Abstract
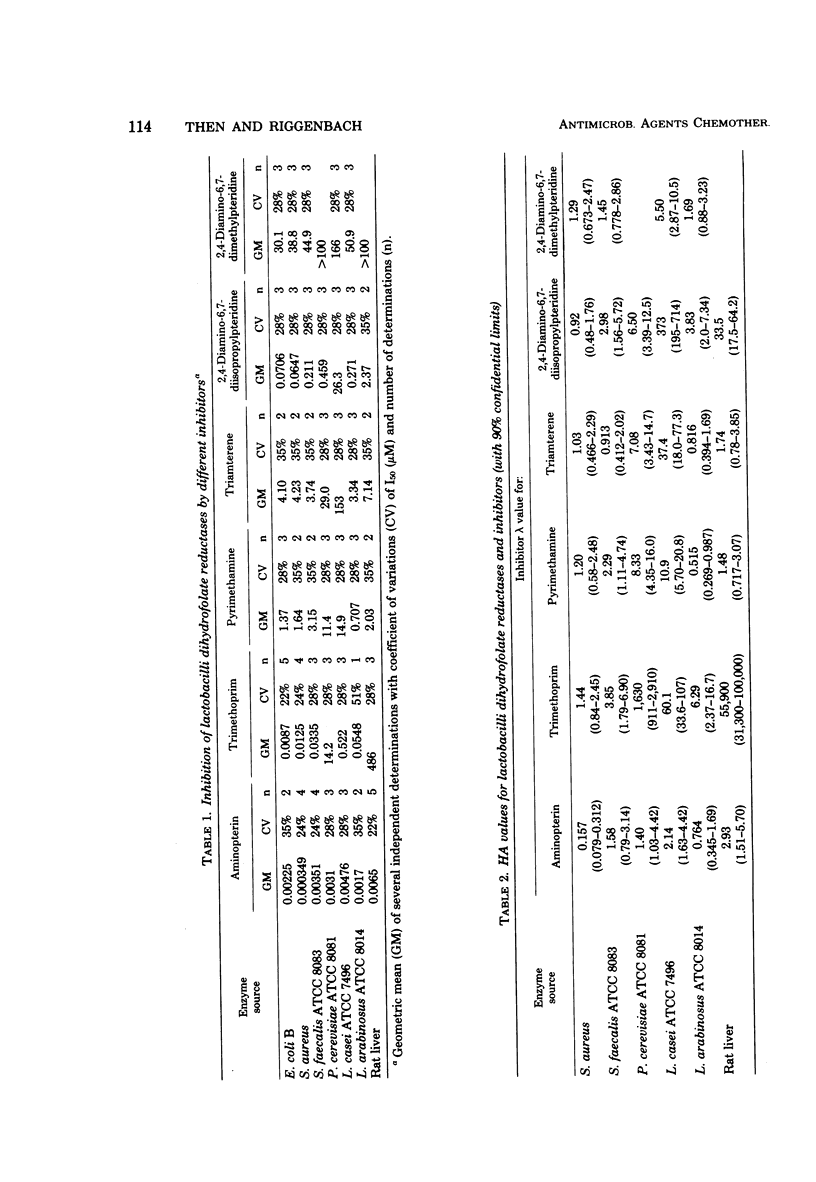
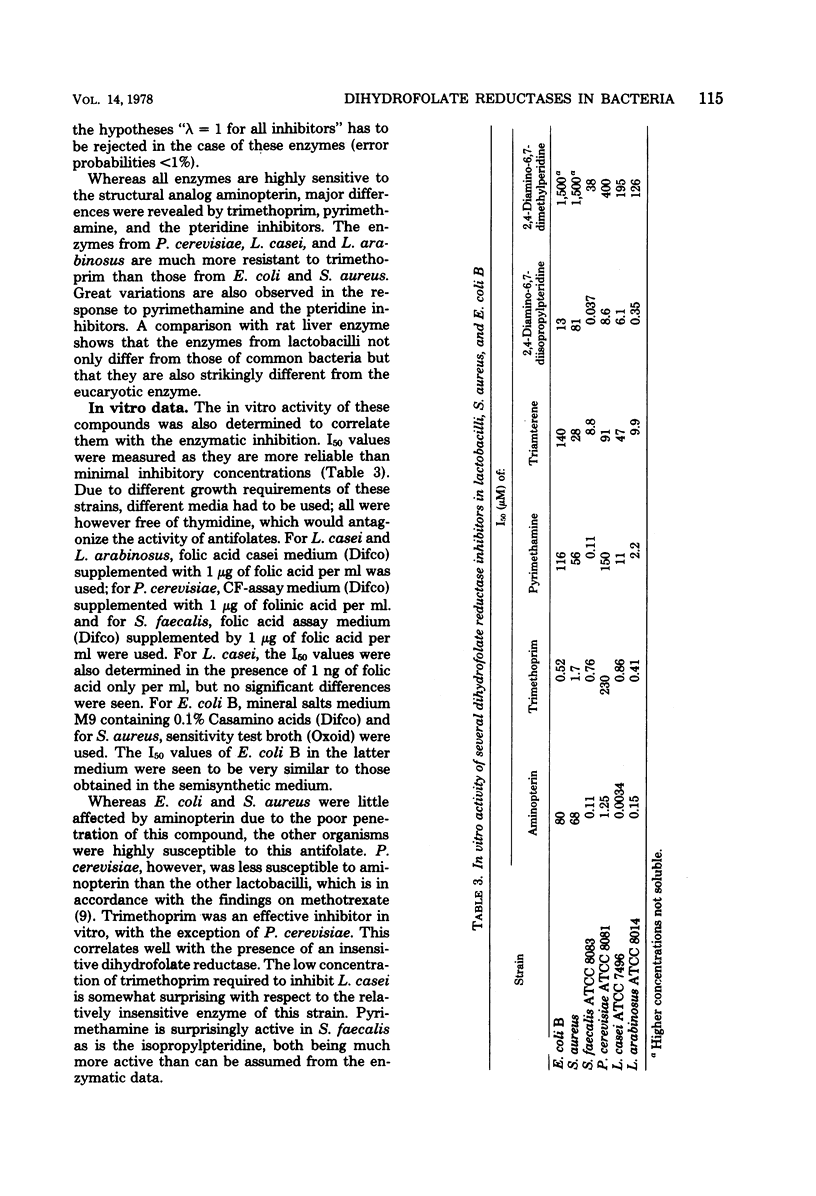
Dihydrofolate reductases from the folate-requiring strains Streptococcus faecalis ATCC 8043, Lactobacillus casei ATCC 7496, and Pediococcus cerevisiae ATCC 8081, as well as from Lactobacillus arabinosus, which is not dependent on exogenous folate, were isolated, and their properties were compared to reductases of Escherichia coli B, Staphylococcus aureus, and rat liver reductase. An inhibition profile with six different inhibitors revealed significant differences among all enzymes. All lactobacilli reductases are less sensitive to trimethoprim than the enzymes of E. coli and S. aureus, the reductase of P. cerevisiae requiring a concentration at least 1,000 times higher for 50% inhibition. Inhibition of growth of S. faecalis by pyrimethamine and 2,4-diamino-6,7-diisopropyl-pteridine was seen to be much stronger than was predicted from the enzymatic data.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batley K. E., Morris H. R. Dihydrofolate reductase from Lactobacillus casei: N-terminal sequence and comparison with the substrate binding region of other reductases. Biochem Biophys Res Commun. 1977 Apr 25;75(4):1010–1014. doi: 10.1016/0006-291x(77)91482-6. [DOI] [PubMed] [Google Scholar]
- Bitar K. G., Blankenship D. T., Walsh K. A., Dunlap R. B., Reddy A. V., Freisheim J. H. Amino acid sequence of dihydrofolate reductase from an amethopterin-resistant strain of Lactobacillus casei. FEBS Lett. 1977 Aug 1;80(1):119–122. doi: 10.1016/0014-5793(77)80420-1. [DOI] [PubMed] [Google Scholar]
- Burchall J. J. Comparative biochemistry of dihydrofolate reductase. Ann N Y Acad Sci. 1971 Nov 30;186:143–152. doi: 10.1111/j.1749-6632.1971.tb46965.x. [DOI] [PubMed] [Google Scholar]
- Burchall J. J., Hitchings G. H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965 Sep;1(2):126–136. [PubMed] [Google Scholar]
- Dewey V. C., Kidder G. W. Pteridine diuretics as biopterin antagonists. Biochem Pharmacol. 1974 Feb 15;23(4):773–779. doi: 10.1016/0006-2952(74)90207-x. [DOI] [PubMed] [Google Scholar]
- Genther C. S., Smith C. S. Antifolate studies. Activities of 40 potential antimalarial compounds against sensitive and chlorguanide triazine resistant strains of folate-requiring bacteria and Escherichia coli. J Med Chem. 1977 Feb;20(2):237–243. doi: 10.1021/jm00212a010. [DOI] [PubMed] [Google Scholar]
- Hynes J. B., Eason D. E., Garrett C. M., Colvin P. L., Jr Quinazolines as inhibitors of dihydrofolate reductase. 4. Classical analogues of folic and isofolic acids. J Med Chem. 1977 Apr;20(4):588–591. doi: 10.1021/jm00214a030. [DOI] [PubMed] [Google Scholar]
- Mandelbaum-Shavit F., Grossowicz N. Carrier-mediated transport of folate in a mutant of Pediococcus cerevisiae. J Bacteriol. 1973 May;114(2):485–490. doi: 10.1128/jb.114.2.485-490.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbaum-Shavit F., Grossowicz N. Dihydrofolate reductase in Pediococcus cerevisiae strains susceptible and resistant to amethopterin. Antimicrob Agents Chemother. 1974 Sep;6(3):369–371. doi: 10.1128/aac.6.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbaum-Shavit F., Grossowicz N. Transport of folinate and related compounds in Pediococcus cerevisiae. J Bacteriol. 1970 Oct;104(1):1–7. doi: 10.1128/jb.104.1.1-7.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbaum-Shavit F. Resistance of Pediococcus cerevisiae to amethopterin as a consequence of changes in enzymatic activity and cell permeability. I. Dihydrofolate reductase, thymidylate synthetase and formyltetrahydrofolate synthetase in amethopterin-resistant and -sensitive strains of Pediococcus cerevisiae. Biochim Biophys Acta. 1976 May 28;428(3):664–673. doi: 10.1016/0304-4165(76)90196-3. [DOI] [PubMed] [Google Scholar]
- Mathews C. K. Evidence that bacteriophage-induced dihydrofolate reductase in a viral gene product. J Biol Chem. 1967 Sep 25;242(18):4083–4086. [PubMed] [Google Scholar]
- McAllister T. A. Resistance to co-trimoxazole. Scand J Infect Dis Suppl. 1976;(8):29–38. [PubMed] [Google Scholar]
- Merkel J. R. Influence of salts on the vibriostatic action of 2,4-diamino-6,7-diisopropyl pteridine. Arch Mikrobiol. 1972;81(4):379–382. doi: 10.1007/BF00412644. [DOI] [PubMed] [Google Scholar]
- Pattishall K. H., Acar J., Burchall J. J., Goldstein F. W., Harvey R. J. Two distinct types of trimethoprim-resistant dihydrofolate reductase specified by R-plasmids of different compatibility groups. J Biol Chem. 1977 Apr 10;252(7):2319–2323. [PubMed] [Google Scholar]
- Peterson D. L., Gleisner J. M., Blakley R. L. The structure of the mutant dihydrofolate reductase from Streptococcus faecium. Amino acid sequence of peptide CNBr 7 and complete sequence of the protein. J Biol Chem. 1975 Jul 10;250(13):4945–4954. [PubMed] [Google Scholar]
- Roberts D., Hall T. C. Drug interactions: pteridine diuretics and antifols. I. The inhibition of dihydrofolate reductase by analogues of 2,4,7-triaminopteridines. J Clin Pharmacol J New Drugs. 1968 Jul-Aug;8(4):217–218. doi: 10.1002/j.1552-4604.1968.tb00097.x. [DOI] [PubMed] [Google Scholar]
- SMITH C. C., IHRIG J. The pharmacological basis for the prolonged antimalarial activity of pyrimethamine. Am J Trop Med Hyg. 1957 Jan;6(1):50–57. doi: 10.4269/ajtmh.1957.6.50. [DOI] [PubMed] [Google Scholar]
- Smith C. C., Genther C. S. Cross-resistance and collateral susceptibility to antifolic antimalarial compounds. Antimicrob Agents Chemother. 1972 Sep;2(3):103–108. doi: 10.1128/aac.2.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D., Phillips A. W., Burchall J. J. The amino-acid sequence of the dihydrofolate reductase of a trimethoprim-resistant strain of Escherichia coli. Eur J Biochem. 1977 Feb;72(3):613–624. doi: 10.1111/j.1432-1033.1977.tb11284.x. [DOI] [PubMed] [Google Scholar]
- WOOD R. C., FERONE R., HITCHINGS G. H. The relationship of cellular permeability to the degree of inhibition by amethopterin and pyrimethamine in several species of bacteria. Biochem Pharmacol. 1961 May;6:113–124. doi: 10.1016/0006-2952(61)90155-1. [DOI] [PubMed] [Google Scholar]
- WOOD R. C., HITCHINGS G. H. A study of the uptake and degradation of folic acid, citrovorum factor, aminopterin, and pyrimethamine by bacteria. J Biol Chem. 1959 Sep;234:2381–2385. [PubMed] [Google Scholar]
- Williams M. N. Effect of N-bromosuccinimide modification on dihydrofolate reductase from a methotrexate-resistant strain of Escherichia coli. Activity, spectrophotometric, fluorescence and circular dichroism studies. J Biol Chem. 1975 Jan 10;250(1):322–330. [PubMed] [Google Scholar]


