Abstract
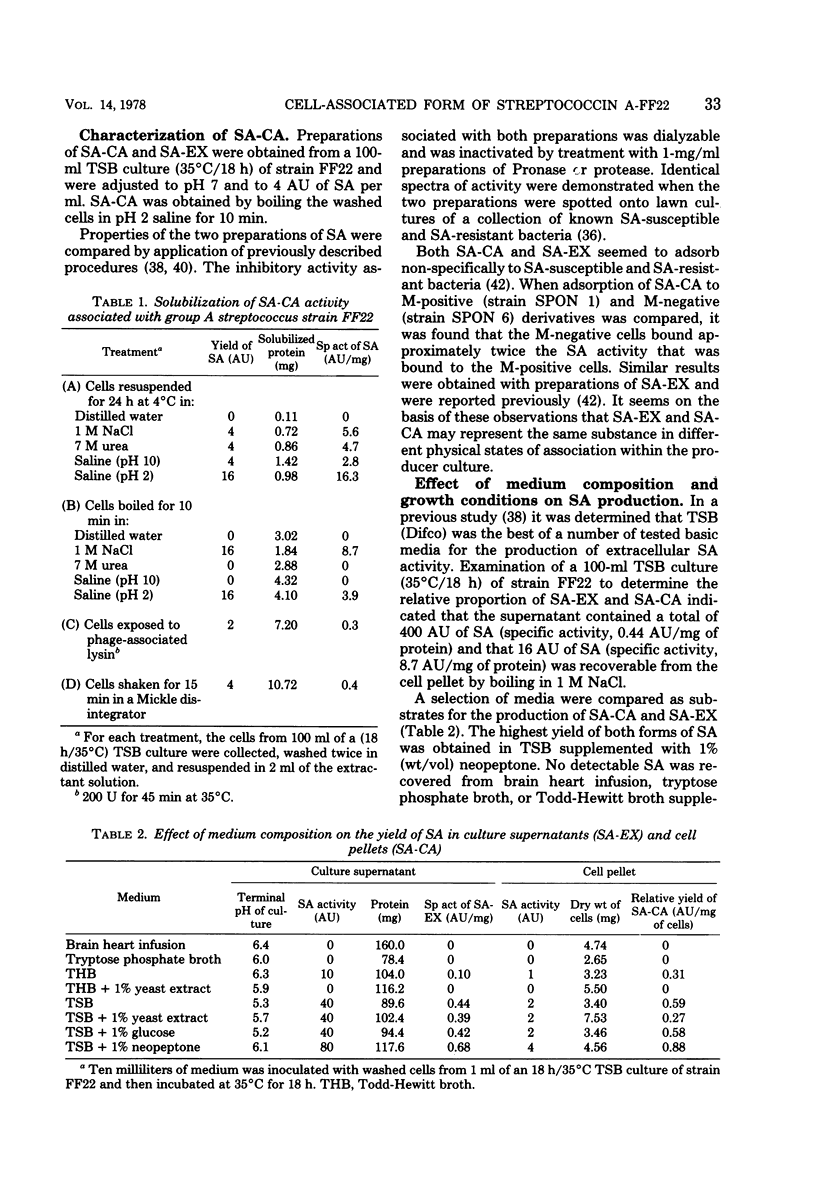
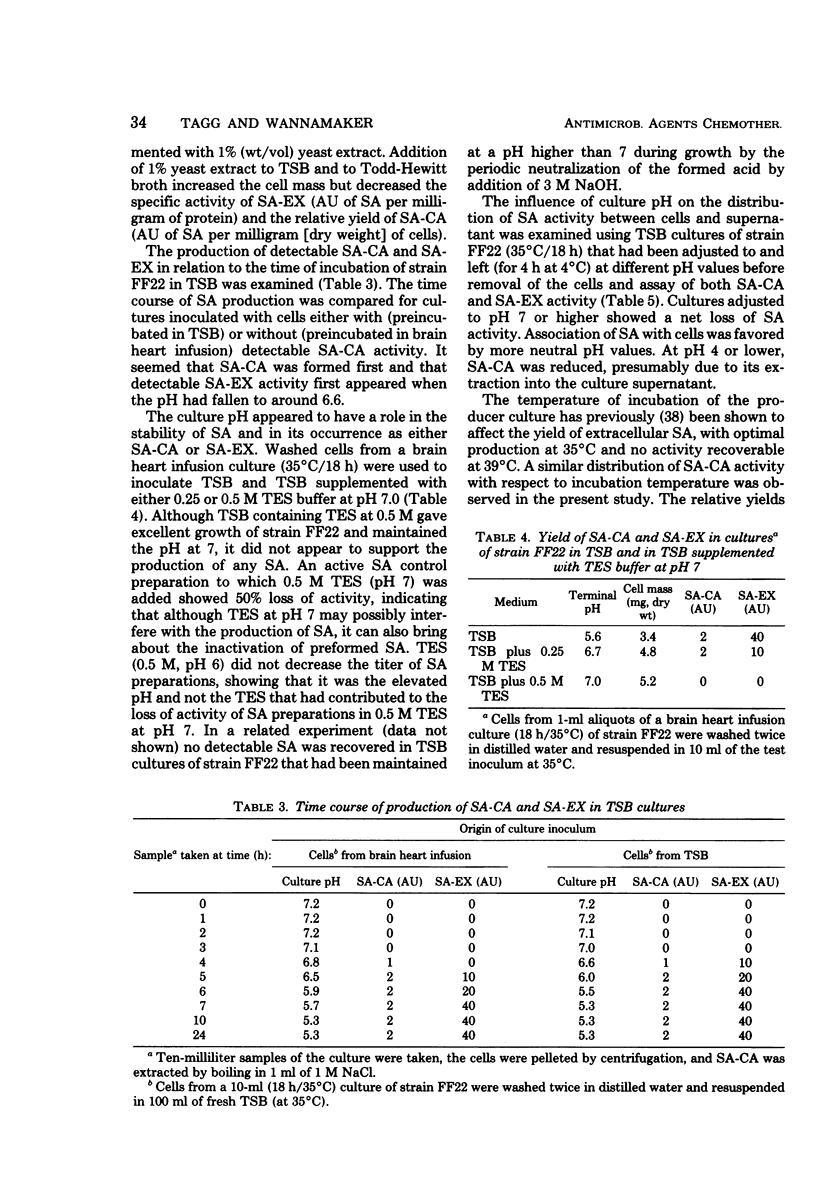
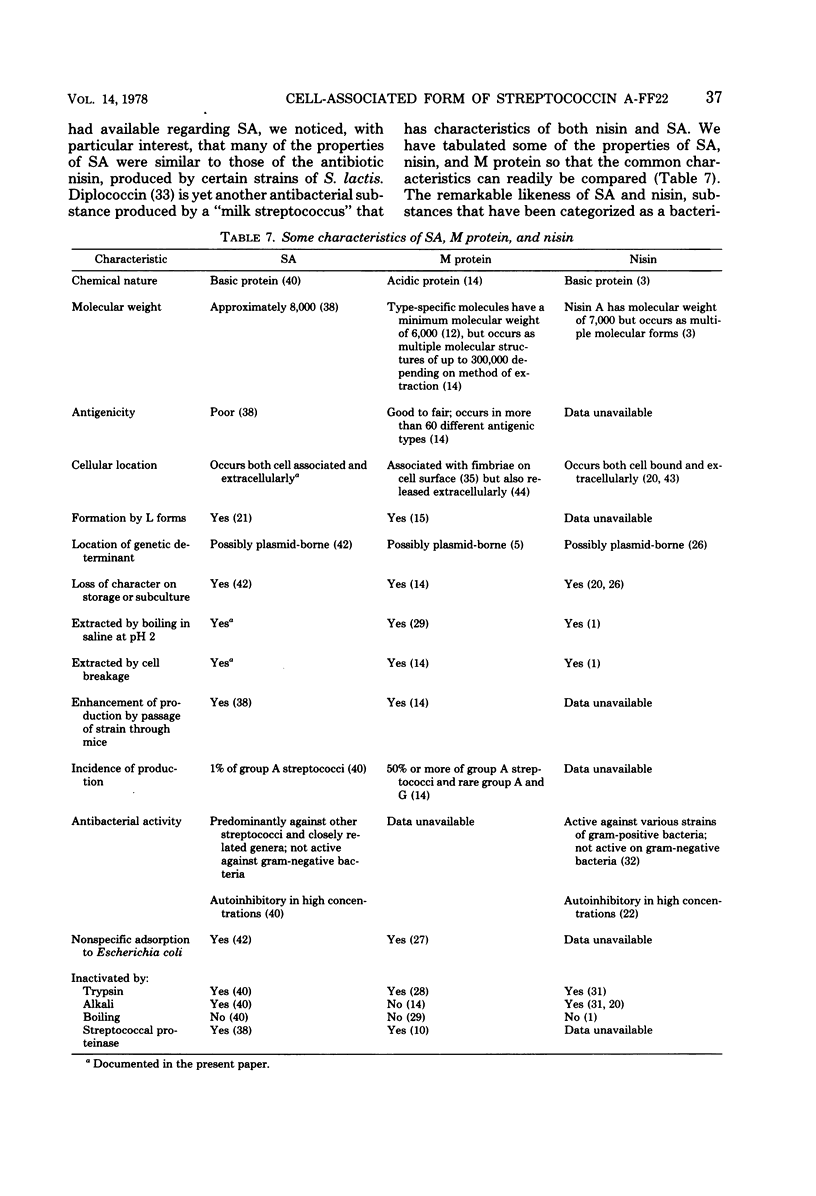
Streptococcin A-FF22 (SA) was shown to occur as both a cell-associated (SA-CA) and an extracellular (SA-EX) component of cultures of the producer bacterium, group A streptococcus strain FF22. SA-CA was solubilized by chemical, enzymatic, and mechanical procedures, similar to those used to release M protein. The independence of SA and M protein in strain FF22 was established by chromatographic separation of the two proteins on the basis of molecular weight and isoelectric point differences between the two substances. Media supporting optimal growth of strain FF22 did not necessarily favor SA production. SA was not produced either at elevated temperatures (39°C) or if the culture was maintained at pH 7 or higher. The release of SA from producer cells was enhanced at lower culture pH values. Much of the SA-CA activity seemed associated with the cell walls of the producer strain, and the nature of the binding appeared to be largely nonspecific in nature and attributable to electrostatic interaction.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARROW G. I. THE NATURE OF INHIBITORY ACTIVITY BY STAPHYLOCOCCUS AUREUS TYPE 71. J Gen Microbiol. 1963 Aug;32:255–261. doi: 10.1099/00221287-32-2-255. [DOI] [PubMed] [Google Scholar]
- Bailey F. J., Hurst A. Preparation of a highly active form of nisin from Streptococcus lactis. Can J Microbiol. 1971 Jan;17(1):61–67. doi: 10.1139/m71-010. [DOI] [PubMed] [Google Scholar]
- CHEESEMAN G. C., BERRIDGE N. J. Observations on the molecular weight and chemical composition of nisin A. Biochem J. 1959 Jan;71(1):185–194. doi: 10.1042/bj0710185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D. J., Robson R. M., Morris J. G. Purification of two Clostridium bacteriocins by procedures appropriate to hydrophobic proteins. Antimicrob Agents Chemother. 1975 Mar;7(3):256–264. doi: 10.1128/aac.7.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary P. P., Johnson Z., Wannamaker L. Genetic instability of M protein and serum opacity factor of group A streptocci: evidence suggesting extrachromosomal control. Infect Immun. 1975 Jul;12(1):109–118. doi: 10.1128/iai.12.1.109-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. O. Effect of culture medium composition and pH on the production of M protein and proteinase by group A Streptococci. J Bacteriol. 1969 Sep;99(3):737–744. doi: 10.1128/jb.99.3.737-744.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani A. S., Tom M. C., Law D. J. Viridins, bacteriocins of alpha-hemolytic streptococci: isolation, characterization, and partial purification. Antimicrob Agents Chemother. 1976 Jan;9(1):81–88. doi: 10.1128/aac.9.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandeu J. P. Chemical and immunological study of colicins e(1), k, a, and q. Infect Immun. 1971 Jan;3(1):1–9. doi: 10.1128/iai.3.1.1-9.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue H. D. Properties and comparative starch-gel electrophoresis of megacins from several Bacillus megaterium strains. J Gen Microbiol. 1972 Oct;72(3):473–483. doi: 10.1099/00221287-72-3-473. [DOI] [PubMed] [Google Scholar]
- FREIMER E. H., KRAUSE R. M., McCARTY M. Studies of L forms and protoplasts of group A streptococci. I. Isolation, growth, and bacteriologic characteristics. J Exp Med. 1959 Dec 1;110:853–874. doi: 10.1084/jem.110.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Bernheimer A. W. Purification and physical properties of group C streptococcal phage-associated lysin. J Exp Med. 1971 May 1;133(5):1105–1117. doi: 10.1084/jem.133.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Siviglia G., Zabriskie J. B. Streptococcal M protein extracted by nonionic detergent. I. Properties of the antiphagocytic and type-specific molecules. J Exp Med. 1976 Jul 1;144(1):32–53. doi: 10.1084/jem.144.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. Purification and partial characterization of a bacteriocin from Serratia marcescens. J Bacteriol. 1972 Jun;110(3):1001–1009. doi: 10.1128/jb.110.3.1001-1009.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N. M proteins of group A streptococci. Bacteriol Rev. 1974 Mar;38(1):57–86. doi: 10.1128/br.38.1.57-86.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano V. J., Hinsdill R. D. Characterization of a Staphylococcus aureus bacteriocin. J Bacteriol. 1970 Oct;104(1):117–125. doi: 10.1128/jb.104.1.117-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W. F. The nature of the colicin K of Escherichia coli K235. Proc Natl Acad Sci U S A. 1973 Mar;70(3):854–858. doi: 10.1073/pnas.70.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH A. Growth and nisin production of a strain of Streptococcus lactis. J Gen Microbiol. 1951 Feb;5(1):208–221. doi: 10.1099/00221287-5-1-208. [DOI] [PubMed] [Google Scholar]
- Hale E. M., Hinsdill R. D. Characterization of a bacteriocin from Staphylococcus aureus strain 462. Antimicrob Agents Chemother. 1973 Dec;4(6):634–640. doi: 10.1128/aac.4.6.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Hryniewicz W., Tagg J. R. Bacteriocin production by group a streptococcal L-forms. Antimicrob Agents Chemother. 1976 Dec;10(6):912–914. doi: 10.1128/aac.10.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A., Kruse H. Effect of secondary metabolites on the organisms producing them: effect of nisin on Streptococcus lactis and enterotoxin B on Staphylococcus aureus. Antimicrob Agents Chemother. 1972 Mar;1(3):277–279. doi: 10.1128/aac.1.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Konisky J. Electron microscopy of colicin I-producing cells. J Bacteriol. 1973 Jan;113(1):452–460. doi: 10.1128/jb.113.1.452-460.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Nature and properties of a Staphylococcus epidermidis bacteriocin. J Bacteriol. 1972 Oct;112(1):243–250. doi: 10.1128/jb.112.1.243-250.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D., de Windt F. Production and purification of a Staphylococcus epidermidis bacteriocin. J Bacteriol. 1972 Oct;112(1):235–242. doi: 10.1128/jb.112.1.235-242.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar W., Rajchert-Trzpil M., Dobrzański W. T. The effect of proflavin, ethidium bromide and an elevated temperature on the appearance of nisin-negative clones in nisin-producing strains of Streptococcus lactis. J Gen Microbiol. 1974 Aug;83(2):295–302. doi: 10.1099/00221287-83-2-295. [DOI] [PubMed] [Google Scholar]
- Krasner R. I., Heitmann R. The presence of a virulence factor in group A streptococcal acid extracts. 2. The enhancement of virulence of heterologous streptococci and other organisms by these extracts. J Infect Dis. 1968 Feb;118(1):39–46. doi: 10.1093/infdis/118.1.39. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C., PERLMANN G. E. Preparation and properties of type-specific M antigen isolated from a group A, type 1 hemolytic streptococcus. J Exp Med. 1952 Jul;96(1):71–82. doi: 10.1084/jem.96.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahmoud S. A., El-Sadek G. M., Dawood A. H. Mode of action of nisin on some representative bacterial spores. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1976;131(3):212–221. doi: 10.1016/s0044-4057(76)80091-3. [DOI] [PubMed] [Google Scholar]
- Oxford A. E. Diplococcin, an anti-bacterial protein elaborated by certain milk streptococci. Biochem J. 1944;38(2):178–182. doi: 10.1042/bj0380178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotta J., Krause R. M., Lancefield R. C., Everly W., Lackland H. New approaches for the laboratory recognition of M types of group A streptococci. J Exp Med. 1971 Nov 1;134(5):1298–1315. doi: 10.1084/jem.134.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Hsu K. C., Gotschlich E. C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969 Nov 1;130(5):1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocin of a group B streptococcus: partial purification and characterization. Antimicrob Agents Chemother. 1975 Jun;7(6):764–772. doi: 10.1128/aac.7.6.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W., Gray E. D. Group A streptococcal bacteriocin. Production, purification, and mode of action. J Exp Med. 1973 Nov 1;138(5):1168–1183. doi: 10.1084/jem.138.5.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., McGiven A. R. Assay system for bacteriocins. Appl Microbiol. 1971 May;21(5):943–943. doi: 10.1128/am.21.5.943-943.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Read R. S., McGiven A. R. Bacteriocin of a group A streptococcus: partial purification and properties. Antimicrob Agents Chemother. 1973 Sep;4(3):214–221. doi: 10.1128/aac.4.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Skjold S., Wannamaker L. W. Transduction of bacteriocin determinants in group A streptococci. J Exp Med. 1976 Jun 1;143(6):1540–1544. doi: 10.1084/jem.143.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Wannamaker L. W. Genetic basis of streptococcin A-FF22 production. Antimicrob Agents Chemother. 1976 Aug;10(2):299–306. doi: 10.1128/aac.10.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J., Hurst A. The location of nisin in the producer organism, Streptococcus lactis. J Gen Microbiol. 1968 Sep;53(2):171–179. doi: 10.1099/00221287-53-2-171. [DOI] [PubMed] [Google Scholar]
- Widdowson J. P., Maxted W. R., Grant D. L., Pinney A. M. The relationship between M-antigen and opacity factor in group A streptococci. J Gen Microbiol. 1971 Jan;65(1):69–80. doi: 10.1099/00221287-65-1-69. [DOI] [PubMed] [Google Scholar]



