Abstract
Background : Imbalance of glutamatergic neurotransmission has been proposed as a key mechanism underlying symptoms of schizophrenia. The neuropetide N-acetylaspartylglutamate (NAAG) modulates glutamate release. NAAG provides a component of the proton magnetic resonance spectrum (1H-MRS) in humans. The signal of NAAG, however, largely overlaps with its precursor and degrading product N-acetylaspartate (NAA) that by itself does not act in glutamatergic neurotransmission. Methods: We quantified NAAG and NAA separately from the 1H-MRS signal in 20 patients with schizophrenia and 20 healthy comparison subjects on a 3.0 Tesla MR scanner. The 1H-MRS voxels were positioned in the anterior cingulate cortex (ACC) and in the left frontal lobe. Psychopathological symptoms and cognitive performance were assessed. Results: In the ACC, the ratio NAAG/NAA was increased (P = .041) and NAAG was increased at a trend level (P = .066) in patients, while NAA was reduced (P = .030). NAA correlated with attention performance in patients (r = .64, P = .005) in the ACC. There was no group difference of NAAG, NAA, or NAAG/NAA in the frontal lobe but an inverse correlation of NAAG with negatives symptoms (Positive and Negative Symptoms Scale [PANSS] negative, r = −.58, P = .018) and with the total symptom score (PANSS total, r = −.50, P = .049). In addition, there was a positive correlation of frontal lobe NAAG (r = .53, P = .035) and NAAG/NAA (r = .54, P = .030) with episodic memory in patients. Conclusions: In this study, we present the first in vivo evidence for altered NAAG concentration in patients with schizophrenia.
Keywords: schizophrenia, N-acetylaspartate, N-acetylasp-artylglutamate, magnetic resonance spectroscopy, anterior cingulate gyrus, frontal lobe
Introduction
The “glutamatergic hypothesis “ of schizophrenia originates from the induction of schizophrenia-like symptoms in animals and volunteers by glutamate release stimulating compounds such as the N-methyl-D-aspartate receptor (NMDAR) antagonists phencyclidine (PCP) and ketamine.1 The presynaptic metabotropic glutamate receptor 3 (mGluR3) inhibits glutamate release. The mGluR2/3 agonist LY404039 reverses PCP induced behavior in animals and has shown clinical effects by reducing psychotic symptoms in patients with schizophrenia.2 The neuropeptide N-acetylaspartylglutamate (NAAG) is an endogenous agonist of the mGluR3.3,4 Inhibition of the NAAG degrading enzymes glutamate carboxypeptidase II (GCP II) and III is associated with a reduction of schizophrenia-like symptoms in animal models.5,6 This qualifies inhibitors of NAAG degrading enzymes as novel drug candidate for the treatment of schizophrenia.7 Despite the proposed relevance of NAAG in pathophysiology and treatment, it is still unknown, whether NAAG levels in patients with schizophrenia are actually altered. Today only postmortem investigations are available, which provide evidence for both, increased and reduced NAAG concentration.8,9
The proton magnetic resonance signal of the methyl group of NAAG corresponds to a component at 2.04 ppm in cerebral spectra of humans in vivo. However, the signal of NAAG strongly overlaps with the signal of the methyl group of its precursor and degrading product N-acetylaspartate (NAA). Despite the narrow spectral gap between the 2 peaks, they can clearly be separated at high field strength in vitro.10 In human studies in vivo, only the combined [NAA+NAAG] peak is usually measured and reported.
There are numerous reports on the combined [NAA+NAAG] peak in schizophrenia. Common observations are signal reductions in the frontal lobe and in the anterior cingulate cortex (ACC).11–13 The reduction of the combined [NAA+NAAG] signal is conventionally interpreted as an indicator of reduced NAA concentration only because NAA is highly concentrated in the brain. NAA levels are considered to reflect mitochondrial function of neurons because the synthesis of NAA is exclusively located in neurons and NAA concentration correlates with adenosine diphosphate levels.14,15 The metabolic role of NAA in the nervous system is not fully understood. Involvement in myelination, osmoregulation, and axon-glial signaling has been proposed.15 NAA does not act in neurotransmission. Thus, the separation of the NAAG from the NAA signal is required to study NAAG as a component of glutamatergic neurotransmission.
To date, 2 1H-MRS studies with separate measures of NAAG and NAA in healthy volunteer have been reported. These showed that the combined [NAA+NAAG] peak is composed of roughly 90% NAA and 10% NAAG. Considerably, higher NAAG fractions were observed in parietal and occipital white matter.16,17
In the present 1H-MRS study, we separately quantified NAAG and NAA and determined the NAAG/NAA ratio in patients with schizophrenia and compared these measures with those of healthy individuals. We also assessed the correlation of NAAG, NAA, and NAAG/NAA with cognitive measures and psychopathological symptoms. We chose the left frontal lobe and the ACC as the target regions as both are considered to be functionally altered in schizophrenia.
Methods
Subjects
We recruited 20 patients with schizophrenia according the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) from the inpatient unit of the Department of Psychiatry and Psychotherapy, University of Bonn. All were on stable atypical antipsychotic medication (11 on monotherapy and 9 on combination therapy) and in partial or full remission with regard to positive symptoms. The mean time since first episode was 7.2 years (SD: 8.5). Current symptoms were assessed with the Positive and Negative Symptoms Scale (PANSS).18 Demographic data and PANSS scores are listed in table 1.
Table 1.
Demographic Data and Neuropsychological Measures (Mean and SD)
| Patients (n = 20) | Controls (n = 20) | Difference | |
| Sex (m/f) | 14/6 | 11/9 | P = .514 |
| Age (years) | 34.5 (10.2) | 30.7 (9.1) | P = .217 |
| PANSS positive | 10.8 (3.5) | ||
| PANSS negative | 13.9 (3.9) | ||
| PANSS general | 26.4 (6.2) | ||
| PANSS total | 51.0 (11.3) | ||
| VLMT, immediate recall (trial 1–5) | 50.1 (11.5) | 61.9 (7.6) | P = .001 |
| VLMT, delayed recall (trial 6) | 9.3 (3.2) | 13.9 (1.1) | P < .001 |
| VLMT, recognition (trail 7) | 11.8 (3.7) | 14.1 (1.8) | P = .017 |
| Digit span | 13.1 (4.3) | 19.1 (2.9) | P < .001 |
| Block span | 13.5 (3.4) | 17.9 (2.8) | P < .001 |
| Letter-number-sequencing | 13.6 (4.2) | 18.5 (3.2) | P < .001 |
| DSST | 46.1 (13.8) | 69.0 (7.1) | P < .001 |
| TMT A (s) | 36.5 (19.6) | 25.2 (10.4) | P = .032 |
| TMT B (s) | 76.9 (42.6) | 50.9 (17.6) | P = .018 |
| Composite episodic memory score | −0.66 (0.96) | 0.56 (0.48) | P < .001 |
| Composite working memory score | −0.67 (0.86) | 0.47 (0.61) | P < 0.001 |
| Composite attention score | −0.54 (1.04) | 0.46 (0.49) | P < 0.001 |
Note: PANSS, Positive and Negative Symptoms Scale; VLMT, Auditory Verbal Learning and Memory Test; DSST, Digit symbol substitution test; TMT, Trail making test.
Twenty comparison subjects were recruited from the general population by advertisement. None of these control subjects suffered from any psychiatric or neurological disorder at present or in the past or was taking any medication. The demographic data are listed in table 1.
All participants gave written informed consent prior to the study. The protocol was approved by the local ethical committee.
Neuropsychological Assessment
A neuropsychological test battery was applied in all participants to correlate cognitive performance with 1H-MRS data. The battery included the Auditory Verbal Learning and Memory Test (AVLT),19 the digit span test 20 and block span test 21 of the revised Wechsler Memory Scale, the letter-number-sequencing test,22 the digit symbol substitution test (DSST),20 and the trail making test (TMT) A and B.23
We created composite scores for 3 cognitive domains by averaging z-transformed raw scores. These composite scores were (1) a verbal memory score derived from AVLT trials 1 to 5, 6, and 7 (Cronbach Alpha: .95), (2) a working memory score derived from the digit span test, the block span tests, and the letter-number-sequencing test (Cronbach Alpha: .94), and (3) an attention score composed of TMT A, TMT B, and DSST (Cronbach Alpha: .92). All neuropsychological raw data and the composite scores are listed in table 1.
Proton MR-Spectroscopy (1H-MRS)
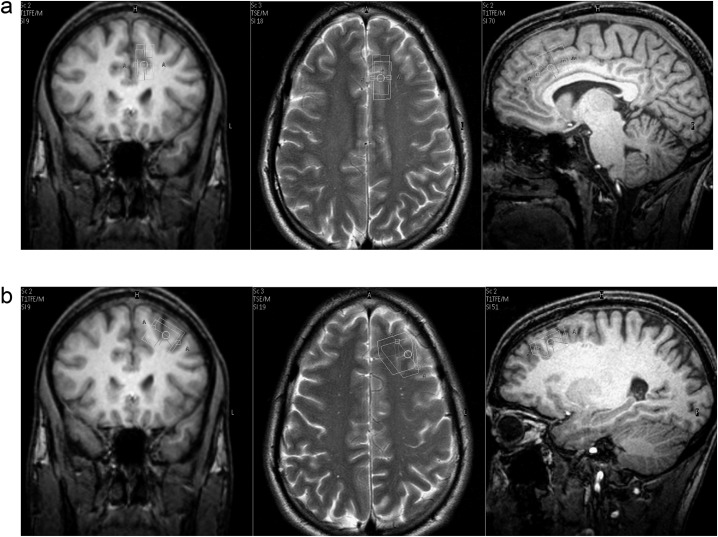
The single voxel 1H-MRS study was performed on a 3.0 Tesla MR scanner (Achieva 3.0, Philips Healthcare) using a 12-element birdcage quadrature head coil (diameter 28 cm and length 23 cm) for signal transmission and reception. We positioned one voxel within the ACC (6-ml voxel size) and one voxel within the left frontal lobe (8-ml voxel size) (figure 1). The ACC voxel, placed just superior to the genu of the corpus callosum and parallel to its surface, was constrained to the left hemisphere to reduce susceptibility broadening of the spectral lines caused by inclusion of the hemispheric fissure. As a consequence, the lateral extension was reduced to only 12 mm to avoid spatial overlap with the other voxel. The second voxel was positioned in the medial lateral part of the left frontal lobe. Patient movement was restrained by foam pads lateral to the head and by fixing the forehead with Velcro straps, and the magnetic resonance imaging (MRI) scans used for voxel planning were repeated after completion of the MRS acquisitions to detect possible shifts of the head position. Data of sufficient quality from the frontal lobe voxel were only obtained in 18 patients, while in the other 2 cases, the spectral data from this region had to be discarded due to patient movement.
Fig. 1.
Position of the 1H-MRS voxels in the (a) anterior cingulate cortex and the (b) left frontal lobe.
Water-suppressed 1H-MR spectra with a repetition time (TR) of 2000 ms and echo times (TE) of 30 ms and 140 ms were obtained from these voxels using image-guided PRESS localization. NAAG and NAA measures were taken from the spectra at TE 140 ms because at TE 30 ms, the overlapping signal of the 3CH2 protons of glutamate (Glu) and glutamine (Gln) in the range 2.00–2.07 ppm contributes about 10% to the peak as determined by density-matrix calculations with the nuclear magnetic resonance (NMR)-spectra calculation using operators (SCOPE) (NMR-SCOPE) module of the magnetic resonance user interface (MRUI) software package.24 At the longer TE, this signal contribution was calculated to be less than 3% at the NAAG position due to dephasing of the coupled spin system of glutamate/glutamine, and it is further slightly reduced by the shorter T2 relaxation time of these components. These calculations were confirmed by phantom experiments using model solutions containing NAA, Glu, and Gln in concentrations approximately twice the cerebral values but with concentration ratios 1:0.75:0.3 closely matching the in vivo ratios for human brain.25 While at TE 30 ms, the Glx component at 2.08 ppm indeed has a 16% peak area relative to NAA with considerable residue at the NAAG frequency of 2.04 ppm and at TE 80 ms, the residual Glx signal at 2.05 ppm has already decreased to 3% of NAA intensity and is no longer detectable above noise at the TE of 140 ms (figure 2a).
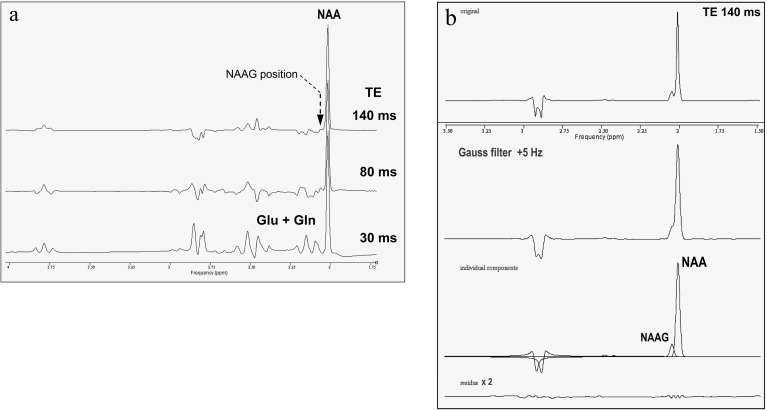
Fig. 2.
1H-MRS spectra from phantoms containing model solutions of (a) NAA (25 mmol/l), glutamate (19 mmol/l), and glutamine (8 mmol/l). At a TE of 140 ms (top row), no spectral components from Glu or Gln are detectable at the position of the NAAG methyl resonance (arrow), whereas at shorter TE, spectral overlap with these components is considerable. (b) NAA (25 mmol/l) and NAAG (3 mmol/l). Additional Gaussian broading of the TE 140 ms spectrum by 5 Hz was applied to match in vivo conditions and increased the original linewidths of NAA and NAAG from 2.1 Hz (top row) to 3.5 Hz (middle row). Nominal NAAG/NAA ratio 0.12 was well reproduced also in the line broadened spectrum.
In vivo spectra were sampled with 1024 data points at 2-kHz bandwidth, yielding a noninterpolated frequency resolution of 2 Hz/pt and were averaged over 128 signal acquisitions. Signal averaging was performed online but possible line broadening and phase shifts caused by patient movement during the MRS scan were suppressed by measuring the Larmor frequency in each TR interval for drift correction. Absolute metabolite concentrations in mmol/l brain tissue were determined by referencing the [NAA+NAAG] signal to the internal tissue water signal from unsuppressed 1H-MRS, immediately accomplished with 32 signal averages after acquiring the TE 140-ms spectrum. These acquisitions of both [NAA+NAAG] and water signal were performed with TR 4000 ms and an intermediate TE of 80 ms to minimize errors due to T1 saturation, T2 relaxation, and underlying glutamate/glutamine 3CH2 components in the metabolite spectra. The metabolite relaxation times for frontal lobe and ACC at 3.0 T required in the extrapolation to TE 0 were taken from earlier data,26 as T2 values for [NAA+NAAG] we used 279 ms (frontal lobe) and 254 ms (ACC), respectively. Further, it was assumed that the relaxation times of the acetyl moiety in NAA and NAAG are not significantly different.17 The absolute concentrations of [NAA+NAAG] were then corrected for partial cerebro spinal fluid (CSF) volume, which was obtained from biexponential 4-parameter fitting of the T2 values and the relative fractions of brain tissue water and CSF to an additional series of unsuppressed spectra, with TR 6000 ms and TE increasing in 7 steps from 35 to 800 ms. The concentrations of the other metabolites choline compounds (Cho), total creatine ((P)Cr), and myo-inositol (MI) were derived by referencing against [NAA+NAAG] in the water-suppressed spectra obtained at TE 140 ms (Cho, (P)Cr) and at TE 30 ms (MI), respectively. For MI, an effective T2 of 75 ms was used in the extrapolation from TE 30 ms to 0 TE taking into account the modeled signal decay due to dephasing of the J-coupled C1/C3 and C4/C6 protons as well as T2 relaxation.
The metabolic ratios Glx/(P)Cr and Gln/(P)Cr were determined from the short-TE spectra at 30 ms without relaxation correction, with Glx defined as the summation over the overlapping glutamate/glutamine/GABA-CH2 components in the 2.1- to 2.4-ppm frequency range and Gln taken from the 4CH2 resonance of glutamine at 2.47 ppm. Table 2 lists the respective 1H-MRS sequences at with which the metabolite concentrations and ratios were determined.
Table 2.
1H-MRS Sequences Used for Quantification of Metabolite Concentrations
| TR (ms) TE (ms) | 2000 30 ws | 2000 140 ws | 4000 80 us | 6000 35/60/100/140/280/500/800 us |
| [NAA+NAAG] | x | |||
| [NAAG] | x | |||
| [NAA] | x | |||
| NAAG/NAA | x | |||
| [Cho] | x | |||
| [(P)Cr | x | |||
| [MI] | x | |||
| Glx/(P)Cr | x | |||
| Gln/(P)Cr | x | |||
| T2 (H2O) and % CSF in MRS voxel | x |
Note: NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; Cho, Choline compounds; (P)Cr, Total creatine; MI, Myo-inositol; Glx, Glutamate/glutamine/GABA-CH2; Gln, Glutamine (ws = H2O-suppressed, us = unsuppressed).
Spectra were preprocessed by apodization with matched Lorentz-Gauss filtering (5-Hz Gaussian broadening followed by −6-Hz Lorentzian narrowing) and time-domain quantified using the advanced method for accurate, robust and efficient spectral fitting (AMARES) algorithm of the MRUI software package.24,27 Mean full with half maximum (FWHM) linewidth (after apodization) of the NAA methyl resonance in the long-TE spectra was 4.4 Hz for the frontal lobe voxel and 4.2 Hz in the ACC, thus allowing independent fitting of the NAAG component separated from NAA by 4.6 Hz at 3.0 T (figure 3). This frequency spacing was introduced as fixed value in the AMARES fit, and the linewidths of both components were kept equal. To improve the reliability of the results, only spectra with resulting NAA linewidths less than 6 Hz and the small NAAG component fitted by AMARES with an accuracy (Cramér-Rao lower bounds [CRLB]) better than 50% were further included in the evaluation of NAAG/NAA ratios and NAAG and NAA concentrations. Following both of these criteria, the NAAG data from both voxels in one patient and from the frontal lobe voxel of one control had to be omitted. NAAG could be determined in both target regions with mean CRLB of only 13%. Because the CRLB values for NAA were less than 2.5% in all cases (mean value 1.4%), also the accuracy of the ratios NAAG/NAA determined by error propagation is almost entirely defined by the CRLB of NAAG.
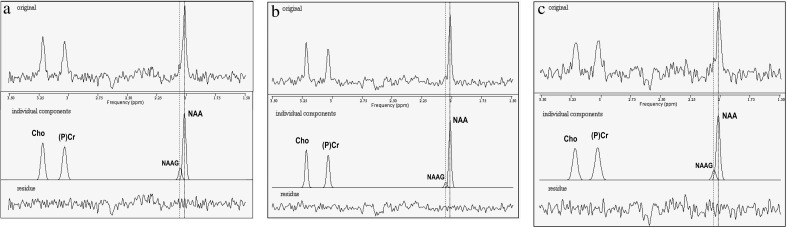
Fig. 3.
Examples of 1H-MRS spectra (TE = 140 ms) from (a) the anterior cingulate cortex (ACC) of a patient with (from top to bottom) the raw spectrum, the fitted components, and the residues. The frequency positions of the methyl resonances of NAAG (2.04 ppm) and NAA (2.01 ppm) are marked with dashed and dotted lines, respectively. The linewidth (FWHM) of NAAG and NAA was 3.8 Hz and the ratio NAAG/NAA determined by the AMARES fit was 0.15. Signal-to-noise ratio was 5.3 for NAAG and 32.1 for NAA in the raw spectrum, and fitting accuracy (CRLB) of NAAG and NAA were 8% and 1.3%, respectively. (b) The ACC of a healthy control. NAAG and NAA linewidth was 3.4 Hz in this case, and the NAAG/NAA ratio was 0.08. (c) The left frontal lobe of a patient, with NAAG and NAA linewidth of 4.7 Hz and a NAAG/NAA ratio of 0.14.
The reliability of the separate determination of NAAG and NAA concentrations by the applied quantification method was also tested in phantom experiments using aqueous solutions with 3 mmol/l NAAG and 25 mmol/l NAA. While phantom T1 (1280 ms for NAA) resembled the in vivo values,25 phantom T2 was much longer (>750 ms for both NAAG and NAA) and measured NAAG/NAA ratios reproduced the nominal concentration ratio 0.12 within 7% at all TE 30/80/140 ms. CRLB of NAAG and NAA were 3.2% and 0.5%, respectively, at TE 140 ms and did not increase after additional 5-Hz Gaussian broadening to match in vivo linewidths (figure 2b).
Statistics
We compared all metabolic concentrations and ratios between patients and comparison subjects with a MANOVA including all metabolic concentrations and ratios. In addition, we tested the correlations of the concentrations of NAAG and NAA and of the metabolic ratio NAAG/NAA with the PANSS subscores in patient group with the Pearson correlation coefficient. The correlations of the concentrations of NAAG and NAA and of the metabolic ratio NAAG/NAA with the 3 cognitive composite scores were tested for both groups separately by Pearson correlation. All analyses were performed for each 1H-MRS voxel separately.
Results
Means and SDs of the absolute concentrations of NAA, NAAG, Cho, (P)Cr, and MI and of the ratios NAAG/NAA, Glx/(P)Cr, and Gln/(P)Cr from the patients and the comparison subjects are listed in table 3.
Table 3.
1H-MRS Measures (Mean, SD)
| Patients | Controls | Difference | |
| Anterior cingulate gyrus | |||
| NAA+NAAG (mmol/l) | 11.42 (1.02) | 11.83 (0.81) | n.s. |
| NAAG (mmol/l) | 1.36 (0.55) | 1.05 (0.44) | P = 0.067 |
| NAA (mmol/l) | 10.16 (0.91) | 10.77 (0.79) | P = .030 |
| NAAG/NAA | 0.14 (0.06) | 0.10 (0.04) | P = .046 |
| Cho (mmol/l) | 2.14 (0.27) | 1.99 (0.21) | n.s. |
| (P)Cr (mmol/l) | 9.73(1.09) | 9.47 (0.85) | n.s. |
| MI (mmol/l) | 5.17 (0.71) | 5.34 (0.60) | n.s. |
| Glx/(P)Cr | 2.11 (0.30) | 2.06 (0.33) | n.s. |
| Gln/(P)Cr | 0.28 (0.08) | 0.25 (0.08) | n.s. |
| Left frontal lobe | |||
| NAA+NAAG (mmol/l) | 11.54 (0.53) | 11.60 (0.88) | n.s. |
| NAAG (mmol/l) | 0.99 (0.33) | 0.99 (0.49) | n.s |
| NAA (mmol/l) | 10.58 (0.59) | 10.64 (1.02) | n.s |
| NAAG/NAA, | 0.10 (0.05) | 0.09 (0.05) | n.s |
| Cho (mmol/l) | 1.96 (0.21) | 1.87 (0.22) | n.s. |
| (P)Cr (mmol/l) | 9.05 (0.91) | 8.98 (0.69) | n.s. |
| MI (mmol/l) | 4.96 (0.91) | 5.10 (0.68) | n.s. |
| Glx/(P)Cr | 1.93 (0.23) | 1.89 (0.25) | n.s. |
| Gln/(P)Cr | 0.24 (0.06) | 0.24 (0.05) | n.s. |
Note: NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; Cho, Choline compounds; (P)Cr, Total creatine; MI, Myo-inositol; Glx, Glutamate/glutamine/GABA-CH2; Gln, Glutamine. The statistical methods are reported in the text. NAA is reduced in patients and the ratio NAAG/NAA is increased. NAAG is increased in patients at a trend level.
Anterior Cingulate Cortex
The MANOVA over all metabolic measures revealed a significant group effect (P = .044). A significant increase in patients was observed for the ratio NAAG/NAA (P = .041) and the NAAG concentration at a statistical trend level (P = .066). NAA in the patient was reduced (P = .030). The other metabolic concentrations or metabolic ratios did not differ between both groups. None of the PANSS subscores correlated significantly with the concentration of either NAAG or NAA or with the metabolic ratio NAAG/NAA. The attention composite score correlated with the concentration of NAA (r = .64, P = .005) in patients. In the comparison subjects, there was no significant correlation of the concentrations of NAAG and NAA or the ratio NAAG/NAA with cognitive measures.
Frontal Lobe
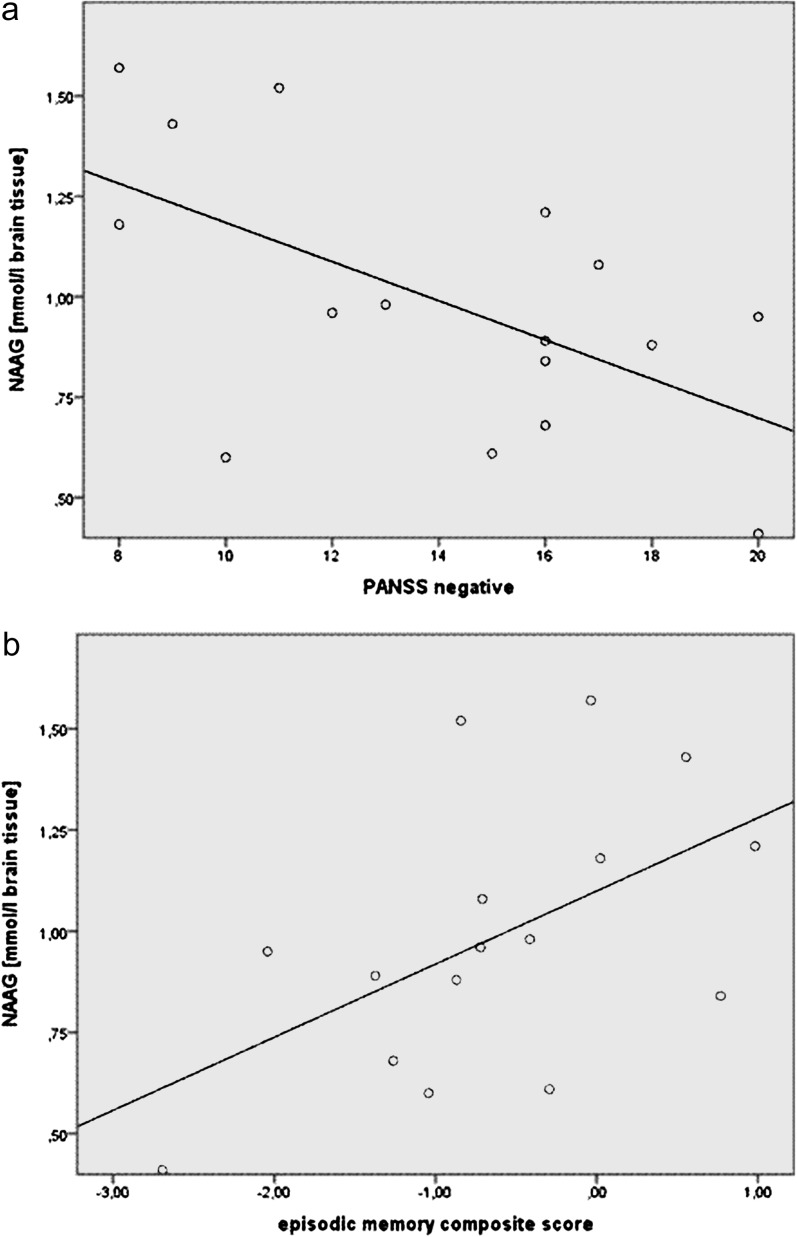
The group effect of the MANOVA was not significant, and there were no differences in any metabolic concentration or metabolic ratio between both groups within the frontal lobe voxel. In patients, the concentration of NAAG correlated inversely with the PANSS negative scale (r = −.58, P = 0.018) (figure 4) and with the PANSS total score (r = −.50, P = .049). In patients, there was a positive correlation of the episodic memory composite score with the concentration of NAAG (r = .53, P = .035) and with the ratio NAAG/NAA (r = .54, P = .030). In the comparison subjects, there was no significant correlation of the concentrations of NAAG and NAA or the ratio NAAG/NAA with any cognitive measure.
Fig. 4.
Correlation of NAAG in the frontal lobe with (a) PANSS negative scores and (b) the episodic memory score in the patient group.
Discussion
The determination of NAAG concentration is crucial for gaining further insight into the glutamatergic dysfunction in schizophrenia and for assessing modes of action of novel antipsychotic drugs candidates.7 In this first 1H-MRS report on NAAG in patients with schizophrenia, we found an increased NAAG/NAA ratio and a trend toward an increased concentration of NAAG in the ACC. In the left frontal lobe, we observed an inverse correlation of NAAG concentration with negative symptoms and a positive correlation of NAAG with episodic memory in patients. Our data contribute to earlier postmortem studies that delivered partly controversial results. Tsai et al8 found increased NAAG in the hippocampus of patients with schizophrenia but not in the cingulate cortex or the frontal lobe. Nudmamud et al9 reported reduced NAAG levels in the superior temporal gyrus in patients and no difference in the frontal lobe compared with a control sample. In recent postmortem gene expression studies, mGluR3 was lower in the prefrontal cortex in patients,28 while the concentration of the NAAG degrading enzyme GCP II has been found to be both higher and lower, suggesting altered regulation of NAAG levels.29,30
Based on the ameliorating effect of inhibitors of the NAAG degrading enzyme GCP II on schizophrenia-like symptoms in animals5,6 and the antipsychotic efficacy of the mGluR2/3 agonist LY404039 in humans,2 reduced NAAG concentration has been proposed as a pathological condition associated with symptoms of schizophrenia in vivo.28,30
Recent experiments in neuroblastoma cells revealed that antipsychotic treatment may increase NAAG concentration, suggesting reversal of low NAAG in patients by antipsychotic treatment.31 This may explain increased NAAG levels that have been observed in postmortem brain tissue of patients with schizophrenia.8 The effects of antipsychotics in patients may also explain the increased NAAG levels within the ACC observed in the present study because all were on stable antipsychotic treatment and in partial or full remission with regard to positive symptoms. High NAAG concentration in the frontal lobe correlated with less negative symptoms and with better memory performance within the group of patients, suggesting beneficial effects of high NAAG levels. This finding supports the concept of increasing NAAG as a potential treatment approach that is derived from animal and human studies.2,5,6
In a recent publication, the role of NAAG as an agonist at the mGluR3 has been questioned.32 While the authors did not observe a direct effect of NAAG on the mGluR3, they did report modulatory effects of NAAG on the mGluR3 response to glutamatergic stimulation. This highlights the need for refined characterization of the action of NAAG in the glutamatergic system. The relevance of NAAG for schizophrenia, which is supported by different lines of evidence, however, is not generally questioned by the uncertainty on the interplay of NAAG, mGluR3, and glutamate.
NAA was reduced in the ACC in patients with schizophrenia in the present study. This is in agreement with many but not all 1H-MRS studies that report a reduction of the combined [NAA+NAAG] peak.13 In addition, we found a correlation of NAA in the ACC with attentional performance in the patient group. As NAA indicates the status of neuronal mitochondria, the correlation of NAA with performance in attention tasks in patients is in agreement with reports on the crucial role of the ACC in attention modulation in schizophrenia.33,34
The increase of NAAG and the decrease of NAA as well as the significant difference of the NAAG/NAA ratio between patients and comparison subjects in the ACC in our study must raise caution, when interpreting the 1H-MRS studies on the commonly used combined [NAA+NAAG] peak. Change of NAAG as a component of glutamatergic neurotransmission and of NAA as a compound that does not act in neurotransmission may mask each other and may induce wrong conclusion about the individual concentration of both in schizophrenia. This potential confound also extends to 1H-MRS reports on other diseases.
We cannot confirm the reports on reduced NAA or [NAA+NAAG] in the frontal lobe. In a meta-analysis of 27 studies on frontal gray matter, [NAA+NAAG] was reported to be lower in patients with schizophrenia even though several individual studies did not find a significant difference between patients and comparison subjects.13 The differences in several parameters of individual studies beyond sample size such as field strength, voxel placement, and patients’ characteristics may contribute to the in part discrepant findings. Also, potential disease-related alterations in metabolite relaxation times may mimic concentration changes and could account for the heterogeneity of the study results. This is also a limitation of our study because with respect to tolerable examination time, it was not possible to measure metabolite T2 times individually and to acquire only fully T1-relaxed spectra. However, by the choice of an intermediate TE of 80 ms and a long TR of 4000 ms for the concentration referencing of [NAA+NAAG] against internal tissue water, we tried to minimize the influence of metabolite relaxation times on absolute concentration values.
We did not detect differences in metabolic ratios that include glutamate or glutamine. Some studies have reported reduced glutamate in different brain areas in schizophrenia,11,35–37 while others found increased concentration37 or no change.38 With regard to glutamine, a reduction in the ACC of chronic patients has been reported in one study.37 Differences in patient characteristics may contribute to the lack of glutamine difference in our data.
Separate measurement of cerebral NAAG and NAA concentrations by 1H-MRS in vivo has thus far only been reported in 2 studies on healthy volunteers.16,17 This is due to the strong overlap of the methyl resonances of both metabolites, which allows discrete quantification only at high magnetic fields, while the majority of 1H-MRS studies on patients with schizophrenia and other disorders has been performed at field strengths less than 3T. Moreover, according to the first study on separate measures of NAAG and NAA, in some brain structures such as the frontal lobe, NAAG is lower than in other cerebral regions, which makes separate quantification even more demanding.16 While in the more recent study17 the investigators discriminated NAAG from NAA by spectral editing in separate acquisitions, where quantification accuracy strongly depends on the degree of coediting the respective other component, our time-domain-fitting procedure is similar to that of the first report on separate measures of NAAG and NAA16 who used LC model fitting39 of NAAG and NAA signals at 2T. The NAAG fractions of 0.08 and 0.18 obtained in that study for frontal gray and white matter, respectively, are in agreement with our NAAG/NAA ratio of 0.10 in the healthy control group in our data.
Overall, it needs to be stressed that in vivo measurement of NAAG and NAA separately is highly challenging, and replication of our data in patients with schizophrenia is needed.
Our study is limited by the inclusion of patients on stable medication only as opposed to untreated subjects. Ultimately, 1H-MRS studies in patients before and after the initiation of antipsychotic treatment will disclose whether a reduction of NAAG is a phenomenon of schizophrenia per se and whether an increase of NAAG is related to improvement of symptoms by antipsychotic treatment.
We conclude that the concentrations of NAAG and NAA act differently in schizophrenia, which impacts on the interpretation of the large body of literature on the [NAA+NAAG] peak in this disorder and in other diseases. The opportunity to measure NAAG in patients creates a new and promising approach for intensified research on the glutamatergic systems and on effects of novel antipsychotic drugs candidates.
Acknowledgments
The study was conducted within in-house financial resources. F.J., N.F., A.M.S., K.-U.K., N.P., W.M., H.-H.S., W.B., M.W., and F.T. have reported no biomedical financial interest or potential conflict of interest.
References
- 1.Paz RD, Tardito S, Atzori M, Tseng KY. Glutamatergic dysfunction in schizophrenia: from basic neuroscience to clinical psychopharmacology. Eur Neuropsychopharmacol. 2008;18:773–786. doi: 10.1016/j.euroneuro.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 3.Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174–181. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- 4.Cartmell J, Adam G, Chaboz S, et al. Characterization of [3H]-(2S,2'R,3'R)-2-(2',3'-dicarboxy-cyclopropyl)glycine ([3H]-DCG IV) binding to metabotropic mGlu2 receptor-transfected cell membranes. Br J Pharmacol. 1998;123:497–504. doi: 10.1038/sj.bjp.0701647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olszewski RT, Bukhari N, Zhou J, et al. NAAG peptidase inhibition reduces locomotor activity and some stereotypes in the PCP model of schizophrenia via group II mGluR. J Neurochem. 2004;89:876–885. doi: 10.1111/j.1471-4159.2004.02358.x. [DOI] [PubMed] [Google Scholar]
- 6.Olszewski RT, Wegorzewska MM, Monteiro AC, et al. Phencyclidine and dizocilpine induced behaviors reduced by N-acetylaspartylglutamate peptidase inhibition via metabotropic glutamate receptors. Biol Psychiatry. 2008;63:86–91. doi: 10.1016/j.biopsych.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, Neale JH, Pomper MG, Kozikowski AP. NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat Rev Drug Discov. 2005;4:1015–1026. doi: 10.1038/nrd1903. [DOI] [PubMed] [Google Scholar]
- 8.Tsai G, Passani LA, Slusher BS, et al. Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Arch Gen Psychiatry. 1995;52:829–836. doi: 10.1001/archpsyc.1995.03950220039008. [DOI] [PubMed] [Google Scholar]
- 9.Nudmamud S, Reynolds LM, Reynolds GP. N-acetylaspartate and N-Acetylaspartylglutamate deficits in superior temporal cortex in schizophrenia and bipolar disorder: a postmortem study. Biol Psychiatry. 2003;53:1138–1141. doi: 10.1016/s0006-3223(02)01742-0. [DOI] [PubMed] [Google Scholar]
- 10.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Bustillo JR, Rowland LM, Mullins P, et al. (1)H-MRS at 4 tesla in minimally treated early schizophrenia. Mol Psychiatry. 2010;15:629–636. doi: 10.1038/mp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jessen F, Scherk H, Träber F, et al. Proton magnetic resonance spectroscopy in subjects at risk for schizophrenia. Schizophr Res. 2006;87:81–88. doi: 10.1016/j.schres.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30:1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- 14.Pan JW, Takahashi K. Interdependence of N-acetyl aspartate and high-energy phosphates in healthy human brain. Ann Neurol. 2005;57:92–97. doi: 10.1002/ana.20317. [DOI] [PubMed] [Google Scholar]
- 15.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pouwels PJ, Frahm J. Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR Biomed. 1997;10:73–78. doi: 10.1002/(sici)1099-1492(199704)10:2<73::aid-nbm448>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Edden RA, Pomper MG, Barker PB. In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 Tesla. Magn Reson Med. 2007;57:977–982. doi: 10.1002/mrm.21234. [DOI] [PubMed] [Google Scholar]
- 18.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 19.Helmstaedter C, Lendt M, Lux S. Verbaler Lern- und Merkfähigkeitstest (VLMT) Göttingen, Germany: Beltz; 2001. [Google Scholar]
- 20.Tewes U. Hamburg-Wechsler-Intelligenztest für Erwachsene, Revision 1991 (HAWIE-R) Bern, Switzerland: Huber; 1991. [Google Scholar]
- 21.Härting C, Neufeld H, Kessler J, et al. Die Wechsler Memory Scale-Revised (WMS-R). Deutsche Version. Göttingen, Germany: Hogrefe; 2000. [Google Scholar]
- 22.Gold JM, Carpenter C, Randolph T, Weinberger D. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- 23.Reitan RM. Trail Making Test (TMT) Weinheim, Germany: Beltz; 1979. [Google Scholar]
- 24.Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 25.Ratiney H, Sdika M, Coenradie Y, et al. Time-domain semi-parametric estimation based on a metabolite basis set. NMR Biomed. 2005;18:1–13. doi: 10.1002/nbm.895. [DOI] [PubMed] [Google Scholar]
- 26.Träber F, Block W, Lamerichs R, Gieseke J, Schild HH. 1H metabolite relaxation times at 3.0 tesla: measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging. 2004;19:537–545. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- 27.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 28.Ghose S, Gleason KA, Potts BW, Lewis-Amezcua K, Tamminga CA. Differential expression of metabotropic glutamate receptors 2 and 3 in schizophrenia: a mechanism for antipsychotic drug action? Am J Psychiatry. 2009;166:812–820. doi: 10.1176/appi.ajp.2009.08091445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guilarte TR, Hammoud DA, McGlothan JL, et al. Dysregulation of glutamate carboxypeptidase II in psychiatric disease. Schizophr Res. 2008;99:324–332. doi: 10.1016/j.schres.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai SJ. Central N-acetyl aspartylglutamate deficit: a possible pathogenesis of schizophrenia. Med Sci Monit. 2005;11:HY39–H45. [PubMed] [Google Scholar]
- 31.Arun P, Madhavarao CN, Moffett JR, Namboodiri AM. Antipsychotic drugs increase N-acetylaspartate and N-acetylaspartylglutamate in SH-SY5Y human neuroblastoma cells. J Neurochem. 2008;106:1669–1680. doi: 10.1111/j.1471-4159.2008.05524.x. [DOI] [PubMed] [Google Scholar]
- 32.Fricker AC, Mok MH, de la Flor R, et al. Effects of N-acetylaspartylglutamate (NAAG) at group II mGluRs and NMDAR. Neuropharmacology. 2009;56:1060–1067. doi: 10.1016/j.neuropharm.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Wang K, Fan J, Dong Y, Wang CQ, Lee TM, Posner MI. Selective impairment of attentional networks of orienting and executive control in schizophrenia. Schizophr Res. 2005;78:235–241. doi: 10.1016/j.schres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Camchong J, Dyckman KA, Austin BP, Clementz BA, McDowell JE. Common neural circuitry supporting volitional saccades and its disruption in schizophrenia patients and relatives. Biol Psychiatry. 2008;64:1042–1050. doi: 10.1016/j.biopsych.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tayoshi S, Sumitani S, Taniguchi K, et al. Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy (1H-MRS) Schizophr Res. 2009;108:69–77. doi: 10.1016/j.schres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Lutkenhoff ES, van Erp TG, Thomas MA, et al. Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry. 2010;15:308–318. doi: 10.1038/mp.2008.87. [DOI] [PubMed] [Google Scholar]
- 37.Théberge J, Al-Semaan Y, Williamson PC, et al. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry. 2003;160:2231–2233. doi: 10.1176/appi.ajp.160.12.2231. [DOI] [PubMed] [Google Scholar]
- 38.Olbrich HM, Valerius G, Rüsch N, et al. Frontolimbic glutamate alterations in first episode schizophrenia: evidence from a magnetic resonance spectroscopy study. World J Biol Psychiatry. 2008;9:59–63. doi: 10.1080/15622970701227811. [DOI] [PubMed] [Google Scholar]
- 39.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]