Abstract
α-Klotho was first identified as the responsible gene in a mutant mouse line whose disruption results in a variety of premature aging-related phenotypes. α-Klotho has been shown to participate in the regulation of parathyroid hormone secretion and trans-epithelial transport of Ca2+ in the choroid plexus and kidney. α-Klotho, acting as a cofactor for FGF23, is also a major regulator of vitamin D biosynthesis and phosphate reabsorption in the kidney. These suggest that α-Klotho is a key player that integrates a multi-step regulatory system of calcium and phosphate homeostasis. Collectively, the molecular function of α-Klotho reveals a new paradigm that may change current concepts in mineral homeostasis and give rise to new insights in this field.
Keywords: mineral homeostasis, α-Klotho, FGF23, PTH, Vitamin D, Na+, K+-ATPase
Introduction
In Greek mythology, life span is controlled by the three daughters of Zeus and Themis, namely, Klotho who combs and spins the thread of life, Lachesis who determines the length of life by measuring the length of thread, and Athropos who cuts the string to bring a life to an end. In science, the name Klotho was conferred to a gene that was fortuitously discovered in 1997.
From the analysis of insertion mutant mouse lines, we generated the klotho mutant mouse, a short-lived model mouse that displays a variety of premature aging-related phenotypes.1) A homologue was subsequently identified and named β-klotho.2) To avoid confusion, we refer to the original gene as α-klotho (α-kl). The α-kl gene is predominantly expressed in tissues that are involved in the regulation of calcium and phosphate homeostasis,1),3) and α-kl mutant mice exhibited multiple phenotypes related to disruption of mineral homeostasis including ectopic calcification in a variety of soft tissues, decreased bone mineral density, hypercalcemia and severe hyperphosphatemia in association with increased concentrations of 1,25(OH)2D1),4) and serum FGF23.5) These led to the prediction that α-Klotho (α-Kl) is involved in calcium and phosphorus homeostasis.
Calcium ion (Ca2+) is a key mineral composition because of its diverse intra-and extracellular roles.6) Among intracellular Ca2+, the cytosolic free calcium concentration ([Ca2+]i) is an important second messenger and is the cofactor for proteins and enzymes that regulate a variety of cellular functions, including hormonal secretion, neuro-transmission, muscle contraction, cell motility, glycogen metabolism and cellular proliferation.6) The [Ca2+]i in resting cells is ~100 nM. It is regulated by a series of channels, pumps, and other transport mechanisms that control the movements of Ca2+ into and out of the cell and between various intracellular compartments.7) Consonant with its role as a second messenger, the [Ca2+]i in activated cells can rise by 10- to 100-fold due to the uptake of extracellular Ca2+ and/or release of Ca2+ from cellular stores, such as the endoplasmic reticulum. Despite the importance of intracellular Ca2+ in cellular metabolism, this compartment comprises less than 1% of the total body calcium content in mammalian species.8)
Extracellular Ca2+ ([Ca2+]0) serves as a cofactor for adhesion molecules, clotting factors, and other proteins; regulates neuronal excitability; and is an essential part of the mineral phase of bone. Particularly, the cloning of a G protein-coupled calcium sensing receptor (CaR) has proved that the calcium ion can indeed serve as an extracellular first messenger.9) In contrast to intracellular Ca2+, the [Ca2+]o is tightly controlled at ~1{1–3 mM in mammals.8),10) Such accurate control of the [Ca2+]o ensures a steady supply of Ca2+ for intracellular functions. The total amount of soluble extracellular Ca2+, however, constitutes only a minute fraction of the total calcium content (e.g. mammals ~0.1%). The highest fraction of total body calcium resides as calcium phosphate salts within the skeleton where it provides a structural framework that protects critical bodily structures and facilitates locomotion as well as constituting a large reservoir of calcium and phosphate ions for times when intestinal absorption and renal conservation of these ions are not sufficient for maintenance of constancy of the extracellular Ca2+ and phosphate concentrations.8),10),11)
A complex homeostatic system has evolved in mammalian species, which is designed to maintain near constancy of the [Ca2+]o.8),10)–12) This system includes two essential components. The first component is comprised of cell types that sense changes in the [Ca2+]o and respond with appropriate alterations in their secretion of Ca2+-regulating hormones. The parathyroid glands are key sensors of variations in the [Ca2+]o in mammalian species, responding with changes in parathyroid hormone (PTH) secretion that are inversely related to the ambient ionized calcium concentration.13) In contrast to the parathyroid cell, high [Ca2+]o stimulate hormonal release from calcitonin (CT)-secreting parafollicular or C-cells of the thyroid gland.14) The second essential component of the homeostatic system is comprised of the effecter systems, specialized cells in the kidneys, bone and intestine that respond to these calcitropic hormones with changes in the transport of mineral ions so as to restore the [Ca2+]o toward the normal state.
Calcium homeostasis is largely regulated by the actions of two major hormones, namely PTH and 1,25-dihydroxyvitamin D (1,25(OH)2D) that trigger the response of target cells by binding to their receptors and subsequently controlling the intake, metabolism, and excretion of calcium. Furthermore, the synthesis and secretion of these hormones are mutually activated or suppressed by each other, and are also controlled by [Ca2+]o which is monitored by calcium sensing receptors.15),16) In response to slight decrements in the level of the [Ca2+]o, PTH release rapidly increases within second order.17)–19) Renal actions of PTH include a reduction in tubular reabsorption of phosphate ions as well as an increase in distal tubular reabsorption of Ca2+, which takes place within minutes.15) PTH also acts on bone cells to enhance the release of Ca2+ from the skeleton within 1–2 h.8),10),11),20),21) When hypocalcemia is more prolonged, elevation of PTH levels persist for several hours or more and then the circulating 25-hydroxyvitamin D [25(OH)D] is activated by the renal 25-hydroxyvitamin D-1α-hydroxylase (Cyp27b1) to form 1,25(OH)2D22)–24) that in turn acts on specific receptors in the intestine25),26) to promote gastrointestinal absorption of Ca2+ and phosphate ions. Hypercalcemia produces the opposite series of changes in the Ca2+ homeostatic system. Reductions in the circulating levels of PTH promote renal Ca2+ wasting and phosphate retention as well as diminished skeletal release of mineral ions and eventually leads to reduction of gastrointestinal absorption of these ions through decreased synthesis of 1,25(OH)2D. Therefore, as with hypocalcemia, the response of the homeostatic mechanisms to hypercalcemia tends to restore [Ca2+]o concentrations toward the normal state.
The maintenance of normal phosphate (Pi) homeostasis is critical for diverse physiologic processes including cell signaling, nucleic acid synthesis, energy homeostasis, formation of lipid bilayers, and bone formation.27)–31) Owing to the importance of Pi in diverse biological processes, decrements in serum Pi concentration and negative Pi balance lead to serious diseases. Acute decreases in serum Pi concentration result in myopathy, cardiac dysfunction, abnormal neutrophil function, platelet dysfunction and red-cell membrane fragility.32) Its importance is also illustrated by chronic disorders characterized by hypophosphatemia due to excessive renal phosphate loss.31) In fact, affected patients develop rickets with diminished bone strength, deformity, short structure, and bone pain. Conversely, high phosphate levels may have adverse physiologic effects. Patients with chronic kidney disease often develop hyperphosphatemia due to impaired renal clearance, and subsequently progress to hyperparathyroidism and renal osteodystrophy.33)
In mammals, absorption and reabsorption of Pi take place primarily in the intestine and kidney, respectively. Because the movement of Pi into the cell does not occur by simple diffusion, various H+- or Na+-coupled Pi cotransporters mediate the transport of Pi across the cell membrane.34) The Na+-coupled Pi (NaPi) co-transporters that are important in Pi uptake in vertebrates belong to two large families, the NaPi type II and the NaPi type III families. The major role of NaPi type II has been clearly demonstrated in Npt2−/− mice. Compared to wild-type mice, the Npt2−/− mice display a higher urinary excretion of Pi and lower NaPi co-transport activity (mainly mediated by NaPi-IIa and additionally by NaPi-IIc) in the brush border membranes (BBM) of renal proximal tubules.35) This suggests that the regulation of Pi reabsorption is achieved mainly by controlling the apical expression of NaPi-IIa in BBM of renal proximal tubules.36),37) Consistently, phosphaturic factors reduce the expression of NaPi-IIa, whereas factors that increase renal Pi reabsorption increase NaPi-IIa in BBM. Therefore, control mechanisms that regulate apical expression and membrane retrieval of NaPi-IIa are essential to our understanding of how Pi homeostasis is achieved.
NaPi co-transport activity and organ specific Pi absorptive processes are regulated by PTH and 1,25(OH)2D, which interact in a coordinate fashion to regulate Pi homeostasis. In the kidney, various factors, most importantly PTH, influence the efficiency of renal Pi reabsorption.38) Animals fed with a low-Pi diet have decreased serum Pi concentrations that are associated with a reciprocal increase in circulating Ca2+ concentrations. The increase in serum Ca2+ inhibits PTH release, which in turn reduces the renal excretion of Pi into the urine. In addition, a low-Pi diet and reductions in serum Pi are associated with increased 1,25(OH)2D synthesis,39) which consequently restores serum Pi concentrations to normal state by increasing intestinal (mediated via NaPi-IIb) and renal Pi recoveries. It is, however, difficult to discriminate between direct versus indirect effects of 1,25(OH)2D on renal Pi reabsorption, as in vivo, the vitamin D status is closely associated with alterations in plasma calcium and PTH concentrations. Conversely, when animals are fed a high-Pi diet, serum Ca2+ concentrations decrease, and PTH release is increased. Recently, Martin et al. suggested that changes in PTH secretion in response to dietary Pi occur rapidly (within 10 min) and independently of changes in serum Pi or Ca2+ concentrations,40) suggesting that a signal emanating from the intestine may affect PTH secretion.41) An elevation in serum Pi after a high-Pi meal also reduces 1,25(OH)2D synthesis and intestinal Pi absorption.
Other than PTH and 1,25(OH)2D, several phospaturic peptides such as fibroblast growth factor 23 (FGF23), secreted frizzled related protein 4, matrix extracellular phosphoglycoprotein, and FGF7 have been demonstrated to inhibit the activity of NaPi-IIa co-transporters in renal epithelial cells.38),42)–46) Furthermore, current studies have strongly suggested the importance of the network of proteins interacting with NaPi-IIa co-transporters in apical membrane expression of NaPi-IIa co-transporters and the fate of endocytosed NaPi-IIa co-transporters.47)
Intensive study on calcium homeostasis over the past several decades has established a systematized understanding of its roles in living phenomena, leaving us with the impression that this field is fairly defined and understood. On the contrary, our present knowledge about phosphate homeostasis is fragmentary and still in a premature stage. However, the currently demonstrated molecular function of α-Kl has given new insights into this field. Here, the biological roles and molecular functions of α-Kl in calcium and phosphate homeostasis are summarized.
The discovery of α-Klotho
α-kl mutant mice display a variety of accelerated aging-related disorders, including hypoactivity, sterility, skin thinning, decreased bone mineral density, vascular calcifications, ectopic calcification in various soft tissues (lung, kidney, stomach, heart, and skin), defective hearing, thymus atrophy, pulmonary emphysema, ataxia, and abnormality of pituitary gland, as well as hypoglycemia, hypercalcemia and severe hyperphosphatemia in association with increased concentrations of 1,25(OH)2D.1) The responsible gene for these phenotypes was termed α-kl, and shown to encode a type I membrane protein with an extra-cellular domain that exhibits significant similarity to β-glycosidases, enzymes involved in digestion of the sugar moiety of substrates.48)α-Kl was therefore predicted to be present on the cell surface, however large amounts of α-Kl are detectable as dual forms in the cytoplasm. The 120 kDa premature form of α-Kl resides mainly in the ER, while the 135 kDa is a mature protein that is cleaved and secreted into the blood, cerebrospinal fluid (CSF), and urine.49),50) This suggests α-Kl may have dual actions depending on its intracellular and secreted forms.
The α-kl gene is predominantly expressed in the parathyroid glands, kidney and the choroid plexus.1),3) The parathyroid glands play a key role in systemic calcium homeostasis by monitoring the concentration of [Ca2+]o through the CaR and secreting appropriate levels of PTH to maintain normal [Ca2+]o concentrations.9),15),16) In the kidney, α-Kl is exclusively co-expressed with calcium permeable Transient Receptor Potential V5 (TRPV5) channels, Na+/Ca2+ exchanger 1 (NCX1) and calbindin-D28K (a vitamin D sensitive intracellular Ca2+ transporting protein) in a specialized region of the nephron segments where transepithelial Ca2+ reabsorption is actively regulated. This co-localization is believed to be important for the homeostatic control of [Ca2+]e by regulating Ca2+ reabsorption in the kidney. Indeed, mice lacking TRPV5 display diminished renal Ca2+ reabsorption, which causes severe hypercalciuria and compensatory intestinal uptake of dietary Ca2+.51) The choroid plexus in many respects functions as “the kidney of the brain”.52) Its main function is the secretion of CSF, which involves the movement of water by osmosis and the unidirectional transport of ions including calcium.53) The expression of α-Kl in tissues involved in calcium metabolism, led to the prediction that α-Kl might be involved in calcium homeostasis.54)
Discovery of α-Klotho mutations in human
Familial tumoral calcinosis is characterized by ectopic calcifications and hyperphosphatemia due to inactivating mutations in Fgf23 or UDP-N-acetyl-α-D-galactosamine: polypeptide N-acetyl-galactosaminyltransferase 3 (GALNT3). FGF23 is a hormone that promotes renal phosphate excretion by decreasing phosphate reabsorption in the proximal tubule and also reduces circulating 1,25(OH)2D by decreasing both the biosynthesis and metabolism of 1,25(OH)2D.55) GALNT 3 is a Golgi-associated enzyme that selectively O-glycosylates a furin-like convertase recognition sequence in FGF23, thereby allowing secretion of intact FGF23 by preventing proteolytic processing.56) Therefore, dysfunction of either FGF23 or GALNT 3 decreases circulating bioactive FGF23. Surprisingly, the α-kl deficient phenotype largely overlaps with the phenotype of Fgf23-null mice.1),55) These indicate functional crosstalk between α-Kl and FGF23 and lead us to speculate that α-kl mutation(s) might also be found in the genome of patients with tumoral calcinosis.
Ichikawa et al. reported a homozygous missense mutation (H193R) in the α-kl gene of a 13-year-old girl who presented with severe tumoral calcinosis with dural and carotid artery calcifications.57) Mapping of H193R mutation onto the crystal structure of myrosinase, a plant homolog of α-Kl, revealed that this histidine residue was at the base of the deep catalytic cleft and mutation of this histidine to arginine should destabilize α-Kl. Intra-cellular abundance and secretion of H193R α-Kl were thus markedly reduced, resulting in the diminished ability of FGF23. Similar to mice deficient in α-kl or Fgf23, this patient exhibited defects in mineral ion homeostasis with hypercalcemia, marked hyperphosphatemia and subsequent ectopic calcifications in soft tissues, as well as the elevation of circulating FGF23, PTH and 1,25(OH)2D in serum.
Conversely, a mutation that causes an increase in soluble α-Kl shows adverse defects in mineral ion homeostasis. Recently, our collaborator Brownstein et al. at Yale University discovered a new human disease featuring hypophosphatemic rickets with marked parathyroid hyperplasia and decrease of circulating 1,25(OH)2D, as well as the elevation of FGF23 and PTH.58) In this patient, higher circulatory FGF23, in combination with α-Kl, might have led to remarkable suppressions both of 1,25(OH)2D synthesis and renal phosphate re-absorption, resulting in the vast wasting of phosphate into urine and the decrease of serum 1,25(OH)2D levels. Furthermore, the increase in serum PTH appears to have led to severe hypo-phosphatemia by promoting the rapid removal of NaPi-IIa from the apical membrane followed by its degradation. As for the possible explanation for the cause of marked increase in serum levels of α-Kl, a de novo translocation with a breakpoint adjacent to α-kl gene was suggested. The inference that the translocation is the cause of the patient’s syndrome is supported by (i) the de novo appearance of this translocation in conjunction with the appearance of a rare syndrome, (ii) the marked alteration in α-Kl levels in association with the mutation, and (iii) corroborating prior evidence that genetic deficiency for α-kl in mice produces the reverse phenotypes. And additionally, (iv) a likely loss of function mutation in humans (H193R missense mutation)57) was associated with adverse symptoms.
The discovery of the above two patients raised several issues (i) The first issue is the elevation of circulating FGF23 levels in both patients. In response to severe hyperphosphatemia and increased 1,25(OH)2D concentrations, the patient with the missense mutation had appropriately elevated FGF23 levels. It is also possible that FGF23 signaling in this patient was severely compromised as a result of the diminished ability of mutant α-Kl to form a ternary FGF23-α-Kl-FGFR1c complex, thereby suggesting that the increase in FGF23 production was compensation for impaired FGF23 signaling. Notably, circulatory levels of FGF23 have been shown to increase in α-kl mutant mice.5) Unexpectedly, the circulating FGF23 levels are also markedly elevated in the patient with high serum α-Kl. Since elevation of FGF23 has been demonstrated to be closely associated with hyperphosphatemia, Brownstein et al. proposed that the elevated α-Kl level mimics aspects of the normal response to hyperphosphatemia and implicates α-Kl in the selective regulation of phosphate levels. However, increased PTH has also been demonstrated to increase FGF23 levels. Therefore it is difficult to discern whether the elevated FGF23 is the result of increased α-Kl or is part of a negative-feedback loop responding to the hyperparathyroidism observed in this patient. It should be also noted that FGF23 levels are markedly elevated with α-kl deficiency.5) These suggest complex regulation of FGF23, which remain to be clarified. (ii) The second issue is why parathyroid hyperplasia is observed in both patients. It is difficult to make a consistent explanation from the serum data of both patients, since serum concentrations of phosphate and 1,25(OH)2D are high in the patient with missense mutation, but low in the patient with high serum α-Kl, while both patients commonly showed high serum levels of FGF23. Notably elevated serum phosphate has been reported to promote proliferation of parathyroid cells and enhances both PTH secretion and mRNA stability; these effects have been speculated to be mediated by reduced serum Ca2+ levels, but the mechanism is still uncertain.59)–62) Conversely, high Ca2+ observed in the patient with missense mutation and 1,25(OH)2D are well known to inhibit PTH production, and FGF23 has recently been shown to inhibit expression of PTH mRNA and secretion of PTH from parathyroid cells.63),64) (iii) It should be also noted that subtotal parathyroidectomy normalized serum calcium and PTH levels even in a homozygous missense mutation in a-kl gene.57) How can we reconcile this evidence with current observations? Serum PTH levels are maintained at basal levels under normocalcemic conditions, and are quickly increased when [Ca2+]o is lowered by the active and regulated secretion of PTH. In this context, FGF23 is predicted to be involved in the regulation of PTH storage and basal levels of PTH in serum63),64) and the active and regulated PTH secretion is characterized by its reliance on the α-Kl/Na+,K+-ATPase complex, low [Ca2+]o mediated stimuli and a rapid time-course response.54) However, the role of FGF23 in the active and regulated secretion of PTH has yet to be defined.63),64) As for the PTH secretion in this patient, while the extent of functional disorder of α-Kl in the parathyroid glands is not clear, it is possible that these two PTH regulatory pathways are malfunctioning since they are dependent on α-Kl. Indeed, serum PTH concentrations are maintained at very low levels after subtotal parathyroidectomy. However, serum calcium levels remain at nearly the upper limit of normal range.57) Since calcium metabolism is known to be regulated by many factors such as dietary calcium intake, influence by other regulatory factors and phosphate concentrations, an unknown mechanism that compensates for impaired PTH secretion is assumed to exist, but evidence of such a system remains to be found.
α-Klotho/Na+,K+-ATPase complex formation
The Na+,K+-adenosine triphosphatase (Na+,K+-ATPase) is a well-studied heteromer consisting of a larger α subunit responsible for ion transport and catalytic activity, and of a smaller glycosylated β subunit required for the regulation of the catalytic action of the α subunit and for the control of cell surface recruitment of Na+,K+-ATPase complexes.65) Although Na+,K+-ATPase is ubiquitously expressed, its expression level varies among tissues; the expression level of Na+,K+-ATPase is significantly higher in the kidney DCT cells, choroid plexus and the parathyroid glands. This suggests that highly expressed Na+,K+-AT-Pase in these α-Kl expressing cells plays a specific role in cooperation with α-Kl.
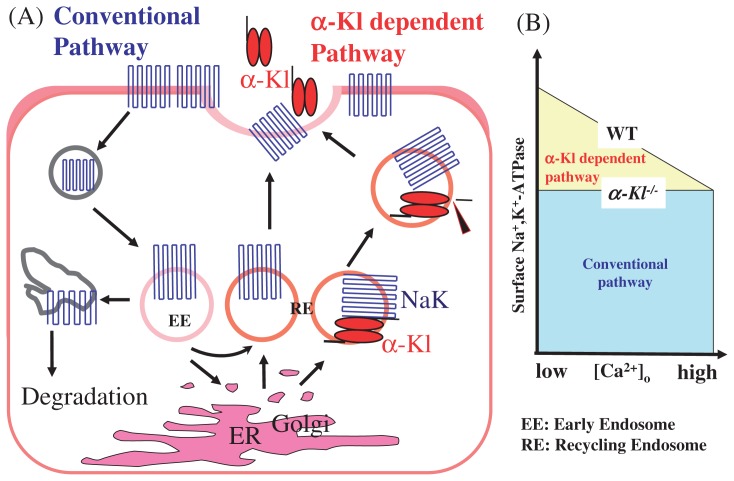
Na+,K+-ATPase was isolated as a binding protein of two intracellular forms of α-Kl, namely, 120 kDa premature form and 135 kDa mature form. Sub-cellular fractionation revealed that the complexes of premature α-Kl and Na+,K+-ATPase are found in the ER fraction, while mature α-Kl and Na+,K+-ATPase complexes accumulate in the endosome and Golgi apparatus/cell membrane fractions. From cell surface biotinylation analyses, it was also confirmed that although α1-and β1-subunits of Na+,K+-ATPase are readily detectable both in the cell surface and cytoplasmic fractions, α-Kl is primarily detected in the cytoplasm but not in the cell surface fraction. Taken together, this suggests that a subset of Na+K+-ATPase traffics from the ER to the cell surface in conjunction with α-Kl and α-Kl/Na+,K+-ATPase complexes located in the ER and Golgi apparatus, and abundantly accumulates in the endosome fraction including the recycling endosome, in preparation for the recruitment to the cell surface (Fig. 1A).54)
Fig. 1.
Surface recruitment of Na+,K+-ATPase in correlation with the cleavage and secretion of α-Kl. In α-Kl expressing cells, Na+,K+-ATPase is recruited to the cell surface by a combination of “the conventional pathway” and “the α-Kl dependent pathway”. Low Ca2+ induces the massive recruitment of Na+,K+-ATPase to the plasma membrane. In contrast, high Ca2+ leads to the decline of such additional recruitment. Accordingly, [Ca2+]e regulates this additional recruitment of Na+,K+-ATPase to the plasma membrane in correlation with the cleavage and secretion of α-Kl.
Surface recruitment of Na+,K+-ATPase in response to [Ca2+]o
The interaction of α-Kl and Na+,K+-ATPase raised an hypothesis that α-Kl directly affects Na+,K+-ATPase activity. Based on the facts that (i) the α-Kl and Na+,K+-ATPase complexes are preferentially localized intracellularly and (ii) the catalytic action of Na+,K+-ATPase is an event that is regulated on the cell surface, the regulation of catalytic efficiency by α-Kl was ruled out as unlikely, and instead focus was put on its regulatory role in the recruitment of Na+,K+-ATPase to the cell surface.
The activity of Na+,K+-ATPase in the choroid plexus epithelial cells is inversely correlated with [Ca2+]o. When incubated in a low Ca2+ solution, the activity of Na+,K+-ATPase increased rapidly, while it decreased in a high Ca2+ solution. Consistent with this observation, low Ca2+ levels in media significantly increased the surface amount of Na+, K+-ATPase and conversely high Ca2+ levels decreased the amount of Na+,K+-ATPase (Fig. 1B). The fluctuation of the amount of Na+,K+-ATPase at the epithelial cell surface rapidly changes and becomes detectable within 30 sec after the shift of [Ca2+]o. This result demonstrates that Na+,K+-ATPase activity is correlated with its amount on the cell surface, both of which respond to [Ca2+]o, leading us to conclude that the shift of [Ca2+]o triggers a rapid response that recruits Na+,K+-ATPase to the plasma membrane (Fig. 1A,B). As expected, α-Kl is required for the rapid and regulated recruitment of Na+,K+-ATPase to the cell surface. Furthermore, secretion of α-Kl is induced in response to low [Ca2+]o in the kidney, parathyroid glands and the choroid plexus, suggesting that [Ca2+]o mediates cleavage of α-Kl in conjunction with the rapid response that recruits Na+,K+-ATPase to the cell surface (Fig. 1A, Fig. 3A).54)
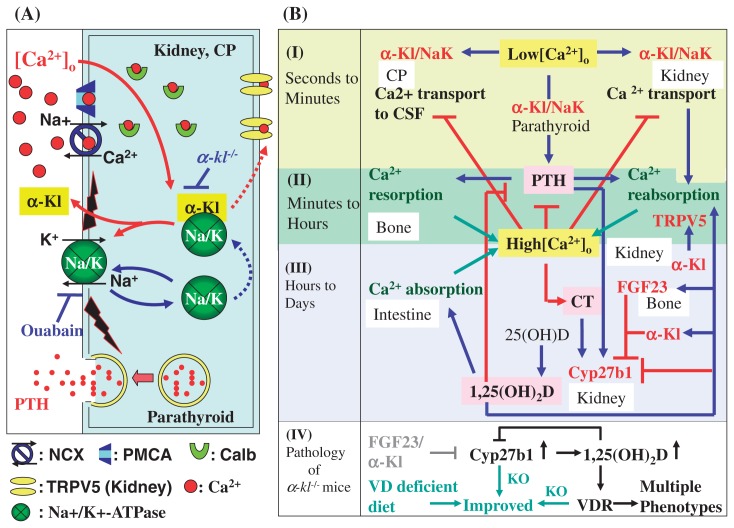
Fig. 3.
Roles of α-Kl and FGF23 in calcium homeostasis. α-Kl binds to Na+,K+-ATPase and regulates the recruitment of Na+,K+-ATPase to the cell surface membrane in response to lowered [Ca2+]o. α-Kl controls calcium re-absorption in the kidney DCT cells, calcium transportation across the choroid plexus into the CSF, and PTH secretion in the parathyroid glands. α-Kl is also involved in the signal transduction of Fgf23 that suppresses the gene expression of Cyp27b1 in the DCT nephrons that leads to negative regulation of 1,25(OH)2D3 synthesis. Calcium metabolism is governed by complicated reciprocal actions and feedback mechanisms and thus calcium concentrations in serum, body fluid and CSF are maintained within strictly narrow ranges.
Novel Na+,K+-ATPase recruiting system
The surface expression of Na+,K+-ATPase is controlled by the balance of recruitment to the plasma membrane and internalization of Na+,K+-ATPase (the conventional pathway).66) In α-Kl expressing cells, Na+,K+-ATPase is recruited to the cell surface by a combination of “the conventional pathway” and “the α-Kl dependent pathway” (Fig. 1A). The latter is characterized by its Ca2+-dependency and rapid response. A low [Ca2+]o induces further recruitment of Na+,K+-ATPase to the plasma membrane. In contrast, high Ca2+ leads to the decline of such additional recruitment. Under normocalcemic conditions, a certain amount of Na+,K+-ATPase is additionally recruited by the α-Kl dependent pathway. However, the recruitment to the plasma membrane of Na+,K+-ATPase in α-kl−/− mice is solely dominated by the conventional pathway and probably represents ‘basal’ recruitment. Therefore, the amount/activity of surface Na+,K+-ATPase in wild type mice is significantly higher than that of α-kl−/− mice. Accordingly, [Ca2+]o regulates this additional recruitment of Na+,K+-ATPase to the plasma membrane in correlation with the cleavage and secretion of α-Kl (Fig. 1A,B).54)
Reconstruction of Na+,K+-ATPase recruitment system
To define the components of the cell surface machinery that links the fluctuation of [Ca2+]o and the efficiency of additional recruitment of Na+,K+-ATPase to the plasma membrane, we analyzed candidate molecules that fulfilled the following criteria: (i) expressed in the choroid plexus and (ii) active at low Ca2+ concentrations. Among several candidates, we focused on a cation channel, TRPV4, since (i) TRPV4 is highly expressed in the apical membrane of choroid plexus epithelial cells and (ii) low Ca2+ concentrations in the extracellular fluid induce larger cation permeability when activated with agonist. To analyze the role of TRPV4, 4α-PDD (4α-phorbol 12,13 didecanoate), a specific agonist for TRPV4, was used to activate TRPV4 at normal [Ca2+]o (1.25 mM). In choroid plexuses prepared from WT mice, 4α-PDD administration induced a rapid increase of the cell surface expression of Na+,K+-ATPase. However, in α-kl−/− mice, the administration of 4α-PDD did not induce such a phenomenon. Therefore, α-Kl appears to be required for this TRPV4 signaling pathway. Consistent with this result, the amount of secreted α-Kl was induced up to 2–3 fold over the control upon 4α-PDD administration. Moreover, addition of 2 mM EGTA diminished the effect of 4α-PDD, indicating that extracellular Ca2+ is essential for this system.54) Based on this evidence, we reconstructed the Na+,K+-ATPase recruitment system in HeLa cells by transfection of plasmids expressing TRPV4 together with various forms of α-Kl. Na+,K+-ATPase recruitment and its activity was found to be induced by the expression of intact α-Kl and TRPV4, and by 4α-PDD administration. Consistently, secretion of the extracellular domain of intact α-Kl was rapidly induced by the administration of 4α-PDD.54) Although these results do not prove that TRPV4 per se plays a role in sensing Ca2+ levels, TRPV4 and the success in reconstruction provide a good tool for studying the role of α-Kl in Na+,K+-ATPase recruitment.
α-Klotho/Na+,K+-ATPase complex in the transepithelial Ca2+ transport
Na+,K+-AT-Pase is a membrane-bound pump that transports two K+ in and three Na+ out of the cell.65) Na+,K+-ATPase activity influences many kinds of cellular and physiological events which take place near the cell membrane by the regulation of electrochemical gradients in the plasma membrane, since the fluctuations of Na+,K+-ATPase activity reconcile the activities of other ATPases, ion channels, and ion exchangers including Ca2+/cation anti-porter superfamily members such as NCX and NCKX.67),68) Particularly, NCXs comprise three members (NCX1; most extensively studied, and broadly expressed with particular abundance in heart, brain and kidney, NCX2 expressed in brain, and NCX3 expressed in brain and muscle) and the NCX proteins sub-serve a variety of roles depending upon the site of expression. These include cardiac excitation-contraction coupling, neuronal signaling and Ca2+ reabsorption in the kidney,69) raising the possibility that the higher Na+ gradient created by the Na+,K+-ATPase activity may drive the trans-epithelial calcium transport in the choroid plexus and kidney (Fig. 3A).
In the choroid plexus, the importance of the α-Kl/Na+,K+-ATPase complex in Ca2+ transport was suggested by (i) surface amounts of Na+,K+-ATPase in α-kl−/− mice were lower than that of WT, and consistently, (ii) the concentration of total calcium in CSF in α-kl−/− mice was considerably lower than that of WT mice. These results imply that calcium homeostasis is impaired in the CSF of α-kl−/− mice and that α-Kl is involved in the regulation of calcium concentrations in the CSF (Fig. 3A). Similarly, the responsive increase of surface Na+,K+-ATPase expression may play a pivotal role in Ca2+ reabsorption in the kidney DCT cells (Fig. 3A). The calcium transport across various nephron segments was actively examined in the second half of the last century.70)–75) In parallel, the involvement of Ca2+-ATPase76) and Ca2+/Na+ antiport77) in Ca2+ transport was suggested, and Shareghi and Stoner found that Ca2+ reabsorption in the DCT and the granular portion of the cortical collecting duct was significantly enhanced by PTH.78) In 1993, Brown et al. proposed a molecular basis for the above observations by the discovery of the calcium sensing receptor (CaR).9) CaR senses high [Ca2+]o concentrations and inhibits tubular reabsorption of calcium when [Ca2+]o is increased. Thus, serum Ca2+ levels are excessively elevated in CaR knockout mice due to enhanced renal tubular calcium reabsorption in addition to marked increase of serum PTH and resultant stimulated bone resorption.79) However, as for the transcellular Ca2+ transport in the DCT nephron and choroid plexus, the involvement of CaR seems to be limited or unlikely, if any, because the major site of intra-renal CaR expression is the thick ascending limb of Henle’s loop but not in DCT cells,80) and because CaR is not detectable in the choroid plexus.54) Furthermore, the response of CaR to increasing [Ca2+]o concentration is well recognized, but the response of CaR to lowered [Ca2+]o is not as well defined.16) On the other hand, α-Kl and Na+,K+-ATPase are involved in the response to a wide range of [Ca2+]o. Indeed, transepithelial Ca2+ transport in the DCT nephron and choroid plexus is efficiently induced in response to low [Ca2+]o concentrations and suppressed when Ca2+ concentrations are increased. This indicates that the α-Kl/Na+,K+-ATPase system regulates Ca2+ transport, at least in part, in a CaR independent manner. Taken together, all of the above suggest the importance of calcium sensor machinery that is distinct from CaR.
These studies suggested that Ca2+ reabsorption is enhanced directly in response to low [Ca2+]o stimuli in the distal nephron as well in a cell autonomous manner independent of calcium-regulating hormones. Kronenberg et al. described their prediction in a textbook as follows: “Ca2+ reabsorption is enhanced directly by tendency to hypocalcemia, which is detected by calcium-sensing receptors in Henle’s loop and possibly also in the distal nephron that control transepithelial calcium movements independent of PTH or 1,25(OH)2D”.81) Although its molecular basis has long remained unknown, the following mechanism may be the first satisfactory explanation for the prediction proposed by Kronenberg et al. That is, the transepithelial transport of Ca2+ is directly triggered and processed by the increased Na+ gradient in cooperation with TRPV5, Calbindin D28k, and NCX-1, all of which are exclusively co-expressed with α-Kl in the nephron segment responsible for the active and regulated Ca2+ reabsorption (Fig. 3A). Indeed, regulated reabsorption of calcium in the kidney is impaired in α-kl−/− mice, resulting in the excess excretion of calcium into urine.82)
α-Klotho/Na+,K+-ATPase complex in the regulated PTH secretion
In the parathyroid glands, PTH secretion is stimulated by the decline of [Ca2+]o. In fact, a substantial decrease in serum Ca2+ concentration corresponded to a marked increase in serum PTH in WT mice. However, the serum PTH response in α-kl−/− mice was significantly lower than that of WT mice, suggesting that α-Kl is essential for the regulated secretion of PTH. Furthermore, the extent of PTH release in WT samples treated with ouabain, a specific inhibitor of Na+,K+-ATPase, is intriguingly similar to that seen in the specimens from α-kl−/− mice without ouabain, and ouabain treatment induced no additional inhibitory effects on PTH secretion in the parathyroid glands from α-kl−/− mice.54) Collectively, current and previous observations suggest the following regulatory scheme of PTH secretion. When [Ca2+]o is lowered, Na+,K+-ATPase is quickly recruited to the plasma membrane through the “α-Kl dependent pathway” in correlation with the cleavage and secretion of α-Kl (Fig. 1A). An electrochemical gradient across the cell membrane created by increased Na+,K+-ATPase may cause the activation of unidentified signal(s), leading to the release of PTH. In the PTH-secreting system, α-Kl and Na+,K+-ATPase work as players of a common signaling pathway and thus, if this pathway is disrupted either by α-kl deficiency or by administration of ouabain, the regulated secretion of PTH is equivalently suppressed (Fig. 3A).54) In turn, when parathyroid CaR is activated in response to the increase of [Ca2+]o, inositol-1,4,5-triphosphate (IP3) accumulates and intracellular calcium rises because of the release of calcium from intracellular stores and of the opening of plasma membrane calcium channels. This increase in the intracellular calcium subsequently leads to a decrease in PTH secretion.16) Since Brown et al. first suggested the importance of Na+,K+-ATPase in regulating the secretion of PTH, the crucial question of how Na+,K+-ATPase activity is regulated in response to low Ca2+ stimuli in the parathyroid glands has remained unanswered.83) The identification of α-Kl as a key regulator in the surface recruitment of Na+,K+-ATPase in response to low Ca2+ stimuli offers an answer (Fig. 3A). However, the intra-cellular mechanism that actively induces PTH secretion when [Ca2+]o is lowered remains to be clarified.
α-Kl as an enzyme β-glucuronidase
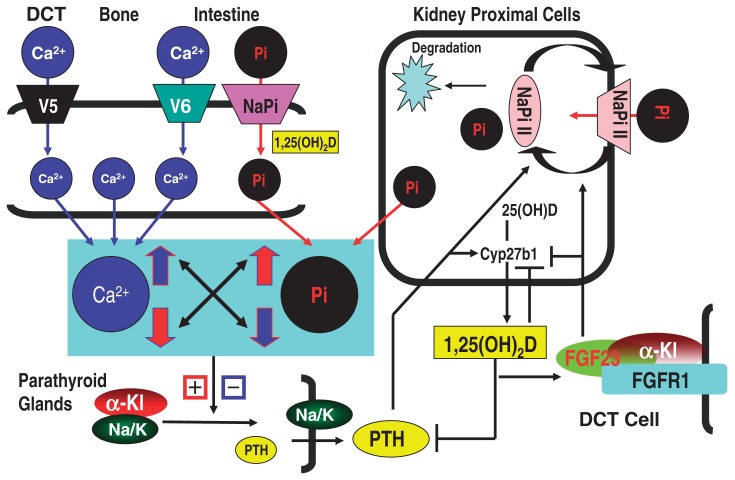
The high similarity of α-Kl with the β-glucosidase family, suggested that it may possess a β-glucosidase activity. However, this has been questioned because α-Kl lacks the glutamic acid residues that are responsible for the catalytic activity of this enzyme family. Nonetheless, β-glucuronidase activity for α-Kl was demonstrated.84) In addition to β-glucuronides, α-Kl was found to hydrolyze the extracellular sugar residues of TRPV5.50) As described above, TRPV5 is exclusively co-expressed with calbindin D28k, NCX-1 and α-Kl in the DCT epithelial cells which are responsible for the active and regulated Ca2+ reabsorption in the kidney. Ca2+ enters into the cell at the luminal membrane (apical) via TRPV5 and/or TRPV6 and is sequestered by calbindin-D28k or -D9k. Bound Ca2+ then diffuses to the basolateral cell surface where Ca2+ is extruded into the blood compartment via NCX1 and PMCA1b (Plasma Membrane Calcium ATPase 1b) (Fig. 3A).51) High Ca2+ influx is possibly obtained by prolonged channel durability at the cell surface, because, regardless of [Ca2+]o, both TRPV5 and TRPV6 are constitutively active as cation channels. The question is how the TRPV5 and TRPV6 channel abundance is controlled. Chang et al. reported a novel mechanism that regulates the abundance of TRPV5 at the luminal cell surface:50)α-Kl in urine, as a β-glucuronidase, increases TRPV5 channel abundance at the luminal cell surface by hydrolyzing the N-linked extracellular sugar residues of TRPV5 without affecting the Ca2+ uptake activity of the TRPV5 channel (Fig. 3A). More recently, it was also reported that α-Kl in urine increases TRPV5 channel abundance at the luminal cell surface by participating in specific removal of α2,6-linked sialic acids from glycan chains of TRPV5.85)
This maintains a durable calcium channel activity and membrane calcium permeability in the kidney, resulting in an increased Ca2+ influx from the lumen to preserve normal blood Ca2+ levels by reduction of Ca2+ loss via urine (Fig. 3A).
Ca2+ enters at the luminal membrane and is sequestered by calcium binding proteins. The bound Ca2+ is then extruded into the blood compartment (Fig. 3A). In this scheme, Ca2+ efflux at basolateral membrane is quickly controlled in response to the fluctuations of [Ca2+]o, and Ca2+ efflux at the basolateral membrane might be mutually balanced by Ca2+ influx at the apical membrane since the excessive influx of Ca2+ over the efflux ability results in a cytotoxic overflow of Ca2+ in the cytoplasm. Importantly, α-Kl plays critical roles in both the influx and efflux of Ca2+ by regulating the abundance of TRPV5 channels on the luminal cell surface50),85) and the recruitment of Na+,K+-ATPase to the basolateral cell surface,54) respectively (Fig. 3A). This leads to a prediction that α-Kl may play the critical role in the coordinate and balanced regulation of influx and efflux of Ca2+ that takes place at both sides of DCT cells.
α-Kl in combination with FGF23 regulates vitamin D metabolism
FGF23, in combination with α-Kl, negatively regulates the metabolism of vitamin D in the kidney. In this pathway, α-Kl is assumed to be necessary for the recognition of FGF23 by target cells. As Urakawa et al. reported, α-Kl binds to FGF23 and converts the canonical FGF receptor 1c (FGFR1c) to a receptor specific for FGF23.5) This enables the high affinity binding of FGF23 to the cell surface of DCT cells where α-Kl is expressed. Subsequently, α-Kl enhances the ability of FGF23 to induce phosphorylation of FGF receptor substrate and extracellular signal-regulated kinase (ERK) in DCT cells. As for the regulation of Cyp27b1 (1α-hydroxylase) gene expression, it is reasonable to speculate that either (i) one or more signal mediators from the distal to the proximal tubule would be required or (ii) some paracrine action of secreted α-Kl would be necessary for this signal transduction to take place because the Cyp27b1 gene is preferentially expressed in proximal convoluted tubule (PCT) cells, but not in DCT cells where FGF23/α-Kl signal is transduced.
1,25(OH)2D, the end-product of this pathway, has prominent effects on the kidney, intestine and bone (Fig. 3B). 1,25(OH)2D enhances calcium up-take in the intestine. In the kidney, 1,25(OH)2D activates the vitamin D receptor (VDR) by binding to its ligand binding domain and negatively regulating the expression of Cyp27b1, while positively regulating Cyp24 and α-Kl expression.4) In the bone, 1,25(OH)2D binds to VDR and induces FGF23 synthesis in osteocytes86) and osteoblasts.87) In turn, secreted FGF23 suppresses 1,25(OH)2D synthesis and the expression of α-Kl in the kidney to adjust extra-cellular concentrations of Ca2+ and Pi. Based on these findings, we proposed a comprehensive regulatory scheme of mineral homeostasis that is illustrated by the mutually regulated positive/negative feedback actions of α-Kl, FGF23 and 1,25(OH)2D.
FGF23, in combination with α-Kl, regulates renal phosphate reabsorption
The kidney plays a predominant role in the regulation of serum Pi levels. The majority of Pi reabsorption takes place in the proximal tubule, and is mainly mediated by NaPi-IIa and additionally by the related protein NaPi-IIc.88) Serum Pi levels are also regulated by intestinal absorption of Pi that is mediated via Na+Pi-IIb transporter, the actions of which are affected by dietary Pi content and 1,25(OH)2D3. The regulated Pi reabsorption in the kidney is affected by various factors such as PTH, dietary Pi, 1,25(OH)2D and FGF23. PTH has been considered to be the major regulator of NaPi-IIa and NaPi-IIc abundance in the apical membranes of PCT cells, promoting the rapid removal of NaPi-II from the membrane and its subsequent degradation.88)–90) As a feedback loop, an increment in Pi is known to promote (i) proliferation of parathyroid cells, (ii) enhancement of PTH secretion, and (iii) stabilization of PTH mRNA, and thereby Pi concentration can be enhanced. However, this evidence does not suggest the presence of a Pi receptor, since the effects of high Pi concentrations are possibly indirect and have been speculated to be mediated by means of reduced serum Ca2+ levels.59)–62) [Ca2+]o and Pi levels are inversely regulated and thus the increase in serum Pi results in the decrease in [Ca2+]o that trigger the increase of PTH activity. Other than PTH, FGF23 also down-regulates serum Pi levels, due to decreased NaPi-IIa abundance in the apical membrane (Fig. 2). In fact, homozygous loss of function of FGF23 results in severe hyperphosphatemia and conversely, gain of mutation of FGF23 results in hypophosphatemia.91)–93) Of particular interest, α-Kl is essential for the above two pathways since FGF23 signaling and regulated secretion of PTH depend on the actions of α-Kl (Fig. 2). Thus, renal and intestinal Na+Pi transporter activities and expression levels of the type IIa, IIb, and IIc NaPi transporter proteins were significantly increased in the α-kl/α-kl mice (originally established mutant line) and the NaPi IIa transporter was localized exclusively to the apical membrane of PCT cells in α-kl/α-kl mice.94),95)
Fig. 2.
PTH and FGF23/α-Kl suppress renal phosphate reabsorption. The majority of phosphate reabsorption occurs in the PCT cells in the kidney. PTH and FGF23/α-Kl promote the rapid removal of NaPi-IIa from the membrane. An increase in serum phosphate promotes proliferation of parathyroid cells, PTH secretion and stabilization of PTH mRNA. The increased phosphate signal might be mediated by means of reduced serum Ca2+ levels. +, −: indicate positive and negative regulations, respectively.
The roles of α-Klotho and FGF23 in multistep regulation of calcium homeostasis
The comprehensive scheme of calcium homeostasis and a global image of α-Kl function in the regulatory network of calcium homeostasis are illustrated in Fig. 3B. As shown in Fig. 3B, calcium homeostasis is maintained by a “multi-step response system” categorized according to the time course, into (I) seconds to minutes, (II) minutes to hours and (III) hours to day(s) order regulations. In response to hypocalcemic stimuli, transepithelial Ca2+ transport in the choroid plexus and the α-Kl-expressing nephron segments, and PTH secretion in the parathyroid glands are all triggered by electrochemical gradients created by Na+,K+-ATPase (Fig. 3B-I).54) Because these responses begin immediately after a decline in Ca2+ concentration and persist for a short time, they can be placed into the “seconds to minutes order regulation” (Fig. 3B-I). The PTH-mediated increase of Ca2+, such as Ca2+ reabsorption in the kidney and Ca2+ reabsorption in the bone continues for hours and thus belong to the “minutes to hours order regulation” (Fig. 3B-II). The production of 1,25(OH)2D and subsequent 1,25(OH)2D-mediated intestinal calcium uptake are in the order of “hours to day(s) regulation” (Fig. 3B-III). In addition, 1,25(OH)2D enhances the expression and function of TRPV5 present on the apical membrane of DCT cells, and thereby up-regulates Ca2+ reabsorption in the kidney.96) In turn, increased calcium suppresses the transepithelial Ca2+ transport and PTH secretion. The increased VDR ligand, 1,25(OH)2D, suppresses Cyp27b1 gene expression and enhances the expression of FGF23. Subsequently, secreted FGF23, in combination with α-Kl, suppresses Cyp27b1 gene expression in the kidney (Fig. 3B-III).
Taken together, calcium metabolism is governed by complicated reciprocal actions along with feedback mechanisms in the time course from seconds to minutes, hours and day(s) order regulations. Thus, extra-cellular calcium concentration is rapidly adjusted and continuously maintained within strictly narrow ranges. In this system, α-Kl is involved in “seconds to minutes order regulation” of transepithelial Ca2+ transport and secretion of PTH. Subsequently, α-Kl participates in “minutes to hours order regulation” and “hours to day(s) order regulation” through the action of secreted PTH and PTH-mediated production of 1,25(OH)2D, respectively. α-Kl also participates in the signal transduction of FGF23 to adjust calcium concentration by down-regulating the production of 1,25(OH)2D (Fig. 3B). Furthermore, α-Kl in urine increases TRPV5 channel abundance at the luminal cell surface by hydrolyzing the sugar residues of TRPV5, which also increase Ca2+ reabsorption in the kidney (Fig. 3B).50),85),96) Therefore, α-Kl is the key player that integrates a “multi-step calcium control system” and should be categorized into a class of protein distinct from the known regulatory molecules (PTH, 1,25(OH)2D and CT) and from the direct handling molecules for transepithelial calcium transport such as TRPV5, Calbindin D28k, and NCX-1.
Major cause of phenotypes observed in a-kl−/− and Fgf23−/− mice
The overproduction of 1,25(OH)2D and altered mineralion homeostasis are believed to be the major causes of the premature aging-like phenotypes observed in α-kl−/− and Fgf23−/− mice, because the lowering of 1,25(OH)2D activity by (i) dietary restriction (a regimen in which α-kl−/− mice are fed a vitamin D-deficient diet),4) (ii) genetic ablation of 1α-hydroxylase in α-kl−/− mice (unpublished data) or in Fgf23−/− mice,97) or (iii) genetic ablation of the VDR gene in α-kl−/−mice (unpublished data) are all able to rescue the premature aging-like phenotypes and enable these mice to survive normally without obvious abnormalities (Fig. 3B-IV). These independently established conclusions consistently indicate that α-Kl is the key regulator of calcium and phosphate homeostasis and clearly explain the major cause of phenotypes observed in α-kl−/− mice and the reason why α-Kl is expressed in the tissues related to calcium and phosphate regulation. Furthermore, the reduced blood glucose and insulin concentrations observed in α-kl−/− mice can be strikingly improved in both male and female α-kl−/− mice when they are fed a vitamin D-deficient diet, suggesting that the impaired glucose metabolism in α-kl−/− mice is a secondary effect caused by increased vitamin D activity.4),98) Importantly, the patient carrying a missense mutation of α-kl gene and the patient with high serum α-Kl exhibited severe defects in mineral ion homeostasis, but normal fasting glucose and insulin concentrations.57),58) These studies provide compelling evidence that α-Kl has a minimal, if any, effect on glucose metabolism and does not support a previous study reporting that α-Kl interferes with insulin or insulin-like growth factor signal transduction.99) The next question that needs to be resolved is how hypervitaminosis D and the subsequently altered mineral-ion balance lead to the multiple premature ageing-like phenotypes, as documented in both α-kl and Fgf23 null mice.
Conclusions
Recent advances that have given rise to marked progress in mineral homeostasis can be summarized as follows; (i) α-Kl binds to Na+,K+-ATPase, and Na+,K+-ATPase is recruited to the plasma membrane by a novel α-Kl dependent pathway in correlation with cleavage and secretion of α-Kl in response to [Ca2+]o fluctuation. (ii) The increased Na+ gradient created by Na+,K+-ATPase activity drives the transepithelial transport of Ca2+ in cooperation with NCX or NCKX in the choroid plexus and the kidney, a mechanism that is defective in α-kl−/− mice. (iii) The regulated PTH secretion in the parathyroid glands is triggered via recruitment of Na+,K+-ATPase to the cell surface in response to lowered [Ca2+]o concentrations. (iv) α-Kl, in combination with FGF23, regulates the production of 1,25(OH)2D in the kidney. In this pathway, α-Kl binds to FGF23, and α-Kl converts the canonical FGF receptor 1c to a specific receptor for FGF23, enabling the high affinity binding of FGF23 to the cell surface of the distal convoluted tubule where α-Kl is expressed. (v) FGF23 signal down-regulates serum Pi levels due to decreased NaPi-IIa abundance in the apical membrane of the kidney proximal tubule cells. (vi) α-Kl increases TRPV5 channel abundance at the luminal cell surface by hydrolyzing the N-linked extracellular sugar residues of TRPV5, resulting in increased Ca2+ influx from the lumen. (vii) The phenotype exhibited by a gain of function mutation in humans is the opposite to that found in loss of function mutations in humans and mice. (viii) Discoveries of human α-kl mutations provide compelling evidence that α-Kl is a pivotal regulator of calcium and phosphate homeostasis not only in rodents but also in humans.
Collectively, recent findings reveal a new paradigm that may change current concepts in mineral homeostasis and give rise to new insight into this field, although this concept requires further verification in the light of related findings.
Profile
Yo-ichi Nabeshima was born in 1946 and started his research career in 1972 with studies on the biosynthesis of proteins in the liver in the Department of Biochemistry, after graduating from the School of Medicine at the Niigata University. He obtained his Doctor in Medical Science from The Niigata University in 1976, working on the metabolism of ribosomal proteins. He was promoted to assistant professor in 1976 and lecturer in 1979 at The Niigata University, and researcher of the Department of Biochemistry, Japanese Foundation of Cancer Research, Cancer Research Institute. In 1988, he moved to the National Institute of Neuroscience, National Center for Neurology and Psychiatry to start his own research group as Head of Division of Molecular Genetics. Just after moving to the Molecular and Cellular Biology Institute Osaka University in 1997, he was promoted to professor of Department of Pathology and Tumor Biology Graduate School of Medicine Kyoto University in 1998. He was appointed Vice Dean of Graduate School of Medicine Kyoto University (2005–2008), the Head of Genome Research Center Kyoto University (2005–2008), and the Head of Career Path Promotion Unit for Young Life Scientists Kyoto University (2007–2008). Over the past 30 years, he has focused on molecular genetics and his works are summarized into two main thesis; firstly, genetic program which regulate animal development and maturation modeling with skeletal muscles and neural cells, and secondary, the discovery of Klotho mutant mice and identification of its causative gene which contributed to a clarification of genetic program of homeostasis maintenance. The elucidations of alternative splicing mechanism (1984), the roles of myogenin in muscle development (1993), the molecular mechanism of asymmetric cell division of neuronal stem cells (1995, 1997), the roles of STEF, a member of Rho family small G protein family in neuronal cell migration (1997, 2003, 2006), the molecular functions of Ptf1a in neuronal cell fate determination (2005), induction of skeletal muscle cells from bone marrow stromal cells (2005), the establishment of live-imaging systems of undifferentiated spermatogonia cells (2007), and discoveries and characterizations of α-Klotho and β-Klotho genes (1997, 2005, 2007) are the highlights of his study. He was awarded the Baelz Prize in 1998, the Uehara Prize in 2005, and the TAKEDA Medical Award in 2007. At present he is a Member of Science Council of Japan.

References
- 1).Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsuki T., et al. (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 [DOI] [PubMed] [Google Scholar]
- 2).Ito S., Fujimori T., Furuya A., Satoh J., Nabeshima Y., Nabeshima Y. (2005). Impaired negative feedback suppression of bile acid synthesis in mice lacking βKlotho. J. Clin. Invest. 115, 2202–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Takeshita K., Fujimori T., Kurotaki Y., Honjo H., Tsujikawa H., Yasui K., et al. (2004) Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation 109, 1776–1782 [DOI] [PubMed] [Google Scholar]
- 4).Tsujikawa H., Kurotaki Y., Fujimori T., Fukuda K., Nabeshima Y. (2003) Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 17, 2393–2403 [DOI] [PubMed] [Google Scholar]
- 5).Urakawa I., Yamazaki Y., Shimada T., Iijima K., Hasegawa H., Okawa K., et al. (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 [DOI] [PubMed] [Google Scholar]
- 6).Brown E.M. (1991) Extracellular Ca2+ sensing, recognition of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol. Rev. 71, 371–411 [DOI] [PubMed] [Google Scholar]
- 7).Borle A.B. (1981) Control, modulation, and regulation of cell calcium. Rev. Physiol. Biochem. Pharmacol. 90, 13–153 [DOI] [PubMed] [Google Scholar]
- 8).Parfitt A.M., Kleerekoper M. (1980) The divalent ion homeostatic system: physiology and metabolism of calcium, phosphorus, magnesium, and bone. InClinical Disorders of Fluid and Electrolyte Metabolism (3rd ed) (eds. by Maxwell M.H., Kleeman C.R.). McGraw-Hill, New York, pp. 269–398 [Google Scholar]
- 9).Brown E.M., Gamba G., Riccardi D., Lombardi M., Butters R., Kifor O., et al. (1993) Cloning and characterization of an extracellular Ca2+ sensing receptor from bovine parathyroid. Nature 366, 575–580 [DOI] [PubMed] [Google Scholar]
- 10).Stewart A.F., Broadus A.E. (1987) Mineral metabolism. In Endocrinology and Metabolism (2nd ed)(eds. Felig P., Baxter J.D., Broadus A.F., Frohman L.A.). New York, McGraw-Hill, pp. 1317–1453 [Google Scholar]
- 11).Aurbach G.D., Marx S.J., Spiegel A.M. (1985) Parathyroid hormone, calcitonin, and the calciferols. InTextbook of Endocrinology (7th ed) (eds. Wilson J.D., Foster D.W.). PA: Saunders, Philadelphia, pp. 1137–1217 [Google Scholar]
- 12).Brown E.M. (1983) Regulation of the synthesis, secretion and actions of parathyroid hormone. InContemporary Issues in Nephrology. Divalent Cation Homeostasis (eds. Brenner B.M., Stein J.H.). Churchhill-Livingstone; vol. 11, New York, pp. 151–188 [Google Scholar]
- 13).Brown E.M., Chen C.J., Leboff M.S., Kifor O., El-Hajj G. (1989) Mechanisms underlying the inverse control of parathyroid hormone secretion by calcium. InSecretion and Its Control (eds. Oxford G., Armstrong C.M.). Rockfeller Univ. Press, New York, pp. 252–268 [PubMed] [Google Scholar]
- 14).Austin L.A., Heath H., III (1981) Calcitonin: Physiology and pathophysiology. N. Engl. J. Med. 304, 269–278 [DOI] [PubMed] [Google Scholar]
- 15).Bilezikian J.P., Marcus R., Levine M.A. (2003) Section II: Physiological aspects of the parathyroid. InThe Parathyroids: Basic and Clinical Concepts (2nd ed) (eds. Bilezikian J.P., Marcus R., Levine M.A.). Academic Press, New York, pp. 167–291 [Google Scholar]
- 16).Brown E.M., MacLead R.J. (2001) Extracellular calcium sensing and extra cellular calcium signaling. Physiol. Rev. 81, 239–297 [DOI] [PubMed] [Google Scholar]
- 17).Blum J.W., Trescel U., Born W., Tobler P.H., Taylor C.M., Binswanger U., et al. (1983) Rapidity of plasma 1,25-dihydroxyvitamin D responses to hypo- and hypercalcemia in steers. Endocrinology 113, 523–526 [DOI] [PubMed] [Google Scholar]
- 18).Brown E.M., Leombruno R., Thatcher J., Burrowes M. (1985) The acute secretory response to alterations in the extracellular calcium concentration and dopamine in perfused bovine parathyroid cells. Endocrinology 116, 1123–1132 [DOI] [PubMed] [Google Scholar]
- 19).Wallfelt C., Lindh E., Larsson R., Johansson H., Rastad J., Akerstrom G., et al. (1988) Kinetic evidence for cytoplasmic calcium as an inhibitory messenger in parathyroid hormone release. Biochim. Biophys. Acta 969, 257–262 [DOI] [PubMed] [Google Scholar]
- 20).Raisz L.G. (1965) Bone resorption in tissue culture: factors influencing the response to parathyroid hormone. J. Clin. Invest. 44, 103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Raisz L.G., Kream B.E. (1983) Regulation of bone formation. N. Engl. J. Med. 309, 35–396855852 [Google Scholar]
- 22).Fraser D.R., Kodicek E. (1973) Regulation of 25-hydroxycholecalciferol-hydroxylase activity in kidney by parathyroid hormone. Nature New Biol. 241, 163–166 [DOI] [PubMed] [Google Scholar]
- 23).Hughes M.R., Baylink D., Jones P.G., Haussler M.R. (1976) Radioreceptor assay for 25-hydroxyvitamin D2/D3 and 1,25-dihydroxy-vitamin D2/D3. Application to hypervitaminosis. D. J. Clin. Invest. 58, 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Hughes M.R., Brumbaugh P.F., Haussler M.R., Wergedal J.E., Baylink J. (1975) Regulation of serum 1,25-dihydroxyvitamin D3 by calcium and phosphate in the rat. Science Wash. DC 190, 578–590 [DOI] [PubMed] [Google Scholar]
- 25).Brumbaugh P.F., Hughes M.R., Haussler M.R. (1975) Cytoplasmic and nuclear binding components for 1,25-dihydroxyvitamin D3 in chick parathyroid glands. Proc. Natl. Acad. Sci. USA 72, 4871–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Marx S.J., Liberman U.A., Eil C.A. (1983) Calciferols: actions and deficiencyes in actions. Vitam. Horm. 40, 235–308 [DOI] [PubMed] [Google Scholar]
- 27).Cohen P. (1989) The structure and regulation of protein phosphatases. Annu. Rev. Biochem. 58, 453–508 [DOI] [PubMed] [Google Scholar]
- 28).Hubbard S.R., Till J.H. (2000) Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 69, 373–398 [DOI] [PubMed] [Google Scholar]
- 29).Hunter T., Cooper J. A. (1985) Protein-tyrosine kinase. Annu. Rev. Biochem. 54, 897–930 [DOI] [PubMed] [Google Scholar]
- 30).Krebs E.G., Beavo J.A. (1979) Phosphorylation-depphosphorylation of enzymes. Annu. Rev. Biochem. 48, 923–959 [DOI] [PubMed] [Google Scholar]
- 31).Neuman W. (1980) Bone material and calcification mechanisms. InFundamental and Clinical Bone Physiology (ed. Urist M.). J.B. Lippincott Co, Philadelphia, pp. 83–107 [Google Scholar]
- 32).Knochel J.P. (1977) The pathophysiology and clinical characteristics of severe hypophosphatemia. Arch. Intern. Med. 137, 203–220 [PubMed] [Google Scholar]
- 33).Slatopolsky E. (2003) New developments in hyper-phopsatemia management. J. Am. Soc. Nephrol. 14, 297–299 [DOI] [PubMed] [Google Scholar]
- 34).Werner A., Kinne R.K. (2001) Evolution of the Na-Pi cotransport systems. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R301–R312 [DOI] [PubMed] [Google Scholar]
- 35).Beck L., Karaplis A.C., Amizuka N., Hewson A.S., Ozawa H., Tenenhouse H.S. (1998) Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl. Acad. Sci. USA 95, 5372–5377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Murer H., Hernando N., Forster I., Biber J. (2003) Regulation of Na/Pi transporter in the proximal tubule. Annu. Rev. Physiol. 65, 531–542 [DOI] [PubMed] [Google Scholar]
- 37).Hernando N., Biber J., Forster I., Murer H. (2005) Recent advances in renal phosphate transport. Ther. Apher. Dial. 9, 323–327 [DOI] [PubMed] [Google Scholar]
- 38).Berndt T., Kumar R. (2007) Phosphatonins and the regulation of phosphate homeostasis. Annu. Rev. Physiol. 69, 341–359 [DOI] [PubMed] [Google Scholar]
- 39).Condamine L., Keutmann H.T., Niall H.D., Friedlander G., Garabedian M. (1994) Local action of phosphate depletion and insulin-like growth factor 1 on in vitro production of 1,25 dihydroxyvitamin D by cultured mammalian kidney cells. J. Clin. Invest. 94, 1673–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Martin D.R., Ritter C.S., Slatopolsky E., Brown A.J. (2005) Acute regulation of parathyroid hormone by dietary phosphate. Am. J. Physiol. Endocrinol. Metab. 289, E729–E734 [DOI] [PubMed] [Google Scholar]
- 41).Landsman A., Lichtstein D., Bacaner M., Ilani A. (2001) Dietary phosphate-dependent growth is not mediated by changes in plasma phosphate concentration. Br. J. Nutr. 86, 217–223 [PubMed] [Google Scholar]
- 42).ADHR Consortium (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26, 345–348 [DOI] [PubMed] [Google Scholar]
- 43).Rowe P.S., de Zoysa P.A., Dong R., Wang H.R., White K.F. (2000) MEPE, a new gene expressed in bone marrow and causing osteomalacia. Genomics 67, 54–68 [DOI] [PubMed] [Google Scholar]
- 44).Berndt T., Craig T.A., Bowe A.E., Vassiliadis J., Reczek D. (2003) Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J. Clin. Invest. 112, 785–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).De Beur S.M., Finnegan R.B., Vassiliadis J., Cook B., Barberio D. (2002) Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J. Bone Miner. Res. 17, 1102–1120 [DOI] [PubMed] [Google Scholar]
- 46).Carpenter T.O., Ellis B.K., Insogna K.L., Philbrick W.M., Sterpka J., Shimkets R. (2005) FGF7: an inhibitor of phosphate transport derived from oncogenic osteomalacia-causing tumors. J. Clin. Endocrinol. Metab. 90, 1012–1020 [DOI] [PubMed] [Google Scholar]
- 47).Hernando N., Gisler S.M., Pribanic S., Deliot N., Capuano P., Wagner C.A., et al. (2005) NaPi-IIa and interacting partners. J. Physiol. 567, 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Henrissat B., Davies G. (1997) Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 7, 637–644 [DOI] [PubMed] [Google Scholar]
- 49).Imura A., Iwano A., Kita N., Thoyama O., Fujimori T., Nabeshima Y. (2004) Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 565, 143–147 [DOI] [PubMed] [Google Scholar]
- 50).Chang Q., Hoefs S., van der Kemp A.W., Topala C.N., Bindels R.J., Hoenderop J.G. (2005) The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310, 490–493 [DOI] [PubMed] [Google Scholar]
- 51).Hoenderop J.G., Leeuwen J.P., Eerden B.C., Kersten F.F., Emp A.W., Merillat A.M., et al. (2003) Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Invest. 112, 1906–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Strange K. (1992) Regulation of solute and water balance and cell volume in the central nervous system. J. Am. Soc. Nephrol. 3, 12–27 [DOI] [PubMed] [Google Scholar]
- 53).Somjen G.G. (2002) Ion regulation in the brain: implications for pathophysiology. Neuroscientist 8, 254–267 [DOI] [PubMed] [Google Scholar]
- 54).Imura A., Tsuji Y., Murata M., Maeda R., Kubota K., Iwano A., et al. (2007) α-Klotho as a regulator of calcium homeostasis. Science 316, 1615–1618 [DOI] [PubMed] [Google Scholar]
- 55).Shimada T., Kakitani M., Yamazaki Y., Hasegawa H., Takeuchi Y., Fujita T., et al. (2004) Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 113, 561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Kato K., Jeanneau C., Tarp M.A., Benet-Pagès A., Lorenz-Depiereux B., Bennett E.P., et al. (2006) Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem. 281, 18370–18377 [DOI] [PubMed] [Google Scholar]
- 57).Ichikawa S., Imel E.A., Kreiter M.L., Yu X., Mackenzie D.S., Sorenson A.H., et al. (2007) A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Invest. 117, 2684–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Brownstein C.A., Adler F., Nelson-Williams C., Iijima J., Li P., Imura A., et al. (2008) A translocation causing increased α-Klotho level results in hypophosphatemic rickets and hyper-parathyroidism. Proc. Natl. Acad. Sci. 105, 3455–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Kilav R., Silver J., Naveh-Many T. (2001) A conserved cis-acting element in the parathyroid hormone 3′-untranslated region is sufficient for regulation of RNA stability by calcium and phosphate. J. Biol. Chem. 276, 8727–8733 [DOI] [PubMed] [Google Scholar]
- 60).Moallem E., Kilav R., Silver J., Naveh-Many T. (1998) RNA-protein binding and post-transcriptional regulation of parathyroid hormone gene expression by calcium and phosphate. J. Biol. Chem. 273, 5253–5259 [DOI] [PubMed] [Google Scholar]
- 61).Bell O., Silver J., Naveh-Many T. (2005) Identification and characterization of cis-acting elements in the human and bovine PTH mRNA 3′-untranslated region. J. Bone Miner. Res. 20, 858–866 [DOI] [PubMed] [Google Scholar]
- 62).Silver J., Kilav R., Sela-Brown A., Naveh-Many T. (2000) Molecular mechanisms of secondary hyperparathyroidism. Pediatr. Nephrol. 14, 626–628 [DOI] [PubMed] [Google Scholar]
- 63).Ben-Dov I., Galitzer H., Lavi-Moshayoff V., Goetz R., Kuro-o M., Mohammadi M., et al. (2007) The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 117(12), 4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Krajisnik T., Bjorklund P., Marsell R., Ljunggren O., Akerstrom G., Jonsson K.B., et al. (2007) Fibroblast growth factor-23 regulates parathyroid hormone and 1a-hydroxylase expression in cultured bovine parathyroid cells. J. Endocri. 195, 125–131 [DOI] [PubMed] [Google Scholar]
- 65).Skou J.C. (1988) The Na+,K+-pump. Methods Enzymol. 156, 1–25 [DOI] [PubMed] [Google Scholar]
- 66).Kaplan J.H. (2002) Biochemistry of Na+,K+-ATPase. Annu. Rev. Biochem. 71, 511–535 [DOI] [PubMed] [Google Scholar]
- 67).Skou J.C., Esmann M. (1992) The Na+,K+-ATPase. J. Bioenerg. Biomembr. 24, 249–261 [DOI] [PubMed] [Google Scholar]
- 68).Lytton J. (2007) Na+/Ca+ exchangers: three mammalian gene families control C2+ transport. Biochem. J. 406, 365–382 [DOI] [PubMed] [Google Scholar]
- 69).Blaustein M.P., Lederer W.J. (1999) Sodium/calcium exchange: Its physiologicalimplications. Physiol. Rev. 79, 763–854 [DOI] [PubMed] [Google Scholar]
- 70).Lassiter W.C., Gottschalk C.W., Mylle M. (1963) Micropuncture study of renal tubular reabsorption of calcium in normal rodents. Am. J. Physiol. 204, 771–775 [Google Scholar]
- 71).Rocha A.S., Magaldi J.B., Kokko J.P. (1977) Calcium and phosphate transport in isolated segments of rabbit Henle’s loop. J. Clin. Invest. 59, 975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Suki W.N. (1979) Calcium transport in the nephron. Am. J. Physiol. 237, F1–F6 [DOI] [PubMed] [Google Scholar]
- 73).Quamme G.A. (1980) Effect of calcitonin on calcium and magnesium transport in rat nephron. Am. J. Physiol. 238, E573–E578 [DOI] [PubMed] [Google Scholar]
- 74).Quamme G.A. (1982) Effect of hypercalcemia on renal tubular handling of calcium and magnesium. Can. J. Physiol. Pharmacol. 60, 1275–1280 [DOI] [PubMed] [Google Scholar]
- 75).Sutton R.A., Wong N.L., Quamme G.A., Dirks J.H. (1983) Renal tubular calcium transport: effects of changes in filtered calcium load. Am. J. Physiol. 245, F515–F520 [DOI] [PubMed] [Google Scholar]
- 76).Kinne-Saffran E., Kinne R. (1974) Localization of a calcium stimulated ATPase in the basal-lateral plasma membranes of the proximal tubule of rat kidney cortex. J. Membr. Biol. 17, 263–274 [DOI] [PubMed] [Google Scholar]
- 77).Ullrich K.J., Rumrich J.G., Klöss S. (1976) Active Ca2+ reabsorption in the proximal tubule of the rat kidney. Pfugers. Arch. 364, 223–228 [DOI] [PubMed] [Google Scholar]
- 78).Shareghi G.R., Stoner L.C. (1978) Calcium transport across segments of the rabbit distal nephron in vitro. Am. J. Physiol. 235, F367–F375 [DOI] [PubMed] [Google Scholar]
- 79).Ho C., Conner D.A., Pollack M.R., Ladd D.J., Kifor O., Warren H.B., et al. (1995) A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat. Genet. 11, 389–394 [DOI] [PubMed] [Google Scholar]
- 80).Riccardi D., Park J., Lee W.S., Gamba G., Brown E.M., Hebert S.C. (1995) Cloning and functional expression of a rat kidney extra-cellular calcium-sensing receptor. Proc. Natl. Acad. Sci. USA 92, 131–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81).Wilson J.D., Foster D.W., Kronenberg H.M., Larsen P.R. (2003): Hormones and disorders of mineral metabolism. InWilliams Textbook of Endocrinology (eds. Bringhurst F.R., Demay M.B., Kronenberg H.M.). Saunders, Philadelphia, pp. 1303–1371 [Google Scholar]
- 82).Tsuruoka S., Nishiki K., Ioka T., Ando H., Saito Y., Kurabayashi M., et al. (2006) Defect in parathyroid-hormone-induced luminal calcium absorption in connecting tubules of Klotho mice. Nephrol. Dial. Transplant. 21, 2762–2767 [DOI] [PubMed] [Google Scholar]
- 83).Brown E.M., Watson E.J., Thatcher J.G., Koletsky R., Dawson-Hughes B.F., Posillico J.T., et al. (1987) Ouabain and low extracellular potassium inhibit PTH secretion from bovine parathyroid cells by a mechanism that does not involve increases in the cytosolic calcium concentration. Metabolism 36, 36–42 [DOI] [PubMed] [Google Scholar]
- 84).Tohyama O., Imura A., Iwano A., Freund J.N., Henrissat B., Fujimori T., et al. (2004) Klotho is a novel β-glucuronidase capable of hydrolyzing steroid β-glucuronides. J. Biol. Chem. 273, 9777–9784 [DOI] [PubMed] [Google Scholar]
- 85).Cha S.K., Ortega B., Kurosu H., Rosenblatt K.P., Kuro-o M., Huang C.L. (2008) Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc. Natl. Acad. Sci. USA 105(28), 9805–9810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Liu S., Zhou J., Tang W., Jiang X., Rowe D.W., Quarles L.D. (2006). Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 291, E38–E49 [DOI] [PubMed] [Google Scholar]
- 87).Kolek O.I., Hines E.R., Jones M.D., LeSueur L.K., Lipko M.A., Kiela P.R., et al. (2005) 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest Liver Physiol. 289, G1036–G1042 [DOI] [PubMed] [Google Scholar]
- 88).Tenenhouse H. (2005) Regulation of phosphorus homeostasis by the type IIa Na/phosphate co-transporter. Annu. Rev. Nutr. 25, 197–214 [DOI] [PubMed] [Google Scholar]
- 89).Forster I.C., Hernando N., Biber J., Murer H. (2006) Proximal tubular handling of phosphate: A molecular perspective. Kidney Int. 70, 1548–1559 [DOI] [PubMed] [Google Scholar]
- 90).Pfister M.F., Ruf I., Stange G., Ziegler U., Lederer E., Biber J., et al. (1998) Parathyroid hormone leads to the lysosomal degradation of the renal type II Na/Pi cotransporter. Proc. Natl. Acad. Sci. USA 95, 1909–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91).Larsson T., Marsell R., Schipani E., Ohlsson C., Ljunggren O., Tenenhouse H.S., et al. (2004) Transgenic mice expressing fibroblast growth factor 23 under the control of the α1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 145(7), 3087–3094 [DOI] [PubMed] [Google Scholar]
- 92).Bai X., Miao D., Li J., Goltzman D., Karaplis A.C. (2004) Transgenic mice overex-pressing human fibroblast growth factor 23(R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology 145, 5269–5279 [DOI] [PubMed] [Google Scholar]
- 93).Shimada T., Hasegawa H., Yamazaki Y., Muto T., Hino R., Takeuchi Y., et al. (2004) FGF23 is a potent regulator of the vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 19, 429–435 [DOI] [PubMed] [Google Scholar]
- 94).Segawa H., Yamanaka S., Ohno Y., Onitsuka A., Shiozawa K., Aranami F., et al. (2007) Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am. J. Physiol. Renal Physiol. 292, 769–779 [DOI] [PubMed] [Google Scholar]
- 95).Miyamoto K., Ito M., Tatsumi S., Kuwahara M., Segawa H. (2007) New aspect of renal phosphate reabsorption: The Type IIc sodium-dependent phosphate transporter. Am. J. Nephrology 27, 503–515 [DOI] [PubMed] [Google Scholar]
- 96).Schoeber J.P.H., Hoenderop J.G.J., Bindels R.J.M. (2007) Concerted action of associated proteins in the regulation of TRPV5 and TRPV6. Biochemical Society Transduction 35, 115–119 [DOI] [PubMed] [Google Scholar]
- 97).Razzaque M.S., Lanske B. (2006) Hyper-vitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol. Med. 12, 298–305 [DOI] [PubMed] [Google Scholar]
- 98).Razzaque M.S., Sutara D., Taguchi T., St-Arnaud R., Lanske B. (2006) Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 20, 720–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99).Kurosu H., Yamamoto M., Clark A.J.D., Pastor J.V., Nandi A., Grunani P., et al. (2005) Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]