Abstract
The functional impact of pulmonary C-fiber activation on upper airway biomechanics has not been evaluated. Here, we tested the hypothesis that pulmonary C-fiber activation alters the respiratory-related control of tongue movements. The force produced by tongue movements was quantified in spontaneously breathing, anesthetized adult rats before and after stimulation of pulmonary C fibers via intrajugular delivery of capsaicin (0.625 and 1.25 μg/kg). Brief occlusion of the trachea was used to increase the respiratory drive to the tongue muscles, and hypoglossal (XII) nerve branches were selectively sectioned to denervate the protrusive and retrusive tongue musculature. Tracheal occlusion triggered inspiratory-related tongue retrusion in rats with XII nerves intact or following section of the medial XII nerve branch, which innervates the genioglossus muscle. Inspiratory-related tongue protrusion was only observed after section of the lateral XII branch, which innervates the primary tongue retrusor muscles. The tension produced by inspiratory-related tongue movement was significantly attenuated by capsaicin, but tongue movements remained retrusive, unless the medial XII branch was sectioned. Capsaicin also significantly delayed the onset of tongue movements such that tongue forces could not be detected until after onset of the inspiratory diaphragm activity. We conclude that altered neural drive to the tongue muscles following pulmonary C-fiber activation has a functionally significant effect on tongue movements. The diminished tongue force and delay in the onset of tongue movements following pulmonary C-fiber activation are potentially unfavorable for upper airway patency.
Keywords: upper airway patency, pharyngeal, hypoglossal
the position and stiffness of the mammalian tongue is partly determined by activation of the extrinsic tongue muscles. These muscles insert into the body of the tongue and can be functionally classified as either protrusive or retrusive. The primary protrudor muscle is the genioglossus, which is innervated by the medial hypoglossal branch (XIIMED), and the primary retractor muscles are the styloglossus and hyoglossus, which are innervated by the lateral hypoglossal branch (XIILAT) (24, 25, 47). The respiratory-related discharge of these muscles has been extensively studied in both humans and animals (2, 4, 8, 25–27, 36, 63, 64). Although the genioglossus muscle has been the focus of the majority of experimental studies, both the protrudor and retractor muscles can show phasic inspiratory activity in humans (46), as well as a rat experimental model (8, 25, 36, 38). Inspiratory bursting in protrudor and retractor muscles increases with respiratory drive and is stimulated by negative upper airway pressure in both humans and rats (25, 46, 62–64). In addition, the rat model has been used to show that coactivation of tongue protrudor and retractor muscles can reduce upper airway collapsibility more effectively than isolated contraction of the genioglossus (22, 25, 27).
The intense interest in the neural regulation of the tongue muscles derives from the potential contribution of the tongue to obstructive sleep apnea/hypopnea syndrome (OSAHS). Many studies suggest that reduction of extrinsic tongue muscle activity during sleep is a contributing factor to OSAHS (15, 31, 60). While other factors, including altered pharyngeal biomechanics, are important (67), the significant role of tongue muscle activity is widely accepted (15, 31, 60). Accordingly, it is of interest to determine whether factors associated with OSAHS alter the regulation of XII motor output. Much work has focused on the impact of intermittent hypoxia and/or apnea on XII motor output. However, recent evidence suggests that OSAHS is also associated with chronic inflammation (29, 30, 32, 49, 52), and systemic inflammation can impair the expression of respiratory neuroplasticity (68). Another possibility is that inflammatory mediators (e.g., reactive oxygen species, cyclooxygenase metabolites, cytokines) can directly alter the regulation of breathing via activation of pulmonary C fibers (40, 42, 43, 61, 69). These neurons are the main chemo-sensitive afferents in the lung, and activation of these afferents can have differential effects on the neural drive to tongue protrudor vs. retrusor muscles (36). During normocapnic conditions, both the XIIMED and XIILAT nerve branches display a bursting pattern, which is synchronized with inspiratory phrenic discharge (36). This type of XII activity is defined as phasic inspiratory activity and is considerably attenuated by capsaicin-induced pulmonary C-fiber activation (36). However, a group of previously silent motoneurons with axons in the XIIMED branch are selectively activated by capsaicin treatment, but not in a phasic pattern (36). Rather, these recruited cells fire with a nonrhythmic, tonic bursting pattern. In contrast, this response does not occur in recordings made from the XIILAT nerve branch (36). Selective activation of tongue protrudors in response to a respiratory-related stimulus has been reported in at least one prior study (66), but the majority of prior studies have reported that tongue protrudor and retractor muscles respond in parallel (25, 46, 62). Given the biomechanical complexity of tongue movements (47), the potential for differential responses of the extrinsic tongue muscles to pulmonary C-fiber activation (36), and the potential for inflammation and associated activation of pulmonary C-fibers to alter respiratory control (40, 42, 43, 61, 69), we conducted the present experiments to determine the mechanical response of the tongue to capsaicin. We hypothesized that stimulation of pulmonary C fibers with capsaicin would alter the respiratory-related control of tongue movement. This hypothesis was tested using a rat model, which permits quantification of the magnitude and direction of tongue movements during breathing (27).
MATERIALS AND METHODS
Fifteen adult male Wistar rats (404 ± 11 g) obtained from the laboratory animal facility at National Taiwan University were studied. All experimental procedures were approved by the Institutional Animal Care and Use Committee at National Taiwan Normal University. Animals were divided into three groups as follows. The XII nerves were intact in group 1 (N = 5, XII intact). To more selectively examine the influence of capsaicin on tongue movements, the medial (group 2, XIIMED section; N = 5) and lateral XII branches (group 3, XIILAT section; N = 5) were sectioned bilaterally. Since the XIIMED innervates the primary tongue protrudor muscle, the genioglossus, and the XIILAT innervates the primary retrusor muscles, the stylo- and hyoglossus, these nerve section experiments enable more selective examination of tongue protrusion vs. retrusion (25).
Experimental preparation.
Animals were pretreated with atropine (0.5 mg/kg im, Sigma, St. Louis, MO) to reduce airway mucus secretion during the surgical procedures and then anesthetized with urethane (1.2 g/kg ip, Sigma). Our previous studies (25, 36, 38) have demonstrated that both the phrenic and XII nerves display clear respiratory activity, and that tongue movements can be quantified under this anesthesia protocol. Surgical procedures were not initiated until animals were unresponsive to deep pressure applied to the paws by a hemostat. Rectal temperature of the animal was monitored by a thermometer and maintained at 37 ± 1°C with an electric blanket. The trachea was cannulated below the larynx with tubing [PE-240, outer diameter (OD): 2.42 mm, inner diameter (ID): 1.67 mm, Clay Adams, division of Becton Dickinson, Parsippany, NJ]. Catheters were inserted into the left femoral artery (PE-50, OD: 1.52 mm, ID: 0.86 mm) and right jugular vein (PE-100, OD: 1.52 mm, ID: 0.86 mm) for blood pressure measurement and drug administration, respectively. The diaphragm electromyographic (EMG) activity was recorded by a pair of fine wires (no. 793500, PFA-coated stainless steel, seven strands, diameter: 0.006 in. bare, 0.009 in. coated, A-M Systems, Carlsborg, WA). The coating of the wire was removed around 2–3 mm on the tip and then sutured on the ventral midline diaphragm, as previously described (44). The diaphragm EMG signals were band-pass filtered (0.3–3 kHz) and amplified with an AC preamplifier (Grass, P5111, Quincy, MA) and then integrated (time constant = 50 ms). The sampling rate of all signals was 1 kHz.
Measurement of the tongue force.
The procedures for measuring the forces associated with tongue movement were based on a previous study (25). That prior study from Fuller et al. (25) provides a detailed description of the methods used to assess tongue force, along with a diagram of the experimental preparation. After confirming a stable and surgically adequate plane of anesthesia, the animal was placed in a supine position. The lower jaw was secured in place, and a small surgical retractor was positioned to hold the mouth open. A fine suture (suture size: 3–0, SC163, UNIK, Taiwan) was then made through the tip of the tongue, and a short length of thread was used to connect to an isometric force transducer (sensitivity: 150 μVg, no. 7004, Ugo Basile, Comerio, Italy) fixed on a manipulator. The transducer was calibrated in every experiment. The position of the tongue and thread was maintained perpendicular to the force transducer. With this arrangement, tongue movement could load or unload the force transducer during retraction and protrusion of the tongue, respectively. The XII nerves and their branches were then exposed via a ventral approach, as previously described (34, 36–39). The XII branches innervating the geniohyoid muscle were sectioned to prevent this muscle from altering the tongue force measurements.
The amount of tension in the line that connects the tongue to the force transducer is a critical factor in this experimental setup (25), and this was standardized across animals as follows. The whole XII nerve was placed on the bipolar stainless-steel electrode connected with the stimulator (Grass, S8800, Quincy, MA). The tongue was pulled in a stepwise increase of 1-mm length, and electrical stimulation current (pulse duration: 0.1 ms, current: 25–70 μA) was delivered to induce contraction of the tongue muscles. With this approach, an approximate “length-tension” curve was generated for each condition, and the stimulation experiments were conducted at the position at which the peak force could be measured (25).
Experimental protocol.
After establishment of the optimal position of the tongue for force measurements, a short baseline period was recorded, and then respiratory drive was increased by clamping the tracheal tube during the expiratory period (∼25 s). Previous work established that this procedure induces robust and quantifiable tongue movements (25). Following the initial baseline occlusion trial, the procedure was repeated with both low (0.625 μg/kg) and high (1.25 μg/kg) doses of capsaicin. The capsaicin doses were randomly injected into the right jugular vein at 10 s after the onset of the tracheal occlusion. These doses of capsaicin selectively activate vagal pulmonary C fibers in the rat (35, 36, 38). At the end of the experiment, the XII nerves were cut caudal to the branch point, and tracheal occlusion was repeated. In all cases, tongue movements recorded by the force transducer were abolished by this procedure. This protocol ensured that tongue movement induced by tracheal occlusion was due to contraction of tongue muscle via activation of the XII nerve. At the end of the experimental protocol, animals were euthanized with an acute intravenous bolus injection of urethane (1.2 g/kg).
Data analysis.
All data were recorded using a Power lab system (ADI Instrument Pty NES). Activity of the diaphragm was determined by the maximal amplitude of integrated diaphragm EMG signals during inspiratory duration and expressed relative to the peak value during tracheal occlusion treatment (%occlusion). The tongue force was determined by the difference between maximum and minimum value in a respiratory cycle and then calibrated by the force generated after bilateral section of the whole XII nerve. The magnitude of tongue force was expressed relative to the peak value measured during tracheal occlusion without capsaicin treatment (%occlusion) and presented as the absolute unit (mN). The onset difference between the initiation of diaphragm inspiratory bursting and tongue force was also calculated, similar to our previous reports (34, 36–38). Briefly, the onset of tongue force and diaphragm activity was considered as the time point when the force and integrated EMG signals rose above the “baseline” established in the preceding expiratory period. The onset difference was defined as the period between these two time points (i.e., diaphragm activity onset time minus tongue force onset time). Thus a negative value indicated that tongue force was detected earlier than the inspiratory diaphragm EMG signal.
The occurrence of rhythmic tongue force production or phasic diaphragm activity during tracheal occlusion was calculated per minute (min−1). There was no significant difference in frequency across three experimental groups, and accordingly data from these groups were combined to assess the effect of capsaicin. Frequency data were averaged every 5 s before and after capsaicin injection.
The influence of capsaicin administration on the tongue force was analyzed by one-way ANOVA with repeated measurement and then followed by Student-Newman-Keuls modified t-test. Two-way ANOVA followed by Student-Newman-Keuls modified t-test was used to examine the frequency of tongue force production and the differential influence of capsaicin administration on the response of diaphragm and tongue force development. Data are presented as means ± SE. A P value < 0.05 was considered to represent a statistically significant difference.
RESULTS
Tongue movements evoked by XII nerve stimulation or tracheal occlusion.
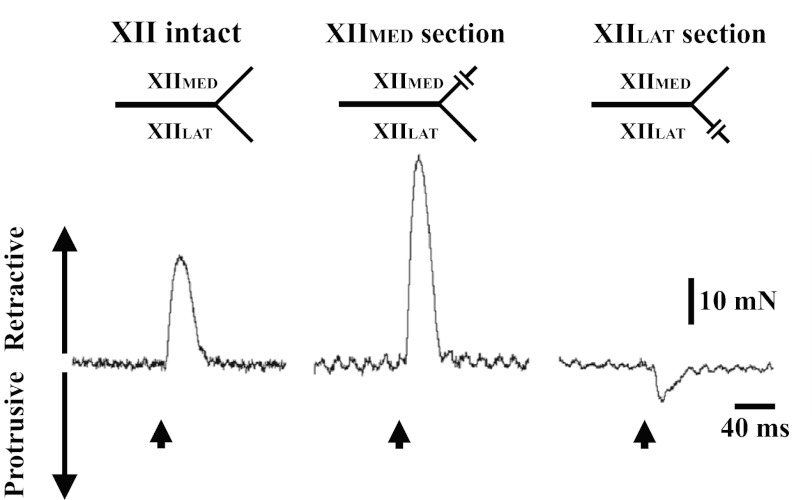
Initial experiments established tongue movements during baseline conditions without capsaicin. Consistent with a prior report (25) using this model, the tongue retracted during electrical stimulation of the main XII nerve (Fig. 1). When nerve stimulation was repeated after section of the XIIMED, the tongue retracted (Fig. 1). Tongue protrusion was evoked only when the XII nerve was stimulated after section of the XIILAT (Fig. 1). Tongue movements were not clearly observable during spontaneous breathing at baseline. This observation is similar to a report from human subjects showing that, during quiet breathing, tongue muscle activity (21) and the anteroposterior movements are minimal (14). However, end-expiratory tracheal occlusion caused a progressive increase in tongue retrusive movements, with tongue force reaching a peak value at the end of the occlusion (Fig. 2). Similar to the XII nerve stimulation results (Fig. 1), tracheal occlusion stimulus induced retrusive tongue force in XII-intact and XIIMED-section animals, whereas protrusion of the tongue was observed in XIILAT-section animals. As illustrated by Fig. 3, respiratory-related tongue movements began before the onset of the diaphragm inspiratory burst (Fig. 3). Specifically, tongue movements preceded the diaphragm inspiratory burst by 69.2 ± 26.0 and 33.5 ± 19.5 ms in XII-intact and XIIMED-section animals, respectively. Similarly, the onset of the protrusive tongue force preceded the diaphragm burst onset by 18.6 ± 2.5 ms.
Fig. 1.
Retrusive and protrusive tongue force induced by electrical stimulation of the hypoglossal (XII) nerve. Stimulation of the XII nerve resulted in retraction of the tongue when the nerve was intact and also following section of the medial branch (XIIMED). Protrusive tongue force was evoked in animals following section of the lateral XII branch (XIILAT). The upward arrow indicates the time that the electrical stimulation was delivered to the XII nerve.
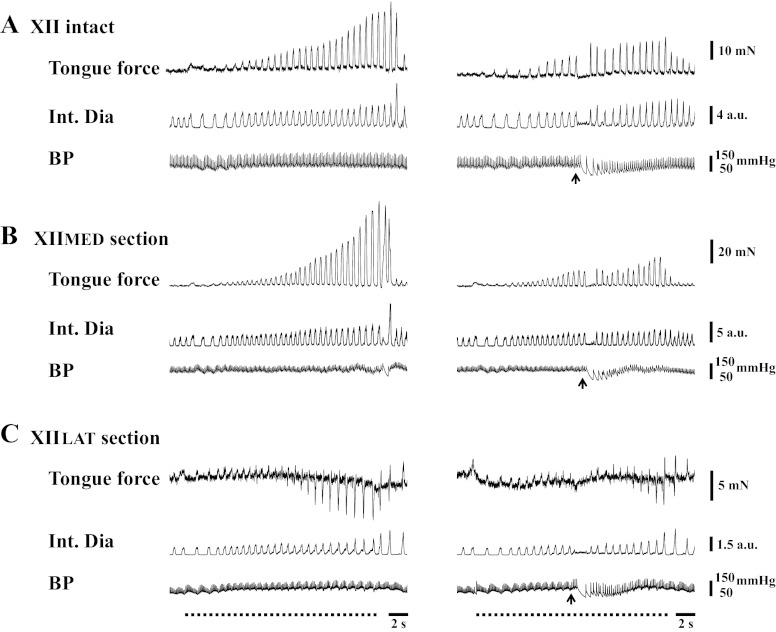
Fig. 2.
Representative examples of the tongue force and integrated diaphragm (Int Dia) activity in response to capsaicin administration in spontaneously breathing rats. The capsaicin was delivered during tracheal occlusion in rats with XII nerves intact (A), or following selective section of the XIIMED (B) or XIILAT branches (C). During tracheal occlusion, retrusive tongue force progressively increased in both XII-intact and XIIMED-section rats. Protrusive tongue movement occurred in XIILAT-section rats at ∼10 s after tracheal occlusion. The upward arrow indicates the time of capsaicin injection. The horizontal dotted line represents the period of airway occlusion. BP, blood pressure; au, arbitrary units.
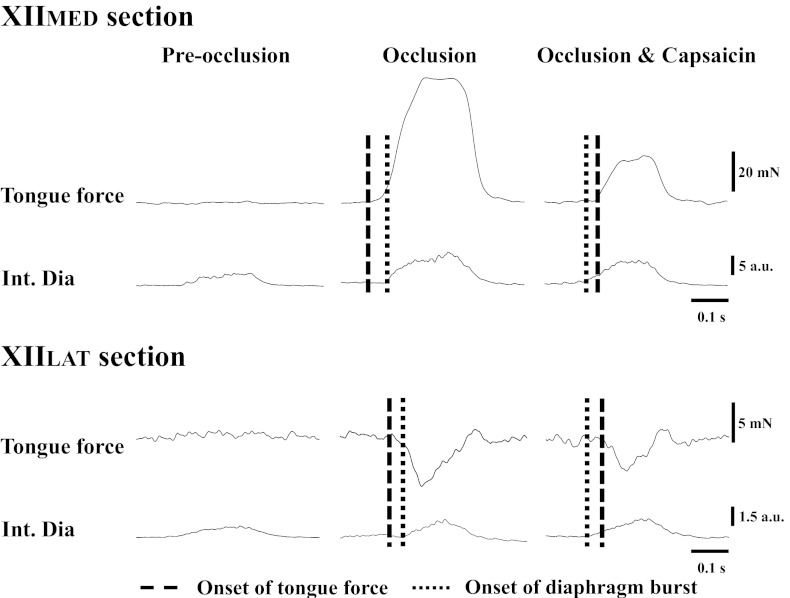
Fig. 3.
Representative example showing the temporal relationship between the forces developed by tongue movements and the onset of the Int Dia burst. Tongue force could not be clearly observed during the baseline period of spontaneous breathing (left, “Pre-occlusion”). Following tracheal occlusion, the onset of retrusive tongue force in the XIIMED-sectioned rat and protrusive force in XIILAT-sectioned rat occurred before the diaphragm burst (middle, “Occlusion”). In contrast, following capsaicin treatment (right, “Occlusion & Capsaicin”), the onset of the tongue force was delayed and occurred after the diaphragm burst.
Intrajugular capsaicin reduces tongue movements.
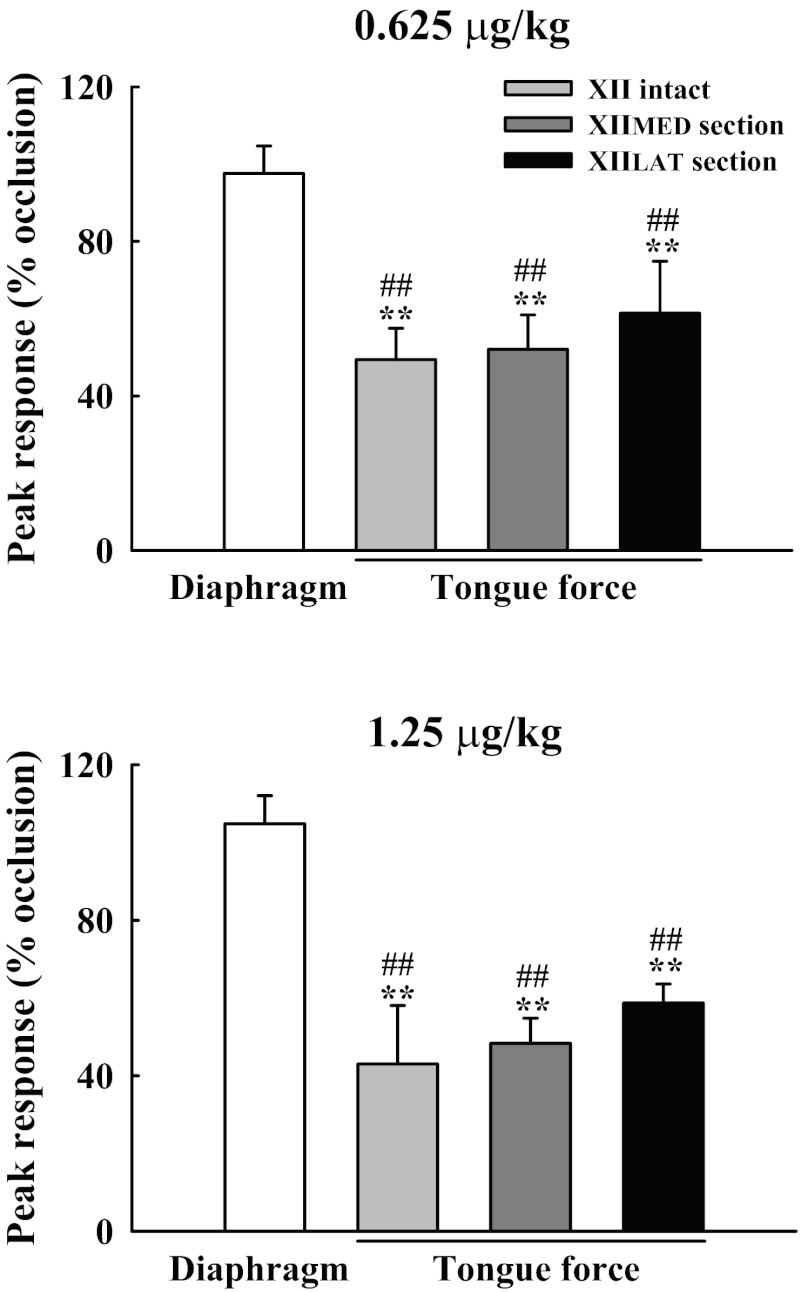
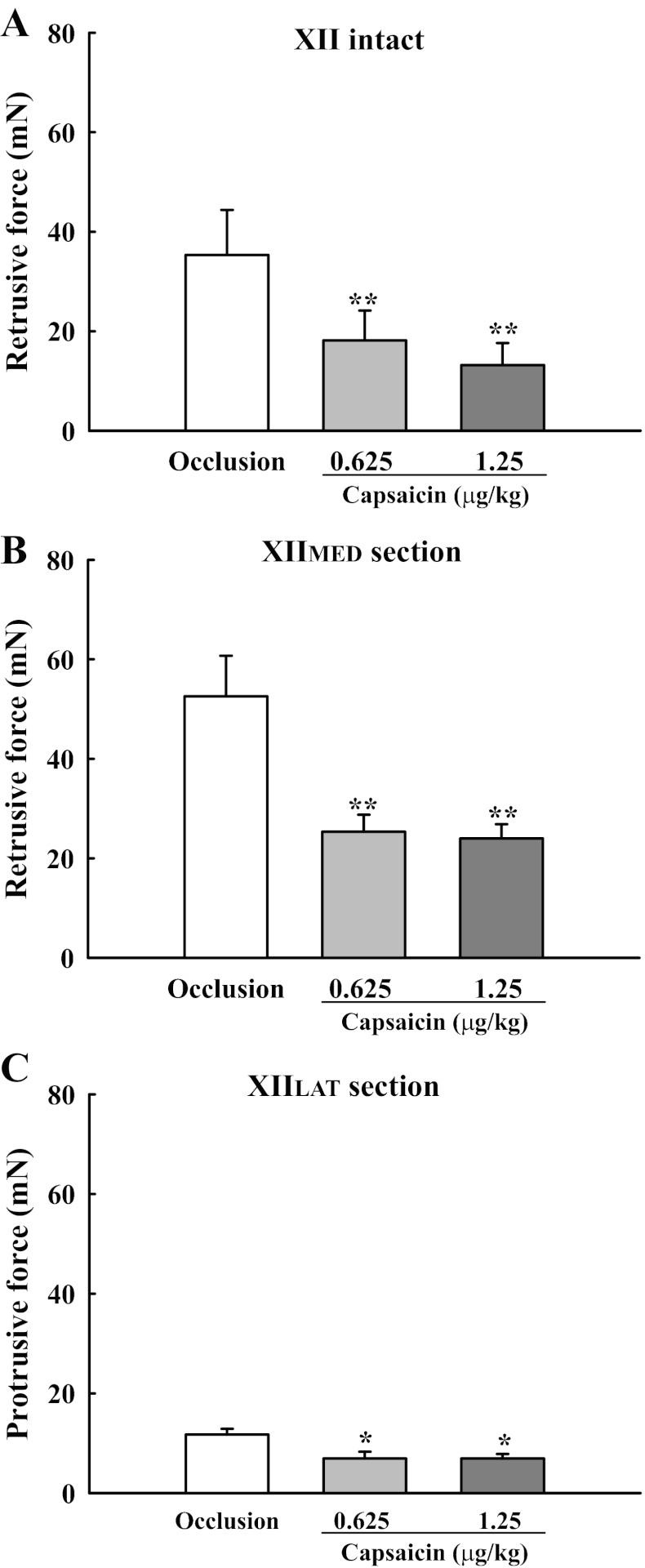
Delivery of capsaicin during tracheal occlusion caused decreases in the force produced by tongue movements in all of the experimental groups. A representative example is provided in Fig. 2 (right). To quantify the differential influence of capsaicin on tongue force and diaphragm activity, the data were normalized to the peak response during tracheal occlusion (%occlusion). The peak retrusive tongue force was significantly reduced by 49 ± 8 and 43 ± 15% in XII-intact rats following the low and high doses of capsaicin, respectively (Figs. 3 and 4). However, the tongue did not protrude during these conditions (e.g., Fig. 2), indicating that tonic increases in genioglossus muscle activity (36) did not “override” the influence of the tongue retrusor muscles. Capsaicin also reduced the protrusive and retrusive forces produced by tongue muscle contraction following section of the XIIMED and XIILAT nerve branches, respectively (Figs. 3–5). In contrast to the tongue response, peak diaphragm activity was minimally affected by capsaicin injection (Fig. 4). Thus capsaicin selectively reduced XII vs. diaphragm motor output.
Fig. 4.
Differential impact of pulmonary C-fiber activation on the peak response of tongue force and diaphragm activity during tracheal occlusion. Both doses of capsaicin significantly attenuated the tongue force in all groups, but did not significantly alter the diaphragm activity. Values are means ± SE. **P < 0.01 compared with the value during tracheal occlusion but without capsaicin treatment. ##P < 0.01, significant difference between response of the diaphragm and tongue force.
Fig. 5.
Effects of capsaicin administration on the tongue force in XII-intact (A), XIIMED-section (B), and XIILAT-section (C) rats during tracheal occlusion. Both doses of capsaicin significantly attenuated the retrusive (A and B) and protrusive (C) tongue force. Values are means ± SE. *P < 0.05 and **P < 0.01 compared with the value during tracheal occlusion, but without capsaicin treatment.
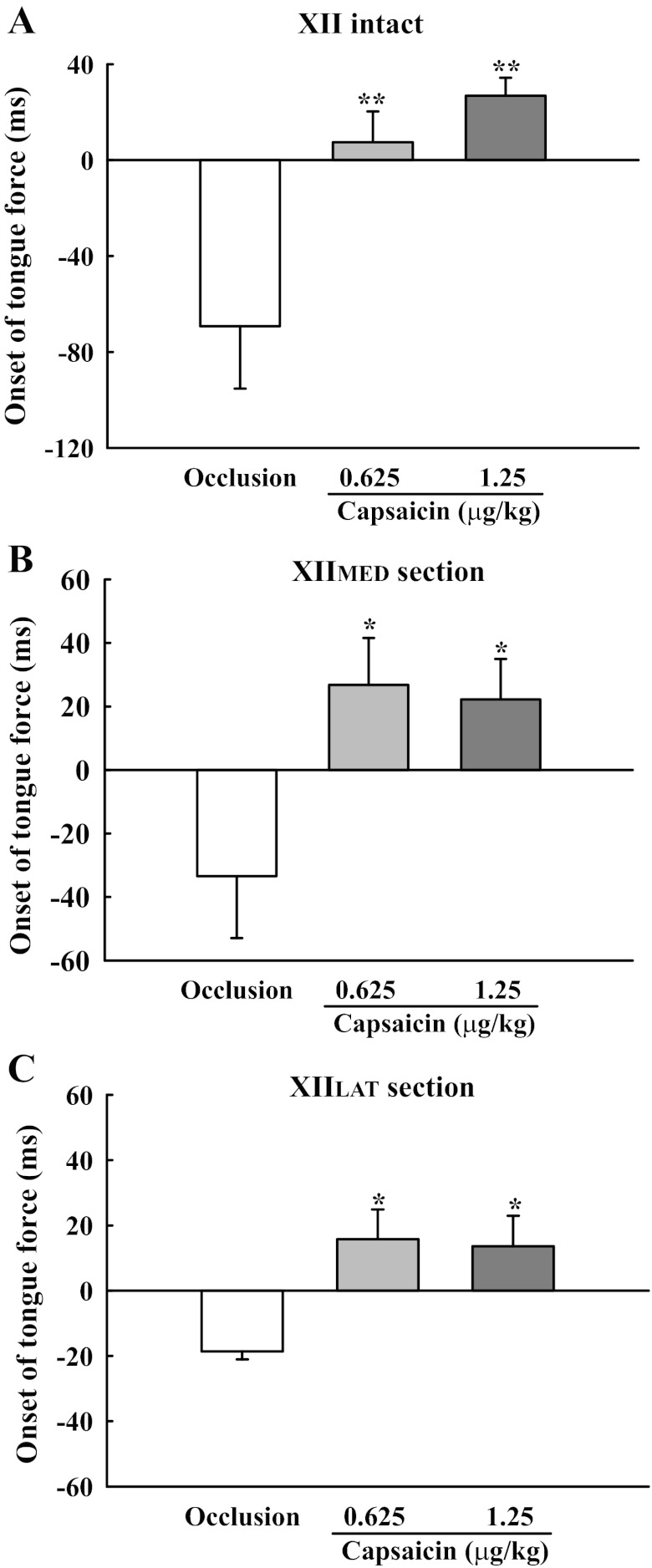
The temporal relationship between the onset of respiratory-related tongue force and the diaphragm inspiratory burst was altered following intrajugular capsaicin. A representative example is shown in Fig. 3. In XII-intact animals, the onset of the tongue force was delayed from −69.2 ± 26.0 ms (i.e., occurring before inspiratory onset) to 7.4 ± 12.9 ms and to 26.8 ± 7.5 ms (i.e., occurring after the inspiratory burst) by the low and high dose of capsaicin, respectively (P < 0.01, Fig. 6A). Similar delays in the onset of tongue protrusive and retrusive forces occurred following section of the XIIMED and XIILAT, respectively (P < 0.05, Fig. 6, B and C).
Fig. 6.
Changes in the relative onset time of tongue force and diaphragm activity following capsaicin administration in XII-intact (A), XIIMED-sectioned (B), and XIILAT-sectioned (C) rats. During tracheal occlusion, the onset of inspiratory-related tongue force occurred before the diaphragm burst, as reflected by negative value of the onset time. However, following both doses of capsaicin, the value of the force onset became positive, indicating that capsaicin delayed the inspiratory tongue force. Values are means ± SE. *P < 0.05 and **P < 0.01 compared with the value during tracheal occlusion, but without capsaicin treatment.
The inspiratory-related frequency of tongue movements was also attenuated by capsaicin. Specifically, frequency was reduced from 96.3 ± 4.5 to 79.5 ± 5.5 and 53.1 ± 5.1 breaths/min in response to 0.625 and 1.25 μg/kg capsaicin administration (P < 0.01), respectively.
DISCUSSION
There are three major findings of the present study. First, tongue movements are profoundly altered by capsaicin-induced pulmonary C-fiber activation, and the tongue continues to retract during inspiratory efforts in the anesthetized rat preparation. Second, the early onset of tongue movements, which is hypothesized to stabilize the upper airway (1), is substantially delayed following intrajugular capsaicin injection. Last, the relationship between tongue force and diaphragm activity is altered by capsaicin, indicating selective inhibition of tongue muscle activity. Taken together, these data suggested that pulmonary C-fiber activation may unfavorably impact upper airway patency through reduction and delay of tongue movements during intense respiratory efforts.
Critique of methods.
The potential caveats associated with this method of assessing tongue movements have been discussed previously (25). It must be acknowledged that the methods employed herein have the potential to influence tongue muscle biomechanics. For example, it was necessary to elevate the lower jaw in these experiments to measure unobstructed tongue movement. In addition, the movements of the tongue are not limited to protrusion and retrusion. Electrical stimulation of the XII nerve and tracheal occlusion can also induce vertical tongue movements (25), which cannot be detected by the force transducer. Finally, detection of protrusive and retrusive tongue force was achieved by unloading and loading the force transducer, respectively. Thus isometric contractions of the tongue retrusor muscles against the fixed force transducer are easier to detect, and the absolute value of the protrusive forces may be underestimated. Despite these caveats, the experimental setup provides a means of reproducible and quantifiable assessment for tongue movements during breathing. In this regard, prior work has shown a significant and linear relationship between tongue force measures and inspiratory tongue muscle EMG activity using this approach (25). Another important consideration is that biomechanical differences likely exist between the rat and human upper airway. The hyoid bone of humans is below the mandible, whereas it is caudal to the mandible in rodents (9). This difference may make retrusive tongue movements more pronounced in the rat. However, it should be emphasized that the literature indicates that the neural regulation of the extrinsic tongue muscles is similar between rat and human (24).
Protrusive and retrusive tongue force.
Coactivation of tongue protrudor and retractor muscles during inspiration has been described in humans (46) and rodent models (25) and, in anesthetized rats, acts to retract the tongue. Thus the tongue force induced by electrical stimulation of the main trunk of the XII nerve and tracheal occlusion with XII nerves intact in the present study are quite similar to previous studies in the human (20) and rat (25, 28). Functionally, the protrusor/retrusor coactivation reduces upper airway collapsibility more effectively than isolated protrusor contraction in both humans (54) and rodent models (22, 25, 27).
While the position of the tongue in the oropharyngeal airway is controlled by the extrinsic muscles (24), the intrinsic muscles that comprise the body of the tongue also play a role (6). Indeed the intrinsic tongue muscles show inspiratory-related activity that can be modulated by respiratory stimuli (i.e., hypercapnia, hypoxia, and airway occlusion) (3, 7). Several reports indicate that the intrinsic tongue muscles probably work in coordination with the extrinsic tongue muscles to optimize pharyngeal airway patency in both human (59) and rat (3, 6, 7). Accordingly, we suggest that retrusive tongue movements following XIIMED section (i.e., protrudor muscle denervation) resulted from coactivation of the extrinsic hyoglossus and styloglossus and intrinsic longitudinal tongue muscle. The protrusive tongue force recorded after XIILAT section (i.e., retrusor muscle denervation) most likely represents activation of the extrinsic genioglossus and intrinsic vertical and transverse muscles.
Reduction of tongue force following capsaicin-induced pulmonary C-fiber activation.
The present results indicate that pulmonary C-fiber activation inhibits tongue force during breathing. These results are in accordance with our previous report showing that inspiratory neural drive to the tongue protrudors and retractors is inhibited by capsaicin in the rat (36). Similarly, DiCarlo et al. (17) and Pickar et al. (58) demonstrated that activation of pulmonary C fibers inhibits somatomotor activity in the rat and cat, respectively. Thus capsaicin-induced pulmonary C-fiber activation may produce a reflex inhibition on somatic motor activity and also on tongue muscle force development.
In addition to a reduction of inspiratory XII activity, our previously observed that tonic bursting in the XIIMED was evoked by capsaicin (36). However, while capsaicin delivery attenuated retrusive force, it did not trigger tongue protrusions in XII-intact animals. Moreover, protrusive tongue movement was also reduced following capsaicin administration in animals with XIILAT section (i.e., retractor muscle denervation). There are several possibilities that can be considered. First, inspiratory XII activity was substantially reduced and tonic bursting was only transiently evoked following capsaicin injection. Therefore, the increase in protrusive force, which may have been induced by short-term tonic bursting, could have been masked by a long-lasting inhibitory effect of capsaicin on inspiratory activity. Furthermore, the extrinsic tongue protrudor muscle is composed of both horizontal and oblique compartments, and these compartments have different innervation patterns and muscle fiber arrangements in the human (50), rodent (48), and canine (51). These two compartments may be responsible for different directional force generation. However, the present measurement apparatus could not detect depressive force, which may be why forces associated with increased tonic activity were not detected following capsaicin administration. Finally, in anesthetized rats, tonic XII bursting reflects recruitment of previously silent XII motoneurons, but not increased bursting of active motoneurons (36). This tonic XII motoneuron bursting may not contribute substantially to protrusive movements of the tongue.
Imbalance between the tongue force development and diaphragm activity following activation of pulmonary vagal C-fiber receptors.
Upper airway patency is determined by a balance between the collapsing force, resulting from negative intraluminal pressures during inspiration, and reduction in pharyngeal compliance, resulting from activation of the tongue and other pharyngeal muscles. For example, selective upper airway muscle paralysis in humans (18) or blockade of XII nerve activity in horses (12) can reduce upper airway patency. Conversely, activation of tongue muscles in rodent models can decrease upper airway compliance and significantly improve patency (5, 22, 23, 27). Also, clinical studies showed that electrical stimulation of the XII nerve or tongue muscles has therapeutic potential for treating OSAHS (19, 20, 53, 55). Our previous report demonstrated that capsaicin-induced pulmonary C-fiber activation can inhibit inspiratory activity of both the phrenic and XII nerves in the mechanical-ventilated rat (36, 38). In contrast, the present data indicate that pulmonary C-fiber activation substantially decreased and delayed inspiratory tongue force development with little change in diaphragm activity (e.g., Fig. 2). This difference probably resulted because pulmonary C fibers were stimulated during a period of tracheal occlusion in the present study. We suggest that tracheal occlusion-induced excitation of the diaphragm can override the inhibitory effects of capsaicin. However, the stimulating effects of tracheal occlusion on tongue motor output were blunted by capsaicin (Fig. 2). Accordingly, we conclude that pulmonary C-fiber activation induced an imbalance between upper airway dilating and collapsing forces, and that this may increase the propensity for the upper airway to narrow or collapse, particularly when respiratory drive is increased.
This conclusion is supported by other studies that have documented divergent responses between upper airway and respiratory “pump” muscle activity. For example, lung inflation or hypoxia triggers different relative changes in phrenic vs. XII motor output (34, 37, 65). In addition, cross-correlation analysis indicates that, at least under some conditions, Bötzinger expiratory neurons selectively inhibit phrenic but not XII motoneurons (56). Last, Peever et al. (57) suggest that phrenic and XII motoneuron output are driven by distinct premotoneuron pools in the rat. Accordingly, we suggest that the available evidence indicates that the respiratory-related output of phrenic and XII motoneurons can be differentially regulated by the central nervous system.
Physiological significance.
The present study demonstrates that both protrusive and retrusive tongue force is significantly attenuated by capsaicin administration. In addition, our previous reports showed capsaicin also inhibits inspiratory neural drives to the other upper airway muscles (i.e., laryngeal and nasal muscles) (35, 45) and evokes glottal closure (44). These results support the hypothesis that pulmonary C-fiber activation can have a functional impact on upper airway patency. Pulmonary C fibers can be activated under some pathological conditions, such as pulmonary edema, pulmonary embolism, and sepsis (13, 16, 33), suggesting that upper airway patency may be compromised under these conditions. Furthermore, OSAHS patients have considerably higher circulating levels of proinflammatory mediators (32, 52), which can activate pulmonary C fibers (41, 61, 69). Interestingly, Carley et al. (10) demonstrated that intraperitoneal capsaicin injection can exacerbate sleep-disordered breathing, as reflected by increase in the total number of apneas, as well as decreased ventilation, during sleep in an animal model. Taken together, we suggest that the available evidence indicates that activation of pulmonary C fibers will increase the risk of the upper airway collapse and could, therefore, contribute to OSAHS. Pharmacological amelioration of pulmonary C-fiber activation and/or hypersensitivity may be of value in the treatment of OSAHS (11).
GRANTS
Support for this work was provided by National Science Council Grants NSC100-2320-B-110-003-MY2 (K.-Z. Lee) and NSC96-2311-B-003-001, and National Taiwan Normal University Grant 96TOP001 (J.-C. Hwang). D. D. Fuller was supported by National Institutes of Health Grant 1R01HD052682-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.-Z.L. conception and design of research; K.-Z.L. performed experiments; K.-Z.L. analyzed data; K.-Z.L., D.D.F., and J.-C.H. interpreted results of experiments; K.-Z.L. prepared figures; K.-Z.L. drafted manuscript; K.-Z.L., D.D.F., and J.-C.H. edited and revised manuscript; K.-Z.L., D.D.F., and J.-C.H. approved final version of manuscript.
REFERENCES
- 1. Adachi S, Lowe AA, Tsuchiya M, Ryan CF, Fleetham JA. Genioglossus muscle activity and inspiratory timing in obstructive sleep apnea. Am J Orthod Dentofacial Orthop 104: 138–145, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Bailey EF. Activities of human genioglossus motor units. Respir Physiol Neurobiol 179: 14–22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol 96: 440–449, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bailey EF, Fregosi RF. Modulation of upper airway muscle activities by bronchopulmonary afferents. J Appl Physiol 101: 609–617, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bailey EF, Fregosi RF. Pressure-volume behaviour of the rat upper airway: effects of tongue muscle activation. J Physiol 548: 563–568, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bailey EF, Huang YH, Fregosi RF. Anatomic consequences of intrinsic tongue muscle activation. J Appl Physiol 101: 1377–1385, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Bailey EF, Janssen PL, Fregosi RF. PO2-dependent changes in intrinsic and extrinsic tongue muscle activities in the rat. Am J Respir Crit Care Med 171: 1403–1407, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO(2) on upper airway and respiratory pump muscle activity in the rat. J Physiol 532: 525–534, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brooks SM. Perspective on the human cough reflex. Cough 7: 10, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carley DW, Pavlovic S, Malis M, Knezevic N, Saponjic J, Li C, Radulovacki M. C-fiber activation exacerbates sleep-disordered breathing in rats. Sleep Breath 8: 147–154, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Carley DW, Radulovacki M. Pharmacology of vagal afferent influences on disordered breathing during sleep. Respir Physiol Neurobiol 164: 197–203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheetham J, Pigott JH, Hermanson JW, Campoy L, Soderholm LV, Thorson LM, Ducharme NG. Role of the hypoglossal nerve in equine nasopharyngeal stability. J Appl Physiol 107: 471–477, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Chen HF, Kou YR. Vagal and mediator mechanisms underlying the tachypnea caused by pulmonary air embolism in dogs. J Appl Physiol 88: 1247–1253, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Cheng S, Butler JE, Gandevia SC, Bilston LE. Movement of the tongue during normal breathing in awake healthy humans. J Physiol 586: 4283–4294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diaz V, Dorion D, Renolleau S, Letourneau P, Kianicka I, Praud JP. Effects of capsaicin pretreatment on expiratory laryngeal closure during pulmonary edema in lambs. J Appl Physiol 86: 1570–1577, 1999 [DOI] [PubMed] [Google Scholar]
- 17. DiCarlo SE, Collins HL, Chen CY. Vagal afferents reflexly inhibit exercise in conscious rats. Med Sci Sports Exerc 26: 459–462, 1994 [PubMed] [Google Scholar]
- 18. Eikermann M, Vogt FM, Herbstreit F, Vahid-Dastgerdi M, Zenge MO, Ochterbeck C, de Greiff A, Peters J. The predisposition to inspiratory upper airway collapse during partial neuromuscular blockade. Am J Respir Crit Care Med 175: 9–15, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Eisele DW, Schwartz AR, Smith PL. Tongue neuromuscular and direct hypoglossal nerve stimulation for obstructive sleep apnea. Otolaryngol Clin North Am 36: 501–510, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Eisele DW, Smith PL, Alam DS, Schwartz AR. Direct hypoglossal nerve stimulation in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 123: 57–61, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Fogel RB, Malhotra A, Pillar G, Edwards JK, Beauregard J, Shea SA, White DP. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am J Respir Crit Care Med 164: 2025–2030, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Fregosi RF. Influence of tongue muscle contraction and dynamic airway pressure on velopharyngeal volume in the rat. J Appl Physiol 104: 682–693, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Fregosi RF. Influence of tongue muscle contraction and transmural pressure on nasopharyngeal geometry in the rat. J Appl Physiol 111: 766–774, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respir Physiol 110: 295–306, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol 507: 265–276, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fuller DD. Episodic hypoxia induces long-term facilitation of neural drive to tongue protrudor and retractor muscles. J Appl Physiol 98: 1761–1767, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol 519: 601–613, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. J Neurophysiol 74: 547–555, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Hatipoglu U, Rubinstein I. Inflammation and obstructive sleep apnea syndrome pathogenesis: a working hypothesis. Respiration 70: 665–671, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Huxtable AG, Vinit S, Windelborn JA, Crader SM, Guenther CH, Watters JJ, Mitchell GS. Systemic inflammation impairs respiratory chemoreflexes and plasticity. Respir Physiol Neurobiol 178: 482–489, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jordan AS, White DP. Pharyngeal motor control and the pathogenesis of obstructive sleep apnea. Respir Physiol Neurobiol 160: 1–7, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kimoff RJ, Hamid Q, Divangahi M, Hussain S, Bao W, Naor N, Payne RJ, Ariyarajah A, Mulrain K, Petrof BJ. Increased upper airway cytokines and oxidative stress in severe obstructive sleep apnoea. Eur Respir J 38: 89–97, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Lai CJ, Ruan T, Kou YR. The involvement of hydroxyl radical and cyclooxygenase metabolites in the activation of lung vagal sensory receptors by circulatory endotoxin in rats. J Appl Physiol 98: 620–628, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Lee KZ, Fuller DD. Preinspiratory and inspiratory hypoglossal motor output during hypoxia-induced plasticity in the rat. J Appl Physiol 108: 1187–1198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee KZ, Fuller DD, Lu IJ, Ku LC, Hwang JC. Pulmonary C-fiber receptor activation abolishes uncoupled facial nerve activity from phrenic bursting during positive end-expired pressure in the rat. J Appl Physiol 104: 119–129, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Lee KZ, Fuller DD, Lu IJ, Lin JT, Hwang JC. Neural drive to tongue protrudor and retractor muscles following pulmonary C-fiber activation. J Appl Physiol 102: 434–444, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Lee KZ, Fuller DD, Tung LC, Lu IJ, Ku LC, Hwang JC. Uncoupling of upper airway motor activity from phrenic bursting by positive end-expired pressure in the rat. J Appl Physiol 102: 878–889, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Lee KZ, Lu IJ, Ku LC, Lin JT, Hwang JC. Response of respiratory-related hypoglossal nerve activity to capsaicin-induced pulmonary C-fiber activation in rats. J Biomed Sci 10: 706–717, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Lee KZ, Sandhu MS, Dougherty BJ, Reier PJ, Fuller DD. Influence of vagal afferents on supraspinal and spinal respiratory activity following cervical spinal cord injury in rats. J Appl Physiol 109: 377–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee LY. Respiratory sensations evoked by activation of bronchopulmonary C-fibers. Respir Physiol Neurobiol 167: 26–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee LY, Gu Q, Gleich GJ. Effects of human eosinophil granule-derived cationic proteins on C-fiber afferents in the rat lung. J Appl Physiol 91: 1318–1326, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47–65, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Lin YS, Hsu CC, Bien MY, Hsu HC, Weng HT, Kou YR. Activations of TRPA1 and P2X receptors are important in ROS-mediated stimulation of capsaicin-sensitive lung vagal afferents by cigarette smoke in rats. J Appl Physiol 108: 1293–1303, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Lu IJ, Lee KZ, Hwang JC. Capsaicin-induced activation of pulmonary vagal C fibers produces reflex laryngeal closure in the rat. J Appl Physiol 101: 1104–1112, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Lu IJ, Lee KZ, Lin JT, Hwang JC. Capsaicin administration inhibits the abducent branch but excites the thyroarytenoid branch of the recurrent laryngeal nerves in the rat. J Appl Physiol 98: 1646–1652, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Mateika JH, Millrood DL, Kim J, Rodriguez HP, Samara GJ. Response of human tongue protrudor and retractors to hypoxia and hypercapnia. Am J Respir Crit Care Med 160: 1976–1982, 1999 [DOI] [PubMed] [Google Scholar]
- 47. McClung JR, Goldberg SJ. Functional anatomy of the hypoglossal innervated muscles of the rat tongue: a model for elongation and protrusion of the mammalian tongue. Anat Rec 260: 378–386, 2000 [DOI] [PubMed] [Google Scholar]
- 48. McClung JR, Goldberg SJ. Organization of the hypoglossal motoneurons that innervate the horizontal and oblique components of the genioglossus muscle in the rat. Brain Res 950: 321–324, 2002 [DOI] [PubMed] [Google Scholar]
- 49. McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. Am J Respir Crit Care Med 180: 692–700, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Mu L, Sanders I. Human tongue neuroanatomy: nerve supply and motor endplates. Clin Anat 23: 777–791, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mu L, Sanders I. Neuromuscular specializations of the pharyngeal dilator muscles. II. Compartmentalization of the canine genioglossus muscle. Anat Rec 260: 308–325, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol 94: 179–184, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Oliven A, O'Hearn DJ, Boudewyns A, Odeh M, De Backer W, van de Heyning P, Smith PL, Eisele DW, Allan L, Schneider H, Testerman R, Schwartz AR. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol 95: 2023–2029, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Oliven A, Odeh M, Geitini L, Oliven R, Steinfeld U, Schwartz AR, Tov N. Effect of coactivation of tongue protrusor and retractor muscles on pharyngeal lumen and airflow in sleep apnea patients. J Appl Physiol 103: 1662–1668, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Oliven R, Tov N, Odeh M, Gaitini L, Steinfeld U, Schwartz AR, Oliven A. Interacting effects of genioglossus stimulation and mandibular advancement in sleep apnea. J Appl Physiol 106: 1668–1673, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Peever JH, Mateika JH, Duffin J. Respiratory control of hypoglossal motoneurones in the rat. Pflügers Arch 442: 78–86, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Peever JH, Shen L, Duffin J. Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neuroscience 110: 711–722, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Pickar JG. The thromboxane A2 mimetic U-46619 inhibits somatomotor activity via a vagal reflex from the lung. Am J Physiol Regul Integr Comp Physiol 275: R706–R712, 1998 [DOI] [PubMed] [Google Scholar]
- 59. Pittman LJ, Bailey EF. Genioglossus and intrinsic electromyographic activities in impeded and unimpeded protrusion tasks. J Neurophysiol 101: 276–282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44: 931–938, 1978 [DOI] [PubMed] [Google Scholar]
- 61. Ruan T, Ho CY, Kou YR. Afferent vagal pathways mediating respiratory reflexes evoked by ROS in the lungs of anesthetized rats. J Appl Physiol 94: 1987–1998, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Ryan S, McNicholas WT, O'Regan RG, Nolan P. Reflex respiratory response to changes in upper airway pressure in the anaesthetized rat. J Physiol 537: 251–265, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ryan S, Nolan P. Long-term facilitation of upper airway muscle activity induced by episodic upper airway negative pressure and hypoxia in spontaneously breathing anaesthetized rats. J Physiol 587: 3343–3353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Saboisky JP, Jordan AS, Eckert DJ, White DP, Trinder JA, Nicholas CL, Gautam S, Malhotra A. Recruitment and rate-coding strategies of the human genioglossus muscle. J Appl Physiol 109: 1939–1949, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Saito Y, Ezure K, Tanaka I. Difference between hypoglossal and phrenic activities during lung inflation and swallowing in the rat. J Physiol 544: 183–193, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sauerland EK, Mitchell SP. Electromyographic activity of intrinsic and extrinsic muscles of the human tongue. Tex Rep Biol Med 33: 444–455, 1975 [PubMed] [Google Scholar]
- 67. Susarla SM, Thomas RJ, Abramson ZR, Kaban LB. Biomechanics of the upper airway: changing concepts in the pathogenesis of obstructive sleep apnea. Int J Oral Maxillofac Surg 39: 1149–1159, 2010 [DOI] [PubMed] [Google Scholar]
- 68. Vinit S, Windelborn JA, Mitchell GS. Lipopolysaccharide attenuates phrenic long-term facilitation following acute intermittent hypoxia. Respir Physiol Neurobiol 176: 130–135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yu J, Lin S, Zhang J, Otmishi P, Guardiola JJ. Airway nociceptors activated by pro-inflammatory cytokines. Respir Physiol Neurobiol 156: 116–119, 2007 [DOI] [PubMed] [Google Scholar]