Abstract
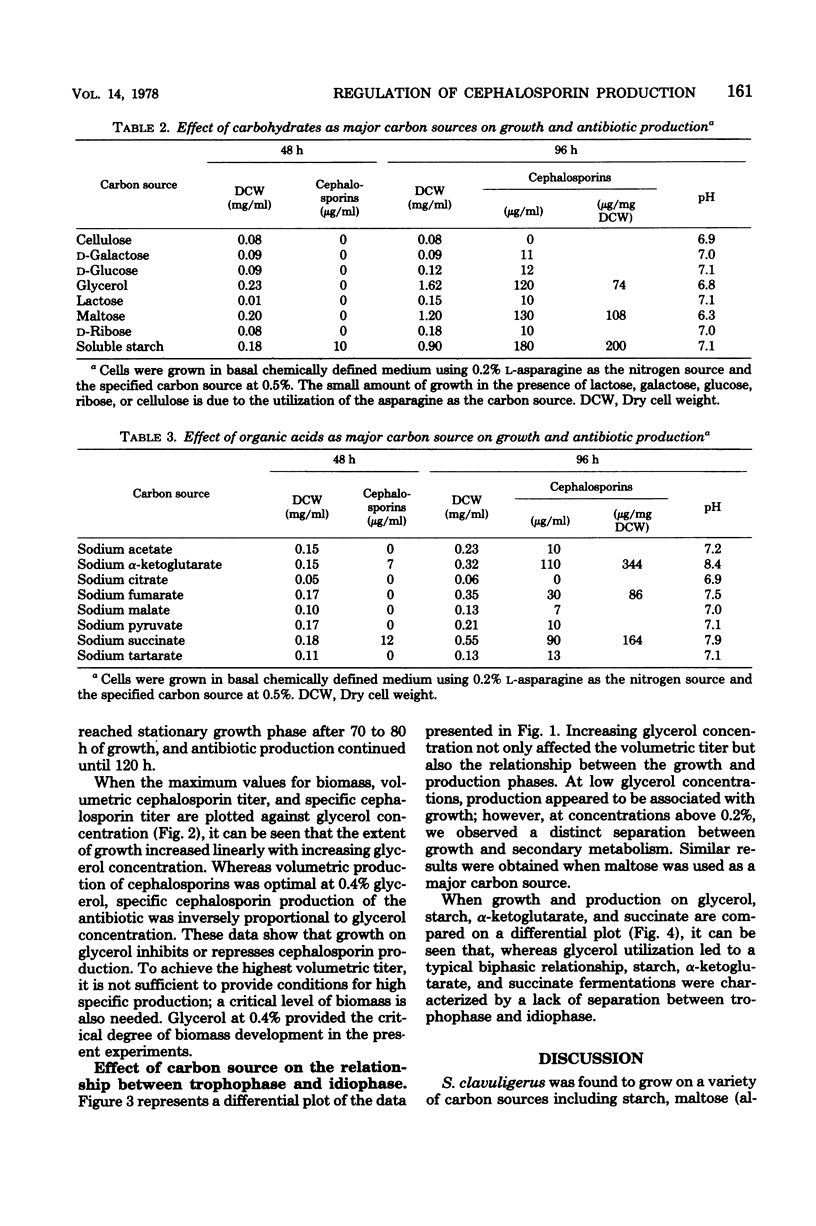
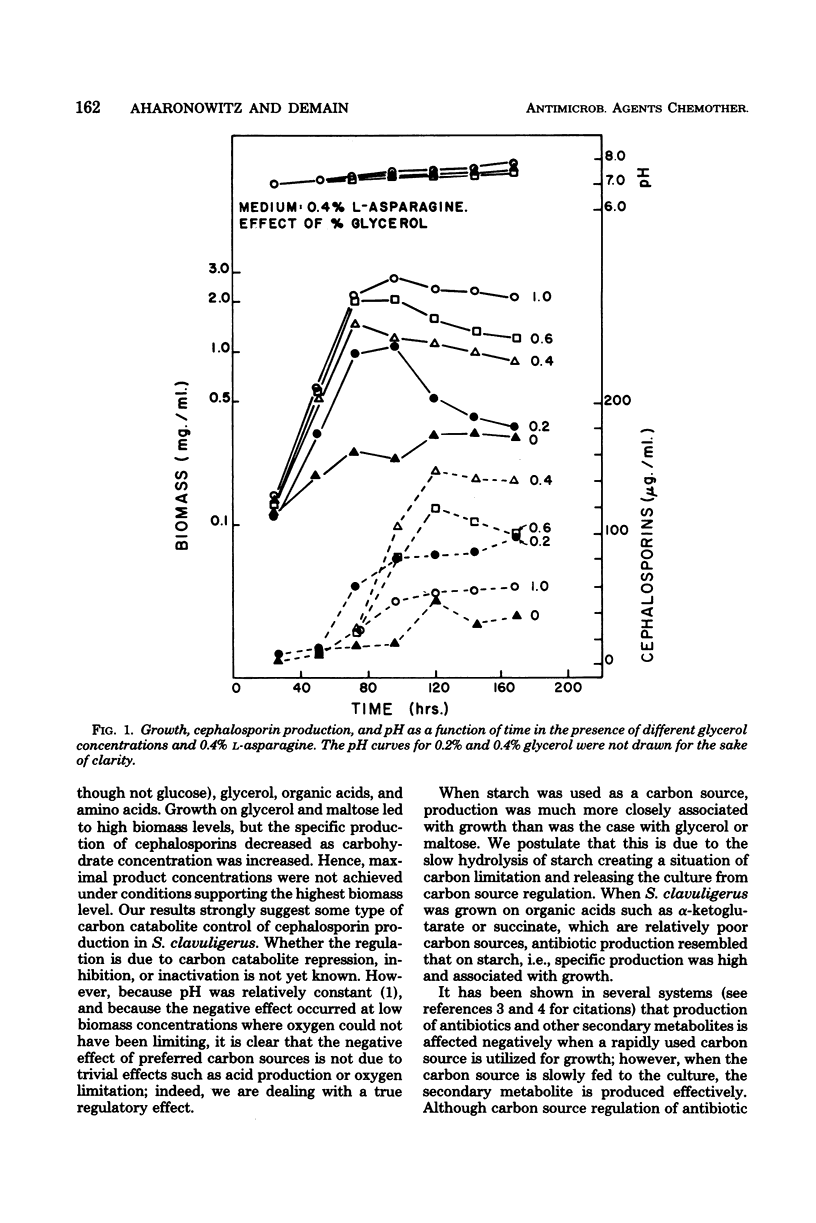
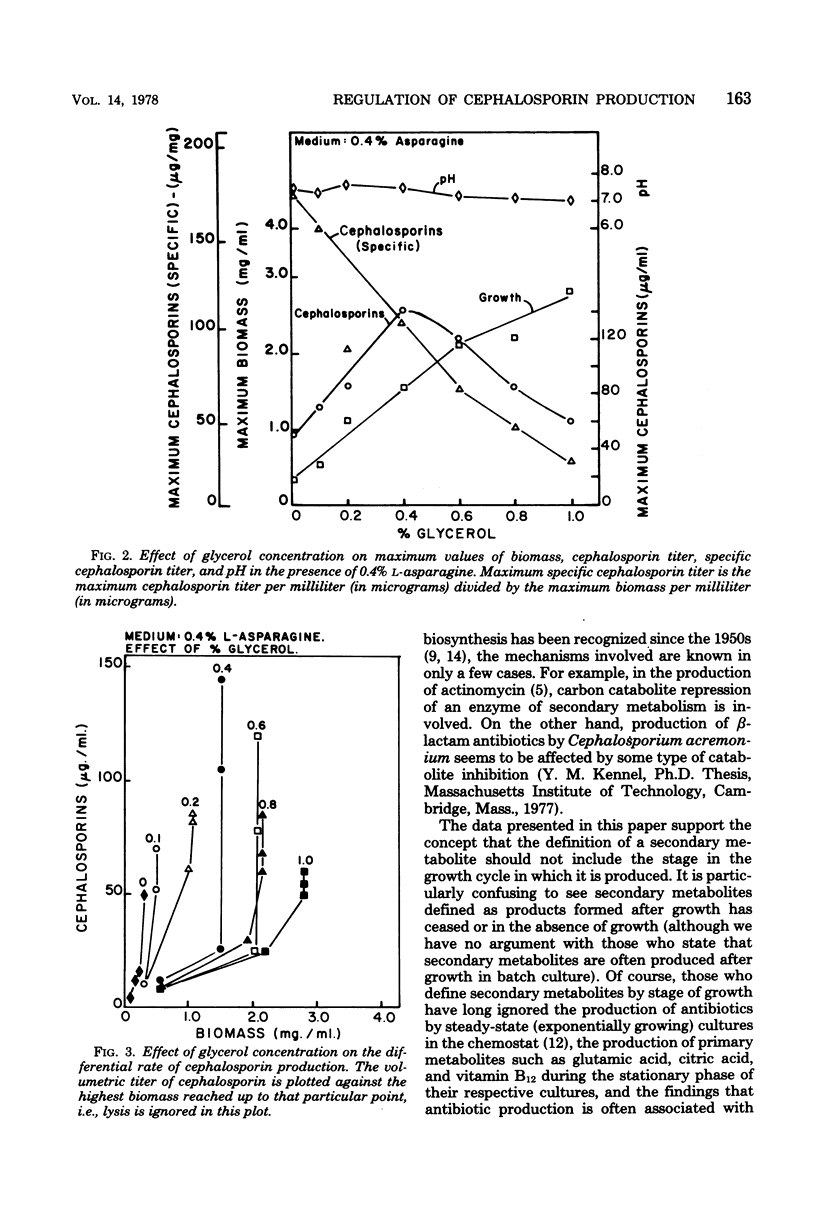
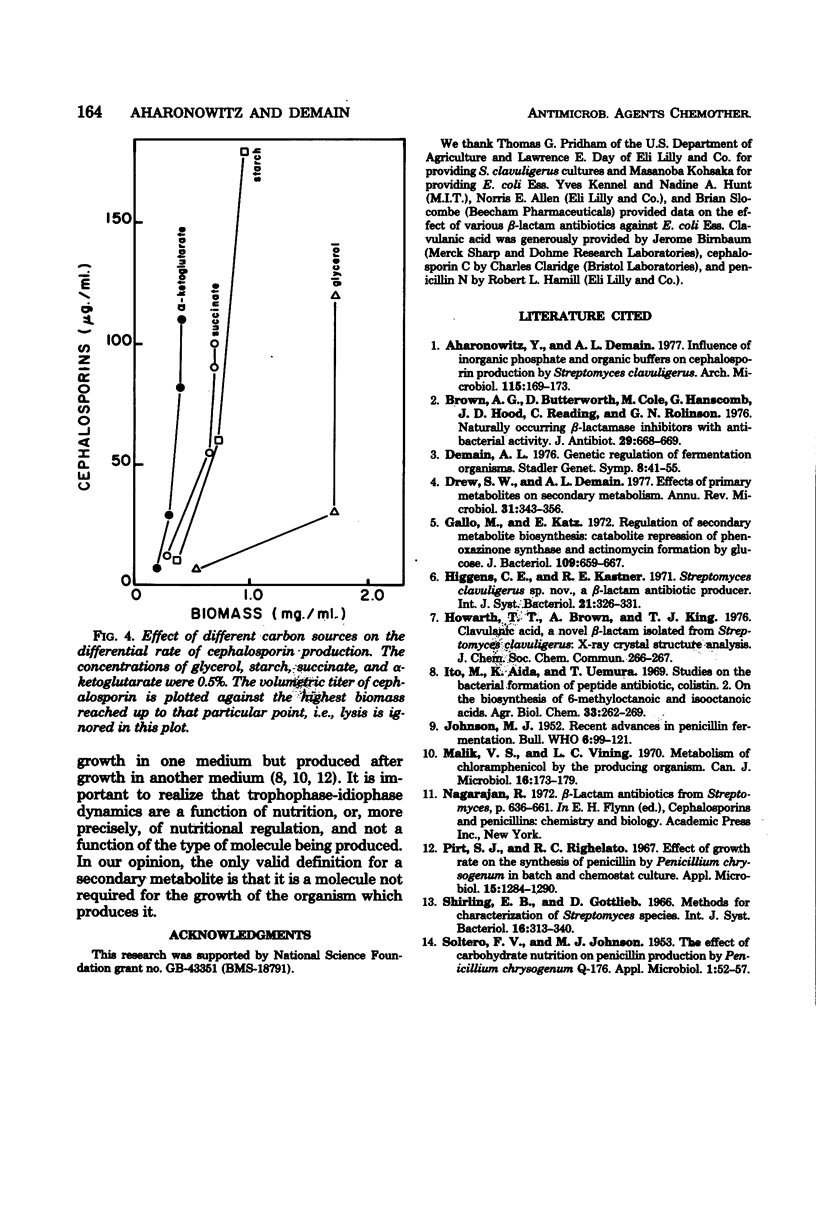
Cephalosporin production by Streptomyces clavuligerus is regulated by some type of carbon catabolite control. Increasing concentrations of preferred carbon sources, such as glycerol and maltose, decreased production of the antibiotics. Poorer carbon sources, such as α-ketoglutarate and succinate, led to high specific production of cephalosporins and shifted the dynamics of fermentation to a greater degree of association with growth. The results support the concept that the phase in which a product is made by a microorganism is not a function of the particular molecule produced, but rather of the nutritional environment presented to the organism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y., Demain A. L. Influence of inorganic phosphate and organic buffers on cephalosporin production by Streptomyces clavuligerus. Arch Microbiol. 1977 Nov 18;115(2):169–173. doi: 10.1007/BF00406371. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Butterworth D., Cole M., Hanscomb G., Hood J. D., Reading C., Rolinson G. N. Naturally-occurring beta-lactamase inhibitors with antibacterial activity. J Antibiot (Tokyo) 1976 Jun;29(6):668–669. doi: 10.7164/antibiotics.29.668. [DOI] [PubMed] [Google Scholar]
- Drew S. W., Demain A. L. Effect of primary metabolites on secondary metabolism. Annu Rev Microbiol. 1977;31:343–356. doi: 10.1146/annurev.mi.31.100177.002015. [DOI] [PubMed] [Google Scholar]
- Gallo M., Katz E. Regulation of secondary metabolite biosynthesis: catabolite repression of phenoxazinone synthase and actinomycin formation by glucose. J Bacteriol. 1972 Feb;109(2):659–667. doi: 10.1128/jb.109.2.659-667.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON M. J. Recent advances in penicillin fermentation. Bull World Health Organ. 1952;6(1-2):99–121. [PMC free article] [PubMed] [Google Scholar]
- Malik V. S., Vining L. C. Metabolism of chloramphenicol by the producing organism. Can J Microbiol. 1970 Mar;16(3):173–179. doi: 10.1139/m70-030. [DOI] [PubMed] [Google Scholar]
- Pirt S. J., Righelato R. C. Effect of Growth Rate on the Synthesis of Penicillin by Penicillium chrysogenum in Batch and Chemostat Cultures. Appl Microbiol. 1967 Nov;15(6):1284–1290. doi: 10.1128/am.15.6.1284-1290.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLTERO F. V., JOHNSON M. J. The effect of the carbohydrate nutrition on penicillin production by Penicillium chrysogenum Q-176. Appl Microbiol. 1953 Jan;1(1):52–57. doi: 10.1128/am.1.1.52-57.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]


