Abstract
Background: Striatal dopaminergic neurotransmission has been postulated to be fundamental to the emergence of key symptoms of schizophrenia, such as psychotic symptoms, and is targeted by currently available dopaminergic drugs. A specific marker of the integrity of presynaptic dopamine neurons in the striatum, the density of striatal dopamine terminals, can be quantified through molecular neuroimaging of the dopamine active transporter (DAT). However, the currently available results using this approach in schizophrenia are inconsistent. Methods: Thirteen Single Photon Emission Tomography or Positron Emission Tomography (PET) studies investigating DAT density in the striatum of schizophrenic patients and matched controls were included in a quantitative meta-analysis. Binding potentials in the striatum, caudate, and putamen, as well as demographic, clinical, and methodological variables, were extracted from each publication. Hedges’ g was used as a measure of effect size. Results: The overall database contained 202 subjects with schizophrenia and 147 controls, well matched with respect to sociodemographic variables. Striatal DAT density was not significantly different between patients and controls. Similar negative findings were regionally confirmed in the putamen and caudate. There was no moderating effect for external factors. Conclusions: Our meta-analysis uncovered no evidence indicating altered density of striatal dopamine terminals in schizophrenia. Moreover, striatal DAT density did not seem to be influenced by antipsychotic medication or illness duration. Our data suggest that altered integrity of striatal dopaminergic synapses is not critical for the emergence of schizophrenia or its treatment. These findings should be useful in further refining dopaminergic hypotheses of schizophrenia.
Keywords: schizophrenia, psychosis, dopamine, neuroimaging, PET, dopamine transporter, DAT, meta-analysis, presynaptic
Introduction
The most enduring neurochemical theory of schizophrenia centers upon dysregulation of dopaminergic neurotransmission.1 The initial “dopamine receptor hypothesis” of a striatal “hyperdopaminergic” state2 was originally put forth on the basis of the seminal works of Carlsson et al3 showing that reserpine blocked the reuptake of dopamine and that neuroleptics affected dopamine metabolism.4 In the 70s, such a theory was further corroborated by the works of Seeman5 and Snyder,2 showing that antipsychotic efficacy was directly linked to their ability to block the dopamine D2 receptor.5,6 The second “hypofrontality” version of the dopamine hypothesis (1991) was based on the emerging evidence that dopamine receptors show different brain distributions, characterized as D1 predominantly cortical and D2 predominantly subcortical.7 Davies et al7 thus suggested that the effects of abnormalities in dopamine function could vary by brain region, with dopaminergic increases in striatum but not in prefrontal cortex. Lesions of dopamine neurons in the prefrontal cortex were found to increase levels of dopamine and D2 receptor density in the striatum, supporting this hypothesis.8 Since then, subcortical and striatal hyperdopaminergia have been postulated to be fundamental to the emergence of psychotic symptoms.1 The striatum receives dense ascending projections from the mesencephalic dopamine neurons of the substantia nigra and ventral tegmental area, and a number of striatal structural and functional alterations have been observed in schizophrenia.9 Since dopaminergic signals in midbrain and striatum are also essential for the signaling of reward and salience, abnormal striatal dopamine release could lead to the emergence of psychosis through aberrant regulation of salience (the new “aberrant salience” dopamine hypothesis of schizophrenia).10 According to this recent interpretation of striatal hyperdopaminergia, delusions and hallucinations would emerge over time as the individual’s own explanation of the experience of aberrant salience.10 Consequently, the locus of dopamine dysregulation shifted from being primarily at the D2 postsynaptic receptor level to being at the presynaptic dopaminergic level, with the abnormal firing of dopamine neurons and the release of dopamine leading to aberrant assignment of salience to innocuous stimuli.1,10
Over the past years, neurochemical imaging techniques have enabled the striatal presynaptic dopaminergic system to be independently characterized in vivo.11 In particular, the development of new radiotracers combined with single photon emission computed tomography (SPECT) or positron emission tomography (PET) techniques has allowed accurate investigations of striatal presynaptic dopaminergic alterations in schizophrenia (for reviews see12–14). Different indexes of presynaptic dopamine neurotransmission such as dopamine synthesis capacity studies15 and dopamine release16/depletion17 studies have generally shown significant alterations in the striatal presynaptic dopaminergic function in schizophrenia. Importantly, elevated dopamine synthesis and release is also seen in subjects at risk for schizophrenia18 and in the prodromal state,8 suggesting that they are part of the risk architecture of schizophrenia. Despite this convergent evidence from molecular neuroimaging, alterations could in principle be due to structural changes (alterations in the number of dopaminergic neurons or the density of the synaptic connections they make in striatum), functional alterations in amount or regulation of dopamine released, bound and taken up into the synapse, or both. We have addressed the functional integrity of striatal presynaptic dopamine neurotransmission in the companion meta-analysis published in this issue.19 We test here the original neurodegenerative model of schizophrenia, which proposed that functional alterations are secondary to a change in the number or density of striatal dopamine terminals over the course of the illness,20,21 similarly to others neurodegenerative diseases such as Parkinson disease. Investigation of the dopamine active transporter (DAT) density with molecular neuroimaging techniques (employing specific radioligands combined with PET or SPECT) allows the measurement of the structural integrity of presynaptic dopamine neuron in the striatum. For example, in neurology, DAT imaging can be clinically used to assess striatal dopamine terminal integrity and support a diagnosis of Parkinson's disease, to detect subclinical dopaminergic dysfunction in subjects at risk of Parkinson's disease, or to exclude syndromes such as dystonic and severe essential tremors and drug-induced and psychogenic parkinsonism.22
The DAT (SLC6A3 [GenBank DQ307031]) is a sodium chloride–dependent protein located on the presynaptic membrane of dopaminergic terminals, which regulates both duration and magnitude of dopamine neurotransmission at the synapse by removing dopamine from the synaptic cleft trough active reuptake (dopamine active reuptake, DAT). The DAT protein is localized in dopaminergic axons, mainly in the mesencephalic dopamine neurons of the substantia nigra and of the ventral tegmental area and is particularly enriched in the striatum (including putamen and caudate nucleus) and nucleus accumbens, with much lower levels in amygdala, hypothalamus, hippocampus, some thalamic nuclei, and neocortex.23 Because striatal DAT are located exclusively on dopamine synthesizing neurons, the measurement of striatal DAT density is a specific marker of presynaptic dopaminergic neuron integrity.24 In the presynaptic dopaminergic neuron, the amino acid L-tyrosine is converted to dihydroxyphenylalanine (L-DOPA). Subsequently, DOPA decarboxylase or aromatic L-amino acid decarboxylase (AAAD) converts L-DOPA to dopamine. Cytoplasmic dopamine is then moved into synaptic vesicles for storage and subsequent release through the vesicular monoamine transporter 2 (VMAT-2). The molecular regulation of presynaptic dopamine neurotransmission is detailed in figure 1 of the accompanying companion paper.19 DAT structure is characterized by 12 α-helical transmembrane domains, which consist of 20–24 amino acids, a large second hydrophilic extracellular loop with 2–4 potential glycosylation sites and intracellular localization of both N- and C-terminals.23 It also has several putative phosphorylation sites for various kinases (for a review on DAT regulation see.25) Phosphorylation and dephosphorylation of DAT at these sites regulate the reuptake of dopamine by controlling the cycle between transporter internalization and recruitment back to the plasma membrane and thus the number of active transporters.22 The gene that encodes the DAT protein is located on human chromosome 5, consists of 15 coding exons, and is roughly 64-kbp long. This gene has a variable number tandem repeat (VNTR) at the 3′ end (rs28363170), and the genetic polymorphism can affect the basal level of expression of the transporter.23 Genetic deletion of the DAT gene in mice results in a persistent increase in extracellular dopamine tone, that is functionally revealed as hyperactivity (for a review of animal DAT models see26) through the lack of another reuptake mechanism.26 Numerous drugs have important pharmacological interactions with DAT.24 In general, these compounds fall into 2 categories: those that block dopamine transport (eg, cocaine, methylphenidate27) and those that serve as substrates for transport (eg, dopamine, amphetamine, and 3,4-methylenedioxymethamphetamine [MDMA or “ecstasy”]). Among other factors influencing DAT density, the investigation of the effect of age on DAT availability has provided consistent results indicating a 6%–8% decline of DAT per decade, using various SPECT or PET radioligands.28
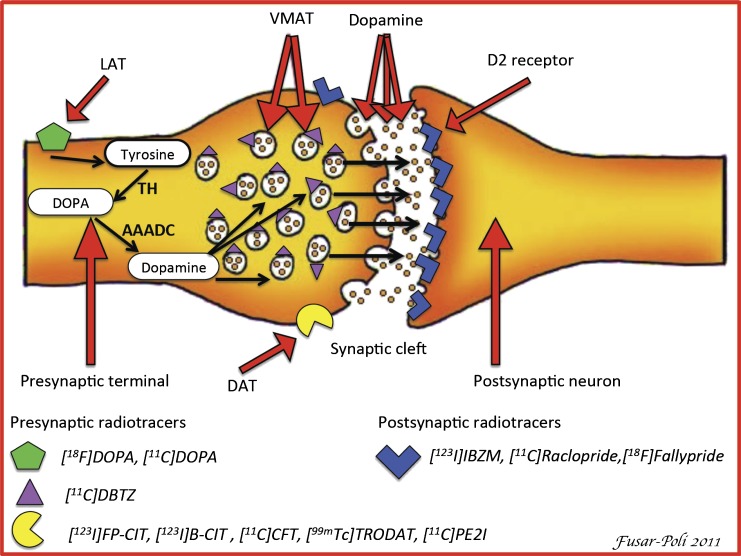
Fig. 1.
Simplified diagram of a striatal dopaminergic synapse. On the presynaptic side, potential markers for imaging of the integrity of dopaminergic neurons are shown. 6-[18F]fluoro-L-3,4-dihydroxyphenylalanine ([18F]DOPA) PET and [11C]DOPA PET provide a measure of the dopamine synthesis capacity. Thus, in the case of [18F]-DOPA PET, the radiotracer is taken up in the dopaminergic neuron via an L-amine acid transporter (LAT) and is then decarboxylated to [18F]fluorodopamine by L-aromatic acid decarboxylase (AAADC) and temporarily stored in vesicles within the nerve terminals. [11C]dihydroxytetrabenazine ([11C]DTBZ) is a commonly used marker for the neuronal synaptic vesicular transporter (vesicular monoaminergic transporter [VMAT-2]) in humans. Substituted (nor)phenyltropanes ([123I]FP-CIT, [123I]β-CIT, [11C]PE2I, [11C]CFT, and [99mTc]TRODAT) are frequently used PET and SPECT tracers for imaging of dopamine transporter (DAT) in humans. Striatal DAT density provides a measure of the density of dopaminergic terminals or innervation into the striatum. Finally, commonly used radiotracers for D2/3 receptors are substituted benzamides ([123I]IBZM, [11C]raclopride, and [18F]fallypride) and are used to address postsynaptic functioning. For convenience, only D2 receptors are shown on the postsynaptic cell.
In the light of the key role played by DAT in regulating presynaptic dopaminergic neurotransmission, a number of SPECT and PET studies addressing DAT density have been published in schizophrenia over the past decade. Although these studies aim at assessing the structural integrity of presynaptic striatal neurons to date, the results are conflicting and inconsistent across centers. Furthermore, the magnitude of striatal DAT density in schizophrenia and the potential confounding role played by external methodological or clinical factors has yet to be consistently measured across different studies.
In the present meta-analysis, we sought to address these issues, focusing on DAT investigations of striatum in schizophrenia. Our first aim was to examine the evidence for an altered striatal presynaptic dopaminergic integrity, as measured across available molecular imaging studies of striatal DAT density. We then estimated the magnitude of differences in DAT density between patients with schizophrenia and matched controls. Finally, we assessed the potential confounding role played by a number of moderators such as sociodemographic characteristics of the sample, illness duration, severity of psychotic symptoms, and exposure to antipsychotics.
Methods
Molecular Imaging the DAT
A brief review of the molecular imaging techniques employed to investigate DAT density in schizophrenia is detailed in the online supplementary material S1 and summarized in figure 1 below here (see online supplementary material S1).
Selection Procedures
Search Strategies.
A systematic search strategy was used to identify relevant studies. First, we carried out a PubMed, Science Direct, and Scopus search to identify putative striatal DAT studies in subjects affected with schizophrenia. The search was conducted in April 2011, and no time span was specified for date of publication. We used the following search terms: “DAT,” “schizophrenia,” “psychosis,” “PET,” “SPECT.” In a second step, the reference lists of the articles included in the review were manually checked for relevant studies not identified by computerized literature searching. Next, the corresponding authors were contacted by e-mail requesting any detail not included in the original manuscripts. There was no language restriction, though all included papers were in English.
Selection Criteria.
Studies were included if they met the following criteria: (a) were reported in an original article in a peer-reviewed journal, (b) had involved subjects affected with DSM/ICD schizophrenia and a matched control group, (c) had analyzed the 2 groups with SPECT or PET techniques assessing striatal DAT density, and (d) had reported the mean (SD) for striatal DAT density in both groups. In cases of 2 or more studies from the same center, we have carefully checked for overlapping samples by contacting the authors to verify that there was not a significant overlap in the samples.
Recorded Variables.
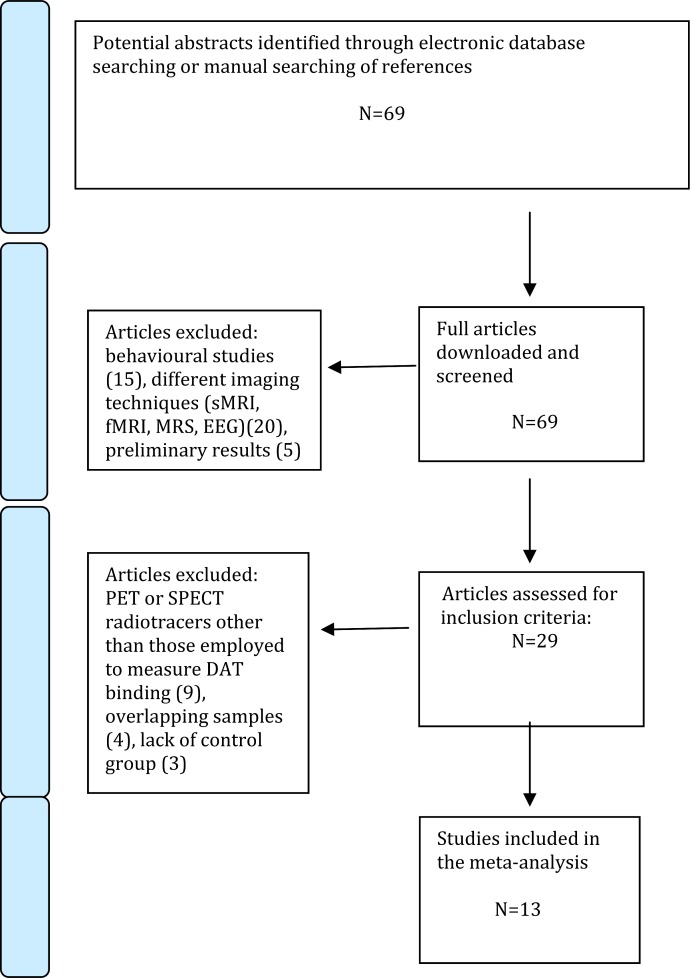
The recorded variables for each article included in the meta-analysis were disease stage (first episode, chronic), illness duration, gender, mean age of participants, exposure to antipsychotics, severity of psychotic symptoms, type of radiotracer used, and statistical significance of the core findings. These data have been reported in tables to assist the reader in forming an independent view on the core findings. To achieve a high standard of reporting, we have adopted “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines and the revised QUOROM Statements (Quality of Reporting of Meta-analyses)29 (see figure 2).
Fig. 2.
Search strategy used for the inclusion of the studies considered in the current meta-analysis.
Statistical Analysis.
Data were then entered in an electronic database and analyzed with a meta-analytical approach by using Comprehensive Meta-Analysis Software version 2 (Biostat, Inc., Englewood, NJ). This package employs the same computational algorithms used by the Cochrane Collaborators to weight studies by the inverse variance method. The meta-analysis of individual studies increases statistical power by reducing the SE of the weighted average effect size. A smaller SE results in a smaller CI around the weighted average effect size, and a small CI increases the likelihood of detecting nonzero population effects. Notably, small CIs also increase the precision of the estimated population effect size.
The primary outcome was striatal DAT density in the patient and in the control group. As a measure of effect size, the Hedges’ g was adopted, ie, the difference between the means of the patient and control groups, divided by the SD and weighted for sample size, in order to correct for bias from small sample sizes.30 This metric is normally computed by using the square root of the mean square error from the ANOVA testing for differences between the 2 groups, as indicated by the formula
where
and
where X is the raw score, M is the mean, and N is the number of cases.30
In a secondary step, we conducted additional meta-analyses to regionally address DAT density in the caudate and putamen. To determine whether categorical factors (type of radiotracer) modified the DAT density subgroup analyses were performed. The influence of continuous moderator variables was tested using meta-regression analyses: age of participants, severity of psychotic symptoms (as measured with the positive and negative syndrome scale [PANSS]), gender (% of females), duration of illness (DOI) (months), and exposure to antipsychotics (% of drug-naïve subjects). The slope of meta-regression (β-coefficient: direct [+] or inverse [−]) of the regression line indicates the strength of a relationship between moderator and outcome. To limit risk of false positive (type I) errors arising from multiple comparisons, we adjusted P < .05 by dividing α with the number of meta-regressions.
We employed random effects models, which are more conservative than fixed-effect models, and argued to better address heterogeneity between studies and study populations, allowing for greater flexibility in parsing effect size variability. Moreover, they are less influenced by extreme variations in sample size. Heterogeneity among study point estimates was assessed with the Q statistic with magnitude of heterogeneity being evaluated with the I 2 index. The possibility of such publication bias was examined by visually inspecting funnel plots and applying the regression intercept of Egger. In this way, we assessed whether there was a tendency for selective publication of studies based on the nature and direction of their results. To assess the robustness of the results, we performed sensitivity analyses by sequentially removing each study and rerunning the analysis.
Results
Studies Found
The combined search strategies yielded a total of 69 articles, of which after a complete full text analysis, 40 were excluded. Of 29 that were considered eligible, 13 studies published between 2000 and 2009 met our inclusion criteria and were included in the present meta-analysis, (figure 2). The overall database contained 202 subjects with schizophrenia (mean age 31.7 y, 30% females) and 147 controls (mean age 34.2 y, 31% females). DOI ranged from few months to several years. Patients and controls were well matched with respect to age and gender (all P > .05). The sociodemographic details of the whole sample are presented in table 1.
Table 1.
List of Studies Addressing DAT Density in Schizophrenia Included in the Meta-Analysis
| Author | Year | Radiotracer | Technique | Disease Stage | DOI | SD | Treatment | Schizophrenia | Controls | SCZ/DAT (1) | |||||||
| N | F | Age | SD | N | F | Age | SD | Value | P | ||||||||
| Laruelle et al30 | 2000 | [123I]β-CIT | SPECT | C | 180 | ? | 8DF,16A | 24 | 2 | 41 | 8 | 22 | 2 | 39 | 8 | 0.93 | ns |
| Laakso et al31 | 2000 | [18F]CFT | PET | FEP | 9 | ? | DN | 9 | 3 | 30 | 7 | 9 | 3 | 30 | 6 | 1.01 | ns |
| Lavalaye et al32 | 2001 | [123I]FP-CIT | SPECT | C,FEP | 21 | 13 | 10DN,3DF,23A | 36 | 4 | 21 | 3 | 10 | 3 | 20 | 1 | 0.98 | ns |
| Laakso et al33 | 2001 | [18F]CFT | PET | C | 119 | ? | A | 8 | ? | 37 | 6 | 8 | ? | 35 | 6 | 0.88 | sig |
| Hsiao et al34 | 2003 | [99mTc]TRODAT | SPECT | FEP | 10 | 13 | DN | 12 | 10 | 26 | 8 | 12 | 10 | 30 | 9 | 1.04 | ns |
| Sjoholm et al35 | 2004 | [123I]β-CIT | SPECT | C | 180 | ? | A | 6 | 0 | 41 | 2 | 5 | 0 | 60 | ? | 1.51 | sig |
| Yang et al36 | 2004 | [99mTc]TRODAT | SPECT | FEP | 16 | 26 | DN | 11 | 5 | 26 | 10 | 12 | 3 | 33 | 13 | 1.01 | ns |
| Yoder et al37 | 2004 | [11C]CFT | PET | C | ? | ? | 1DN,1DF,8A | 10 | 2 | 41 | 11 | 10 | 3 | 45 | 18 | 0.92 | ns |
| Mateos et al38 | 2005 | [123I]FP-CIT | SPECT | FEP | 5 | 2 | A | 20 | 2 | 26 | 5 | 10 | 4 | 27 | 4 | 0.89 | sig |
| Schmitt et al39 | 2006 | [99mTc]TRODAT | SPECT | FEP | ? | ? | DN | 28 | 5 | 31 | 9 | 12 | 2 | 32 | 8 | 0.98 | ns |
| Kim et al40 | 2006 | [123I]IPT | SPECT | C | 24 | 32 | DF | 10 | 8 | 29 | 8 | 10 | 4 | 31 | 6 | 0.93 | ns |
| Mateos et al41 | 2007 | [123I]FP-CIT | SPECT | FEP | 4 | 2 | DN | 20 | 6 | 26 | 5 | 15 | 7 | 29 | 7 | 0.87 | sig |
| Arakawa et al42 | 2009 | [11C]PE2I | PET | C | 32 | 43 | 6DN,2DF | 8 | 2 | 37 | 10 | 12 | 2 | 33 | 12 | 1.09 | ns |
Note: C, chronic schizophrenia; FEP, first-episode schizophrenia; DOI, duration of illness (months); DN, drug naive; DF, drug free; A, treated with antipsychotics; F, females; ns, nonsignificant; sig, significant; PET, Positron Emission Tomography; SPECT, single photon emission computed tomography; DAT, dopamine active transporter; (1) Striatal DAT binding in the schizophrenic group/Striatal DAT binding in the control group.
Psychotic Symptoms
Most studies have assessed psychotic positive and negative symptoms by using the PANSS or the Brief Psychiatric Rating Scale (BPRS) and 3 of them uncovered significant correlations between striatal DAT density and psychotic symptoms.31,38,39 Laruelle et al31 found a trend level association in patients with schizophrenia between low striatal DAT density and severity of negative symptoms.31 Schmitt et al found that hallucinations were inversely correlated with striatal DAT density in the subgroup of schizophrenic patients with predominantly positive symptoms.40 Yoder et al38 uncovered trend-levels inverse correlations between DAT density and negative psychotic symptoms.38
Dopamine Transporter Density in the Striatum
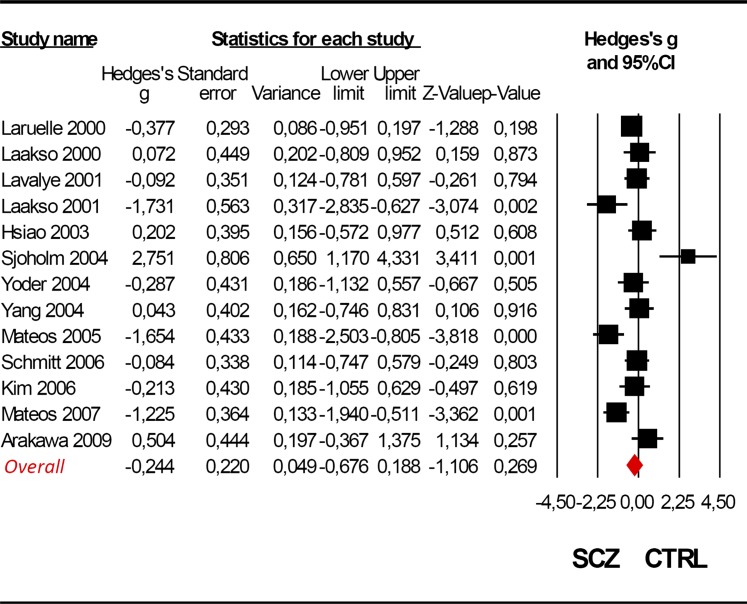
Four of 13 studies reported significant differences in striatal DAT density between the schizophrenic and control group.34 , 36 , 39 , 42 However, the pooled meta-analysis indicated no statistical evidence for a significant difference between the 2 groups (Hedges’ g = −0.244, CI 95% from −0.676 to 0.188, Z = −1.106, P = .269, figure 3). The striatal DAT density schizophrenia/control ratio ranged from 0.87 to 1.51, with an average value of 0.95.
Fig. 3.
Meta-analysis of striatal dopamine transporter density in schizophrenia. Random effect models (Q = 44.075; P < .001; I 2 = 75.082).
Dopamine Transporter Density in the Caudate and Putamen
The meta-analysis of studies investigating the caudate confirmed no significant differences in DAT density between the patient and the control group (Hedges’ g = −0.197, CI 95% from −0.564 to 0.133, Z−0.782, P = .431). Similarly, DAT density in the putamen was not statistically different between schizophrenics and controls (Hedges’ g = −0.187, CI 95% from −0.661 to 0.153, Z = −0.652, P = .549).
Effect of Moderators
The type of radiotracer did not influence the meta-analytical results (Q = 2.740, P = .602). Meta-regressions revealed no significant effects for the examined moderator variables (year of publication β = −.004, CI 95% from −0.084 to 0.077, Z = −0.092, P = .927; age of participants β = .019, CI 95% from −0.017 to 0.056, Z = 1.03, P = .301; DOI β = .013, CI 95% from −0.002 to 0.005, Z = −0.725, P = .468; severity of psychotic symptoms β = .005, CI 95% from −0.018 to 0.008, Z = −0.751, P = .452; exposure to antipsychotics β = .355, CI 95% from 0.0153 to 0.864, Z = 1.371, P = .171; and gender β = −.413, CI 95% from −0.527 to 1.354, Z = 0.861, P = .389) on the findings reported above here.
Tests for Publication Bias, Heterogeneity, and Sensitivity Analysis
Visual inspection of funnel plots revealed no evidence of publication bias. Quantitative evaluation of publication bias, as measured by the Egger intercept, was nonsignificant (P = .392). According to the criteria set by Higgins and Thompson, the heterogeneity in published studies was large in magnitude and statistically significant (Q = 44.075; P < .001; I 2 = 75.082). Robustness of meta-analytic findings was confirmed by sequentially removing each study and reanalyzing the remaining data set (producing a new analysis for each study removed). The results remained essentially unchanged in direction and magnitude.
Discussion
To our knowledge, this is the first comprehensive meta-analysis addressing DAT density in schizophrenia. We found no evidence for an altered integrity of striatal dopamine terminals in schizophrenia. Similarly, no abnormalities were regionally detected in the caudate or putamen. Antipsychotic treatment, DOI, age, gender, and severity of psychotic symptoms had no effect on the meta-analytical findings.
The present meta-analysis tested the hypothesis that striatal hyperdopaminergia in schizophrenia is accompanied by alterations of presynaptic neuronal integrity. Because striatal DAT is located on dopaminergic presynaptic neurons, its measurement with molecular imaging techniques allows the assessment of the density of presynaptic dopaminergic terminals. In principle, the modified dopamine hypothesis of schizophrenia would be compatible with a change in striatal DAT in either direction. If increased striatal dopamine neurotransmission is a primary event in the illness, increases of DAT could accompany an increase in presynaptic dopaminergic terminals. On the other hand, if striatal hyperdopaminergia is a secondary event, it can be compensatory for a reduction of dopamine terminals and itself propagate synapse loss through excitotoxicity. This hypothesis of striatal dopaminergic neurodegeneration in schizophrenia parallels the physiopathology of idiopathic Parkinson disease and parkinsonian syndromes, which are characterized neuropathologically by degeneration of dopaminergic cells, resulting in a loss of dopamine transporters (DATs) in the striatum. Thus, the idea that schizophrenia might be associated with an accelerated loss of DAT over the course of the illness proposed that sustained hyperactivity of dopamine neurons might be secondary to neurotoxic loss of striatal dopamine neurons.20,21
Three studies included in the present database uncovered significant striatal DAT decreases in schizophrenic subjects as compared with controls.34 , 39 , 42 The decreased DAT density was attributed to a higher loss of dopamine neuron integrity, in line with the neurotoxic hypothesis of striatal hyperdopaminergia. Conversely, one study found greater DAT density in schizophrenic patients as compared with controls.36 To preserve the hypothesis of abnormal striatal integrity of dopaminergic terminals, the authors interpreted such a finding as biological adaptation to chronic D2 receptor blockade induced by chronic neuroleptic treatment.36 However, when we tested these finding at a meta-analytical level, in a total sample of around 200 schizophrenic subjects, we found that the DAT density was not significantly different as compared with controls. As the meta-analysis was sufficiently powered, robust and not undermined by significant publication bias, this negative finding is statistically reliable. The finding that DAT is unaffected in schizophrenia is in line with molecular imaging studies investigating other structural striatal presymaptic indexes such as the VMAT-2. These studies did not find evidence supporting the hypothesis that excessive dopamine activity in schizophrenia could be explained by altered density of striatal dopamine terminals.44 Similarly, genetic studies addressing the potential association between schizophrenia and the 40-bp VNTR polymorphism in the 3′-UTR (untranslated region) of the DAT gene (SLC6A3) showed negative results (for a meta-analysis see45). Our finding suggests that the increased presynaptic striatal DA activity in schizophrenia indicated by other imaging parameters (for systematic reviews see12–14) is not secondary to dysfunctional DAT or an alteration in the density of dopaminergic terminals. In the companion paper published in the present issue, we present the first quantitative meta-analysis addressing the functional integrity of striatal presynaptic dopamine neurotransmission.19 The negative results of our meta-analysis are also in line with several postmortem studies of DAT concentration, which reported no alteration in DAT density in striatal tissue of patients with schizophrenia compared with control subjects.46,47 However it is known that the DAT density decreases as people get older, with a decline in dopaminergic neurons of 4% to 8% per decade in the general population.48 Some authors have speculated that loss of striatal dopamine terminals can be associated with evolution of the illness from an active phase associated with positive symptoms to a more stable or phase in which negative symptoms dominate the clinical picture.30 It was therefore assumed that the findings of these postmortem studies did not reflect the early phase of psychotic illness or the acute psychotic state because they were conducted with elderly patients.49 We found no support for such a hypothesis because we found that DOI had no impact on the overall meta-analytical results. Furthermore, the subset (n = 7) of studies included in the present meta-analysis addressing striatal DAT density in first-episode subjects yielded again inconsistent results. Finally, although some individual studies have reported significant associations between striatal DAT density and psychotic symptoms,31,40 no significant correlations were detected at a meta-analytical level.
We did not find any effect of antipsychotic treatment on DAT density in schizophrenia. An early study in chronic schizophrenic who were drug free for at least 3 weeks prior to scanning showed striatal DAT density is unaffected by presence or absence of neuroleptics.16 A subsequent study in subjects suffering from a first or second episode of psychosis revealed again no significant difference between antipsychotic-naïve patients, patients treated with olanzapine, patients treated with risperidone, and controls.33 Two studies included in the present meta-analysis have adopted a longitudinal design to specifically address the antipsychotic effect on striatal DAT density in schizophrenia. The first study found no changes in chronic schizophrenics after 4 weeks of treatment with olanzapine.49 The second study enrolled antipsychotic-naïve first-episode patients and investigated them at baseline and after 4 weeks of risperidone treatment and confirmed a null effect of antipsychotic on striatal DAT density.42 These results are in line with animal models showing that chronic administration of clozapine, risperidone, or haloperidol did not alter DAT density in rats.50,51 These consistent results are in interesting contrast to preclinical findings that show that D2 receptors are a critical regulator of synaptic arborization,52 with pronounced increases in synaptic density seen under chronic D2 blockade 53 and in D2 receptor knockout mice.54 Clinically, D2 blockade is suspected to contribute to striatal volume enlargement in schizophrenia55 and has been shown to have short-term reversible structural effects.56 Given these structural changes, it is important to point out that any inference from measured DAT to structural parameters, such as density of dopaminergic terminals, is limited by the fact that the measurement will be affected both by the amount of synapses and the average amount of transporter protein in the synaptic membrane, and therefore remains indirect. In particular, a downregulation of DAT could in principle be counterbalanced or obscured by a concomitant rise in synaptic abundance. Multimodal approaches with simultaneous fine-grained volumetric assessment and PET would be useful to study this possibility. Barring this possibility, based on these results, we conclude that neither D2 receptor blockade or the increases in presynaptic striatal dopamine release reliably found in schizophrenia12 appear to cause appreciable changes in DAT density, making a role of this molecule, and the integrity of the presynaptic terminals that it labels, in either the pathogenesis or the currently practiced treatment of schizophrenia unlikely.55,56 Conversely, DAT alterations may represent a core feature of other neuropsychiatric disorders such as ADHD and seem sensible to the effect of psychostimulants such as methylphenidate.19
The present study, and the literature it surveys, has several limitations. The absence of a positive finding always raises the question of a type II error, due to sample bias or inadequate experimental power. On average, the studies included in the present meta-analysis have enrolled about 15 patients and 11 controls only. Consequently, although we found no significant findings, we cannot exclude that future studies in larger samples will have the power to detect significant group differences. In particular, the meta-regressions between moderating factors and DAT density are limited by the small sample size and by the cross-sectional nature of the analysis. Thus, the lack of any effect of medication on the DAT density cannot rule out any potential effect of antipsychotics on striatal integrity and structure. In fact, there is evidence indicating exposure to antipsychotics can affect gray matter volume of the basal ganglia, with a differential effect between typical and atypical molecules.57 Furthermore, it has been suggested that high heterogeneity across studies may be the consequence of the variable DAT sensitivity (ie, binding kinetic, time from injection to imaging) across different radiotracers employed. For example, there is evidence indicating that the specific to non specific ratio of labeled cocaine is relatively low and that this radioligand may occupy other binding sites than FP-CIT, altrophane, TRODAT-1 and IPT.58 Also, the binding of a specific radioligand may be influenced by a complex network of interactions,40 including glutamatergic modulations of striatal and extrastriatal DAT. Some advancement in the field may be achieved by dual-isotope imaging techniques, which allow labeling simultaneously the striatal presynaptic DAT density and postsynaptic dopamine D2 receptor. For example, a dual-isotope study with first-episode schizophrenic patients and healthy controls37 found no significant DAT density difference but a significant correlation between DAT and D2 receptor availability, which was not present in the healthy control group. Using the same approach, in an extended cohort of highly acute psychotic first episode never treated schizophrenic patients, other authors replicated the finding of a significant correlation between DAT and D2 receptor availability in the patient but not in the control group.59 Future studies are also needed to elucidate alterations of DAT density in extrastriatal regions. Recent developments of new radioligands with high affinity and selectivity for DAT have allowed the evaluation of thalamic DAT density in schizophrenia.43 Alterations in this region may be of particular interest as neuropathological and in vivo imaging studies in schizophrenia have identified several structural and functional abnormalities in the thalamus, and these changes have been related to cognitive impairments and symptomatology in established and early psychosis.60
Conclusions
The present meta-analysis uncovered no evidence for altered dopamine transporter density in the striatum of patients affected with schizophrenia. Therefore, a change in the integrity of presynaptic dopamine terminals is unlikely to contribute to the pathogenesis course or treatment of schizophrenia. With our meta-analysis available, robust evidence from studies showing altered presynaptic dopamine activity in schizophrenic patients19 likely reflects a true difference in functional status rather than an epiphenomenon of structural or treatment-related changes.
Addendum
At the time of publication of the present meta-analysis, a new study addressing longitudinal changes of striatal DAT binding (as measured by [123I] FP-CIT SPECT) in 14 antisychotic naive schizophrenic patients and in 7 matched controls was published. The authors found that patients' DAT binding had decreased by 2.3% at the 4-year follow-up, whereas controls' DAT binding had decreased by 4.8%, however, no between-groups statistical significant differences were detected. Indeed, these negative longitudinal results further support the core finding of our meta-analysis.61
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
We would like to thanks the reviewers for their valuable comments and for their help in improving the present manuscript. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Howes O, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snyder SH. The dopamine hypothesis of schizophrenia: focus on the dopamine receptor. Am J Psychiatry. 1976;133:197–202. doi: 10.1176/ajp.133.2.197. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180:1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on the formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol. 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 5.Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 6.Creese I, Burt D, Snyder S. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 7.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 8.Fusar-Poli P, Howes OD, Allen P, et al. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch Gen Psychiatry. 2010;67:683–691. doi: 10.1001/archgenpsychiatry.2010.77. [DOI] [PubMed] [Google Scholar]
- 9.Simpson E, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 11.McGuire P, Howes OD, Stone J, Fusar-Poli P. Functional neuroimaging in schizophrenia: diagnosis and drug discovery. Trends Pharmacol Sci. 2008;29:91–98. doi: 10.1016/j.tips.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Howes OD, Montgomery AJ, Asselin M, Murray R, Grasby P, McGuire P. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry. 2007;51:s13–s18. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyon GJ, Abi-Dargham A, Moore H, Lieberman JA, Javitch JA, Sulzer D. Presynaptic regulation of dopamine transmission in schizophrenia. Schizophr Bull. 2011;37:108–117. doi: 10.1093/schbul/sbp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyake N, Thompson J, Skinbjerg M, Abi-Dargham A. Presynaptic dopamine in schizophrenia. CNS Neurosci Ther. 2011;17:104 –109. doi: 10.1111/j.1755-5949.2010.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer-Lindenberg A, Miletich RS, Kohn PD, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 16.Laruelle M, Abi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 18.Huttunen J, Heinimaa M, Svirskis T, et al. Striatal dopamine synthesis in first-degree relatives of patients with schizophrenia. Biol Psychiatry. 2008;63:114–117. doi: 10.1016/j.biopsych.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 19. doi: 10.1093/schbul/sbr180. Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of 18F/11C-DOPA studies. Schizophr Bull. 2011; doi:11.0337/schbul/sbr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieberman JA, Kinon BJ, Loebel AD. Dopaminergic mechanisms in idiopathic and drug-induced psychoses. Schizophr Bull. 1990;16:97–110. doi: 10.1093/schbul/16.1.97. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman JA, Sheitman BB, Kinon BJ. Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology. 1997;17:205–229. doi: 10.1016/S0893-133X(97)00045-6. [DOI] [PubMed] [Google Scholar]
- 22.Brooks DJ. Imaging dopamine transporters in Parkinson's disease. Biomark Med. 2010;4:651–660. doi: 10.2217/bmm.10.86. [DOI] [PubMed] [Google Scholar]
- 23.Piccini PP. Dopamine transporter: basic aspects and neuroimaging. Mov Disord. 2003;18(suppl 7):S3–S8. doi: 10.1002/mds.10571. [DOI] [PubMed] [Google Scholar]
- 24.Meisenzahl EM, Schmitt GJ, Scheuerecker J, Moller HJ. The role of dopamine for the pathophysiology of schizophrenia. Int Rev Psychiatry. 2007;19:337–345. doi: 10.1080/09540260701502468. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- 26.Gainetdinov RR. Dopamine transporter mutant mice in experimental neuropharmacology. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:301–313. doi: 10.1007/s00210-007-0216-0. [DOI] [PubMed] [Google Scholar]
- 27. doi: 10.1176/appi.ajp.2011.11060940. Fusar-Poli P, Rubia K, Rossi G, Sartori G, Balottin U. Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. American Journal of Psychiatry. 2012. In press. [DOI] [PubMed] [Google Scholar]
- 28.Varrone A, Halldin C. Molecular imaging of the dopamine transporter. J Nucl Med. 2010;51:1331–1334. doi: 10.2967/jnumed.109.065656. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedges L, Holkin I. Statistical Methods for Meta-Analysis. New York, NY: Academic press; 1985. [Google Scholar]
- 31.Laruelle M, Abi-Dargham A, van Dyck C, et al. Dopamine and serotonin transporters in patients with schizophrenia: an imaging study with [(123)I]beta-CIT. Biol Psychiatry. 2000;47:371–379. doi: 10.1016/s0006-3223(99)00257-7. [DOI] [PubMed] [Google Scholar]
- 32.Laakso A, Vilkman H, Alakare B, et al. Striatal dopamine transporter binding in neuroleptic-naive patients with schizophrenia studied with positron emission tomography. Am J Psychiatry. 2000;157:269–271. doi: 10.1176/appi.ajp.157.2.269. [DOI] [PubMed] [Google Scholar]
- 33.Lavalaye J, Linszen DH, Booij J, et al. Dopamine transporter density in young patients with schizophrenia assessed with [123]FP-CIT SPECT. Schizophr Res. 2001;47:59–67. doi: 10.1016/s0920-9964(00)00023-2. [DOI] [PubMed] [Google Scholar]
- 34.Laakso A, Bergman J, Haaparanta M, et al. Decreased striatal dopamine transporter binding in vivo in chronic schizophrenia. Schizophr Res. 2001;52:115–120. doi: 10.1016/s0920-9964(00)00095-5. [DOI] [PubMed] [Google Scholar]
- 35.Hsiao MC, Lin KJ, Liu CY, Tzen KY, Yen TC. Dopamine transporter change in drug-naive schizophrenia: an imaging study with 99mTc-TRODAT-1. Schizophr Res. 2003;65:39–46. doi: 10.1016/s0920-9964(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 36.Sjoholm H, Bratlid T, Sundsfjord J. 123I-beta-CIT SPECT demonstrates increased presynaptic dopamine transporter binding sites in basal ganglia in vivo in schizophrenia. Psychopharmacology (Berl) 2004;173:27–31. doi: 10.1007/s00213-003-1700-y. [DOI] [PubMed] [Google Scholar]
- 37.Yang YK, Yu L, Yeh TL, Chiu NT, Chen PS, Lee IH. Associated alterations of striatal dopamine D2/D3 receptor and transporter binding in drug-naive patients with schizophrenia: a dual-isotope SPECT study. Am J Psychiatry. 2004;161:1496–1498. doi: 10.1176/appi.ajp.161.8.1496. [DOI] [PubMed] [Google Scholar]
- 38.Yoder KK, Hutchins GD, Morris ED, Brashear A, Wang C, Shekhar A. Dopamine transporter density in schizophrenic subjects with and without tardive dyskinesia. Schizophr Res. 2004;71:371–375. doi: 10.1016/j.schres.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Mateos JJ, Lomena F, Parellada E, et al. Decreased striatal dopamine transporter binding assessed with [123I] FP-CIT in first-episode schizophrenic patients with and without short-term antipsychotic-induced parkinsonism. Psychopharmacology (Berl) 2005;181:401–406. doi: 10.1007/s00213-005-2250-2. [DOI] [PubMed] [Google Scholar]
- 40.Schmitt GJ, Frodl T, Dresel S, et al. Striatal dopamine transporter availability is associated with the productive psychotic state in first episode, drug-naive schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 2006;256:115–121. doi: 10.1007/s00406-005-0618-2. [DOI] [PubMed] [Google Scholar]
- 41.Kim JS, Lee KS, Han SR, Chung YA. Decreased striatal dopamine transporter binding in a patient with extrapontine myelinolysis. Mov Disord. 2003;18:342–345. doi: 10.1002/mds.10331. [DOI] [PubMed] [Google Scholar]
- 42.Mateos JJ, Lomena F, Parellada E, et al. Lower striatal dopamine transporter binding in neuroleptic-naive schizophrenic patients is not related to antipsychotic treatment but it suggests an illness trait. Psychopharmacology (Berl) 2007;191:805–811. doi: 10.1007/s00213-006-0570-5. [DOI] [PubMed] [Google Scholar]
- 43.Arakawa R, Ichimiya T, Ito H, et al. Increase in thalamic binding of [(11)C]PE2I in patients with schizophrenia: a positron emission tomography study of dopamine transporter. J Psychiatr Res. 2009;43:1219–1223. doi: 10.1016/j.jpsychires.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Taylor SF, Koeppe RA, Tandon R, Zubieta JK, Frey KA. In vivo measurement of the vesicular monoamine transporter in schizophrenia. Neuropsychopharmacology. 2000;23:667–675. doi: 10.1016/S0893-133X(00)00165-2. [DOI] [PubMed] [Google Scholar]
- 45.Costa A, Riedel M, Muller U, Moller HJ, Ettinger U. Relationship between SLC6A3 genotype and striatal dopamine transporter availability: a meta-analysis of human single photon emission computed tomography studies. Synapse. 2011;65:998–1005. doi: 10.1002/syn.20927. [DOI] [PubMed] [Google Scholar]
- 46.Knable MB, Hyde TM, Herman MM, Carter JM, Bigelow L, Kleinman JE. Quantitative autoradiography of dopamine-D1 receptors, D2 receptors, and dopamine uptake sites in postmortem striatal specimens from schizophrenic patients. Biol Psychiatry. 1994;36:827–835. doi: 10.1016/0006-3223(94)90593-2. [DOI] [PubMed] [Google Scholar]
- 47.Pearce RK, Seeman P, Jellinger K, Tourtellotte WW. Dopamine uptake sites and dopamine receptors in Parkinson's disease and schizophrenia. Eur Neurol. 1990;30(suppl 1):9–14. doi: 10.1159/000117168. [DOI] [PubMed] [Google Scholar]
- 48.Booij J, Bergmans P, Winogrodzka A, Speelman JD, Wolters EC. Imaging of dopamine transporters with [123I]FP-CIT SPECT does not suggest a significant effect of age on the symptomatic threshold of disease in Parkinson's disease. Synapse. 2001;39:101–108. doi: 10.1002/1098-2396(200102)39:2<101::AID-SYN1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 49.Kim C, Lee M, Lee P, Choe W, Pyo S. Correlation between psychopathology and dopamine transporter density in striatum before and after taking olanzapine assessed with IPT-SPECT in first episode schizophrenia. Clin Psychopharmacol Neurosci. 2006;4:24–31. [Google Scholar]
- 50.Lavalaye J, Knol RJ, de Bruin K, Reneman L, Janssen AG, Booij J. [123I]FP-CIT binding in rat brain after acute and sub-chronic administration of dopaminergic medication. Eur J Nucl Med. 2000;27:346–349. doi: 10.1007/s002590050044. [DOI] [PubMed] [Google Scholar]
- 51.Reader TA, Ase AR, Huang N, Hebert C, van Gelder NM. Neuroleptics and dopamine transporters. Neurochem Res. 1998;23:73–80. doi: 10.1023/a:1022405621365. [DOI] [PubMed] [Google Scholar]
- 52.Goldman RG, Alexander GE, Zemishlany Z, Mukherjee S, Sackeim H, Prohovnik I. Acute effects of haloperidol on cerebral cortex blood flow in normal and schizophrenic subjects. Biol Psychiatry. 1996;40:604–608. doi: 10.1016/0006-3223(95)00391-6. [DOI] [PubMed] [Google Scholar]
- 53.Parish CL, Stanic D, Drago J, Borrelli E, Finkelstein DI, Horne MK. Effects of long-term treatment with dopamine receptor agonists and antagonists on terminal arbor size. Eur J Neurosci. 2002;16:787–794. doi: 10.1046/j.1460-9568.2002.02132.x. [DOI] [PubMed] [Google Scholar]
- 54.Parish CL, Finkelstein DI, Drago J, Borrelli E, Horne MK. The role of dopamine receptors in regulating the size of axonal arbors. J Neurosci. 2001;21:5147–5157. doi: 10.1523/JNEUROSCI.21-14-05147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldman AL, Pezawas L, Mattay VS, et al. Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry. 2008;63:475–483. doi: 10.1016/j.biopsych.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Tost H, Braus DF, Hakimi S, et al. Acute D2 receptor blockade induces rapid, reversible remodeling in human cortical-striatal circuits. Nat Neurosci. 2010;13:920–922. doi: 10.1038/nn.2572. [DOI] [PubMed] [Google Scholar]
- 57.Smieskova R, Fusar-Poli P, Allen P, et al. The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia?—a systematic review. Curr Pharm Des. 2009;15:2535–2549. doi: 10.2174/138161209788957456. [DOI] [PubMed] [Google Scholar]
- 58.Krause J. SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2008;8:611–625. doi: 10.1586/14737175.8.4.611. [DOI] [PubMed] [Google Scholar]
- 59.Schmitt GJ, la Fougere C, Dresel S, Frodl T, Hahn K, Moller HJ, Meisenzahl EM. Dual-isotope SPECT imaging of striatal dopamine: first episode, drug naive schizophrenic patients. Schizophr Res. 2008;101:133–141. doi: 10.1016/j.schres.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 60.Fusar-Poli P, Stone J, Broome M, et al. Thalamic glutamate levels predicts cortical response during executive functioning in subjects at high risk for psychosis. Arch Gen Psychiatry. 2011;68:881–890. doi: 10.1001/archgenpsychiatry.2011.46. [DOI] [PubMed] [Google Scholar]
- 61.Mane A, Gallego J, Lomena F, Mateos JJ, Fernandez-Egea E, Horga G, Cot A, Pavia J, Bernardo M, Parellada E. A 4-year dopamine transporter (DAT) imaging study in neuroleptic-naive first episode schizophrenia patients. Psychiatry Res. 2011;194:79–84. doi: 10.1016/j.pscychresns.2011.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.