Abstract
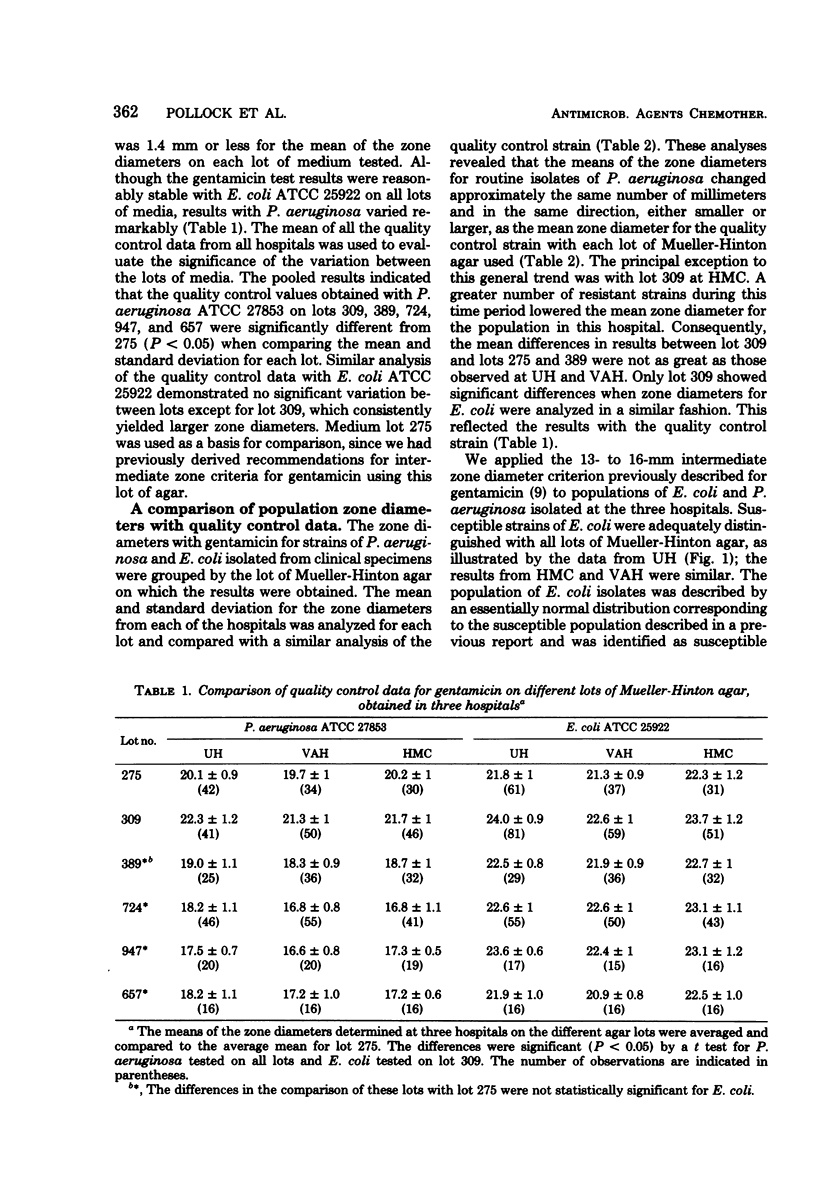
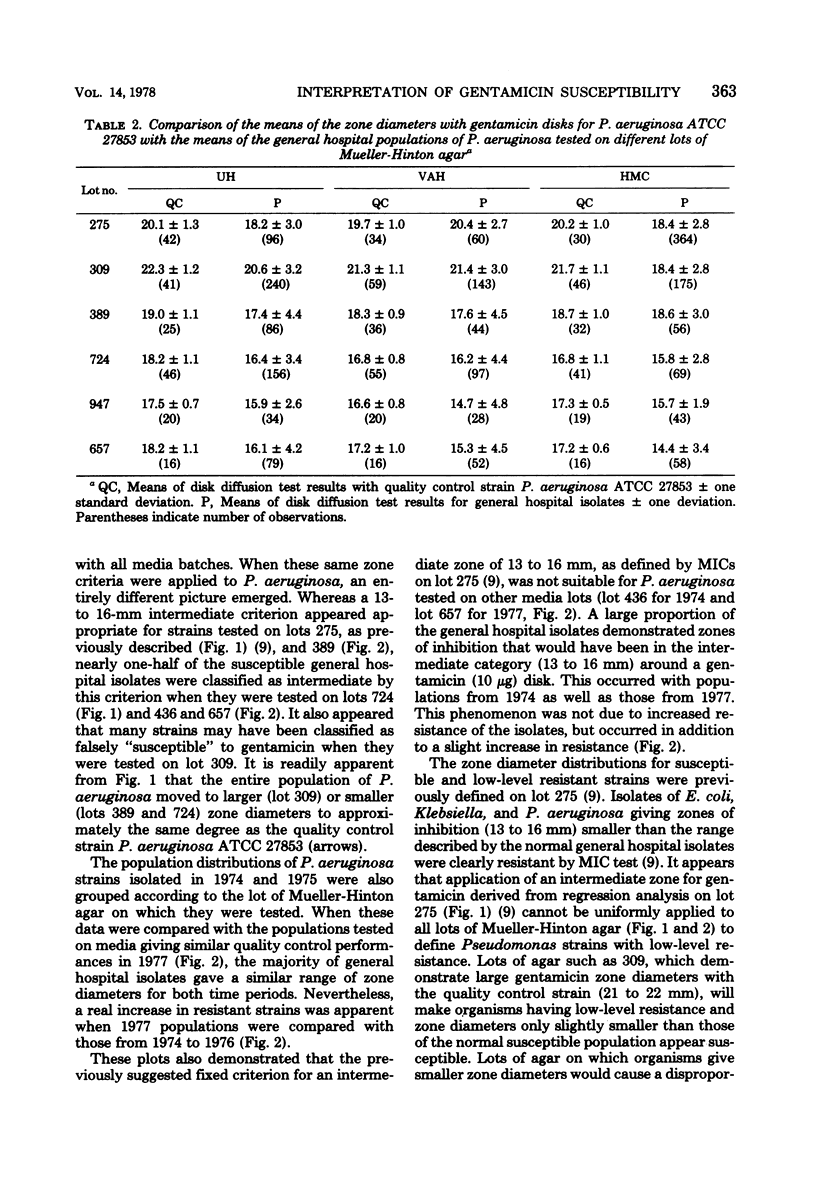
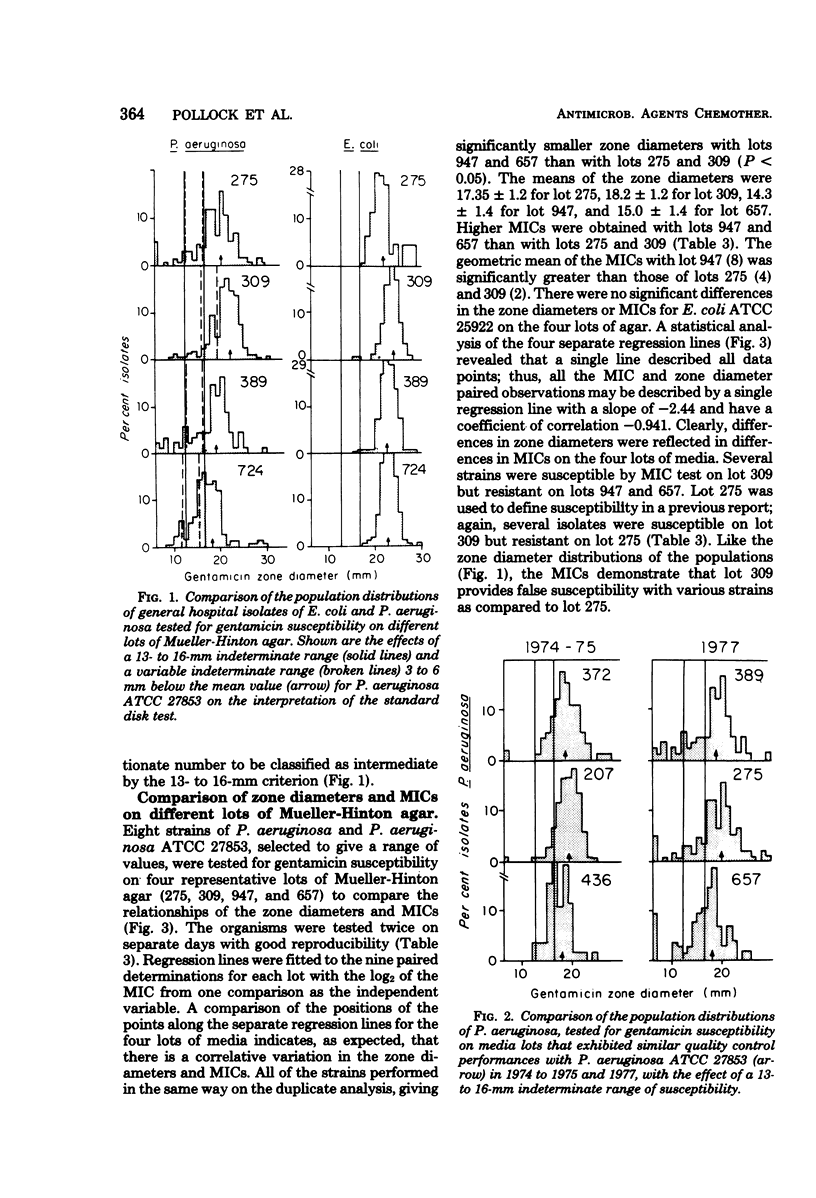
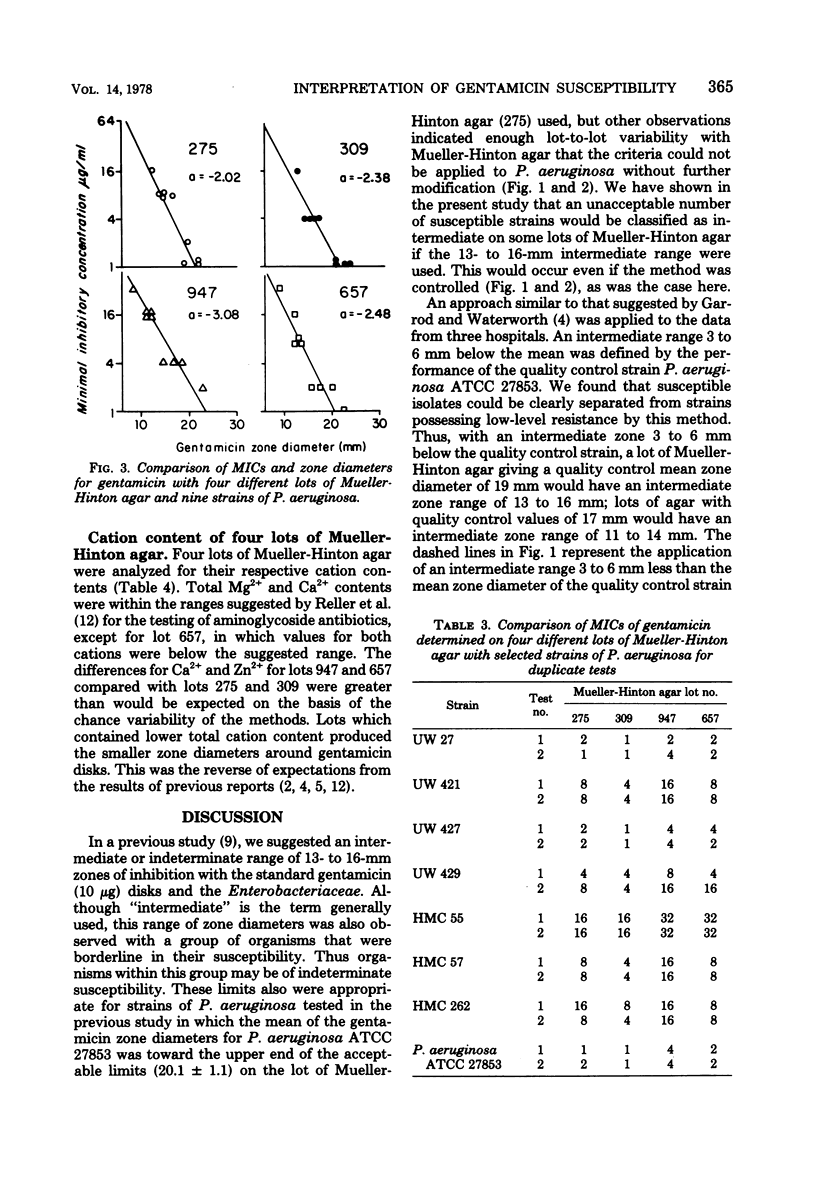
Population distributions and quality control data for strains of Pseudomonas aeruginosa tested for gentamicin susceptibility on six lots of Mueller-Hinton agar were analyzed. The lots of agar were used in three University of Washington hospitals from April 1975 through October 1977. The analyses indicated that the performance of members of the P. aeruginosa populations in each hospital closely followed the performance of the quality control strain, P. aeruginosa ATCC 27853, when tested on each lot of Mueller-Hinton medium. The variability of zone diameters with the P. aeruginosa populations and the quality control strain indicated that a fixed indeterminate range (13 to 16 mm) of gentamicin susceptibility was not applicable to these organisms as it was with the Enterobacteriaceae. Variability in gentamicin susceptibility results was demonstrated in both minimal inhibitory concentration and disk diffusion tests when eight selected P. aeruginosa strains and the quality control strain were tested on each lot of medium. This variation in susceptibility to gentamicin was not related to the total Ca2+, Mg2+, or Zn2+ content of each lot of medium. The data demonstrated that a moving indeterminate range of gentamicin susceptibility, 3 to 6 mm below the mean zone diameter of the quality control strain, was a suitable criterion for strains tested on a single medium lot. These results illustrate the importance of defining stringent performance standards for media used in the susceptibility testing of P. aeruginosa with gentamicin and other aminoglycoside antibiotics.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs W. H., Andrews F. A. Role of ionic strength in salt antagonism of aminoglycoside action on Escherichia coli and Pseudomonas aeruginosa. J Infect Dis. 1976 Nov;134(5):500–504. doi: 10.1093/infdis/134.5.500. [DOI] [PubMed] [Google Scholar]
- D'amato R. F., Thornsberry C., Baker C. N., Kirven L. A. Effect of calcium and magnesium ions on the susceptibility of Pseudomonas species to tetracycline, gentamicin polymyxin B, and carbenicillin. Antimicrob Agents Chemother. 1975 May;7(5):596–600. doi: 10.1128/aac.7.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson H. M., Sherris J. C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1+–1+. [PubMed] [Google Scholar]
- Garrod L. P., Waterworth P. M. Effect of medium composition on the apparent sensitivity of Pseudomonas aeruginosa to gentamicin. J Clin Pathol. 1969 Sep;22(5):534–538. doi: 10.1136/jcp.22.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. N., Kutscher E., Ireland P., Barnett J. A., Sanford J. P. Effect of the concentrations of magnesium and calcium on the in-vitro susceptibility of Pseudomonas aeruginosa to gentamicin. J Infect Dis. 1971 Dec;124 (Suppl):S37–S45. doi: 10.1093/infdis/124.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- Meret S., Henkin R. I. Simultaneous direct estimation by atomic absorption spectrophotometry of copper and zinc in serum, urine, and cerebrospinal fluid. Clin Chem. 1971 May;17(5):369–373. [PubMed] [Google Scholar]
- Minshew B. H., Pollock H. M., Schoenknecht F. D., Sherris J. C. Emergence in a burn center of populations of bacteria resistant to gentamicin, tobramycin, and amikacin: evidence for the need for changes in zone diameter interpretative standards. Antimicrob Agents Chemother. 1977 Dec;12(6):688–696. doi: 10.1128/aac.12.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pybus J. Determination of calcium and magnesium in serum and urine by atomic absorption spectrophotometry. Clin Chim Acta. 1969 Feb;23(2):309–317. doi: 10.1016/0009-8981(69)90046-1. [DOI] [PubMed] [Google Scholar]
- Reller L. B., Schoenknecht F. D., Kenny M. A., Sherris J. C. Antibiotic susceptibility testing of Pseudomonas aeruginosa: selection of a control strain and criteria for magnesium and calcium content in media. J Infect Dis. 1974 Nov;130(5):454–463. doi: 10.1093/infdis/130.5.454. [DOI] [PubMed] [Google Scholar]
- Snelling C. F., Ronald A. R., Cates C. Y., Forsythe W. C. Resistance of gram-negative bacilli to gentamicin. J Infect Dis. 1971 Dec;124 (Suppl):S264–S270. doi: 10.1093/infdis/124.supplement_1.s264. [DOI] [PubMed] [Google Scholar]
- Traub W. H. Susceptibility of Pseudomonas aeruginosa to gentamicin sulfate in vitro: lack of correlation between disc diffusion and broth dilution sensitivity data. Appl Microbiol. 1970 Jul;20(1):98–102. doi: 10.1128/am.20.1.98-102.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau D. L., Freier E. F. Determination of calcium in urine and serum by atomic absorption spectrophotometry (AAS). Clin Chem. 1967 Feb;13(2):101–114. [PubMed] [Google Scholar]
- Washington J. A., 2nd, Snyder R. J., Kohner P. C., Wiltse C. G., Ilstrup D. M., McCall J. T. Effect of cation content of agar on the activity of gentamicin, tobramycin, and amikacin against Pseudomonas aeruginosa. J Infect Dis. 1978 Feb;137(2):103–111. doi: 10.1093/infdis/137.2.103. [DOI] [PubMed] [Google Scholar]


