Abstract
The purpose of this study was to examine the role of tumor necrosis factor receptor 1 (TNFR1) in the airway hyperresponsiveness characteristic of obese mice. Airway responsiveness to intravenous methacholine was measured using the forced oscillation technique in obese Cpefat mice that were either sufficient or genetically deficient in TNFR1 (Cpefat and Cpefat/TNFR1−/− mice) and in lean mice that were either sufficient or genetically deficient in TNFR1 [wild-type (WT) and TNFR1−/− mice]. Compared with lean WT mice, Cpefat mice exhibited airway hyperresponsiveness. Airway hyperresponsives was also greater in Cpefat/TNFR1−/− than in Cpefat mice. Compared with WT mice, Cpefat mice had increases in bronchoalveolar lavage fluid concentrations of several inflammatory moieties including eotaxin, IL-9, IP-10, KC, MIG, and VEGF. These factors were also significantly elevated in Cpefat/TNFR1−/− vs. TNFR1−/− mice. Additional moieties including IL-13 were also elevated in Cpefat/TNFR1−/− vs. TNFR1−/− mice but not in Cpefat vs. WT mice. IL-17A mRNA expression was greater in Cpefat/TNFR1−/− vs. Cpefat mice and in TNFR1−/− vs. WT mice. Analysis of serum indicated that obesity resulted in systemic as well as pulmonary inflammation, but TNFR1 deficiency had little effect on this systemic inflammation. Our results indicate that TNFR1 is protective against the airway hyperresponsiveness associated with obesity and suggest that effects on pulmonary inflammation may be contributing to this protection.
Keywords: bronchoalveolar lavage, airway responsiveness, TNF-α, inflammation, IL-17A
obesity is emerging as an important risk factor for asthma. Epidemiological data indicate increases in the prevalence and incidence of asthma in obese and overweight individuals. Obesity impairs the efficacy of controller medications and may even increase asthma severity (see reviews in Refs. 6, 20, 46, 66). In obese non-atopic asthmatics, weight loss improves airway hyperresponsiveness (AHR), a characteristic feature of asthma (19). Animal models also support a relationship between obesity and asthma: regardless of how they become obese, obese mice exhibit AHR (28–31, 48, 68, 71, 84).
The mechanistic basis for the relationship between obesity and asthma has not yet been established, but obesity-related systemic inflammation may contribute. In both obese humans and obese mice, even in the absence of any overt inflammatory insult, there is chronic, low-grade, systemic inflammation characterized by increased circulating leukocytes and increased serum concentrations of soluble cytokine receptors, chemokines, acute phase proteins, as well as certain cytokines (65, 75). The source of many of these inflammatory moieties appears to be the adipose tissue itself, which becomes infiltrated with activated macrophages in the obese (83), although intestinal inflammation secondary to obesity-related alterations in commensal bacteria may also play a role (18). The systemic inflammation of obesity is likely to be functionally important, since serum concentrations of inflammatory markers correlate with the presence of obesity-related conditions such as Type 2 diabetes and atherosclerosis (4, 60, 72, 82). The observation that the onset of systemic inflammation corresponds temporally with the onset of AHR in obese Cpefat mice (30) suggests that this inflammation may also be important for asthma.
Of the various inflammatory moieties that are increased in the blood of obese individuals, augmented tumor necrosis factor (TNF)-α (35, 52, 84, 88) may be particularly important for asthma. TNF-α can induce AHR when administered exogenously, both in humans (73) and in other species (37). Attenuating endogenous TNF-α signaling also improves AHR in some animal models of asthma (12, 13, 33, 36, 51, 53, 54, 69), although protective roles for TNF-α or its receptors have also been observed (32, 63). In addition, risk estimates for asthma as a function of body mass index (BMI) are higher in subjects with the G/A or A/A TNF-α-308 polymorphisms that lead to higher TNF-α expression in G/G subjects, especially among those with nonatopic asthma (9). Finally, serum concentrations of TNF receptor (sTNFR) 1 are inversely related to lung function in subjects with obesity (43).
TNF-α mediates its effects by binding to either of two receptors, TNFR1 and TNFR2, that differ in several respects (21, 38, 50, 55, 64, 81). For example, TNFR1 has a greater ability than TNFR2 to induce inflammation, whereas TNFR2 has greater pro-angiogenic effects. TNFR1 has a death domain that promotes apoptosis, whereas TNFR2 does not. TNFR2 interacts mainly with membrane-associated but not -soluble TNFα, whereas TNFR1 interacts with both forms of TNF-α. We have previously reported that signaling via TNFR2 contributes to obesity-related AHR (84). The purpose of this study was to examine the role of TNFR1 in these events. To do so, we generated obese Cpefat mice that are also genetically deficient in the TNFR1 receptor (Cpefat/TNFR1−/− mice). Cpefat mice are genetically deficient in carboxypeptidase E (Cpe), an enzyme involved in processing prohormones and proneuropeptides that are important for appetite regulation and energy expenditure (15, 44). Absence of Cpe leads to obesity (29, 30). Airway responsiveness to intravenous methacholine was assessed in otherwise unchallenged Cpefat/TNFR1−/− mice along with wild-type (WT), Cpefat, and TNFR1−/− mice. Bronchoalveolar lavage (BAL) was performed, and serum was obtained to assess the role of TNFR1 in the systemic inflammation of obesity and to determine whether there were TNFR1-dependent inflammatory changes within the lungs. We also evaluated pulmonary mRNA expression of several genes that may contribute to AHR and are altered in lungs of obese mice.
METHODS
Animals.
This study was approved by the Harvard Medical Area Standing Committee on Animals. Cpefat mice are infertile. Therefore, heterozygous Cpe+/− mice were purchased from Jackson Labs (Bar Harbor, ME). Cpe+/− mice were mated with TNFR1−/− mice, also purchased from Jackson Labs. Both types of mice were on a C57BL/6 background. Cpe+/−/TNFR1+/− offspring were bred back to TNFR1−/− mice. Cpe+/−/TNFR1−/− mice from this mating were then bred together to obtain Cpefat/TNFR1−/− mice and TNFR1−/− controls. Heterozygous Cpe+/− mice were mated with each other to generate WT and Cpefat mice. All mice were fed standard mouse chow diets. Mice were 11–16 wk of age at the time of study, and there was no significant difference in average age among groups.
Protocol.
Two cohorts of WT, Cpefat, TNFR1−/−, and Cpefat/TNFR1−/− mice were used. In the first, mice were anesthetized and instrumented for the measurement of pulmonary mechanics and airway responsiveness to intravenous methacholine followed by bronchoalveolar lavage (BAL). In the second cohort, mice were euthanized with an overdose of anesthetic. Blood was collected by cardiac puncture, and serum was generated.
Pulmonary mechanics and airway responsiveness.
Mice were anesthetized with pentobarbital sodium (50 mg/kg) and xylazine (7 mg/kg). The trachea was cannulated with a tubing adaptor, and the tail vein was also cannulated for the delivery of acetyl-β-methylcholine chloride (methacholine). The mice were ventilated using a specialized ventilator (flexiVent, SCIREQ, Montreal, Canada), as previously described (30). Once ventilation was established, the chest wall was opened bilaterally to expose the lungs to atmospheric pressure and thus exclude any chest wall contributions to pulmonary mechanics. A positive end-expiratory pressure (PEEP) of 3 cmH2O was applied by placing the expiratory line under water. The tail vein was cannulated for the delivery of methacholine. The lungs were first inflated to three times tidal volume (Vt) to standardize volume history. The static elastance (Estat) of the lungs was then assessed from the deflation limb of quasistatic pressure volume curves as previously described (29). Finally, baseline pulmonary mechanics and responses to intravenous methacholine were measured using the forced oscillation technique, as previously described (29, 30). Briefly, 1 min after an inflation to three times Vt, mice were given a 1 μl/g bolus of PBS. Pulmonary resistance (RL) was measured every eighth breath for the next 1–2 min until RL peaked. At that point, a forcing function containing frequencies ranging from 0.25 to 19.63 Hz was applied by the ventilator, and lung impedance was measured and used to obtain Newtonian resistance (Rn), which largely reflects the conducting airways and the coefficients of lung tissue damping (G) and lung tissue elastance (H), which reflect changes in the small airways and lung tissue, as previously described (29, 30). The procedure was repeated using increasing doses of methacholine chloride dissolved in PBS increasing in half-log intervals from 0.03 to 1.0 mg/ml at a dose of 1 μl/g, with 5 min between doses of methacholine.
BAL.
The lungs were lavaged, and total cell numbers and differentials were assessed as previously described (30). Lavage supernatants were frozen at −80°C until analyzed for sTNFR1 by ELISA. Serum was isolated and stored at −20°C until assayed for TNF-α, leptin, sTNFR1, and sTNFR2 by ELISA. Sources of ELISA assays were as follows: EBioscience (San Diego, CA) for TNF-α and R&D systems (Minneapolis, MN) for sTNFR1, sTNFR2, and leptin. In addition, serum and BAL were analyzed by Bioplex assay for 35 different cytokines, chemokines, and growth factors (Eve Technologies, Calgary, Alberta, Canada).
RNA extraction and real-time PCR.
RNA was extracted from lungs and cDNA was generated as previously described (70). TrkB, endothelin-1, fractalkine, adipsin, and IL-17 mRNA expression were quantified by real-time PCR (7300 Real-Time PCR Systems, Applied Biosystems) using SYBR-green detection. The delta Ct (ΔCt) was obtained by subtracting from the gene of interest, the Ct values for a housekeeping gene, 18S. Primers have been previously described (34, 84). Changes in mRNA were expressed relative to values from the WT mice to obtain ΔΔCt values. Expression was calculated by the ΔΔCt method (47).
Statistics.
Differences in BAL and serum moieties and gene expression were assessed by factorial ANOVA using Cpe genotype and TNFR1 genotype as main effects. Effects on airway responsiveness were assessed using repeated-measures ANOVA. The Fisher Least Significant Difference test was used for post hoc comparisons. Serum eotaxin, G-CSF, IL-6, IL-13, IP-10, KC, MCP-1, and MIG as well as BAL eotaxin, IFN-γ, and MIG were log transformed before statistical analysis to conform to a normal distribution. BAL G-CSF, IL-10, and MIP-2 were not normally distributed even after log transformation. For these outcomes, we used a Mann-Whitney U-test to assess the overall effect of Cpe genotype and TNFR1 genotype, and a Kruskal Wallis test to assess differences within the four groups. In the case of results from the Bioplex assay, samples below the limit of detection of the assay were assigned a value of 0. Statistical analyses were carried out using SAS software (SAS Institute, Cary, NC). Results presented are means ± SE unless otherwise indicated. P < 0.05 was considered statistically significant.
RESULTS
Body weight.
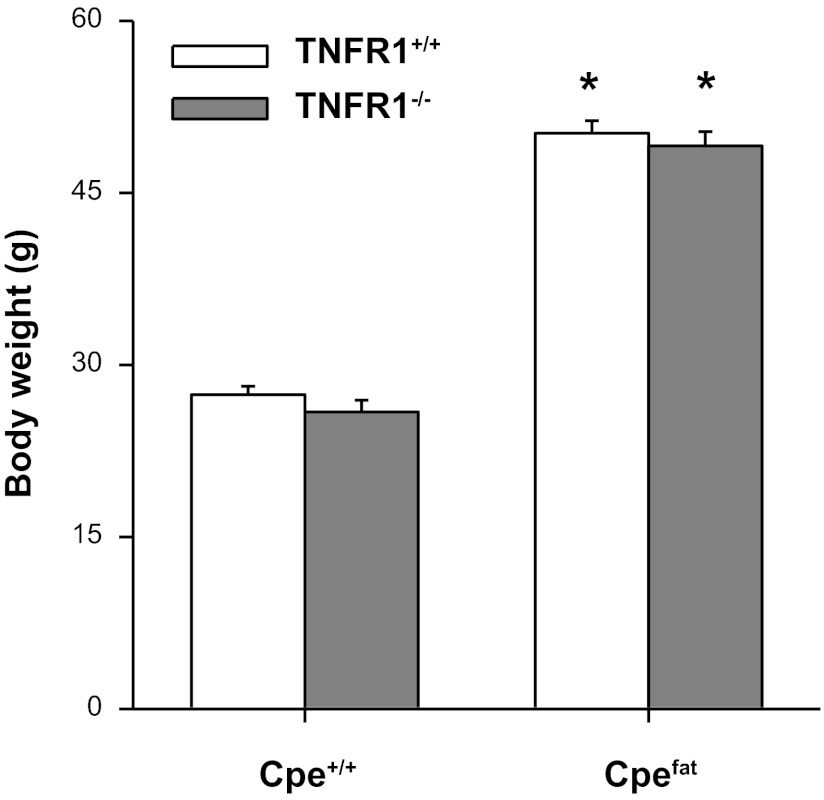
Factorial ANOVA indicated a significant (P < 0.001) effect of Cpe genotype on body weight, whereas TNFR1 genotype had no effect. Cpefat mice, whether TNFR1 deficient or not, weighed approximately twice as much as controls (Fig. 1).
Fig. 1.
Effect of tumor necrosis factor (TNF) receptor (TNFR) 1 deficiency on body weight. Cpefat mice, mice with a genetic deficiency in the carboxypeptidase E (Cpe) gene; Cpe+/+ mice, mice genetically sufficient for Cpe; TNFR1−/− mice, mice genetically deficient in the TNFR1 gene. TNFR1+/+ mice, mice genetically sufficient for TNFR1. Results are means ± SE of data from 23–30 mice in each group and include data from both the mice used in the measurements of airway responsiveness and the mice used for collection of serum. *Significant difference compared with TNFR1 genotype-matched lean mice (P < 0.001).
Pulmonary mechanics and airway responsiveness.
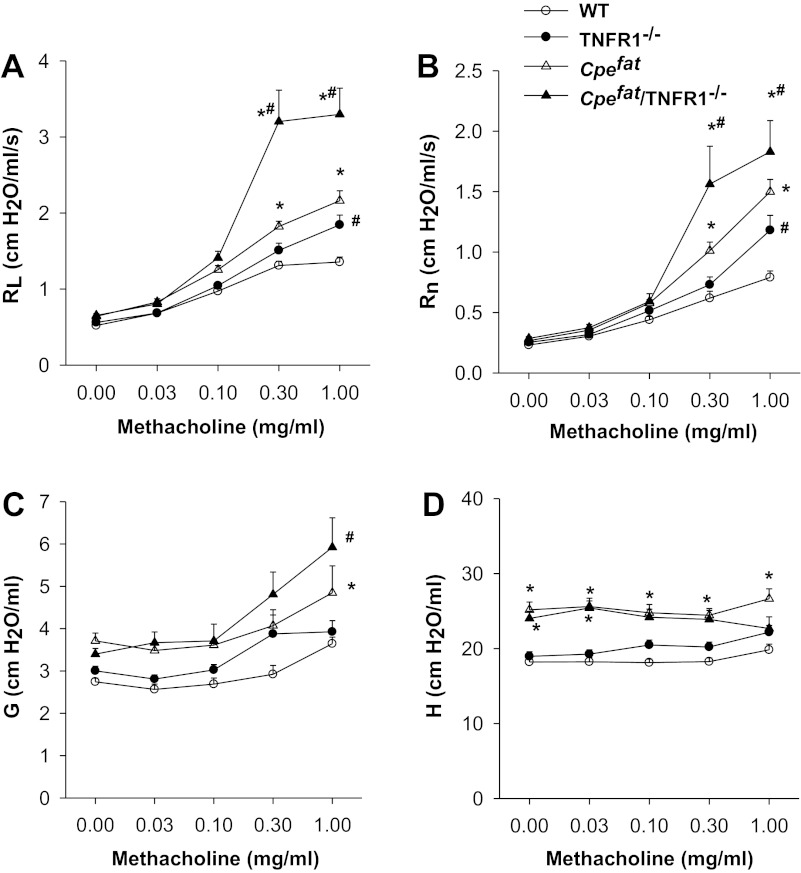
Estat was ∼30% higher in Cpefat vs. WT mice (17.6 ± 0.3 vs. 13.6 ± 0.4 cmH2O/ml), consistent with previous observations (29), but there was no effect of TNFR1 genotype on Estat (17.8 ± 0.6 and 14.8 ± 0.6 cmH2O/ml in Cpefat/TNFR1−/− and TNFR1−/− mice, respectively). Repeated-measures ANOVA also indicated a significant effect of genotype on pulmonary mechanics for RL, Rn, G, and H (P < 0.001 in each case). Methacholine-induced changes in RL were significantly greater in Cpefat and Cpefat/TNFR1−/− mice than in their respective lean controls (Fig. 2A). Surprisingly, airway responsiveness was significantly greater in Cpefat/TNFR1−/− than in Cpefat mice. In contrast, airway responsiveness was not different in lean TNFR1−/− vs. WT mice, except at the highest concentration of methacholine. Essentially similar results were obtained for Rn (Fig. 2B). In contrast, much more modest changes in G occurred following methacholine (Fig. 2C). For G, there was an effect of obesity at the highest concentration of methacholine for both Cpefat/TNFR1−/− and Cpefat mice, but there was no difference between these two genotypes. For H, there was an effect of obesity on the baseline values, consistent with the changes in Estat, but methacholine had virtually no effect on H (Fig. 2D). The results are consistent with the airways rather than the lung tissue being the primary locus of the Cpefat/TNFR1 interaction.
Fig. 2.
Airway responsiveness to intravenous methacholine in lean wild-type (WT) and TNFR1−/− mice and in obese Cpefat and Cpefat/TNFR1−/− mice. Cpefat/TNFR1−/− mice, mice genetically deficient in both Cpe and TNFR1. Results are means ± SE of data from 7–13 mice per group. Outcome indicators are: pulmonary resistance (RL; A); Newtonian resistance (Rn; B); and the coefficients of lung tissue damping (G; C) and elastance (H; D). Results are from male and female mice combined. Similar results were obtained in each gender. *Significant difference compared with TNFR1 genotype-matched lean mice (P < 0.05). #Signficant difference compared with Cpe genotype-matched TNFR1-sufficient mice (P < 0.05).
Pulmonary inflammation.
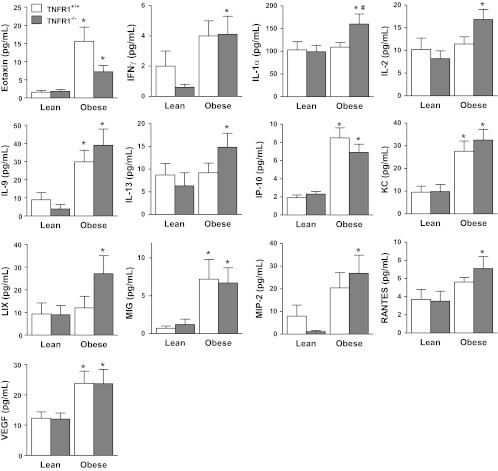
Neither obesity nor TNFR1 genotype had any effect on the inflammatory cell populations recovered from BAL fluid (Table 1). In TNFR1-sufficient mice, there were obesity-related increases in BAL fluid eotaxin, IL-9, IP-10, KC, MIG, and VEGF (Fig. 3). These factors were also significantly elevated in TNFR1−/− obese mice. BAL IFN-γ, IL-1α, IL-2, IL-13, LIX, RANTES, and MIP-2 were also elevated in Cpefat/TNFR1−/− vs. TNFR1−/− mice. BAL sTNFR1 was also elevated in BAL of Cpefat vs. WT mice (574 ± 117 vs. 294 ± 16 pg/ml, respectively; P < 0.01), consistent with previous reports in Cpefat mice as well as other types of obese mice (42, 48, 67).
Table 1.
Bronchoalveolar lavage cells in WT and Cpefat mice with and without TNFR1
| Cell Type | WT/WT | WT/TNFR1−/− | Cpefat/WT | Cpefat/TNFR1−/− |
|---|---|---|---|---|
| Macrophages | 23.6 ± 3.1 | 25.2 ± 5.2 | 29.6 ± 5.7 | 22.2 ± 4.2 |
| PMNs | 0.15 ± 0.05 | 0.10 ± 0.02 | 0.16 ± 0.04 | 0.09 ± 0.01 |
| Lymphocytes | 1.3 ± 1.1 | 1.1 ± 1.3 | 0.8 ± 0.7 | 0.3 ± 0.2 |
Results are means ± SE of data from 6–10 mice per group. Results are expressed as cells ×104/ml. WT, wild-type mice; TNFR, tumor necrosis factor receptor; PMNs, polymorphonuclear neutrophils.
Fig. 3.
Bronchoalveolar lavage (BAL) fluid concentrations of eotaxin, IFN-γ, IL-1α, IL-2, IL-9, IL-13, IP-10, KC, LIX, MIG, MIP-2, RANTES, and VEGF in lean (Cpe sufficient) and obese (Cpefat) mice that were either TNFR1 sufficient (TNFR1+/+) or deficient (TNFR1−/−). Results are means ± SE of data from 7–9 mice in each group. IL-7, G-CSF, IL-10, and IL-12 p40 were not affected by either obesity or TNFR1 deficiency. GM-CSF, IL-3, IL-4, IL-1β, IL-5, IL-6, IL-12 p70, IL-15, IL-17, LIF, MCP-1, MIP-1α, MIP-1β, and TNFα were below the limit of detection in most samples. *Significant difference vs. TNFR1 genotype-matched lean mice (P < 0.05). #Signficant difference vs. Cpe genotype-matched TNFR1+/+ mice (P < 0.05).
Pulmonary gene expression.
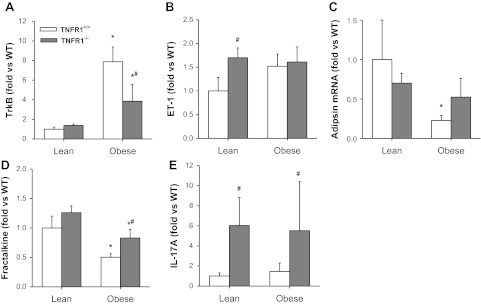
We recently reported results of a microarray analysis on lungs of obese Cpefat vs. lean WT mice (84). Several of the obesity-affected genes including endothelin, trkB, adipsin, and fractalkine have the capacity to impact airway responsiveness either directly (5, 41, 61, 74) or indirectly (40). Moreover, reductions in obesity-related AHR in TNFR2−/− mice were associated with reductions in obesity-related elevations in both trkB and endothelin. To determine whether TNFR1 deficiency impacted the expression of these genes, we performed RT-PCR on RNA generated from lungs of WT, TNFR1−/−, Cpefat, and Cpefat/TNFR1−/− mice (Fig. 4). Consistent with our previous observations, TrkB and endothelin mRNA expression were increased, whereas adipsin and fractalkine expression were decreased in Cpefat vs. WT mice, although the increase in endothelin did not reach statistical significance (Fig. 4B). Notably, TNFR1 deficiency caused a significant attenuation of the effects of obesity on expression of these genes. TrkB expression was significantly reduced in Cpefat/TNFR1−/− vs. Cpefat mice (Fig. 4A), and obesity-related reductions in adipsin and fractalkine expression were reduced or abolished in TNFR1−/− mice (Fig. 4, C and D). The results indicate that obesity-related changes in the pulmonary expression of these genes is dependent on TNF-α acting at least in part through TNFR1.
Fig. 4.
Pulmonary mRNA expression of TrkB (A), endothelin (ET-1; B), adipsin (C), fractalkine (D), and IL-17 (E) in lean (Cpe sufficient) and obese (Cpefat) mice that were either TNFR1 sufficient (TNFR1+/+) or deficient (TNFR1−/−). Results are means ± SE of data from 8–12 mice per group. *Significant difference vs. TNFR1 genotype-matched WT mice (P < 0.01).
Because of increasing evidence of a role for IL-17 in AHR (39), we also examined IL-17 mRNA expression in the lung tissue of these mice. Although we did not observe any significant effect of obesity on IL-17 expression, there was a marked and significant increase in IL-17 mRNA expression in TNFR1-deficient mice regardless of whether or not they were obese (Fig. 4E).
Systemic inflammation.
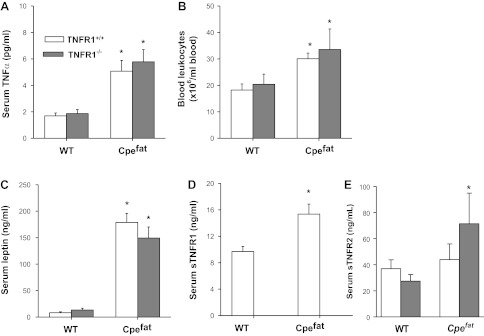
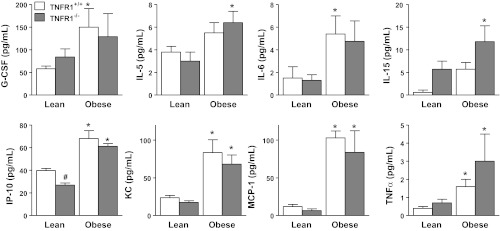
We have previously postulated that the innate AHR that characterizes obese mice is related to low-grade systemic inflammation that exists in the obese state (29, 30). Therefore, we assessed the effect of attenuating TNFR1 signaling on this systemic inflammation. Compared with WT controls, serum TNF-α was significantly increased in the obese mice (Fig. 5A), but there was no effect of TNFR1 genotype on serum TNF-α. Bioplex assay also confirmed increases in serum TNF-α in the obese mice, regardless of TNFR1 genotype (Fig. 6). Blood leukocytes, and serum concentrations of leptin and sTNFR1 were also elevated in obese mice (Fig. 5, B and D), but we observed no significant effect of TNFR1 genotype on any of these markers of systemic inflammation (except for sTNFR1, which was reduced to background levels in the TNFR1-deficient mice). Factorial ANOVA indicated a significant effect of obesity on serum G-CSF, IL-5, IL-6, IL-15, IP-10, KC, MCP-1, and TNF-α, and a significant effect of TNFR1 deficiency on serum IP-10 and IL-15 (Fig. 6). TNFR1 deficiency resulted in a significant reduction in serum IP-10 in lean mice and a significant overall increase in serum IL-15 (P < 0.02) when lean and obese mice were considered together.
Fig. 5.
Serum TNF-α (A), leptin (C), soluable TNFR1 (sTNFR1; D), sTNFR2 (E), and total blood leukocytes (B) in lean (Cpe sufficient) and obese (Cpefat) mice that were either TNFR1 sufficient (TNFR1+/+) or deficient (TNFR1−/−). Results are means ± SE of data from 8–12 mice in each group. *Significant difference compared with TNFR1 genotype-matched lean mice (P < 0.05).
Fig. 6.
Serum G-CSF, IL-5, IL-6, IL-15, IP-10, KC, MCP-1, and TNF-α assessed by Bioplex assay in lean (Cpe sufficient) and obese (Cpefat) mice that were either TNFR1 sufficient (TNFR1+/+) or deficient (TNFR1−/−). Results are means ± SE of data from 6–8 mice per group. There was no significant effect of obesity or TNFR1 genotype on serum concentrations of IL-1α, IL-7, IL-9, IL-13, IL-17, MIG, MIP-2, RANTES, and eotaxin. Serum concentrations of GM-CSF, IFN-γ, IL-1β, IL-2, IL-3, IL-4, IL-10, IL-12 (p40 and p70), LIF, M-CSF, MIP-1β, and VEGF were at or below the limit of detection in the majority of obese and lean samples. Serum LIX was above the range of the standard curve in all samples. *Significant difference vs. TNFR1 genotype-matched lean mice (P < 0.05). #Significant difference vs. obesity-matched TNFR1+/+ mice (P < 0.05).
DISCUSSION
We have previously reported that obese mice have hyperresponsive airways (28–31, 48, 68, 71, 84). We now report that TNFR1 deficiency exacerbates this innate AHR (Fig. 2), suggesting that the TNFR1 receptor plays a protective role in these mice. We also report obesity- and TNFR1-related elevations in inflammatory mediators that may contribute to obesity-related AHR (Table 1; Fig. 3). Surprisingly, given the importance of TNFR1 for TNF-α-mediated inflammation (50, 55), TNFR1 was, if anything, protective against obesity-related inflammation.
Compared with WT mice, obese Cpefat mice exhibited AHR, consistent with previous reports using these mice (29, 30, 84) and other types of obese mice (28, 31, 48, 68). Acute exogenous administration of TNF-α has been shown to increase airway responsiveness (37, 73). TNF-α also has been shown to act directly on airway smooth muscle, increasing its contractility (11). Furthermore, other obesity-related conditions are ameliorated in obese mice lacking TNF-α or TNF receptors (7, 17, 45, 57, 79, 80). Consequently, we examined the hypothesis that obesity-related elevations in TNF-α might be contributing to obesity-related AHR. We examined obese mice deficient in TNFR1 because others have shown that the insulin resistance, the enhanced hepatic tumorigenesis, and the hepatic steatosis of obesity are mediated by TNF-α acting via TNFR1 (57, 79). In addition, TNF-α-induced increases in airway smooth muscle contractility are mediated via TNFR1 (2, 11). Many of the pro-inflammatory effects of TNF-α are also mediated predominantly via TNFR1 (50, 55, 58, 81). However, instead of reducing airway responsiveness, TNFR1 deficiency further elevated airway responsiveness in Cpefat mice (Fig. 2). This increase in airway responsiveness was not the result of greater obesity in the Cpefat/TNFR1−/− mice, since body weight was virtually identical in the two strains (Fig. 1). Although obesity did alter the elastic properties of the lung, which could impact airway responsiveness, the augmented responsiveness in Cpefat/TNFR1−/− mice was not the result of such differences, since Estat was the same in Cpefat and Cpefat/TNFR1−/− mice. Previous data from our laboratory indicate that obesity-related increases in Estat are likely the result of smaller lung size in these mice (84).
There are three possible explanations for increased airway responsiveness in Cpefat/TNFR1−/− vs. Cpefat mice (Fig. 2). First, in obese mice, sustained, albeit small, elevations in circulating TNF-α (Figs. 5 and 6), acting via TNFR1, may exert effects that are protective against obesity-related AHR. Other protective roles for TNF-α have been reported in murine disease models. For example, TNF-α protects against skin lesions in a model of psoriasis (49), against nephritis in a lupus model (26), and against insulitis in a Type 1 diabetes model (25). Some investigators (32, 63) have also reported a role for TNF-α in protection against allergen-induced AHR, although others (13, 36, 51, 53, 54) have shown that blocking TNF-α attenuates allergen-induced AHR. Chronic administration of TNF-α attenuates T-cell receptor signaling, and others have suggested that this may account for the ability of TNF-α to suppress pathogenesis in T-cell-dependent conditions, such as those described above (16). Indeed, chronic elevations in TNF-α, such as those that occur in obesity, may have differing effects on TNF-α signaling outcomes (i.e., apoptosis vs. NF-κB activation) rather than acute elevations in TNF-α (14).
Second, the absence of TNFR1 signaling may alter the profile of bacteria species that comprise the intestinal flora. Recent reports indicate that, in mice, commensal bacteria can alter both the complement of T-cell subsets present in the lungs and the induction of allergen-induced AHR (23, 56). There is also evidence of alterations in the gut microbiome in obesity (76, 77).
Finally, we have previously reported that obese mice lacking TNFR2 fail to develop AHR (84), and it may be that TNFR2 is exclusively required for effects of TNF-α on obesity-related AHR. Consistent with this hypothesis, we have previously reported that TNFR2 but not TNFR1 is required for ozone-induced AHR in lean mice (69). Although, at first glance, one would expect no effect of TNFR1 deficiency on AHR under such circumstances, it is important to note that sTNFR1 was elevated both in serum and BAL of Cpefat mice, consistent with previous reports in these mice as well as in other types of obese mice (42, 48, 67). sTNFR1 is the extracellular domain of the TNFR1. It is released by proteolytic cleavage from the cell surface and can neutralize TNF-α, limiting TNF-α effects (22, 78, 87). Thus obesity-related elevations in sTNFR1 may serve to neutralize TNF-α, preventing it from acting on TNFR2. Such a hypothesis is consistent with the observation that, when TNFR1 (and consequently sTNFR1) was deleted, airway responsiveness increased in obese mice (Fig. 2). Such TNF-α neutralizing effects of obesity-related elevations in sTNFR1 might be expected to result in increased BAL and/or serum concentrations of TNF-α in obese TNFR1-deficient vs. -sufficient mice, which was not observed (Figs. 3 and 6). However, these assays assess soluble TNF-α. Interactions of TNFR2 with membrane-bound TNF-α (the primary form of TNF-α recognized by this receptor) might still be inhibited by increased sTNFR1.
Because we previously reported that the attenuated AHR in Cpefat/TNFR2−/− vs. Cpefat mice was associated with differences in pulmonary inflammation and gene expression (84), we assessed BAL cells, a panel of cytokines, chemokines, and growth factors, and also examined expression of relevant pulmonary genes in these TNFR1−/− mice. We sought to determine whether there were effects of TNFR1 deficiency that might be contributing to the enhanced AHR observed in Cpefat/TNFR1−/− mice. BAL concentrations of many cytokines and chemokines were higher in obese than in lean mice (Fig. 3), and in some cases significant obesity-related changes were only observed in the TNFR1-deficient mice. Notably, BAL IL-13 was significantly elevated in obese vs. lean TNFR1-deficient but not TNFR1-sufficient mice (Fig. 3). IL-13 has been implicated in AHR induced by a variety of other stimuli, including allergen, viral infection, and ozone (24, 59, 85, 86). Although BAL IL-17A was below the limit of detection of the Bioplex assay in most samples, pulmonary IL-17A mRNA expression was also increased by TNFR1 deficiency, regardless of whether the mice were obese or lean (Fig. 4E). IL-17A acts alone to promote AHR by direct effects on airway smooth muscle contractility (39), but IL-17A also synergizes with IL-13 to promote AHR by augmenting IL-13-dependent signaling (40). Hence, it is possible that IL-17A and IL-13 contribute to the augmented AHR observed in Cpefat/TNFR1−/− vs. Cpefat mice, although further experiments are required to assess the roles of these cytokines.
Although we observed obesity-related elevations of BAL concentrations of chemokines such as KC and eotaxin that have the capacity to recruit neutrophils and eosinophils, we did not observe any increase in BAL neutrophils in obese vs. lean mice (Table 1), and eosinophils were not observed. We have previously reported reduced expression of the adhesion molecule VCAM in lungs of obese vs. lean mice (84), and it is possible that such changes limit recruitment of leukocytes into the lungs despite increases in BAL chemotactic factors.
Because obesity-related increases in both airway responsiveness and TrkB expression were attenuated in Cpefat mice lacking TNFR2 (84), and because signaling through TrkB can lead to AHR (5, 8), we previously suggested that obesity-related increases in pulmonary TrkB expression might be contributing to obesity-related AHR. Our present results do not support this hypothesis: obesity-related increases in TrkB were reduced in both TNFR1- (Fig. 4A) and TNFR2 (84)-deficient mice, indicating that TNF-α has an important role in obesity-related changes in the expression of this gene. However, TNFR1 and TNFR2 deficiency had opposing effects on AHR in Cpefat mice (Fig. 2; Ref. 84). We also observed reduced fractalkine and adipsin mRNA expression in lungs of Cpefat vs. WT mice consistent with previous observations (84), and TNFR1 deficiency significantly attenuated these obesity-dependent effects (Fig. 4).
TNF-α induces expression of numerous adipocyte genes, including many of the cytokines and chemokines that are elevated in the serum in obesity (10). Hence, we considered the possibility that the observed effects of TNFR1 deficiency on AHR (Fig. 2) were related to interactions between TNF-α and other aspects of the systemic inflammation of obesity. To address this issue, we analyzed serum from WT, Cpefat, TNFR1−/−, and Cpefat/TNFR1−/− mice. Our results confirm previous observations, indicating that total blood leukocytes, IL-6, MCP-1, leptin, G-CSF, KC, TNF-α, and sTNFR1 are increased in serum of obese Cpefat mice (Figs. 5 and 6) (29, 30, 67, 84) and extend them now to include IL-5, IL-15, IP-10, and MIP-1α. Of the various factors examined in serum of obese mice, only IL-15 was significantly affected by TNFR1 deficiency in obese mice (Fig. 6), suggesting that signaling through TNFR1 does not have a major impact on the systemic inflammation of obesity. We were surprised to observe an increase in IL-15 in serum of obese vs. lean mice (Fig. 6.), since others have reported reduced rather than elevated IL-15 in serum of obese human subjects (3). It is conceivable that absence of Cpe rather than obesity per se accounts for our observations, although adipose tissue is capable of IL-15 expression under certain conditions (1). It is unclear whether augmented serum IL-15 in TNFR1−/− mice (Fig. 5) could account for the augmented AHR observed in Cpefat/TNFR1−/− mice (Fig. 2). However, IL-15 does promote γδ T cells (62), and others have reported roles for these cells in regulating AHR, particularly in the context of TNF-α (27). γδ T cells constitute an important source of IL-17A both in the adipose tissue (89) and in the lungs (34).
In summary, our results indicate that TNFR1 is protective against the airway hyperresponsiveness associated with obesity, although it remains to be determined whether it is sTNFR1 or TNFR1 itself that mediates these effects. Although studies with mice may not necessarily be translatable to human subjects, our data showing augmented AHR in obese TNFR1 deficient mice but reduced AHR in obese TNFR2-deficient mice (84) suggest potentially complex effects of currently available pan-TNF-blocking strategies in obesity-related asthma.
GRANTS
This work was supported by National Institutes of Health Grants HL-084044, ES-013307, and ES-00002.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.Z., A.S.W., and S.A.S. conception and design of research; M.Z., A.S.W., L.C., A.W., and E.S.W. performed experiments; M.Z., A.S.W., L.C., A.W., E.S.W., and S.A.S. analyzed data; M.Z., A.S.W., and S.A.S. interpreted results of experiments; M.Z., A.S.W., L.C., and S.A.S. prepared figures; M.Z. and S.A.S. drafted manuscript; M.Z., A.S.W., L.C., and S.A.S. edited and revised manuscript; M.Z., A.S.W., and S.A.S. approved final version of manuscript.
REFERENCES
- 1. Ajuwon KM, Jacobi SK, Kuske JL, Spurlock ME. Interleukin-6 and interleukin-15 are selectively regulated by lipopolysaccharide and interferon-gamma in primary pig adipocytes. Am J Physiol Regul Integr Comp Physiol 286: R547–R553, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Amrani Y, Panettieri RA, Jr, Frossard N, Bronner C. Activation of the TNF alpha-p55 receptor induces myocyte proliferation and modulates agonist-evoked calcium transients in cultured human tracheal smooth muscle cells. Am J Respir Cell Mol Biol 15: 55–63, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Barra NG, Reid S, MacKenzie R, Werstuck G, Trigatti BL, Richards C, Holloway AC, Ashkar AA. Interleukin-15 contributes to the regulation of murine adipose tissue and human adipocytes. Obesity (Silver Spring) 18: 1601–1607, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab 87: 2084–2089, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Bennedich Kahn L, Gustafsson LE, Olgart Hoglund C. Brain-derived neurotrophic factor enhances histamine-induced airway responses and changes levels of exhaled nitric oxide in guinea pigs in vivo. Eur J Pharmacol 595: 78–83, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 175: 661–666, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bouter B, Geary N, Langhans W, Asarian L. Diet-genotype interactions in the early development of obesity and insulin resistance in mice with a genetic deficiency in tumor necrosis factor-alpha. Metabolism 59: 1065–1073, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Braun A, Lommatzsch M, Neuhaus-Steinmetz U, Quarcoo D, Glaab T, McGregor GP, Fischer A, Renz H. Brain-derived neurotrophic factor (BDNF) contributes to neuronal dysfunction in a model of allergic airway inflammation. Br J Pharmacol 141: 431–440, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castro-Giner F, Kogevinas M, Imboden M, de Cid R, Jarvis D, Machler M, Berger W, Burney P, Franklin KA, Gonzalez JR, Heinrich J, Janson C, Omenaas E, Pin I, Rochat T, Sunyer J, Wjst M, Anto JM, Estivill X, Probst-Hensch NM. Joint effect of obesity and TNFA variability on asthma: two international cohort studies. Eur Respir J 33: 1003–1009, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett 582: 117–131, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen H, Tliba O, Van Besien CR, Panettieri RA, Jr, Amrani Y. TNF-α modulates murine tracheal rings responsiveness to G-protein-coupled receptor agonists and KCl. J Appl Physiol 95: 864–872; discussion 863, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Cho HY, Zhang LY, Kleeberger SR. Ozone-induced lung inflammation and hyperreactivity are mediated via tumor necrosis factor-alpha receptors. Am J Physiol Lung Cell Mol Physiol 280: L537–L546, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Cho JY, Pham A, Rosenthal P, Miller M, Doherty T, Broide DH. Chronic OVA allergen challenged TNF p55/p75 receptor deficient mice have reduced airway remodeling. Int Immunopharmacol 11: 1038–1044, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark J, Vagenas P, Panesar M, Cope AP. What does tumour necrosis factor excess do to the immune system long term? Ann Rheum Dis 64, Suppl 4: iv70–iv76, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coleman DL, Eicher EM. Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. J Hered 81: 424–427, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Cope AP, Liblau RS, Yang XD, Congia M, Laudanna C, Schreiber RD, Probert L, Kollias G, McDevitt HO. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med 185: 1573–1584, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Taeye BM, Novitskaya T, McGuinness OP, Gleaves L, Medda M, Covington JW, Vaughan DE. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am J Physiol Endocrinol Metab 293: E713–E725, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLos One 5: e12191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, Irvin CG. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol 128: 508–515, e502, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol 115: 897–909; quiz 910, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Goukassian DA, Qin G, Dolan C, Murayama T, Silver M, Curry C, Eaton E, Luedemann C, Ma H, Asahara T, Zak V, Mehta S, Burg A, Thorne T, Kishore R, Losordo DW. Tumor necrosis factor-alpha receptor p75 is required in ischemia-induced neovascularization. Circulation 115: 752–762, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Hale KK, Smith CG, Baker SL, Vanderslice RW, Squires CH, Gleason TM, Tucker KK, Kohno T, Russell DA. Multifunctional regulation of the biological effects of TNF-alpha by the soluble type I and type II TNF receptors. Cytokine 7: 26–38, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Herbst T, Sichelstiel A, Schar C, Yadava K, Burki K, Cahenzli J, McCoy K, Marsland BJ, Harris NL. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med 184: 198–205, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Holtzman MJ, Byers DE, Benoit LA, Battaile JT, You Y, Agapov E, Park C, Grayson MH, Kim EY, Patel AC. Immune pathways for translating viral infection into chronic airway disease. Adv Immunol 102: 245–276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacob CO, Aiso S, Michie SA, McDevitt HO, Acha-Orbea H. Prevention of diabetes in nonobese diabetic mice by tumor necrosis factor (TNF): similarities between TNF-alpha and interleukin 1. Proc Natl Acad Sci USA 87: 968–972, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacob CO, McDevitt HO. Tumour necrosis factor-alpha in murine autoimmune ‘lupus’ nephritis. Nature 331: 356–358, 1988 [DOI] [PubMed] [Google Scholar]
- 27. Jin N, Roark CL, Miyahara N, Taube C, Aydintug MK, Wands JM, Huang Y, Hahn YS, Gelfand EW, O'Brien RL, Born WK. Allergic airway hyperresponsiveness-enhancing gammadelta T cells develop in normal untreated mice and fail to produce IL-4/13, unlike Th2 and NKT cells. J Immunol 182: 2002–2010, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol 104: 1727–1735, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Johnston RA, Theman TA, Shore SA. Augmented responses to ozone in obese carboxypeptidase E-deficient mice. Am J Physiol Regul Integr Comp Physiol 290: R126–R133, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Johnston RA, Zhu M, Hernandez CB, Williams ES, Shore SA. Onset of obesity in carboxypeptidase E-deficient mice and effect on airway responsiveness and pulmonary responses to ozone. J Appl Physiol 108: 1812–1819, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, Shore SA. Allergic airway responses in obese mice. Am J Respir Crit Care Med 176: 650–658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kanehiro A, Lahn M, Makela MJ, Dakhama A, Fujita M, Joetham A, Mason RJ, Born W, Gelfand EW. Tumor necrosis factor-alpha negatively regulates airway hyperresponsiveness through gamma-delta T cells. Am J Respir Crit Care Med 164: 2229–2238, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Kanehiro A, Lahn M, Makela MJ, Dakhama A, Joetham A, Rha YH, Born W, Gelfand EW. Requirement for the p75 TNF-alpha receptor 2 in the regulation of airway hyperresponsiveness by gamma delta T cells. J Immunol 169: 4190–4197, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Kasahara DI, Kim HY, Williams AS, Verbout NG, Tran J, Si H, Wurmbrand AP, Jastrab J, Hug C, Umetsu DT, Shore SA. Pulmonary inflammation induced by subacute ozone is augmented in adiponectin-deficient mice: role of IL-17A. J Immunol 188: 4558–4567, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katsuki A, Sumida Y, Murashima S, Murata K, Takarada Y, Ito K, Fujii M, Tsuchihashi K, Goto H, Nakatani K, Yano Y. Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 83: 859–862, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Kim J, McKinley L, Natarajan S, Bolgos GL, Siddiqui J, Copeland S, Remick DG. Anti-tumor necrosis factor-alpha antibody treatment reduces pulmonary inflammation and methacholine hyper-responsiveness in a murine asthma model induced by house dust. Clin Exp Allergy 36: 122–132, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Kips JC, Tavernier J, Pauwels RA. Tumor necrosis factor causes bronchial hyperresponsiveness in rats. Am Rev Respir Dis 145: 332–336, 1992 [DOI] [PubMed] [Google Scholar]
- 38. Kishore R, Tkebuchava T, Sasi SP, Silver M, Gilbert HY, Yoon YS, Park HY, Thorne T, Losordo DW, Goukassian DA. Tumor necrosis factor-alpha signaling via TNFR1/p55 is deleterious whereas TNFR2/p75 signaling is protective in adult infarct myocardium. Adv Exp Med Biol 691: 433–448, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, Sheppard D. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nature Med 18: 547–554, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol 11: 928–935, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Landgraf RG, Jancar S. Endothelin A receptor antagonist modulates lymphocyte and eosinophil infiltration, hyperreactivity and mucus in murine asthma. Int Immunopharmacol 8: 1748–1753, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Lang JE, Williams ES, Mizgerd JP, Shore SA. Effect of obesity on pulmonary inflammation induced by acute ozone exposure: role of interleukin-6. Am J Physiol Lung Cell Mol Physiol 294: L1013–L1020, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Lecube A, Sampol G, Munoz X, Ferrer R, Hernandez C, Simo R. TNF-alpha system and lung function impairment in obesity. Cytokine 54: 121–124, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Leibel RL, Chung WK, Chua SC., Jr The molecular genetics of rodent single gene obesities. J Biol Chem 272: 31937–31940, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Liang H, Yin B, Zhang H, Zhang S, Zeng Q, Wang J, Jiang X, Yuan L, Wang CY, Li Z. Blockade of tumor necrosis factor (TNF) receptor type 1-mediated TNF-alpha signaling protected Wistar rats from diet-induced obesity and insulin resistance. Endocrinology 149: 2943–2951, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Litonjua AA, Gold DR. Asthma and obesity: common early-life influences in the inception of disease. J Allergy Clin Immunol 121: 1075–1084; quiz 1085–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol 290: L856–L865, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Ma HL, Napierata L, Stedman N, Benoit S, Collins M, Nickerson-Nutter C, Young DA. Tumor necrosis factor alpha blockade exacerbates murine psoriasis-like disease by enhancing Th17 function and decreasing expansion of Treg cells. Arthritis Rheum 62: 430–440, 2010 [DOI] [PubMed] [Google Scholar]
- 50. MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal 14: 477–492, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Maillet I, Schnyder-Candrian S, Couillin I, Quesniaux VF, Erard F, Moser R, Fleury S, Kanda A, Dombrowicz D, Szymkowski DE, Ryffel B. Allergic lung inflammation is mediated by soluble TNF and attenuated by dominant-negative TNF biologics. Am J Respir Cell Mol Biol 45: 731–739, 2011 [DOI] [PubMed] [Google Scholar]
- 52. Moon YS, Kim DH, Song DK. Serum tumor necrosis factor-alpha levels and components of the metabolic syndrome in obese adolescents. Metabolism 53: 863–867, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, Galli SJ. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol 120: 48–55, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Nakae S, Lunderius C, Ho LH, Schafer B, Tsai M, Galli SJ. TNF can contribute to multiple features of ovalbumin-induced allergic inflammation of the airways in mice. J Allergy Clin Immunol 119: 680–686, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Naude PJ, den Boer JA, Luiten PG, Eisel UL. Tumor necrosis factor receptor cross-talk. FEBS J 2011 [DOI] [PubMed] [Google Scholar]
- 56. Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336: 489–493, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140: 197–208, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol 160: 943–952, 1998 [PubMed] [Google Scholar]
- 59. Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, DeKruyff RH, Shore SA, Umetsu DT. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med 205: 385–393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 40: 1286–1292, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Richter M, Cloutier S, Sirois P. Endothelin, PAF and thromboxane A2 in allergic pulmonary hyperreactivity in mice. Prostaglandins Leukot Essent Fatty Acids 76: 299–308, 2007 [DOI] [PubMed] [Google Scholar]
- 62. Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol 9: 480–490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rudmann DG, Moore MW, Tepper JS, Aldrich MC, Pfeiffer JW, Hogenesch H, Tumas DB. Modulation of allergic inflammation in mice deficient in TNF receptors. Am J Physiol Lung Cell Mol Physiol 279: L1047–L1057, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Sasi SP, Yan X, Enderling H, Park D, Gilbert HY, Curry C, Coleman C, Hlatky L, Qin G, Kishore R, Goukassian DA. Breaking the ‘harmony’ of TNF-alpha signaling for cancer treatment. Oncogene 31: 4117–4127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 55: 1537–1545, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther 110: 83–102, 2006 [DOI] [PubMed] [Google Scholar]
- 67. Shore SA, Lang JE, Kasahara DI, Lu FL, Verbout NG, Si H, Williams ES, Terry RD, Lee A, Johnston RA. Pulmonary responses to subacute ozone exposure in obese vs. lean mice. J Appl Physiol 107: 1445–1452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol 95: 938–945, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Shore SA, Schwartzman IN, Le Blanc B, Murthy GG, Doerschuk CM. Tumor necrosis factor receptor 2 contributes to ozone-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med 164: 602–607, 2001 [DOI] [PubMed] [Google Scholar]
- 70. Shore SA, Williams ES, Chen L, Benedito LA, Kasahara DI, Zhu M. Impact of aging on pulmonary responses to acute ozone exposure in mice: role of TNFR1. Inhal Toxicol 23: 878–888, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shore SA, Williams ES, Zhu M. No effect of metformin on the innate airway hyperresponsiveness and increased responses to ozone observed in obese mice. J Appl Physiol 105: 1127–1133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Teramoto S, Yamamoto H, Ouchi Y. Increased C-reactive protein and increased plasma interleukin-6 may synergistically affect the progression of coronary atherosclerosis in obstructive sleep apnea syndrome. Circulation 107: e40, 2003 [DOI] [PubMed] [Google Scholar]
- 73. Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med 152: 76–80, 1995 [DOI] [PubMed] [Google Scholar]
- 74. Tighe RM, Li Z, Potts EN, Frush S, Liu N, Gunn MD, Foster WM, Noble PW, Hollingsworth JW. Ozone inhalation promotes CX3CR1-dependent maturation of resident lung macrophages that limit oxidative stress and inflammation. J Immunol 187: 4800–4808, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 75. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6: 772–783, 2006 [DOI] [PubMed] [Google Scholar]
- 76. Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3: 213–223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature 457: 480–484, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ulich TR, Yin S, Remick DG, Russell D, Eisenberg SP, Kohno T. Intratracheal administration of endotoxin and cytokines. IV. The soluble tumor necrosis factor receptor type I inhibits acute inflammation. Am J Pathol 142: 1335–1338, 1993 [PMC free article] [PubMed] [Google Scholar]
- 79. Uysal KT, Wiesbrock SM, Hotamisligil GS. Functional analysis of tumor necrosis factor (TNF) receptors in TNF-alpha-mediated insulin resistance in genetic obesity. Endocrinology 139: 4832–4838, 1998 [DOI] [PubMed] [Google Scholar]
- 80. Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389: 610–614, 1997 [DOI] [PubMed] [Google Scholar]
- 81. Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol 5: 392–399, 1995 [DOI] [PubMed] [Google Scholar]
- 82. Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res 9: 414–417, 2001 [DOI] [PubMed] [Google Scholar]
- 83. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Williams AS, Chen L, Kasahara DI, Si H, Wurmbrand AP, Shore SA. Obesity and airway responsiveness: role of TNFR2. Pulm Pharmacol Therap. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Williams AS, Nath P, Leung SY, Khorasani N, McKenzie AN, Adcock IM, Chung KF. Modulation of ozone-induced airway hyperresponsiveness and inflammation by interleukin-13. Eur Respir J 32: 571–578, 2008 [DOI] [PubMed] [Google Scholar]
- 86. Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma [see comments]. Science 282: 2258–2261, 1998 [DOI] [PubMed] [Google Scholar]
- 87. Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, Nahmias Y, Goldwasser Y, Kitagawa Y, Tilles AW, Tompkins RG, Parekkadan B, Yarmush ML. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther 18: 1857–1864, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yamakawa T, Tanaka S, Yamakawa Y, Kiuchi Y, Isoda F, Kawamoto S, Okuda K, Sekihara H. Augmented production of tumor necrosis factor-alpha in obese mice. Clin Immunol Immunopathol 75: 51–56, 1995 [DOI] [PubMed] [Google Scholar]
- 89. Zuniga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, Butcher EC. IL17 regulates adipogenesis, glucose homeostasis, obesity. J Immunol 185: 6947–6959, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]