Abstract
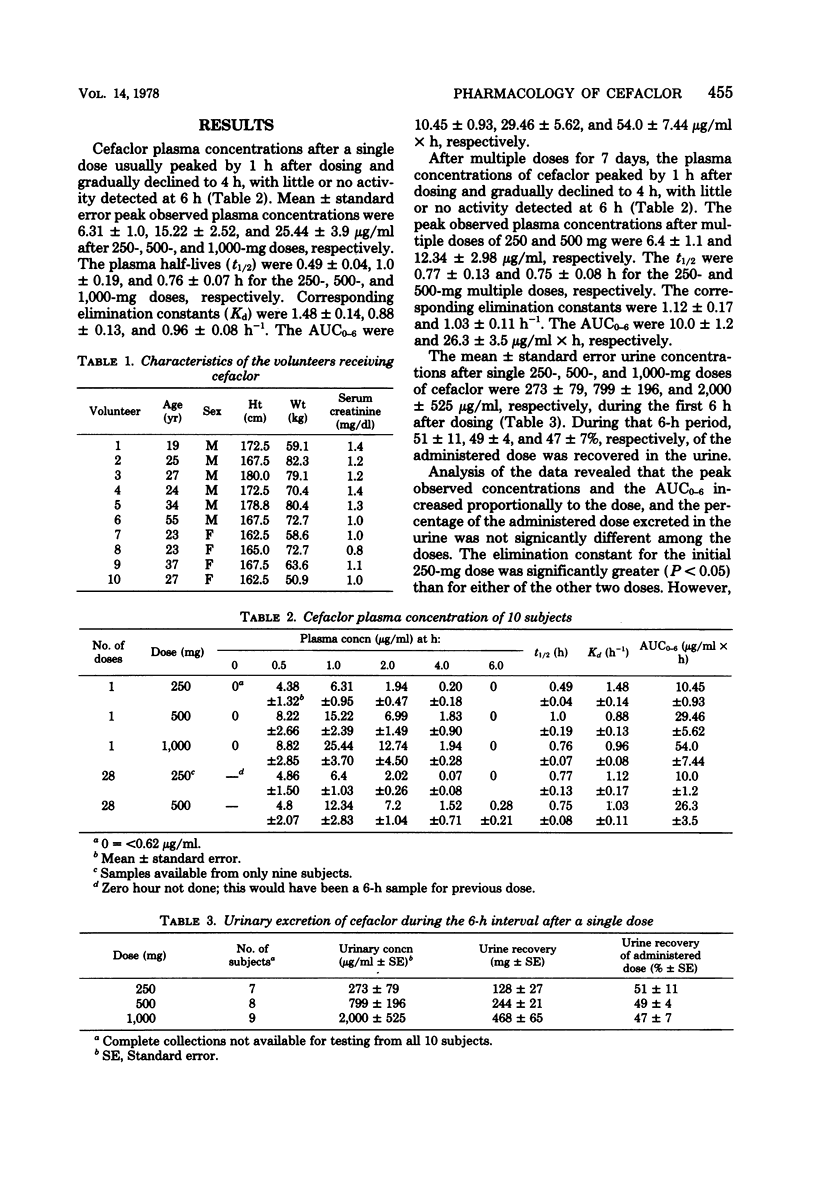
The plasma and urine concentrations of cefaclor were measured after oral administration of single and multiple doses to volunteers. Cefaclor was rapidly absorbed, rapidly excreted in the urine, well tolerated without toxicity, and failed to accumulate in the plasma with chronic dosing.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bill N. J., Washington J. A., 2nd Comparison of in vitro activity of cephalexin, cephradine, and cefaclor. Antimicrob Agents Chemother. 1977 Mar;11(3):470–474. doi: 10.1128/aac.11.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniowski O. M., Scheld W. M., Sande M. A. Comparative pharmacology of cefaclor and cephalexin. Antimicrob Agents Chemother. 1977 Aug;12(2):157–162. doi: 10.1128/aac.12.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. In vitro studies with cefaclor, a new oral cephalosporin. Antimicrob Agents Chemother. 1977 Oct;12(4):490–497. doi: 10.1128/aac.12.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro J., Levison M. E. In vitro activity of cefaclor, a new orally administered cephalosporin antibiotic. Antimicrob Agents Chemother. 1977 Sep;12(3):442–443. doi: 10.1128/aac.12.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Korzeniowski O. M., Sande M. A. In vitro susceptibility studies with cefaclor and cephalexin. Antimicrob Agents Chemother. 1977 Aug;12(2):290–292. doi: 10.1128/aac.12.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadomy S., Wagner G., Carver M. In vitro activities of five oral cephalosporins against aerobic pathogenic bacteria. Antimicrob Agents Chemother. 1977 Nov;12(5):609–613. doi: 10.1128/aac.12.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]


