Abstract
Irradiated or injured cells enter apoptosis, and in turn, promote proliferation of surrounding unaffected cells. In Drosophila, apoptotic cells have an active role in proliferation, where the caspase Dronc and p53 induce mitogen expression and growth in the surrounding tissues. The Drosophila p53 gene structure is conserved and encodes at least two protein isoforms: a full-length isoform (Dp53) and an N-terminally truncated isoform (DΔNp53). Historically, DΔNp53 was the first p53 isoform identified and was thought to be responsible for all p53 biological activities. It was shown that DΔNp53 induces apoptosis by inducing the expression of IAP antagonists, such as Reaper. Here we investigated the roles of Dp53 and DΔNp53 in apoptosis and apoptosis-induced proliferation. We found that both isoforms were capable of activating apoptosis, but that they each induced distinct IAP antagonists. Expression of DΔNp53 induced Wingless (Wg) expression and enhanced proliferation in both ‘undead cells' and in ‘genuine' apoptotic cells. In contrast to DΔNp53, Dp53 did not induce Wg expression in the absence of the endogenous p53 gene. Thus, we propose that DΔNp53 is the main isoform that regulates apoptosis-induced proliferation. Understanding the roles of Drosophila p53 isoforms in apoptosis and in apoptosis-induced proliferation may shed new light on the roles of p53 isoforms in humans, with important implications in cancer biology.
Keywords: Drosophila, apoptosis, regeneration, p53, reaper, hid
Epithelial tissues have the intrinsic capability to repair and regenerate, following irradiation or genetically induced cell death. However, how epithelial cells respond to injury and recover is not well understood. In the past few years, studies from metazoan models, such as Drosophila, forged the concept of apoptosis-induced proliferation, a process by which damaged cells entering apoptosis signal the surrounding unaffected cells to divide so as to recoup the tissue loss.1, 2, 3 Using Drosophila developing imaginal discs as a model, several groups have demonstrated that fly wing imaginal discs submitted to γ-irradiation or genetically induced cell death undergo apoptosis-induced proliferation.4, 5 Apoptosis-induced proliferation requires Drosophila p53 and the caspase Dronc, and involves the release of mitogens such as Wingless (Wg) and Decapentaplegic (Dpp) that induce the growth of the surrounding tissues.6, 7, 8 Apoptosis-induced proliferation has also been observed in hydra, where dying cells express Wnt that is required for cell division.9 A recent study showed that when injected into mice, irradiated mouse embryonic fibroblasts can induce sustained growth of feeder tumour cells.10 Specifically, this study shows that caspase 3, the executioner of apoptosis, stimulates prostaglandin E2 expression and growth of surviving tumour cells. Other studies also demonstrate that mice deficient for the p53 inhibitor, MDM2, develop intestinal hyperplasia due to the activation of the canonical Wnt and EGFR pathways.11 Together, these results suggest that apoptosis-induced proliferation is a fundamental and conserved process by which epithelial tissues recover and regenerate after injury, and that p53 has an active role in both apoptosis and compensatory growth in mice and in Drosophila.
The p53 protein is the product of a well-known tumour suppressor gene, TP53. It is mutated in more than 50% of human cancers. Initial studies of p53 functions have highlighted its key role as a stress-induced factor, particularly in response to DNA damage. The results from decades of studies coined p53 as the ‘guardian of the genome', as it induces DNA repair, cell cycle arrest or apoptosis after exposure to genotoxic stress,12, 13 thus preventing the sequential accumulation of genetic alterations that underpins progression towards neoplasia. However, p53 is present in many lower eukaryotes, including Drosophila, where cancer is not a prevalent biological phenomenon. This paradox leads many to postulate that the tumour suppression function of p53 in vertebrates has probably evolved for some hitherto unappreciated primordial regulatory functions.14, 15, 16 However, the exact nature of such primordial functions has remained elusive.
Until recently, TP53 was thought to be expressed as a single major transcript. This view was radically transformed in the last 15 years by the discovery of two verterbrate p53 paralogs, TP63 and TP73. These two genes encode several protein isoforms with diverse functions in neuronal development, morphogenesis, immune response and responses to specific stress.17 Subsequent to the discovery of the ΔN isoforms of p63 and p73, studies have revealed that p53 express up to 12 protein isoforms generated by alternative splicing sites, codon initiation sites and internal promoter.18, 19
In invertebrate animals, only one gene represents the TP53 gene family. It resembles TP53 more than it resembles TP63 and TP73. In Drosophila, the p53 gene structure is highly conserved compared to its mammalian homologue. Drosophila p53 (Dp53) gene structure was recently reviewed; it contains two alternative promoters and encodes three possible protein isoforms, Dp53, DΔNp53 and Dp53ΔC.19 Experimental evidence only confirms the presence of the full-length Dp53 and DΔNp53. Therefore, in this study, we focused on Dp53 full-length isoform corresponding to the human full-length (TA) p53 that includes a full transactivation domain and DΔNp53, a general counterpart of the N-terminal truncated human p53 forms. DΔNp53 is encoded by an mRNA transcribed from an internal promoter like the N-terminally truncated human Δ133p53, but unlike Δ133p53, it contains a truncated trans-activation domain followed by a complete DNA-binding domain and an oligomerization domain such as that found in the human Δ40Np53.18 These findings raise the possibility that Dp53 and DΔNp53 are the respective functional homologues of the human TAp53 and Δ40/Δ133p53.
DΔNp53 was the first p53 isoform identified in Drosophila and was thought to be the only p53 isoform for several years; it was therefore initially named Dp53 or Dmp53 in earlier publications.20, 21, 22 The subsequent identification of a form of Drosophila p53 matching the mammalian full-length (TA) p53 protein has led to a reassessment of this nomenclature, with the name Dp53 to designate the full-length protein isoform and DΔNp53 for the N-terminal truncated form. Studies on fly primordial germ cells, imaginal discs and adult photoreceptor cells have highlighted the role of DΔNp53 in regulating apoptosis.21, 23, 24, 25, 26 DΔNp53 induces apoptosis through the Reaper-Hid-Grim (RHG) cascade. It was proposed that DΔNp53 directly activates the expression of reaper (rpr), whose protein product activates caspases by inhibiting DIAP1 (Drosophila inhibitor of apoptosis protein).21, 22 In addition to its apoptotic function, the Dp53 locus (Dp53 and/or DΔNp53) regulates several biological functions, such as cell cycle, DNA repair, aging and apoptosis-induced proliferation.3, 27, 28, 29, 30
Here we have investigated the role of Dp53 and DΔNp53 in apoptosis and apoptosis-induced proliferation. We found that both isoforms were capable of activating apoptosis, but that each induced distinct RHG family members to inhibit DIAP1. Strikingly, we observed that DΔNp53 induced wg expression and enhanced proliferation in the wing imaginal disc, suggesting that DΔNp53 promotes apoptosis-induced proliferation. In contrast to DΔNp53, Dp53 did not induce wg expression in the absence of the endogenous p53 gene. Thus, we propose that DΔNp53 is the main p53 isoform that regulates apoptosis-induced proliferation. The physiological consequences of these dual functions of p53 isoforms on apoptosis and apoptosis-induced proliferation are discussed.
Results
Dp53 and DΔNp53 activate distinct RHG genes to induce apoptosis
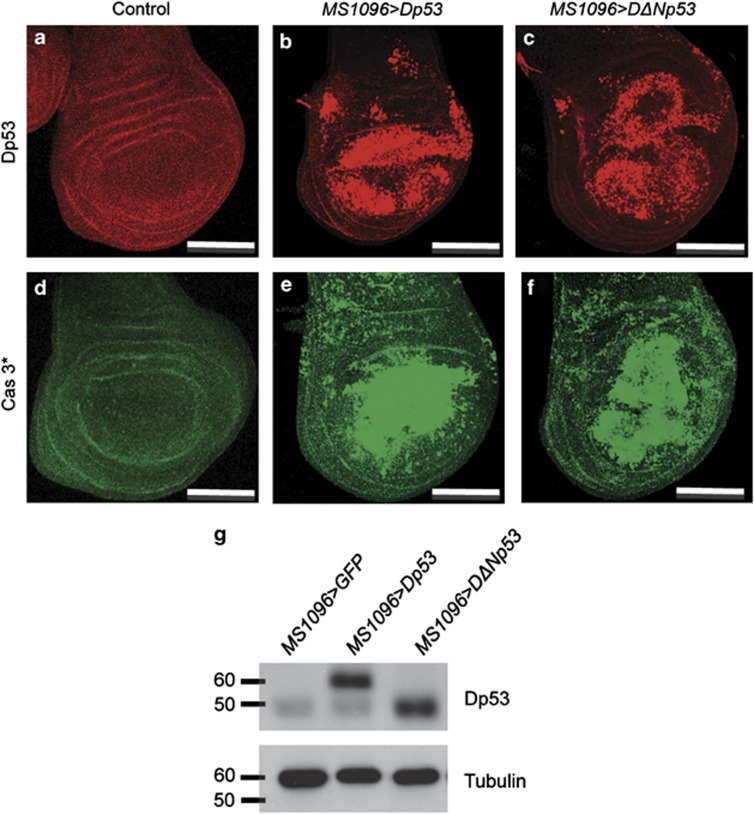
To study the respective functions of Dp53 and DΔNp53, we undertook a gain-of-function approach. We generated UAS-Dp53 and UAS-DΔNp53 Drosophila transgenic lines for tissue-specific expression using the UAS/GAL4 system. To eliminate expression level variations due to position effects,31 we targeted individual UAS-Dp53 and UAS-DΔNp53 insertions to the same chosen genomic region using the site-specific φC31 integrase system. Dp53 and DΔNp53 cDNAs were expressed in the wing imaginal disc using the MS1096 driver that is specific to the wing pouch and hinge areas (Figure 1 and Supplementary Figure 1).32 We observed robust production of Dp53 in wing imaginal discs using an anti-Dp53 antibody that recognizes the DNA-binding domain common to the Dp53 and DΔNp53 isoforms (Figures 1a–c). Similar levels of Dp53 and DΔNp53 isoforms were detected by western blot analysis (Figure 1g). We found that both isoforms induced caspase activation in wing imaginal discs, indicating that their expression leads to apoptosis (Figures 1d–f and Supplementary Figure S2).
Figure 1.
Dp53 or DΔNp53 expression induces caspase activation in wing imaginal discs. (a–f) Ectopic production of Dp53 or DΔNp53 using the MS1096 driver. (a–c) Dp53 and DΔNp53 protein isoforms are detected by immunostaining with an anti-p53 antibody (25F4) directed against the common C terminus domain. (a) MS1096>GFP is used as a negative control. Dp53 (b) and DΔNp53 (c) are detected in the MS1096 domain of expression. (d–f) Wing imaginal discs were stained using an anti-cleaved caspase 3 antibody (Cas 3*). Elevated levels of Dp53 (MS1096>Dp53 in e) or DΔNp53 (MS1096>DΔNp53 in f) induce strong caspase 3 staining in the MS1096 domain. Caspase activation is not detected in control wing discs (MS1096>LacZ in d). (g) Western blot analysis of Dp53 and DΔNp53 in the wing imaginal discs using an anti-p53 antibody (C11) against the common C terminus domain. MS1096>Dp53 and MS1096>DΔNp53 show a band around 60 kDa and 50 kDa, respectively. The endogenous level of DΔNp53 is detected in the wild-type control (MS1096>GFP). Tubulin is used as loading control. Scale bars are 100 μm
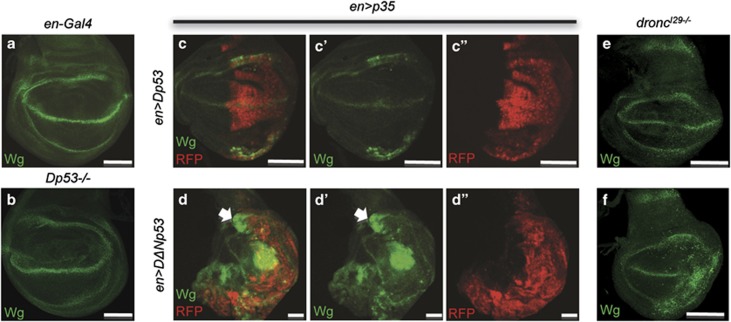
To investigate the mechanisms by which Dp53 and DΔNp53 overexpression lead to apoptosis, we examined rpr and hid expression (Figure 2). We used a rprXRE-lacZ (rprZ) reporter, which carries a 2.2-kb genomic region necessary for rpr induction in response to irradiation.25 Although Dp53 induced robust rprZ activation, DΔNp53 led to only a weak rprZ response in the wing imaginal discs (Figures 2b). To confirm this result, we tested how both Dp53 isoforms can activate p53RE-GFPnls, another rpr activity reporter, which contains a 150-bp rpr enhancer sequence embedding a consensus p53-binding site.15 We found Dp53 induced greater levels of GFP than DΔNp53 (Figures 2e and f). Furthermore, we found that Dp53 production (en>Dp53) led to the formation of blisters in the adult wings (Supplementary Figure S3). Although we currently do not know what p53-related biological process is responsible for the blister formation, we found that the incidence of wing blisters was significantly reduced in an rpr mutant (Supplementary Figure S3 and Table S1a). Because of the pupal lethality induced by the expression of DΔNp53 (en>DΔNp53), we could not test whether rpr mutant reduces wing blisters in this condition. Instead, we showed that rpr mutant partially suppressed pupal lethality induced by DΔNp53 (en>DΔNp53), suggesting that the DΔNp53-mediated phenotype involves rpr (Supplementary Table S1b). Next, we examined hid induction by Dp53 and DΔNp53 using an anti-Hid antibody.4 However, as both Dp53 isoforms lead to rapid elimination of apoptotic cells (data not shown), Hid expression was hard to detect. To overcome this difficulty, we examined the induction of Hid expression by Dp53 isoforms with the engrailed driver in dronc-null wing discs, where the cells were kept ‘undead' (Figures 2h–j). The engrailed driver is expressed in the posterior part of the wing imaginal disc in a clearly delineated domain (Supplementary Figure S1a). We observed much stronger Hid staining in the engrailed domain where DΔNp53 was overexpressed compared with Dp53. Together, these experiments support that rpr is a primary target during Dp53-mediated apoptosis and suggest that Dp53 is responsible for damage-induced transcription of rpr. In contrast, DΔNp53 is a poor activator of rpr and favors hid-mediated apoptosis.
Figure 2.
Dp53 and DΔNp53 use distinct RHG for apoptosis. (a–c) Wing imaginal discs carrying a rprXRE-lacZ (rprZ) was stained for β-galactosidase activity. A strong induction of rprZ is observed upon overproduction of Dp53 (MS1096>Dp53 in b; arrows). In contrast, weak rprZ induction is observed upon overproduction of DΔNp53 (MS1096>DΔNp53 in c; arrows). No rprZ induction is observed in the control disc (MS1096>GFP; a). White stars mark LacZ-positive phagocytes. (d–f) GFP fluorescence is observed in wing imaginal discs carrying p53R-GFPnls (p53 RE). Stronger GFP labelling is observed in Dp53 expression discs (e) compared with DΔNp53 expression discs (f), or in control wing discs (d). (g) Quantification of the rprZ staining area relative to the total wing area. (h and i) Hid protein was visualized by immunostaining in ‘undead cells' with an anti-Hid antibody in wing imaginal discs. Dp53 overproduction induces a mild hid expression (en>Dp53; droncI29−/− in h). DΔNp53 induces a strong hid expression in the engrailed domain of expression (en>DΔNp53; droncI29−/− in i). Scale bars are 100 μm. (j) Quantification of Hid-positive cells per wing in h and i. *P≤0.05, **P≤0.01, in Student's t-test
Dp53 and DΔNp53 differentially regulate apoptosis-induced proliferation
Johnston and colleagues7 have proposed that Dp53 gene promotes the expression of mitogens, such as Wg, which is required for apoptosis-induced proliferation. However, the specific roles of the Dp53 and DΔNp53 isoforms in activating Wg have not been defined. To study the roles of Dp53 and DΔNp53 in apoptosis-induced proliferation, we examined wg expression and cell proliferation after Dp53 or DΔNp53 proteins were produced in the developing wing tissues (Figures 3 and 4). We first used the ‘undead cell' model in which apoptosis is initiated by the expression of Dp53 isoforms but its execution is inhibited by expressing the inhibitor of caspase p35 (Figure 3). We found that DΔNp53 induced strong and widespread wg expression associated with hyperproliferative tissue in a deformed wing disc (Figure 3d). In this context, we determined whether wg expression was induced in neighbouring unaffected cells, namely, in a cell-non-automous manner. We observed that DΔNp53 induced wg expression both inside and outside of the engrailed domain of expression labelled with RFP (Figures 3d). This result indicates that DΔNp53 induces wg expression both in a cell-autonomous and non-autonomous manner. In contrast to DΔNp53, when ectopically expressed, Dp53 was only able to induce a moderate increase of wg that mainly resulted in a thickening of the Wg endogenous pattern of expression within the engrailed domain (Figure 3c). Moreover, Wg expression pattern was completely normal in p53-null wing disc, indicating that endogenous p53 gene does not regulate wg expression (Figures 3a and b). Next, we examined whether the induction of wg by Dp53 isoforms required dronc. We found that in dronc mutant wing discs, DΔNp53 also induced stronger wg expression than Dp53 (Figures 3e and f). From these results, we conclude that the regulation of wg expression by Dp53 isoforms is dronc independent.
Figure 3.
DΔNp53 induced wg expression in undead cells. (a–f) Wg protein was stained with an anti-Wg antibody (green). (a, b) The wg expression in wild-type (a) and in p53-null wing discs (b). (c and d) Double staining Wg (green) and RFP (red). Overproduction of Dp53 or DΔNp53 in wing imaginal discs expressing p35 and RFP (en>p35>RFP). The engrailed domain expression is visualized by RFP. Wg (c′ and d′) and RFP (c″ and d″) single fluorescent channels are shown. (c, c′ and c″) A mild induction of Wg is induced by Dp53 overproduction (en>Dp53) resulting in broadening of the endogenous Wg expression pattern in the engrailed domain. (d, d′ and d″) The overproduction of DΔNp53 (en>DΔNp53) induces a strong and widespread induction of Wg inside and outside the engrailed domain of expression (arrow). (e and f) Overproduction of Dp53 or DΔNp53 in wing imaginal discs mutant for droncI29. (e) A mild induction of Wg is induced by Dp53 overproduction (en>Dp53; droncI29−/−) resulting in broadening of the endogenous Wg expression pattern in the engrailed domain. (f) The overproduction of DΔNp53 (en>DΔNp53; droncI29−/−) induces a strong and widespread induction of Wg. Scale bars are 100 μm
Figure 4.
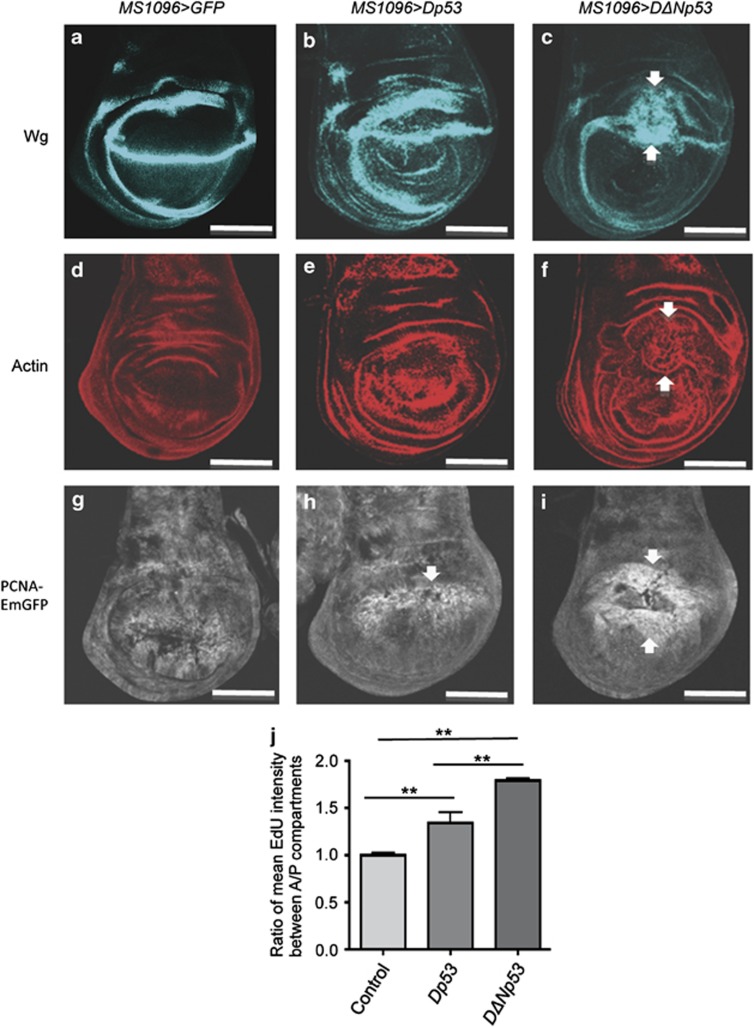
DΔNp53 induced wg expression and enhanced proliferation in wing imaginal discs. (a, d and g) Control wing imaginal discs (MS1096>LacZ). (b, e and h) Wing imaginal discs overproducing Dp53 (MS1096>Dp53). (c, f and i) Wing imaginal discs overproducing DΔNp53 (MS1096>DΔNp53). (a–c) Wg protein was stained with an anti-Wg antibody. (d–f) Actin was stained with phalloidin coupled with TRITC. (g–i) GFP fluorescence in wing imaginal discs carrying PCNA-EmGFP. (c) The overproduction of DΔNp53 leads to a strong increase of wg expression in the MS1096 domain. Increased wg expression by DΔNp53 is associated with tissue folding as visualized with actin staining (f) and with enhanced proliferation visualized with the PCNA-EmGFP reporter (i). Dp53 overproduction does not alter the overall Wg pattern but leads to an apparent thickening of the endogenous Wg domain (b). Dp53 overproduction does not induce tissue folding (e) and only induces some PCNA-EmGFP expression (h). Scale bars are 100 μm. (j) Quantification of EdU staining between the anterior and posterior compartments in control (en>p35) and wing discs overproducing Dp53 (en>Dp53) or DΔNp53 (en>DΔNp53). **P≤0.01, in Student's t-test
Next, we asked if the regulation of wg by Dp53 isoforms can be detected in ‘genuine' apoptotic cells. To achieve this goal, we used the strong MS1096 wing imaginal disc driver (Figure 4). As in the ‘undead' cell model, we observed that DΔNp53 induced strong wg expression in ‘genuine' apoptotic cells (Figure 4c). The increased level of wg expression was clearly detected in the dorsal part of the wing pouch region where the MS1096 is the strongest (Supplementary Figure S1b). The increased wg expression was associated with tissue accumulation and folding, suggesting hyperproliferation (Figures 4c and f). In contrast to DΔNp53, Dp53 expression did not alter the overall pattern of wg expression, but resulted in a thickening of the endogenous wg expression pattern (Figure 4b).
Next, we used a PCNA-EmGFP reporter that monitors E2f1 activity and EdU staining for cell proliferation.33 We observed enhanced PCNA-EmGFP labelling in the presence of DΔNp53, indicating increase cell proliferation (Figure 4i). In contrast, PCNA-EmGFP was only weakly induced by Dp53, suggesting that Dp53 induces little proliferation compared with DΔNp53 (Figures 4h and i). We also evaluated proliferation by EdU, a thymidine analogue that stains cells that have transited to S phase (Figure 4j). Consistent with the PCNA-EmGFP assay result, the EdU staining revealed that DΔNp53 induces more proliferation than Dp53.
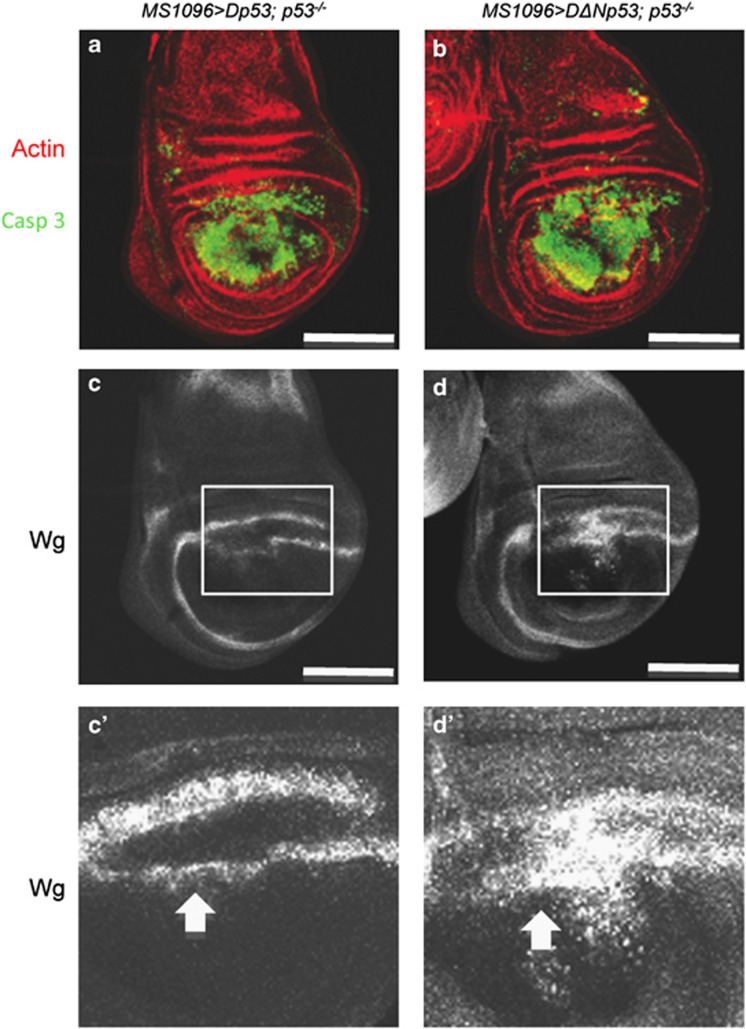
Drosophila p53 gene is proposed to act in a feedback loop to self-amplify and promote apoptosis-induced proliferation.7, 34 Therefore, we wanted to examine how the endogenous p53 gene contributes to the observed overexpression phenotype. We produced DΔNp53 or Dp53 in p53-null flies.35 First, we found that elevated levels of Dp53 or DΔNp53 led to robust caspase activation, indicating that each isoform can induce apoptosis in the absence of the endogenous p53 gene (Figures 5a and b). Next, we observed that DΔNp53 retained the ability to increase wg expression in the p53-null flies (Figures 5d and d′). This suggests that DΔNp53 overexpression alone is sufficient to induce wg expression. In contrast, in p53-null wing discs, we observed that Dp53 expression no longer induced any thickening of wg endogenous expression pattern. Rather, we observed a reduction of wg expression, which could be attributed to apoptosis of Wg-positive cells in this area (Figure 5c′). Together, these results show that DΔNp53, but not Dp53, is the positive regulator of wg expression.
Figure 5.
Dp53 and DΔNp53 differentially regulated wg expression. (a–d) Overexpression of Dp53 or DΔNp53 in p53 mutant flies. Dp53 (a and c) (MS1096>Dp53; p53−/−) and DΔNp53 (b and d) (MS1096>DΔNp53; p53−/−) are expressed in p53 mutant wing imaginal discs. (a and b) Overproduction of Dp53 or DΔNp53 leads to strong active caspase 3 staining (green). (c and c′) The overproduction of Dp53 inhibits wg expression at the dorso-ventral boundary (arrow). (d and d′) The overproduction of DΔNp53 induces wg expression (arrow). c′ and d′ are magnified views of the rectangles shown in c and d, respectively. Scale bars are 100 μm
Discussion
The discovery of multiple p53 isoforms raises the question of their functional specificity in the spectrum of p53-mediated biological responses. In Drosophila, as the first and only p53 isoform identified in almost a decade, the truncated DΔNp53 isoform was initialy presumed responsible for all p53 activities. The identification of the full-length Dp53 isoform that contains a full N-terminal transactivation domain challenged this presumption. Here, using gain-of-function studies, we examined the role of these two isoforms in apoptosis and apoptosis-induced proliferation. We found that both Dp53 isoforms activate apoptosis but preferentially activate different DIAP antagonists (Rpr or Hid) for caspase activation (Figures 1, 2 and Supplementary Figure S2). We showed that DΔNp53 promotes wg expression and cell proliferation, independently of endogenous p53, whereas Dp53 is unable to do so (Figures 3, 4, 5). We also found Dp53 to be primarily responsible for damage-induced transcriptional activation of rpr, whereas DΔNp53 is the p53 isoform dedicated to promoting apoptosis-induced proliferation.
The landmark study of Abrams and colleagues21 showed that DΔNp53 binds a DNA damage response element in the rpr regulatory region, which is responsible for the induction of apoptosis in response to irradiation. Here we showed that in wing imaginal discs, Dp53 is a stronger inducer of rpr expression than DΔNp53 (Figure 2). Moreover, we showed that DΔNp53 strongly induced hid expression, whereas Dp53 was only a weak inducer. Together, these observations suggest that the transcriptional competence of DΔNp53 differs from that of Dp53, and is consistent with a previous study showing that hid is transcriptionally induced by DΔNp53 in eye and wing imaginal discs.25, 28, 34 These results also suggest that some intrinsic ability to distinguish its activity for rpr and hid expressions is embedded in the N-terminus of the full length Dp53. Therefore, we propose that Dp53 is responsible for the damage-mediated activation of rpr for apoptosis, whereas DΔNp53 promotes apoptosis by inducing expression of hid. The physiological consequences of this functional segregation in apoptosis regulation by p53 isoforms remain to be determined.
Previous works have shown that apoptotic cells secrete morphogens that induce proliferation of surrounding cells.4, 36, 37 Although more clearly detected in ‘undead cells', mitogen gene expression and extra proliferation have also been detected in genuine apoptotic cells.4, 36, 38 It was proposed that the initiator caspase Dronc leads to Dp53 expression, which in turn activates mitogen gene expression,7, 34 but the specific roles of Dp53 and DΔNp53 remain to be established. Here we showed that DΔNp53 is a potent inducer of wg expression both in the ‘undead cell' and genuine apoptotic cell models (Figures 3, 4, 5). Specifically, we showed that DΔNp53 induced wg expression independently of dronc (Figure 3f). This indicates that DΔNp53 acts downstream of the apoptotic pathway to induce proliferation via the expression of wg. Thus, like JNK,39 DΔNp53 promotes proliferation independently of the apoptotic cascade. Further analysis will be required to determine the relationship between JNK and p53 isoforms in the induction of proliferation.
Wells et al.7 proposed that in the apoptosis-induced proliferation process, there is a feedback loop that activates wg expression in ‘undead cells' via Dronc and Dp53. Our results are consistent with such a feedback mechanism in which Dp53 and DΔNp53 induce apoptosis via rpr and hid, which in turn amplifies DΔNp53 via Dronc to promote wg expression. Our results also suggest that the feedback loop not only functions in ‘undead cells' but also in genuine apoptotic cells. Together, we propose that p53 isoforms act both upstream and downstream of the apoptotic pathway to promote wg expression and proliferation.
Our results show that DΔNp53 is a potent inducer of wg expression in both wild-type and p53-null wing discs. In contrast, Dp53 only weakly increased wg expression in wild-type but not in p53-null flies (Figures 3, 4, 5). Therefore, the weak induction of wg expression by Dp53 in wild-type disc is likely dependent on the endogenous p53 gene. Further investigations will be required to determine if DΔNp53 is the only p53 isoform regulating wg expression or if another isoform such as Dp53ΔC or the one encoded by the recently annotated p53-RD transcript (Flybase) contribute as well to the regulation of wg expression.
One of the most intensely debated questions regarding Drosophila ΔNp53 isoforms is whether they have their own biological activity or exert a dominant negative activity on p53.40, 41, 42 The fact that DΔNp53 induced Wg expression independently of endogenous p53 gene indicates that DΔNp53 does not require p53 for this function. In vertebrate studies, zebrafish Δ113p53 and human Δ133p53 do not act exclusively in a dominant-negative manner toward p53 but differentially regulate p53 target gene expression to modulate p53 function.41, 42 Similarly, our results show that Drosophila p53 isoforms have the capacity to use distinct targets to orchestrate their biological functions; we have shown that Dp53 promotes rpr expression, whereas DΔNp53 activates Hid and Wg expression in wing epithelium (Figures 2, 3, 4, 5). Overall, we propose that balancing apoptosis and apoptosis-induced proliferation may represent one primordial function of the TP53 gene family, and that this function requires the expression of Dp53 and DΔNp53 isoforms in a tightly controlled manner. In vertebrate, this primordial functional capacity may be differently exploited by TP53, TP63 and TP73 to regulate specific aspects of death/proliferation in the equilibrium, depending upon tissues and physiological contexts.
Material and Methods
UAS-Dp53 and UAS-DΔNp53 transgenic lines
Dp53 and DΔNp53 cDNAs were cloned (Kpn1/Xba1) into a pUAST-w+-attB transgenic fly vector. Best Gene, Inc. (Chino Hills, CA, USA) generated transgenic lines using φC31 integrase-mediated transgenesis. Vector DNA was injected in embryos carrying attP docking sites (strain 9736 at 53B2 and strain 9750 at 65B2). W+ embryos were selected and for establishing stable transgenic fly stocks.
Fly stocks
The following transgenic and mutant fly stocks were used: MS1096-Gal4, en-Gal4, uas-lacZ and uas-RFP (Bloomington stock); uas-GFP,43 rprXRE-lacZ (rprZ) and droncI29 (kind gifts from A Bergmann25); p53R-GFPnls (p53 RE; a generous gift from J Abrams15), rpr87,44 deficiency (3L)H99 (Df(3L)H99, referred to as H9945), PCNA-EmGFP33 and p53-null (p53 [5A-1-4]).35 The following genetic combinations were used to express transgenes in wing imaginal discs: (1) MS1096-Gal4,uas-GFP (MS1096>GFP), (2) MS1096-Gal4;uas-Dp53 (MS1096>Dp53), (3) MS1096-Gal4;uas-DΔNp53 (MS1096> DΔNp53), (4) en-Gal4/uas-Dp53 (en>Dp53), and (5) en-Gal4/uas-DΔNp53 (en>DΔNp53). Flies were raised under standard conditions at 25 °C.
Additional information can be found in the supplemental information.
Acknowledgments
This work was supported by grant from the CNRS (ATIP) and Ligue contre le cancer (Comités de Savoie and Puy-de-Dôme) to BM and NIH R01GM079425 to HDR. Bench fees were funded by a Marie Curie Fellowship ERG (PERG03-GA-2008-230812) to MLDD. FN was supported by a fellowship of the Association Française contre les Myopathies. Work on p53 isoforms by PH and his team is supported by a grant form the French National Cancer Institute (INCa). This work was made possible by the DROSO-TOOLS and PLATIM facilities of the UMS3444, Biosciences, Lyon, France. We thank Virginie Marcel for critical reading of the manuscript. We also thank Carmen Garrido for technical help, and our colleagues and Bloomington centre for fly stocks and reagents.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by M Oren
Supplementary Material
References
- Bergmann A, Steller H. Apoptosis, stem cells, and tissue regeneration. Sci Signal. 2010;3:re8. doi: 10.1126/scisignal.3145re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollereau B, Perez-Garijo A, Bergmann A, Miura M, Gerlitz O, Ryoo HD, et al. Compensatory proliferation and apoptosis-induced proliferation: a need for clarification Cell Death Differ 2012. e-pub ahead of print 22 June 2012; doi: 10.1038/cdd.2012.82 [DOI] [PMC free article] [PubMed]
- Morata G, Shlevkov E, Perez-Garijo A. Mitogenic signaling from apoptotic cells in Drosophila. Dev Growth Differ. 2011;53:168–176. doi: 10.1111/j.1440-169X.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Perez-Garijo A, Shlevkov E, Morata G. The role of Dpp and Wg in compensatory proliferation and in the formation of hyperplastic overgrowths caused by apoptotic cells in the Drosophila wing disc. Development. 2009;136:1169–1177. doi: 10.1242/dev.034017. [DOI] [PubMed] [Google Scholar]
- Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M. DRONC coordinates cell death and compensatory proliferation. Mol Cell Biol. 2006;26:7258–7268. doi: 10.1128/MCB.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells BS, Yoshida E, Johnston LA. Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol. 2006;16:1606–1615. doi: 10.1016/j.cub2006.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends Cell Biol. 2008;18:467–473. doi: 10.1016/j.tcb.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chera S, Ghila L, Wenger Y, Galliot B. Injury-induced activation of the MAPK/CREB pathway triggers apoptosis-induced compensatory proliferation in hydra head regeneration. Dev Growth Differ. 2011;53:186–201. doi: 10.1111/j.1440-169X.2011.01250.x. [DOI] [PubMed] [Google Scholar]
- Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Vega YA, Okano H, Lozano G. The intestinal epithelium compensates for p53-mediated cell death and guarantees organismal survival. Cell Death Differ. 2008;15:1772–1781. doi: 10.1038/cdd.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski R, Hofmann K, Gartner A. Phylogeny and function of the invertebrate p53 superfamily. Cold Spring Harb Perspect Biol. 2010;2:a001131. doi: 10.1101/cshperspect.a001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WJ, Chapo J, Roig I, Abrams JM. Meiotic recombination provokes functional activation of the p53 regulatory network. Science. 2010;328:1278–1281. doi: 10.1126/science.1185640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Tomasini R, McKeon FD, Mak TW, Melino G. The p53 family: guardians of maternal reproduction. Nat Rev Mol Cell Biol. 2011;12:259–265. doi: 10.1038/nrm3086. [DOI] [PubMed] [Google Scholar]
- Dotsch V, Bernassola F, Coutandin D, Candi E, Melino G. p63 and p73, the ancestors of p53. Cold Spring Harb Perspect Biol. 2010;2:a004887. doi: 10.1101/cshperspect.a004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP, et al. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19:2122–2137. doi: 10.1101/gad.1339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel V, Dichtel-Danjoy ML, Sagne C, Hafsi H, Ma D, Ortiz-Cuaran S, et al. Biological functions of p53 isoforms through evolution: lessons from animal and cellular models. Cell Death Differ. 2011;18:1815–1824. doi: 10.1038/cdd.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollmann M, Young LM, Di Como CJ, Karim F, Belvin M, Robertson S, et al. Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell. 2000;101:91–101. doi: 10.1016/S0092-8674(00)80626-1. [DOI] [PubMed] [Google Scholar]
- Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM. Drosophila p53 binds a damage response element at the reaper locus. Cell. 2000;101:103–113. doi: 10.1016/S0092-8674(00)80627-3. [DOI] [PubMed] [Google Scholar]
- Jin S, Martinek S, Joo WS, Wortman JR, Mirkovic N, Sali A, et al. Identification and characterization of a p53 homologue in Drosophila melanogaster. Proc Natl Acad Sci USA. 2000;97:7301–7306. doi: 10.1073/pnas.97.13.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen DG, Peifer M. Puckered, a Drosophila MAPK phosphatase, ensures cell viability by antagonizing JNK-induced apoptosis. Development. 2005;132:3935–3946. doi: 10.1242/dev.01949. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Davis KD, Coffman CR. Programmed cell death of primordial germ cells in Drosophila is regulated by p53 and the outsiders monocarboxylate transporter. Development. 2008;135:207–216. doi: 10.1242/dev.010389. [DOI] [PubMed] [Google Scholar]
- Fan Y, Lee TV, Xu D, Chen Z, Lamblin AF, Steller H, et al. Dual roles of Drosophila p53 in cell death and cell differentiation. Cell Death Differ. 2010;17:912–921. doi: 10.1038/cdd.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CS, Levet C, Chatelain G, Dourlen P, Fouillet A, Dichtel-Danjoy ML, et al. ER stress protects from retinal degeneration. EMBO J. 2009;28:1296–1307. doi: 10.1038/emboj.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassim OW, Fink JL, Cagan RL. Dmp53 protects the Drosophila retina during a developmentally regulated DNA damage response. EMBO J. 2003;22:5622–5632. doi: 10.1093/emboj/cdg543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky MH, Weinert BT, Tsang G, Rong YS, McGinnis NM, Golic KG, et al. Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol. 2004;24:1219–1231. doi: 10.1128/MCB.24.3.1219-1231.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S, Freije WA, Guptan P, Banerjee U. Metabolic control of G1-S transition: cyclin E degradation by p53-induced activation of the ubiquitin-proteasome system. J Cell Biol. 2010;188:473–479. doi: 10.1083/jcb.200912024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Jasper H. It's all about balance: p53 and aging. Aging (Albany NY) 2009;1:884–886. doi: 10.18632/aging.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish MP, Groth AC, Calos MP, Nusse R. Creating transgenic Drosophila by microinjecting the site-specific phiC31 integrase mRNA and a transgene-containing donor plasmid. Nat Protoc. 2007;2:2325–2331. doi: 10.1038/nprot.2007.328. [DOI] [PubMed] [Google Scholar]
- Guillen I, Mullor JL, Capdevila J, Sanchez-Herrero E, Morata G, Guerrero I. The function of engrailed and the specification of Drosophila wing pattern. Development. 1995;121:3447–3456. doi: 10.1242/dev.121.10.3447. [DOI] [PubMed] [Google Scholar]
- Swanhart LM, Sanders AN, Duronio RJ. Normal regulation of Rbf1/E2f1 target genes in Drosophila type 1 protein phosphatase mutants. Dev Dyn. 2007;236:2567–2577. doi: 10.1002/dvdy.21265. [DOI] [PubMed] [Google Scholar]
- Shlevkov E, Morata G. A dp53/JNK-dependant feedback amplification loop is essential for the apoptotic response to stress in Drosophila. Cell Death Differ. 2012;19:457–460. doi: 10.1038/cdd.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong YS, Titen SW, Xie HB, Golic MM, Bastiani M, Bandyopadhyay P, et al. Targeted mutagenesis by homologous recombination in D. melanogaster. Genes Dev. 2002;16:1568–1581. doi: 10.1101/gad.986602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garijo A, Martin FA, Morata G. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development. 2004;131:5591–5598. doi: 10.1242/dev.01432. [DOI] [PubMed] [Google Scholar]
- Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol. 2004;14:1262–1266. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell. 2008;14:399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa Y, Ziv O, Dinur T, Arama E, Gerlitz O. The NAB-Brk signal bifurcates at JNK to independently induce apoptosis and compensatory proliferation. J Biol Chem. 2011;286:15556–15564. doi: 10.1074/jbc.M110.193235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois S, Verhaegh G, North S, Luciani MG, Lassus P, Hibner U, et al. DeltaN-p53, a natural isoform of p53 lacking the first transactivation domain, counteracts growth suppression by wild-type p53. Oncogene. 2002;21:6722–6728. doi: 10.1038/sj.onc.1205874. [DOI] [PubMed] [Google Scholar]
- Aoubala M, Murray-Zmijewski F, Khoury MP, Fernandes K, Perrier S, Bernard H, et al. p53 directly transactivates Delta133p53alpha, regulating cell fate outcome in response to DNA damage. Cell Death Differ. 2011;18:248–258. doi: 10.1038/cdd.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ng SM, Chang C, Zhang Z, Bourdon JC, Lane DP, et al. p53 isoform delta113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev. 2009;23:278–290. doi: 10.1101/gad.1761609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollereau B, Wernet MF, Beaufils P, Killian D, Pichaud F, Kuhnlein R, et al. A green fluorescent protein enhancer trap screen in Drosophila photoreceptor cells. Mech Dev. 2000;93:151–160. doi: 10.1016/s0925-4773(00)00287-2. [DOI] [PubMed] [Google Scholar]
- Moon NS, Di Stefano L, Morris EJ, Patel R, White K, Dyson NJ. E2F and p53 induce apoptosis independently during Drosophila development but intersect in the context of DNA damage. PLoS Genet. 2008;4:e1000153. doi: 10.1371/journal.pgen.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.