in separate cohorts (n = 10). No differences were noted between EAE + 2-PMPA and EAE + vehicle groups regarding the time spent in open arms (mean ± SEM: 34.10 ± 9.22 vs. 35.90 ± 6.99, respectively) and closed arms (mean ± SEM: 224.2 ± 10.07 vs. 225.6 ± 13.65, respectively) of the maze, indicating equal anxiety levels.
2-PMPA Is BBB Penetrable and Alters NAAG in EAE.
Mass spectrometry was used to confirm that 2-PMPA penetrated the CNS and increased brain NAAG levels. 2-PMPA was detected in the cerebellum (mean ± SEM: 0.832 ± 0.056 µg/g tissue), hippocampus (mean ± SEM: 0.835 ± 0.264 µg/g tissue), and frontal cortex (mean ± SEM: 1.193 ± 0.222 µg/g tissue), areas that regulate cognitive function in rodents and humans (21, 22). When measuring NAAG concentrations between EAE + 2-PMPA and EAE + vehicle mice, no difference was detected in the hippocampi (mean ± SEM: 54.98 ± 2.037 vs. 55.54 ± 2.275, respectively), a trend of increase was found in the cerebella (mean ± SEM: 102.4 ± 2.3 vs. 94.23 ± 3.5, respectively; P = 0.0655) and a significant increase was determined in the frontal cortex of EAE + 2-PMPA (mean ± SEM: 32.91 ± 0.93 vs. 26.22 ± 0.8727, respectively; P < 0.001) (Fig. 3E). No differences in NAA levels were detected.
2-PMPA Does Not Alter Lymphocyte Populations in EAE.
Fluorescence-activated cell sorting (FACS) analysis was conducted at the time when cognitive differences were detected between groups, 28 d post-EAE induction, to determine if daily 2-PMPA administration affected immune cell expression. No differences were noted in T lymphocytes in the spleen or brain of EAE + 2-PMPA mice compared with EAE + vehicle mice (Fig. S1).
Hippocampal GCPII Activity Does Not Change in EAE.
Enzymatic activity of GCPII was measured in the hippocampi of control mice and EAE mice treated with either vehicle or 2-PMPA 15 d after immunization. There were no differences detected between control and EAE groups treated with vehicle, indicating that disease state does not affect functional enzymatic activity. GCPII activity in the brains of control and EAE mice treated daily with 2-PMPA did not differ as well. However, within each group, there was a significant decrease in GCPII activity in 2-PMPA–treated mice compared with vehicle-treated mice (Fig. S2).
GCPII Inhibition Has No Effect on Control Mice.
Control (normal, non-EAE) mice treated for at least 4 wk with 2-PMPA did not differ from vehicle-treated normal controls in their Barnes maze, fear conditioning, or elevated plus maze performances. 2-PMPA was detected in the brains of treated mice, but no differences in CNS NAAG levels were observed between control + vehicle and control + 2-PMPA mice.
2-(Phosphonomethyl)Succinic Acid Does Not Affect Cognition and Severity of EAE.
2-(PhosphonoMethyl)Succinic Acid (2-PMSA), a structurally similar but inactive analog of 2-PMPA, was tested in the EAE model to determine if the cognition enhancing effects were specific to GCPII inhibition. Although their structures differ by one methyl group, 2-PMSA is over 1,000-fold less potent for GCPII inhibition (18). Mice were immunized to induce EAE, and daily 2-PMSA treatment did not affect disease severity and elevated plus maze, Barnes maze, and fear conditioning performances (Fig. S3).
Discussion
The present study is unique in suggesting that right hippocampal NAAG/Cr concentrations positively correlate with cognitive function in MS patients. The development of effective treatments for cognitive impairment in MS could benefit over 1 million people worldwide. MS patients with cognitive impairment early in disease progression are more prone to severe learning and memory impairments later in life (23). The average age of MS onset is 20–40 y of age, and MS is the second leading cause of neurological disability in young adults in the United States. An estimated 50–80% of all MS patients are unemployed within 10 y of disease onset (6), and these startlingly high unemployment rates are often attributed to cognitive impairment (24, 25). Therefore, early detection and treatment of cognitive impairment in MS would have a tremendous impact on quality of life.
Based on our initial finding of a positive relationship between hippocampal NAAG/Cr concentration and cognitive function in MS patients, we tested a compound previously demonstrated to increase brain NAAG levels, 2-PMPA (17, 26), in an animal model of MS and measured cognitive performance. Data from the present experiment reveal that up-regulation of CNS NAAG concentration through inhibition of GCPII improves cognitive function in EAE mice. The Barnes maze was used because it is a minimally stressful land maze (27), and EAE mice have impaired performance in this hippocampal-dependent behavioral task compared with healthy wild-type mice (12). Hippocampal degeneration is well documented in MS patients (28), thus making EAE and the Barnes maze the ideal model to study MS-related cognitive impairment. 2-PMPA–mediated GCPII inhibition dramatically enhanced memory, as measured by the Barnes maze and fear conditioning. EAE disease score and maximum maze speed did not differ between EAE + vehicle and EAE + 2-PMPA mice, indicating that the improved performance of 2-PMPA mice in the maze was not due to enhanced physical abilities. To our knowledge, the present study is unique in demonstrating significant amelioration of cognitive function in EAE mice. Historically, potential MS treatments are deemed ineffective and discarded if there is no improvement of EAE disease score. The present study demonstrates that EAE disease score is not the only endpoint for the evaluation of MS therapies and implies the premature abandonment of past treatment options.
The detection of 2-PMPA in the brains of mice treated with the GCPII inhibitor demonstrates that 2-PMPA penetrates the BBB and is responsible for the observed increases in NAAG concentration. The most pronounced increase in NAAG levels occurred in the frontal cortex, which is responsible for executive function and planning of motor movement, two key contributors to successful Barnes maze navigation. The hippocampus also contributes to spatial navigation abilities. Hippocampal NAAG concentrations (right and left) were unaffected by 2-PMPA treatment in EAE mice. We hypothesize that there may be changes in NAAG concentration, activity, and/or turnover in specific regions of the hippocampus (e.g., CA1) (29) that are not detectable in whole fraction analysis. Future studies using more specific analyses of hippocampal substructures are required.
It is noteworthy that 2-PMPA was detected in all brain regions of 2-PMPA–treated control (i.e., normal, non-EAE) mice but did not affect cognitive performance. It is possible that a diseased state is required for 2-PMPA to exert observable, beneficial effects. This conclusion is consistent with studies reporting that GCPII inhibitors have no effect on spatial learning, memory, and basal glutamatergic transmission in normal mice, but selectively mitigate injury-induced changes (18). However, it is possible that administration of a GCPII inhibitor might affect cognitive function in normal mice but was not detectable in our behavior testing paradigms due to the ceiling effect or lack of dose–response testing. Future experiments will investigate this possibility.
The precise mechanism of action of GCPII inhibition in alleviating cognitive impairment in EAE mice is unknown. The substrate and products of GCPII activity are associated with cognitive function and neuronal survival. Availability of extracellular NAA is the rate-limiting feature to NAAG formation in astrocytes (30). Imbalances in NAA concentration, both in cases of elevated NAA (Canavan disease) or hypoacetylaspartia (a single documented human case) (31), lead to cognitive dysfunction. Similarly, insufficient glutamate signaling leads to cognitive dysfunction, whereas excess glutamate signaling is neurotoxic (32). High glutamate concentrations have been observed in the brains (33) and cerebrospinal fluid (34) of MS patients, and excess glutamate signaling has been implicated in a host of neurological disorders including schizophrenia, Parkinson disease, Huntington disease, Alzheimer’s disease, and MS. Whereas decreased glutamate concentration due to GCPII inhibition could certainly contribute to cognitive sparing in EAE, it is hypothesized that NAAG is the crucial signaling messenger that ameliorated cognitive function in the present study. Inhibition of GCPII, which induces a measurable up-regulation in NAAG, has proved to be beneficial in treating various preclinical models of neurological disease (18, 35, 36). Activation of mGluR3 on presynaptic neurons by NAAG decreases glutamate release (37–39), potentially promoting the survival of neurons crucial to cognitive function. Additionally, activation of mGluR3 on astrocytes stimulates the release of transforming growth factor beta, a cytokine with demonstrated in vitro neuroprotective effects (40, 41). It is possible that one or both of these NAAG-mediated pathways regulates cognitive function in MS, requiring future studies to elucidate the exact mechanism. FACS analysis conducted in EAE at the time differences in cognitive function were observed (i.e., 28 d postimmunization) indicate that the spleen and brain lymphocyte populations were not affected by daily 2-PMPA treatment, suggesting that a neural signaling pathway and not immune system regulation is responsible for the improvement of cognitive function in EAE.
The discovery of interdependence between cognitive performance and right but not left hippocampal NAAG concentrations in the present study was unexpected and warrants discussion. Asymmetry of hippocampal size is well documented (right > left) (42, 43), but the roles of right versus left hippocampi in cognitive function are not completely understood. A body of literature is emerging that supports lateralization of hippocampal function. A recent rodent study reported that NAA concentrations positively associate with learning and memory performance, but only in the right hippocampus (44). A human study reported a significant decrease in the level of right hippocampal activity during encoding of successfully recalled items in cognitively impaired MS patients compared with both cognitively preserved MS patients and healthy controls (45). Furthermore, a recent study in healthy volunteers showed a positive correlation between cognitive performance and anterior right but not anterior left hippocampal size (42). Taken together, these studies suggest that the right hippocampus plays a significant role in the maintenance of cognitive function in both healthy subjects and patients with MS. Our results also demonstrate a relationship between cognitive function and the right hippocampus but not the left hippocampus. Whereas others have demonstrated equal bilateral hippocampal volume loss in MS patients over the course of 3–13 y after disease onset (28), our studies focus on metabolite changes in recently diagnosed patients and not gray matter brain structure loss. Therefore, it is possible that the right and left hippocampi have some separate and/or unique functions that may be affected differently by changes in metabolite concentrations.
Neither brain NAAG levels in MS patients nor the relationship of brain NAAG levels to cognition are well defined. To our knowledge only one other MRS study has attempted to examine brain NAAG concentrations in MS patients (46). This study did not measure hippocampal NAAG levels or correlate cognitive function to NAAG levels, making a comparison between studies impossible. A recent preliminary study reported a positive correlation between NAAG concentration and memory in patients with schizophrenia (47). However, the selection of a patient population on atypical antipsychotics, a treatment that up-regulates NAAG production in vitro (48) and improves cognitive function in patients with schizophrenia (49), makes the formation of causative conclusions impossible. Thus, the use of atypical antipsychotics in schizophrenic subjects is a confounding variable that the researchers did not control for, which may have led to a type I error where there is a spurious rather than a causal relationship observed between NAAG levels and cognition.
The present study has limitations that must be addressed. We did not measure hippocampal volume or include healthy controls, making it unclear if there is a bi- or unilateral loss of hippocampal volume in our patient population compared with healthy controls. Additionally, the MS patient population was very small, with only six unique patients included. We applied appropriate statistical corrections to use patient data at two unique time points and applied a more stringent alpha (α = 0.01) to further minimize type I error, but separate baseline and follow-up analyses with larger patient populations are required to confirm the current study. We focused on metabolite measurements in the hippocampi due to reported atrophy in the hippocampi of MS patients compared with healthy controls (50), but other areas of the brain, specifically subcortical gray matter structures, also atrophy in MS patient populations (51). Future human studies will focus on the measurement of metabolite concentrations in deep gray structures, such as the thalamus. Future EAE studies are also required to determine the mechanism of action of GCPII inhibition in cognitive impairment. Because NAAG signaling and GCPII expression are altered in other neurodegenerative diseases, this work also has broad applications to other nondementing CNS disorders. Further work must be done to evaluate the therapeutic potential of GCPII inhibition for the treatment of cognitive impairment in other neurodegenerative disorders.
Materials and Methods
Study Participants.
Participants were a cohort of newly diagnosed MS patients in an ongoing longitudinal study of neuropsychological and neuroimaging outcome measures at the Johns Hopkins Hospital. MS patients who had completed neuropsychological testing and magnetic resonance imaging at a baseline and/or follow-up visit were included in the analyses. Baseline was defined as the visit in which the patient received a positive diagnosis of MS and before any disease-modifying therapy had commenced, whereas follow-up was defined as 6–12 mo following the initial diagnosis. Five MS patients were reviewed at baseline (one male, four female; average age = 44.4 y old) and four participants were reviewed at follow-up (four female; average age = 39.8 y old), three of whom had corresponding baseline data. Of the four follow-up patients, three were on disease-modifying therapies (2 glatiramer acetate, 1 IFNβ-1a). Patient written consent was obtained and procedures were approved by the Johns Hopkins Medicine Institutional Review Board.
Cognitive Testing.
MS patients underwent a modified Minimum Assessment of Cognitive Function In Multiple Sclerosis (MACFIMS) neuropsychological battery (52) at baseline and/or follow-up. Cognitive tests were Symbol Digit Modalities Test, Paced Auditory Serial Addition Test, Verbal Fluency Test, Judgment of Line Orientation, Rey-Osterrieth Complex Figure Copy Test, California Verbal Learning Test II, Brief Visuospatial Memory Test Revised, and the Delis–Kaplan Executive Function System (D-KEFS) Sorting Test.
Magnetic Resonance Spectroscopy.
MR imaging and spectroscopy were performed on a 3-T Philips Intera scanner with a six-channel SENSE head coil as previously described (53). Spectra were analyzed using the LCModel algorithm (54) and quantified in millimolar concentrations using the unsuppressed water signal as an internal intensity reference. Values were only included if Cramér–Rao lower bounds were 50% or less (54).
Animals and Housing.
Seven- to 8-wk-old female C57BL/6 mice were purchased from the National Cancer Institute. All procedures were approved by The Johns Hopkins University Animal Care and Use Committee.
EAE Induction.
Mice were immunized as previously described (55) with the following minor modifications. Mice were immunized with two 50-µL s.c. flank injections, and 300 ng of pertussis toxin was employed on Day 0 and 2.
Treatment Administration and Behavioral Scoring.
Vehicle (50 mM Hepes buffer), 2-PMPA [100 mg/kg in vehicle (pH 7.4) with 10N NaOH], or 2-PMSA [100 mg/kg in vehicle (pH 7.4) with 10N NaOH] was delivered daily via 0.1-mL i.p. injections from the time of EAE immunization until sacrifice. 2-PMPA, 2-PMSA, or vehicle treatment administration on behavior testing days occurred ∼30 min before the first test.
Animals were monitored daily for physical signs of EAE, and scores were assigned as previously described (56).
Barnes Maze.
Mice were immunized to induce EAE and treated daily with either vehicle or 2-PMPA for ∼4 wk and subjected to Barnes maze testing as previously described (57). Paths were recorded and latency to the target hole, path efficiency, speed, and directional times were calculated using ANY-maze software (Stoelting). Different cohorts of mice were tested in 2-PMPA and 2-PMSA studies.
Fear Conditioning.
Following the Barnes maze testing, animals were given 1–2 wk rest then subjected to fear conditioning. Animals were placed in a chamber for 240 s on 3 consecutive days. On day 1, a 2,000-Hz tone was played for 28 s (seconds 180–208), immediately followed by a 2-s scrambled footshock. On day 2, animals were placed back in the same testing environment, but neither sound nor shock was administered. On days 3 and 5, mice were placed in a novel wooden box inside the chamber, and a 2,000-Hz tone was played for 120 s (seconds 121–240). The duration of freezing behavior on each day was measured using FreezeScan software (Clever Sys).
Elevated Plus Maze.
Mice were placed in the center of the maze and given 5 min to freely explore the maze. Time spent in open and closed arms of the maze were tracked using ANY-maze software (Stoelting).
Mass Spectrometry.
Animals were euthanized by cervical dislocation 50 d after EAE immunization and 1 h following the last injection of vehicle or 2-PMPA. Hippocampi, frontal lobe, and cerebellum were dissected, and NAA, NAAG, and 2-PMPA concentrations were measured via mass spectrometry as previously described (58). (Fig. S4).
Perfusion and FACS Immunostaining.
On day 28 postimmunization, mice were anesthetized with isoflurane, spleens were removed, and mice were perfused with HBSS by the intracardiac route to remove circulating leukocytes. Splenocytes and mononuclear cells from the CNS were surface stained with anti-CD4 Peridinin-chlorophyll proteins (PerCP), anti-CD8 Allophycocyanin (APC), anti-CD44 FITC, and anti-CD62L Phycoerythrin (PE) (all from BD Biosciences). Flow cytometry analyses were performed on a FACSCalibur instrument (BD Biosciences) and analyzed using FlowJo software (TreeStar).
GCPII Activity Assay.
Control mice or mice immunized for EAE were treated with vehicle or 2-PMPA for 15 d. Mice were euthanized via cervical dislocation 20 min after their treatment on day 15, and hippocampi were dissected and snap frozen. GCPII activity levels were measured as previously described (58).
Statistical Analyses.
Statistical analyses were completed using GraphPad Prism 5.0 and open source software R. In human studies, Pearson correlations were performed between the metabolites and neurocognitive tests. Generalized estimating equation (GEE) was used to study the association between NAAG and cognitive outcomes and correct for within-subject correlations. Comparisons between groups in the animal studies were made with t tests, whereas two-way ANOVA was completed to measure treatment effects over time. P values <0.01 were considered statistically significant for the human correlation data, whereas P values <0.05 were considered statistically significant in animal data analysis. Statistical support was provided by the Johns Hopkins Institute for Clinical and Translational Research Biostatistics Center.
Supplementary Material
Acknowledgments
The authors thank J. Coughlin, J. Cheng, A. Bakare, A. Riehm, A. Baranowski, and C. Katrinic for their assistance in data collection and technical expertise. This work was supported by the National Institutes of Health Grants K23 MH069702 (to A.I.K.), T32 MH015330 (to K.A.R.), and P41EB015909 (to P.B.B.); the Montel Williams Multiple Sclerosis Foundation; the Nancy Davis Multiple Sclerosis Foundation; the Transverse Myelitis Association; and the Johns Hopkins Brain Science Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209934109/-/DCSupplemental.
References
- 1.Neale JH, et al. Advances in understanding the peptide neurotransmitter NAAG and appearance of a new member of the NAAG neuropeptide family. J Neurochem. 2011;118(4):490–498. doi: 10.1111/j.1471-4159.2011.07338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsukamoto T, Wozniak KM, Slusher BS. Progress in the discovery and development of glutamate carboxypeptidase II inhibitors. Drug Discov Today. 2007;12(17–18):767–776. doi: 10.1016/j.drudis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Lyon L, et al. Fractionation of spatial memory in GRM2/3 (mGlu2/mGlu3) double knockout mice reveals a role for group II metabotropic glutamate receptors at the interface between arousal and cognition. Neuropsychopharmacology. 2011;36(13):2616–2628. doi: 10.1038/npp.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabrese M, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol. 2009;66(9):1144–1150. doi: 10.1001/archneurol.2009.174. [DOI] [PubMed] [Google Scholar]
- 5.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41(5):685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 6.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7(12):1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 7.Benedict RH, Zivadinov R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol. 2011;7(6):332–342. doi: 10.1038/nrneurol.2011.61. [DOI] [PubMed] [Google Scholar]
- 8.Strober LB, et al. Unemployment in multiple sclerosis: The contribution of personality and disease. Mult Scler. 2012;18(5):647–653. doi: 10.1177/1352458511426735. [DOI] [PubMed] [Google Scholar]
- 9.Dutta R, et al. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann Neurol. 2011;69(3):445–454. doi: 10.1002/ana.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emerson MR, Gallagher RJ, Marquis JG, LeVine SM. Enhancing the ability of experimental autoimmune encephalomyelitis to serve as a more rigorous model of multiple sclerosis through refinement of the experimental design. Comp Med. 2009;59(2):112–128. [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkmann V, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277(24):21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 12.Ziehn MO, Avedisian AA, Tiwari-Woodruff S, Voskuhl RR. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab Invest. 2010;90(5):774–786. doi: 10.1038/labinvest.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geurts JJ, et al. MR spectroscopic evidence for thalamic and hippocampal, but not cortical, damage in multiple sclerosis. Magn Reson Med. 2006;55(3):478–483. doi: 10.1002/mrm.20792. [DOI] [PubMed] [Google Scholar]
- 14.Jaarsma D, Veenma-van der Duin L, Korf J. N-acetylaspartate and N-acetylaspartylglutamate levels in Alzheimer’s disease post-mortem brain tissue. J Neurol Sci. 1994;127(2):230–233. doi: 10.1016/0022-510x(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 15.Tsai GC, et al. Reductions in acidic amino acids and N-acetylaspartylglutamate in amyotrophic lateral sclerosis CNS. Brain Res. 1991;556(1):151–156. doi: 10.1016/0006-8993(91)90560-i. [DOI] [PubMed] [Google Scholar]
- 16.Edden RA, Pomper MG, Barker PB. In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 Tesla. Magn Reson Med. 2007;57(6):977–982. doi: 10.1002/mrm.21234. [DOI] [PubMed] [Google Scholar]
- 17.Nagel J, et al. Effects of NAAG peptidase inhibitor 2-PMPA in model chronic pain: Relation to brain concentration. Neuropharmacology. 2006;51(7–8):1163–1171. doi: 10.1016/j.neuropharm.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Slusher BS, et al. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat Med. 1999;5(12):1396–1402. doi: 10.1038/70971. [DOI] [PubMed] [Google Scholar]
- 19.Feng JF, et al. Post-injury administration of NAAG peptidase inhibitor prodrug, PGI-02776, in experimental TBI. Brain Res. 2011;1395:62–73. doi: 10.1016/j.brainres.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23(2):229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 21.Rapoport M, van Reekum R, Mayberg H. The role of the cerebellum in cognition and behavior: A selective review. J Neuropsychiatry Clin Neurosci. 2000;12(2):193–198. doi: 10.1176/jnp.12.2.193. [DOI] [PubMed] [Google Scholar]
- 22.Eichenbaum H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Kujala P, Portin R, Ruutiainen J. The progress of cognitive decline in multiple sclerosis. A controlled 3-year follow-up. Brain. 1997;120(Pt 2):289–297. doi: 10.1093/brain/120.2.289. [DOI] [PubMed] [Google Scholar]
- 24.Morrow SA, et al. Predicting loss of employment over three years in multiple sclerosis: Clinically meaningful cognitive decline. Clin Neuropsychol. 2010;24(7):1131–1145. doi: 10.1080/13854046.2010.511272. [DOI] [PubMed] [Google Scholar]
- 25.Rao SM, et al. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology. 1991;41(5):692–696. doi: 10.1212/wnl.41.5.692. [DOI] [PubMed] [Google Scholar]
- 26.Thomas AG, Bodner A, Ghadge G, Roos RP, Slusher BS. GCP II inhibition rescues neurons from gp120IIIB-induced neurotoxicity. J Neurovirol. 2009;15(5-6):449–457. doi: 10.3109/13550280903350598. [DOI] [PubMed] [Google Scholar]
- 27.Barnes CA. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 28.Sicotte NL, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131(Pt 4):1134–1141. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- 29.Bergeron R, Imamura Y, Frangioni JV, Greene RW, Coyle JT. Endogenous N-acetylaspartylglutamate reduced NMDA receptor-dependent current neurotransmission in the CA1 area of the hippocampus. J Neurochem. 2007;100(2):346–357. doi: 10.1111/j.1471-4159.2006.04253.x. [DOI] [PubMed] [Google Scholar]
- 30.Gehl LM, Saab OH, Bzdega T, Wroblewska B, Neale JH. Biosynthesis of NAAG by an enzyme-mediated process in rat central nervous system neurons and glia. J Neurochem. 2004;90(4):989–997. doi: 10.1111/j.1471-4159.2004.02578.x. [DOI] [PubMed] [Google Scholar]
- 31.Boltshauser E, et al. Follow-up of a child with hypoacetylaspartia. Neuropediatrics. 2004;35(4):255–258. doi: 10.1055/s-2004-821036. [DOI] [PubMed] [Google Scholar]
- 32.Rahn KA, Slusher BS, Kaplin AI. Glutamate in CNS neurodegeneration and cognition and its regulation by GCPII inhibition. Curr Med Chem. 2012;19(9):1335–1345. doi: 10.2174/092986712799462649. [DOI] [PubMed] [Google Scholar]
- 33.Baranzini SE, et al. Genetic variation influences glutamate concentrations in brains of patients with multiple sclerosis. Brain. 2010;133(9):2603–2611. doi: 10.1093/brain/awq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarchielli P, Greco L, Floridi A, Floridi A, Gallai V. Excitatory amino acids and multiple sclerosis: Evidence from cerebrospinal fluid. Arch Neurol. 2003;60(8):1082–1088. doi: 10.1001/archneur.60.8.1082. [DOI] [PubMed] [Google Scholar]
- 35.Majer P, et al. Synthesis and biological evaluation of thiol-based inhibitors of glutamate carboxypeptidase II: Discovery of an orally active GCP II inhibitor. J Med Chem. 2003;46(10):1989–1996. doi: 10.1021/jm020515w. [DOI] [PubMed] [Google Scholar]
- 36.Ghadge GD, et al. Glutamate carboxypeptidase II inhibition protects motor neurons from death in familial amyotrophic lateral sclerosis models. Proc Natl Acad Sci USA. 2003;100(16):9554–9559. doi: 10.1073/pnas.1530168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wroblewska B, Wegorzewska IN, Bzdega T, Olszewski RT, Neale JH. Differential negative coupling of type 3 metabotropic glutamate receptor to cyclic GMP levels in neurons and astrocytes. J Neurochem. 2006;96(4):1071–1077. doi: 10.1111/j.1471-4159.2005.03569.x. [DOI] [PubMed] [Google Scholar]
- 38.Manzoni O, Prezeau L, Sladeczek F, Bockaert J. Trans-ACPD inhibits cAMP formation via a pertussis toxin-sensitive G-protein. Eur J Pharmacol. 1992;225(4):357–358. doi: 10.1016/0922-4106(92)90112-9. [DOI] [PubMed] [Google Scholar]
- 39.Schoepp DD, Johnson BG, Monn JA. Inhibition of cyclic AMP formation by a selective metabotropic glutamate receptor agonist. J Neurochem. 1992;58(3):1184–1186. doi: 10.1111/j.1471-4159.1992.tb09381.x. [DOI] [PubMed] [Google Scholar]
- 40.D’Onofrio M, et al. Neuroprotection mediated by glial group-II metabotropic glutamate receptors requires the activation of the MAP kinase and the phosphatidylinositol-3-kinase pathways. J Neurochem. 2001;78(3):435–445. doi: 10.1046/j.1471-4159.2001.00435.x. [DOI] [PubMed] [Google Scholar]
- 41.Thomas AG, et al. Neuroprotection mediated by glutamate carboxypeptidase II (NAALADase) inhibition requires TGF-beta. Eur J Pharmacol. 2001;430(1):33–40. doi: 10.1016/s0014-2999(01)01239-0. [DOI] [PubMed] [Google Scholar]
- 42.Woolard AA, Heckers S. Anatomical and functional correlates of human hippocampal volume asymmetry. Psychiatry Res. 2012;201(1):48–53. doi: 10.1016/j.pscychresns.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: Meta-analyses of MRI studies. Hippocampus. 2009;19(11):1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- 44.Xi G, et al. Learning and memory alterations are associated with hippocampal N-acetylaspartate in a rat model of depression as measured by 1H-MRS. PLoS ONE. 2011;6(12):e28686. doi: 10.1371/journal.pone.0028686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hulst HE, et al. Functional adaptive changes within the hippocampal memory system of patients with multiple sclerosis. Hum Brain Mapp. 2012;33(10):2268–2280. doi: 10.1002/hbm.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vrenken H, et al. MR spectroscopic evidence for glial increase but not for neuro-axonal damage in MS normal-appearing white matter. Magn Reson Methad. 2005;53(2):256–266. doi: 10.1002/mrm.20366. [DOI] [PubMed] [Google Scholar]
- 47.Jessen F, et al. N-acetylaspartylglutamate (NAAG) and N-acetylaspartate (NAA) in patients with schizophrenia. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arun P, Madhavarao CN, Moffett JR, Namboodiri AM. Antipsychotic drugs increase N-acetylaspartate and N-acetylaspartylglutamate in SH-SY5Y human neuroblastoma cells. J Neurochem. 2008;106(4):1669–1680. doi: 10.1111/j.1471-4159.2008.05524.x. [DOI] [PubMed] [Google Scholar]
- 49.Riedel M, et al. Neurocognition and its influencing factors in the treatment of schizophrenia-effects of aripiprazole, olanzapine, quetiapine and risperidone. Hum Psychopharmacol. 2010;25(2):116–125. doi: 10.1002/hup.1101. [DOI] [PubMed] [Google Scholar]
- 50.Anderson VM, et al. Hippocampal atrophy in relapsing-remitting and primary progressive MS: A comparative study. Mult Scler. 2010;16(9):1083–1090. doi: 10.1177/1352458510374893. [DOI] [PubMed] [Google Scholar]
- 51.Bergsland N, et al. Subcortical and cortical gray matter atrophy in a large sample of patients with clinically isolated syndrome and early relapsing-remitting multiple sclerosis. AJNR Am J Neuroradiol. 2012;33(8):1573–1578. doi: 10.3174/ajnr.A3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benedict RH, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS) J Int Neuropsychol Soc. 2006;12(4):549–558. doi: 10.1017/s1355617706060723. [DOI] [PubMed] [Google Scholar]
- 53.Mohamed MA, et al. Brain metabolism and cognitive impairment in HIV infection: A 3-T magnetic resonance spectroscopy study. Magn Reson Imaging. 2010;28(9):1251–1257. doi: 10.1016/j.mri.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 55.Jones MV, et al. Behavioral and pathological outcomes in MOG 35-55 experimental autoimmune encephalomyelitis. J Neuroimmunol. 2008;199(1-2):83–93. doi: 10.1016/j.jneuroim.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Aheng C, et al. Deletion of UCP2 in iNOS deficient mice reduces the severity of the disease during experimental autoimmune encephalomyelitis. PLoS ONE. 2011;6(8):e22841. doi: 10.1371/journal.pone.0022841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berta S, Gert L, Harald H, Sudarshan P. Barnes maze, a useful task to assess spatial reference memory in the mice. Available at http://dx.doi.org/10.1038/nprot.2007.390. 2007 [Google Scholar]
- 58.Rojas C, et al. Glutamate carboxypeptidase activity in human skin biopsies as a pharmacodynamic marker for clinical studies. J Transl Med. 2011;9:27. doi: 10.1186/1479-5876-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.