Abstract
Regulatory RNAs are being increasingly investigated in neurons, and important roles in brain function have been revealed. Regulatory RNAs are non-protein-coding RNAs (npcRNAs) that comprise a heterogeneous group of molecules, varying in size and mechanism of action. Regulatory RNAs often exert post-transcriptional control of gene expression, resulting in gene silencing or gene expression stimulation. Here, we review evidence that regulatory RNAs are implicated in neuronal development, differentiation, and plasticity. We will also discuss npcRNA dysregulation that may be involved in pathological states of the brain such as neurodevelopmental disorders, neurodegeneration, and epilepsy.
Keywords: Regulatory RNA, non-protein-coding RNA, neurons, brain disorders
1. Introduction
A large body of studies indicates that, in addition to protein coding RNAs (mRNAs), ribosomal RNAs and transfer RNAs, other RNA molecules – generally referred to as non-protein-coding (npc) RNAs – participate in the regulation of a variety of cellular processes (Carninci et al., 2005; Costa, 2007; Erdmann et al., 2001; Kapranov et al., 2007; Mattick and Makunin, 2006; Mattick, 2009; Ponting et al., 2009; Satterlee et al., 2007). Since their discovery in the late sixties and early seventies, the number of identified npcRNAs has increased substantially and, for some of them, evidence of regulatory functions has emerged. In this review, we will first provide a brief introduction on the classification of npcRNAs and will then focus on npcRNAs that have documented regulatory functions in physiological and diseased states in the brain. We refer to these regulatory RNAs as ‘non-protein-coding’ – rather than ‘non-coding’ – RNAs to emphasize that these RNAs carry codes, even if they do not encode specific amino acidic sequences (Brosius and Tiedge, 2004).
Regulatory RNAs have been classified into three relatively distinct groups on the basis of their length: microRNAs (miRNAs) and small interfering RNAs (siRNAs), ranging from 18 to 25 nucleotides; small RNAs with a length of 50-300 nucleotides; and medium, large, and macro RNAs with transcript lengths reaching 100,000 nucleotides (Costa, 2005; Costa, 2007; Eddy, 2001; Shamovsky and Nudler, 2006).
The use of biocomputational analysis, followed by biochemical validation, has contributed significantly to the identification of regulatory RNAs, especially those expressed at low levels (Griffiths-Jones, 2007; Voss et al., 2009; Yoon and De Micheli, 2005a; Yoon and De Micheli, 2005b). For example, the sequence and structure of several regulatory RNAs in yeast have been identified through comparative genomics coupled to thermodynamic stability analysis, and the function of these RNAs has subsequently been experimentally verified (Kavanaugh and Dietrich, 2009). In the human genome, 79 miRNAs regulatory modules – defined as groups of miRNAs and target genes cooperating in post-transcriptional gene regulation – have been identified by computational and experimental approaches (Tran et al., 2008; Yoon and De Micheli, 2005a).
RNA folding/unfolding and secondary structure formation are hierarchical processes as secondary structure determines tertiary structure formation (Onoa and Tinoco, 2004). Since the tertiary structure defines the function of a molecule, computational studies of the regulatory RNA's three-dimensional structure, folding dynamics predictions (Ding et al., 2008b; Frellsen et al., 2009; Mathews and Turner, 2006; Sharma et al., 2008), and analysis of secondary and tertiary structure conservation (Abraham et al., 2008; Xu et al., 2007) can provide insights into their putative functions.
Regulatory RNAs that control the expression of genes located in close proximity are called cis-acting regulatory RNAs, whereas those that act on distant target genes are called transacting regulatory RNAs. Cis-acting regulatory RNAs constitute a growing class of long and macro RNAs that regulate expression of flanking genes (Koerner et al., 2009; Latos and Barlow, 2009). These RNAs show monoallelic expression (i.e. they originate from paternal or maternal allele) and are involved in the epigenetic regulation of chromatin (Kloc et al., 2008; Royo and Cavaille, 2008). A prototypical cis-acting RNA is Xist (Pauler et al., 2007; Wutz and Gribnau, 2007), a large (17 kb long) npcRNA that is located on, and expressed from, the inactive X chromosome. Xist ensures dosage compensation of the X-linked genes in mammals (Brown et al., 1992).
The group of trans-acting regulatory RNAs includes miRNAs (Kocerha et al., 2009b; Kosik, 2006; Krichevsky et al., 2006). In C. elegans, the miRNA lin-4 negatively regulates the level of LIN-14 protein which controls postembryonic development (Lee et al., 1993; Lee and Ambros, 2001). Lin-4 is thus classified as small temporal RNA. Subsequently, more temporal RNAs, such as let-7, have been identified (Eddy, 2001).
Further work on miRNAs has led to the identification, in 58 species, of about 5000 loci, which transcripts are processed into almost 6000 mature miRNA sequences. (Gardner et al., 2009; Griffiths-Jones et al., 2008). miRNAs are involved in many processes such as development and differentiation, apoptosis, tumorigenesis, and brain functions. In this review, we will primarily focus on small and large npcRNAs involved in brain function and disorders. Neuronal functions of miRNAs have been reviewed elsewhere (Eacker et al., 2009; Fineberg et al., 2009; Schratt, 2009) and therefore will not be extensively discussed here.
2. Regulatory RNAs in brain
Recent studies have begun to reveal neuronal functions of regulatory RNAs (Cao et al., 2006; Chang et al., 2009; Mehler and Mattick, 2006; Mehler and Mattick, 2007; Pollard et al., 2006; Satterlee et al., 2007). Several hundreds of miRNAs have been implicated in brain development (Amaral and Mattick, 2008; Krichevsky et al., 2003), neurodevelopmental disorders (Amaral and Mattick, 2008; Chang et al., 2009), and long-term memory storage (Mercer et al., 2008a).
In situ hybridization data on protein-coding transcripts in developing and adult brain (Lein et al., 2007) were also screened for npcRNAs (Mercer et al., 2008b). Expression levels of mRNAs in brain were higher compared to those of regulatory RNAs, but regulatory RNA expression levels in different brain regions were more heterogeneous. These data suggest that many of the 849 npcRNAs identified are specifically associated with brain regions, cell types, and subcellular compartments (Mercer et al., 2008b), and that they may play roles in various nervous system disorders (Bilen et al., 2006a; Bilen et al., 2006b; Mehler and Mattick, 2007; Weinberg and Wood, 2009).
2.1. Regulatory RNAs and gene expression silencing
Post-transcriptional regulation mediated by miRNAs usually results in gene silencing. A miRNA typically targets a specific mRNA and through RNA/RNA interaction either degrades the mRNA or interferes with the translational machinery, and consequently reduces synthesis of the cognate protein (Chekulaeva and Filipowicz, 2009; Valencia-Sanchez et al., 2006).
A brain-relevant example is the suggested role of miRNAs in regulating schizophrenia risk genes (Perkins et al., 2005; Perkins et al., 2007). In drug-induced schizophrenic mice, expression of miRNA-219, a brain-specific RNA in humans and rodents, is strongly reduced, and such reduction can be prevented with antipsychotic treatments (Cheng et al., 2007; Kocerha et al., 2009a; Lukiw, 2007). miRNA-219 regulates expression of CaMKIIγ in cortical cells in in vitro experiments, and it modulates expression of CaMKIIγ protein levels in the prefrontal cortex, suggesting a role in NMDA receptor-mediated signaling cascades associated with neurobehavioral functions (Kocerha et al., 2009a).
2.2. Regulatory RNAs and gene expression stimulation
Recent work has suggested additional mechanisms of gene expression regulation involving regulatory RNAs. These RNAs enhance transcriptional and post-transcriptional activity (e.g. by increasing target mRNA stability), rather than silencing the target gene. Their function is mediated either through RNA/RNA interactions or through RNA/protein interactions.
Kohtz and colleagues identified a developmentally regulated 2.7 kb-long npcRNA, named Evf-1, that is expressed in embryonic dorsal and ventral telencephalon (Kohtz et al., 1998; Kohtz and Fishell, 2004). The Evf-1 gene is a located upstream of the Dlx-6 gene, whose expression pattern it closely resembles. In vertebrates, the family of Dlx comprises six homeodomain transcription factors (Dlx-1 to 6), with critical roles in neuronal differentiation and migration and in epilepsy (Acampora et al., 1999; Anderson et al., 1997; Cobos et al., 2005). Embedded in one of the highly conserved intergenic regions of Dlx-5/6 resides Evf-2 RNA, a 3.8 kb-long npcRNA, a splice variant of Evf-1 (Feng et al., 2006). The authors showed that Evf-2 RNA forms a complex with the Dlx-2 protein. It has been proposed that this complex, interacting with an intergenic enhancing sequence situated between the Dlx-5 and Dlx-6 genes, increases transcriptional activity (Feng et al., 2006).
More recently, the same group has shown that in mice lacking an exon resulting in the absence of Evf-2 RNA, the number of GABAergic interneurons in the dentate gyrus and in the hippocampal CA1 and CA3 regions is reduced by 40-65% during early postnatal stages (Bond et al., 2009). The number of GABAergic interneurons recovers in adult mice, but reduced synaptic inhibition persists, suggesting that GABA-dependent connectivity in adult brain is modulated by the regulatory RNA Evf-2.
Another example of post-transcriptional regulation by an npcRNA has been reported in Alzheimer's disease (AD). A regulatory antisense RNA directed against BACE1 (β-site amyloid precursor protein-cleaving enzyme) mRNA, encoding a vertebrate specific p-secretase, is elevated by six-fold in AD patients (Faghihi et al., 2008; St George-Hyslop and Haass, 2008). The BACE1 antisense transcript (BACE1-AS), a conserved circa 2-kb long RNA, is located on the positive strand of chromosome 11, and on the opposite strand of the BACE1 locus with which it shares a 104-nucleotide complementarity. BACE1-AS positively regulates BACE1 mRNA by increasing its stability: knock-down of BACE1-AS results in a reduction of BACE1 mRNA and consequently of protein abundance in vitro (Faghihi et al., 2008). Cell stressors implicated in AD pathogenesis (e.g. hyperthermia, high glucose) increase BACE1-AS and BACE1 protein, thereby facilitating the “amyloid cascade” (Faghihi et al., 2008).
2.3. Non-protein-coding repeat expansions
Non-protein-coding CTG or CGG expansions, located in an untranslated region of a gene, may result in gain or change of function of an RNA (Daughters et al., 2009; Dick et al., 2006; Ranum and Cooper, 2006). Three notable examples are spinocerebellar ataxia type 8 (SCA8), fragile X tremor ataxia syndrome (FXTAS), and myotonic dystrophy (DM) (Brouwer et al., 2008; Daughters et al., 2009; Ranum and Cooper, 2006).
SCA8 is a progressive degenerative disease (Soong and Paulson, 2007) with a neurodegeneration pattern distinct from other ataxias (Ito et al., 2006). SCA8 ataxia is caused by expansion of CTG repeats that are transcribed as part of SCA8 RNA (Koob et al., 1999; Nemes et al., 2000). SCA8 RNA is expressed primarily in brain but is not translated. The SCA8 transcript is an antisense RNA overlapping with the sense strand of the actin-binding protein KLHL1 gene (Nemes et al., 2000). It has been proposed that neurodegeneration in SCA8 ataxia is caused either by abnormal interaction of SCA8 RNA with RNA binding proteins or by altered SCA8 RNA-mediated regulation of KLHL1 expression (Chen et al., 2008; Mutsuddi et al., 2004; Nemes et al., 2000).
RNA gain-of-function has also been reported in FXTAS. Patients with high number of non-protein-coding CGG expansions in the 5′ UTR region of the FMR1 gene display progressive tremor, ataxia, brain atrophy, and cognitive decline (Greco et al., 2002; Hagerman and Hagerman, 2004; Hagerman et al., 2001). Intranuclear inclusions have been observed in neuronal and astrocytic cells in the cerebral cortex (Greco et al., 2002). In FXTAS, the number of CGG repeats in the 5′ UTR of human FMR1 mRNA ranges from 55 to 200, and the severity of symptoms correlates with increasing repeat number (Leehey et al., 2008). The RNA toxicity hypothesis has been supported by recent work with a mouse model in which 98 CGG triplets contained in the 5′ UTR of murine Fmr1 mRNA are expressed (Hashem et al., 2009). In this model, CGG repeats give rise to the formation of intranuclear inclusions in Purkinje neurons, probably as a result of interaction of the repeats with RNA binding proteins. In post-mortem brain tissues of FXTAS patients, hnRNP A2/B1 and Purα have been identified in intranuclear inclusions (Iwahashi et al., 2006; Jin et al., 2007).
DM is an inherited muscular dystrophy characterized by progressive atrophy of voluntary muscles (Batten and Gibb, 1909). Two types of DM, DM1 and DM2, share the same toxic RNA gain-of-function mechanism but involve two different genes. DM1 is caused by CTG repeat expansion in the 3′ UTR of the myotonic dystrophy protein kinase (DMPK) gene (Brook et al., 1992; Fu et al., 1992), while in DM2, tetranucleotide CCTG expansions occur within intron 1 of the zinc finger 9 (ZNF9) gene (Liquori et al., 2001). Multiple molecular mechanisms have been proposed for DM1 (Cho and Tapscott, 2007; Kaliman and Llagostera, 2008). Repeat expansions in DMPK gene cause decreased expression at the mRNA and protein levels (Carango et al., 1993; Fu et al., 1993; Hofmann-Radvanyi et al., 1993). It is thought that CUG expansions in DMPK mRNA form stem-loop structures (Napierala and Krzyzosiak, 1997) which sequester CUG-binding proteins (CUG-BPs) such as muscleblind-like protein 1 (MBNL1) (Fardaei et al., 2001; Miller et al., 2000; Timchenko et al., 1996). These RNA-protein complexes, trapped in the nucleus, give rise to the ribonuclear foci observed in DM muscle fibers (Liquori et al., 2001; Miller et al., 2000; Wheeler et al., 2007). MBNL1 is homologous to the mbl proteins which in Drosophila are crucial for terminal differentiation of muscle cells and photoreceptors (Artero et al., 1998; Begemann et al., 1997). In a DM mouse model, loss of the MBNL1 gene results in muscle and eye abnormalities similar to DM phenotypes (Kanadia et al., 2003). In addition, the DM mouse model shows splicing dysregulation. Splicing abnormalities as well as sequestration of MBNL1, and other CUG-BPs, by CUG and CCUG repeat expansions seem to contribute to the onset of these disorders.
2.4. Regulatory RNAs and Imprinting
It is becoming increasingly clear that regulatory RNAs are key factors in heterochromatin formation, chromosomal dynamics, epigenetic modification, and genomic imprinting (Bernstein and Allis, 2005; Koerner et al., 2009; O'Neill, 2005; Royo and Cavaille, 2008). Screening of chromatin-state maps in four mouse cell types has revealed over 1,580 large intergenic non-coding RNAs (lincRNAs) (Guttman et al., 2009). These regulatory RNAs show 95% evolutionary conservation across mammals, suggesting that they are required for biological functions yet to be identified. The same approach carried out on 6 human cell types detected over 1,700 new loci encoding lincRNAs (Khalil et al., 2009). A few of these regulatory RNAs have also been found physically associated with chromatin-modifying complexes, for instance the polycomb repressive complex 2 which silences genes involved in development and differentiation, and CoREST, a repressor of neuronal genes (Khalil et al., 2009; Qureshi and Mehler, 2009).
Eighty imprinted genes have been identified in the mouse genome, and many of these are grouped in clusters on chromosome 7 (Barlow and Bartolomei, 2007; Pauler et al., 2007; Verona et al., 2003). Interestingly, the Prader-Willi/Angelman syndrome (PWS/AS) cluster is also located on chromosome 7. This region corresponds to the human 15q11-q13 imprinting domain. The Prader-Willi syndrome (PWS) and the Angelman syndrome (AS) are sister imprinting disorders characterized by neurological and developmental abnormalities, cognitive impairments, obsessive-compulsive symptoms, seizures, and ataxia (Cassidy et al., 2000). The PWS/AS region contains several imprinted genes that encode proteins and npcRNAs. In PWS patients, the paternal expression of the following genes is impaired: protein coding genes MKRN3, MAGEL2, NDN, SNURF – SNRPN, and C/D box small nucleolar RNA (snoRNA) genes (SNORDs) such as HBII-436 (SNORD107), HBII-13 (SNORD64), HBII-437 (SNORD108), HBII-438A (SNORD109A), HBII-85 (SNORD116), HBII-52 (SNORD115), and HBII-438B (SNORD109B) (Cavaille et al., 2000; Runte et al., 2001). In AS patients, maternal expression of the protein coding genes UBE3A and ATP10A is impaired (Herzing et al., 2001; Kishino et al., 1997; Matsuura et al., 1997; Meguro et al., 2001).
Imprinted expression of all these genes is controlled by the bipartite imprinting center (IC), located upstream of the SNURF – SNRPN promoter. The proximal part of the bipartite IC controls paternal expression of the PWS genes while the distal part controls maternal expression of the AS genes (for review see Kantor et al., 2006). It is generally believed that all PWS npcRNAs are processed from the extra long intron-containing U-UBE3A-AS RNA transcript, partially antisense to UBE3A, starting from the U-exons upstream of the SNURF – SNRPN gene and ending in the UBE3A gene (Runte et al., 2001). The UBE3A-AS npcRNA has been reported to negatively regulate expression of the UBE3A gene (Landers et al., 2005). Furthermore, the mature snoRNAs are processed from the introns of this long npcRNA. SNORD115 and SNORD116 are arranged in clusters of 47 and 29 copies, respectively, each interrupted by less conserved exons. The corresponding region on mouse chromosome 7 has a similar structure, except that the mouse has an additional paternally expressed protein coding gene Frat3 and lacks orthologs of SNORD108, SNORD109A, and SNORD109B. The corresponding extra long RNA transcript in mouse is termed LNCAT (Le Meur et al., 2005). Several mouse models for PWS and AS have been generated. The UBE3A gene plays the chief role in the development of AS, which was confirmed by targeted elimination of Ube3a locus in a mouse model (Jiang et al., 1998), and whose loss decreases AMPA receptor expression at the synapses (Greer et al., 2010). The minimal critical region containing cluster of the HBII-85/SNORD116 snoRNA genes is responsible for PWS (de Smith et al., 2009; Ding et al., 2008a; Sahoo et al., 2008; Skryabin et al., 2007). However, other paternally expressed genes are likely to contribute to the phenotype (Kanber et al., 2009).
The second cluster of snoRNAs that is lacking in larger deletions of the PWS locus is HBII-52 (SNORD115) snoRNAs (Cavaille et al., 2000). HBII-52 snoRNAs harbor a guide sequence of 18 nucleotides that is complementary to the transcript encoding the serotonin receptor 2C. However, the modification status of the serotonin receptor 2C transcript is not significantly modulated by the snoRNA (Vitali et al., 2005). A suggested regulation of alternative splicing of the serotonin 2C receptor (Kishore and Stamm, 2006) was not observed in subsequent work (Doe et al., 2009). The question is raised whether the sequence complementarity may simply be fortuitous. Thus, a regulatory role of SNORD115 in serotonin receptor 2C expression remains to be substantiated even though it would fit well in the picture of PWS and other neurodevelopmental disorders. For example, duplication of chromosome 15q11-q13 (which contains the SNORD115 gene cluster among many other genes) is a chromosomal abnormality associated with autism (Bolton et al., 2004; Cook et al., 1997; Gillberg, 1998), a disorder characterized by verbal and nonverbal communication impairments (Consortium, 2001; Wassink and Piven, 2000). A transgenic mouse with duplication of the snoRNA cluster on chromosome 7, mirroring the duplication of human chromosome 15q11-q13, appears to show altered serotonin receptor 2C-mediated responses and autism-like phenotypes (Nakatani et al., 2009). Notably, a subgroup (∼25%) of PWS patients have uniparental disomy (UPD) for maternal chromosome 15 (Fridman and Koiffmann, 2000), and they are likely to manifest autism spectrum disorder (ASD) behaviors, with an estimated comorbidity between 19-36.5% (Descheemaeker et al., 2006; Veltman et al., 2004).
The distal region of the mouse chromosome 12 and on the human chromosome 14 contains an imprinting gene cluster, the Dlk1-Dio3-imprinted domain, that contains seven npcRNAs (Cavaille et al., 2002; Hagan et al., 2009). Deletion or epimutation in this cluster causes uniparental disomy for chromosome 14 (UPD14), a syndrome characterized by moderate to severe mental retardation (Kagami et al., 2005; Kagami et al., 2008). A gene identified in the Dlk1-Dio3 cluster is an ortholog of the human gene MEG3 (maternally expressed gene 3) and generates a polyadenylated npcRNA called Gtl2 (da Rocha et al., 2008). Takahashi and colleagues suggests that Gtl2 is a cis-acting element that plays a critical role in embryonic development and neonatal growth by regulating the Dlk1-Dio3 domain (Takahashi et al., 2009). In addition, Hagan and coworkers suggest a regulatory mechanism on the Dlk1-Dio3 domain, mediated by 52 microRNAs that are embedded in introns of the seven npcRNA genes localized in the Dlk1-Dio3 cluster (Hagan et al., 2009). 80% of these microRNAs are also present in the human orthologous cluster whose loss may be associated with the UPD phenotype (Hagan et al., 2009).
Expression of the gene encoding the insulin-like growth-factor type-2 receptor (Igf2r) represents a well-studied example of regulation by imprinted npcRNAs in brain. The antisense transcript of the Igf2r gene is called Air (antisense Igf2r RNA) and is an imprinted, polyadenylated 108-kb npcRNA (Lyle et al., 2000). The Air promoter lies in the region 2 within the second intron of the Igf2r gene (Sleutels and Barlow, 2001). In non-neuronal somatic cells, imprinting of the Igf2r promoter in the paternal allele and imprinting of the Air promoter in the maternal allele result in expression of Igf2r from the maternal allele and expression of Air from the paternal one (Yamasaki et al., 2005). In contrast, in cultured neurons and in brain, Igf2r is expressed by both parental alleles (Hu et al., 1998; Yamasaki et al., 2005). Biallelic Igf2r expression occurs in the absence of Air expression in neurons, suggesting that Air is critical in Igf2r silencing and that npcRNAs may play a role in neuron-specific epigenetic modifications. Epigenetic mechanisms and chromatin remodeling have recently been discussed as processes important in brain function (Taniura et al., 2007), and more examples of regulatory RNAs involved in neuronal function are likely to be revealed in future work.
2.5. Other mRNA-like RNAs
Some of the npcRNAs mentioned above belong to the class of mRNA-like RNAs which are characterized by multiple spliced exons and by a poly(A) tail at the 3′-terminus; however, they lack an open reading frame (Erdmann et al., 2000; Szell et al., 2008). A further example of these class of npcRNAs is a transcript specifically expressed in brain, named Ntab (non-coding transcript abundantly expressed in brain) RNA (French et al., 2001). This RNA is expressed at high levels in the CA3 area and in the dentate gyrus of the hippocampus. Ntab RNA appears to be localized in dendrites, suggesting a possible role in synaptic activity or plasticity.
Human accelerated regions (HARs) are genome sequences that are conserved in vertebrates and that have been proposed to show a significant accelerated nucleotide substitution rate in humans (Pollard et al., 2006). In a comparative genome analysis, the 118 bp region HAR1 on chromosome 20 appeared as the fastest-evolving region in the human genome: 18 nucleotide substitutions have been estimated to have occurred in human HAR1 since the time of a human– chimpanzee ancestor, whereas for example only 4 nucleotide substitutions were observed between chimpanzee and mouse orthologous HAR1 sequences, and an average of 0.27 substitution was calculated among other amniotes (Pollard et al., 2006). HAR1 is part of the gene HAR1F, the transcript of which has been detected in human brain by in situ hybridization but not by Northern blot analysis (Pollard et al., 2006). Interestingly, the HAR1F transcript is likely an npcRNA, as suggested by analysis of its protein coding potential (Pollard et al., 2006). HAR1F is expressed in the developing human neocortex at critical stages of neuronal differentiation and migration (Pollard et al., 2006). A recent enzymatic and chemical analysis of the secondary structure of HAR1, the probable functional domain of HAR1F RNA, shows that just a few nucleotide substitutions cause the transition from the unstable hairpin structure found in chimpanzees to the cloverleaf-like structure observed in humans (Beniaminov et al., 2008). The distinct localization and structural characteristics of HAR1F RNA in humans suggest an important role of this npcRNA in brain, although its mechanism of action in neurons remains to be elucidated.
2.6. BC RNAs
As discussed above, many of the regulatory functions of npcRNAs impact protein synthetic output through changes of mRNA stability or chromatin modifications. Other npcRNAs directly target the protein synthetic process by interacting with the translational machinery (Chekulaeva and Filipowicz, 2009). One example is the Brain-specific Cytoplasmic (BC) subtype of regulatory RNAs. BC1 RNA and the smaller BC2 transcript were identified in rodents, and both were found expressed almost exclusively in neurons (DeChiara and Brosius, 1987; Milner et al., 1984; Sutcliffe et al., 1982; Sutcliffe et al., 1984). BC1 RNA, and a human counterpart BC200 RNA, are located in somatodendritic domains (Tiedge et al., 1991; Tiedge et al., 1993). BC1 RNA is actively transported from somata to dendrites, in a process that is mediated by a cis-acting targeting element located in the 5′-most domain of the RNA (Muslimov et al., 1997). BC1 RNA inhibits formation of 48S initiation complexes through interaction with poly(A)-binding protein (PABP) and eukaryotic initiation factor 4 A (eIF4A) (Kondrashov et al., 2005; Wang et al., 2002; Wang et al., 2005). BC1 RNA, as well as BC200 RNA, blocks the ATP-dependent helicase activity of eIF4A, decoupling ATP hydrolysis from the mRNA unwinding (Lin et al., 2008).
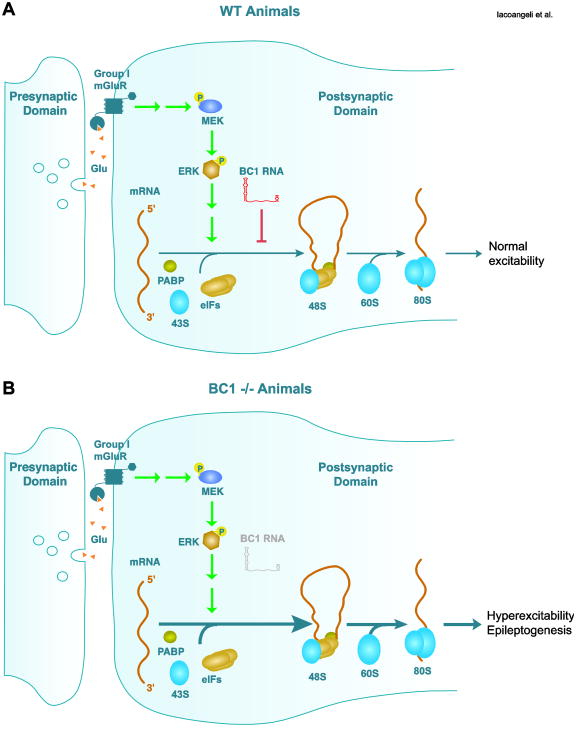
More recently, physiological data have revealed a role of BC1 RNA in epileptogenesis (Zhong et al., 2009). Previous studies have shown that stimulation of group I metabotropic glutamate receptors (mGluRs) in hippocampal slices induces prolonged epileptiform discharges (Merlin and Wong, 1997; Merlin et al., 1998; Wong et al., 1999). Such epileptogenic induction requires activation of the mitogen-activated protein kinase kinase/extracellular regulated kinase (MEK-ERK) pathway (Zhao et al., 2004) and of protein synthesis (Merlin and Wong, 1997; Merlin et al., 1998; Wong et al., 1999). The prolonged epileptiform discharges result from group I mGluR-mediated induction of a voltage-dependent cationic current in hippocampal CA3 pyramidal cells that leads to hyperexcitability of the neuronal population (Bianchi et al., 2009; Chuang et al., 2000; Chuang et al., 2001). The group I mGluR-mediated epileptogenic process is triggered by application of the selective agonist 3,5-dihydroxyphenylglycine (DHPG), but not by synaptically released glutamate. Recent work suggests that group I mGluR-mediated epileptogenesis is normally prevented by BC1 RNA-mediated inhibitory control of translation (Fig. 1A) as in preparations from mice lacking BC1 RNA (BC1-/-), synaptic stimulation of group I mGluRs does induce MEK-ERK- and protein synthesis-dependent epileptogenesis (Fig. 1B) (Zhong et al., 2009). BC1-/- animals also show heightened susceptibility to epilepsy following auditory stimulation (audiogenic seizures). These audiogenic seizures are also suppressed by blockers of the group I mGluR–MEK-ERK–protein synthesis pathway.
Fig. 1. BC1 RNA modulates group I mGluR-dependent excitability in the nervous system.
(A)In wild type (WT) animals, BC1 RNA maintains an appropriate neuronal excitability tone at the synapse by inhibiting (red line) protein synthesis stimulated by the group I mGluR– MEK/ERK pathway (green arrows).
(B)Absence of BC1 RNA in BC1 -/- animals shifts the balance towards group I mGluR-stimulated protein synthesis and precipitates cellular hyperexcitability and epileptogenesis.
mGluR, metabotropic glutamate receptor; MEK/ERK, mitogen-activated protein kinase kinase/extracellular signal-regulated kinase; PABP, poly(A) binding protein; eIF, eukaryotic initiation factor.
Thus, the functional data show that BC1 RNA is a translational regulator of group I mGluR-mediated protein synthesis-dependent excitatory responses in the brain.
3. Concluding Remarks
Increasing evidence that diverse types of npcRNAs contribute to the regulation of gene expression in the nervous system suggests that these RNAs modulate neuronal functions. Abnormal expression or impaired binding properties of regulatory RNAs are associated with impaired brain function, suggesting a role of altered regulatory RNA functionality in brain disorders such as Alzheimer's disease, ataxias, and epilepsy. There is therefore reason to hope that future work in this area may lead to new diagnostic tools and therapeutic approaches.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham M, Dror O, Nussinov R, Wolfson HJ. Analysis and classification of RNA tertiary structures. RNA. 2008;14:2274–89. doi: 10.1261/rna.853208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acampora D, Merlo GR, Paleari L, Zerega B, Postiglione MP, Mantero S, Bober E, Barbieri O, Simeone A, Levi G. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development. 1999;126:3795–809. doi: 10.1242/dev.126.17.3795. [DOI] [PubMed] [Google Scholar]
- Amaral PP, Mattick JS. Noncoding RNA in development. Mamm Genome. 2008;19:454–92. doi: 10.1007/s00335-008-9136-7. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997;19:27–37. doi: 10.1016/s0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- Artero R, Prokop A, Paricio N, Begemann G, Pueyo I, Mlodzik M, Perez-Alonso M, Baylies MK. The muscleblind gene participates in the organization of Z-bands and epidermal attachments of Drosophila muscles and is regulated by Dmef2. Dev Biol. 1998;195:131–43. doi: 10.1006/dbio.1997.8833. [DOI] [PubMed] [Google Scholar]
- Barlow DP, Bartolomei MS. Genomic imprinting in mammals. Vol. CSH Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten FE, Gibb HP. Two Cases of Myotonia Atrophica, showing a peculiar Distribution of Muscular Atrophy. Proc R Soc Med. 1909;2:32–33. doi: 10.1177/003591570900200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann G, Paricio N, Artero R, Kiss I, Perez-Alonso M, Mlodzik M. muscleblind, a gene required for photoreceptor differentiation in Drosophila, encodes novel nuclear Cys3His-type zinc-finger-containing proteins. Development. 1997;124:4321–31. doi: 10.1242/dev.124.21.4321. [DOI] [PubMed] [Google Scholar]
- Beniaminov A, Westhof E, Krol A. Distinctive structures between chimpanzee and human in a brain noncoding RNA. RNA. 2008;14:1270–5. doi: 10.1261/rna.1054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Allis CD. RNA meets chromatin. Genes Dev. 2005;19:1635–55. doi: 10.1101/gad.1324305. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Chuang SC, Zhao W, Young SR, Wong RK. Cellular plasticity for group I mGluR-mediated epileptogenesis. J Neurosci. 2009;29:3497–507. doi: 10.1523/JNEUROSCI.5447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilen J, Liu N, Bonini NM. A new role for microRNA pathways: modulation of degeneration induced by pathogenic human disease proteins. Cell Cycle. 2006a;5:2835–8. doi: 10.4161/cc.5.24.3579. [DOI] [PubMed] [Google Scholar]
- Bilen J, Liu N, Burnett BG, Pittman RN, Bonini NM. MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Mol Cell. 2006b;24:157–63. doi: 10.1016/j.molcel.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Veltman MW, Weisblatt E, Holmes JR, Thomas NS, Youings SA, Thompson RJ, Roberts SE, Dennis NR, Browne CE, Goodson S, Moore V, Brown J. Chromosome 15q11-13 abnormalities and other medical conditions in individuals with autism spectrum disorders. Psychiatr Genet. 2004;14:131–7. doi: 10.1097/00041444-200409000-00002. [DOI] [PubMed] [Google Scholar]
- Bond AM, Vangompel MJ, Sametsky EA, Clark MF, Savage JC, Disterhoft JF, Kohtz JD. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nat Neurosci. 2009;12:1020–7. doi: 10.1038/nn.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, Hunter K, Stanton VP, Thirion JP, Hudson T, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- Brosius J, Tiedge H. RNomenclature. RNA Biol. 2004;1:81–3. doi: 10.4161/rna.1.2.1228. [DOI] [PubMed] [Google Scholar]
- Brouwer JR, Willemsen R, Oostra BA. The FMR1 gene and fragile X-associated tremor/ataxia syndrome. Am J Med Genet B Neuropsychiatr Genet. 2008 doi: 10.1002/ajmg.b.30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Hendrich BD, Rupert JL, Lafreniere RG, Xing Y, Lawrence J, Willard HF. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992;71:527–42. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- Cao X, Yeo G, Muotri AR, Kuwabara T, Gage FH. Noncoding RNAs in the mammalian central nervous system. Annu Rev Neurosci. 2006;29:77–103. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- Carango P, Noble JE, Marks HG, Funanage VL. Absence of myotonic dystrophy protein kinase (DMPK) mRNA as a result of a triplet repeat expansion in myotonic dystrophy. Genomics. 1993;18:340–8. doi: 10.1006/geno.1993.1474. [DOI] [PubMed] [Google Scholar]
- Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, Kodzius R, Shimokawa K, Bajic VB, Brenner SE, Batalov S, Forrest AR, Zavolan M, Davis MJ, Wilming LG, Aidinis V, Allen JE, Ambesi-Impiombato A, Apweiler R, Aturaliya RN, Bailey TL, Bansal M, Baxter L, Beisel KW, Bersano T, Bono H, Chalk AM, Chiu KP, Choudhary V, Christoffels A, Clutterbuck DR, Crowe ML, Dalla E, Dalrymple BP, de Bono B, Della Gatta G, di Bernardo D, Down T, Engstrom P, Fagiolini M, Faulkner G, Fletcher CF, Fukushima T, Furuno M, Futaki S, Gariboldi M, Georgii-Hemming P, Gingeras TR, Gojobori T, Green RE, Gustincich S, Harbers M, Hayashi Y, Hensch TK, Hirokawa N, Hill D, Huminiecki L, Iacono M, Ikeo K, Iwama A, Ishikawa T, Jakt M, Kanapin A, Katoh M, Kawasawa Y, Kelso J, Kitamura H, Kitano H, Kollias G, Krishnan SP, Kruger A, Kummerfeld SK, Kurochkin IV, Lareau LF, Lazarevic D, Lipovich L, Liu J, Liuni S, McWilliam S, Madan Babu M, Madera M, Marchionni L, Matsuda H, Matsuzawa S, Miki H, Mignone F, Miyake S, Morris K, Mottagui-Tabar S, Mulder N, Nakano N, Nakauchi H, Ng P, Nilsson R, Nishiguchi S, Nishikawa S, Nori F, Ohara O, Okazaki Y, Orlando V, Pang KC, Pavan WJ, Pavesi G, Pesole G, Petrovsky N, Piazza S, Reed J, Reid JF, Ring BZ, Ringwald M, Rost B, Ruan Y, Salzberg SL, Sandelin A, Schneider C, Schonbach C, Sekiguchi K, Semple CA, Seno S, Sessa L, Sheng Y, Shibata Y, Shimada H, Shimada K, Silva D, Sinclair B, Sperling S, Stupka E, Sugiura K, Sultana R, Takenaka Y, Taki K, Tammoja K, Tan SL, Tang S, Taylor MS, Tegner J, Teichmann SA, Ueda HR, van Nimwegen E, Verardo R, Wei CL, Yagi K, Yamanishi H, Zabarovsky E, Zhu S, Zimmer A, Hide W, Bult C, Grimmond SM, Teasdale RD, Liu ET, Brusic V, Quackenbush J, Wahlestedt C, Mattick JS, Hume DA, Kai C, Sasaki D, Tomaru Y, Fukuda S, Kanamori-Katayama M, Suzuki M, Aoki J, Arakawa T, Iida J, Imamura K, Itoh M, Kato T, Kawaji H, Kawagashira N, Kawashima T, Kojima M, Kondo S, Konno H, Nakano K, Ninomiya N, Nishio T, Okada M, Plessy C, Shibata K, Shiraki T, Suzuki S, Tagami M, Waki K, Watahiki A, Okamura-Oho Y, Suzuki H, Kawai J, Hayashizaki Y. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–63. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- Cassidy SB, Dykens E, Williams CA. Prader-Willi and Angelman syndromes: sister imprinted disorders. Am J Med Genet. 2000;97:136–46. doi: 10.1002/1096-8628(200022)97:2<136::aid-ajmg5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Cavaille J, Buiting K, Kiefmann M, Lalande M, Brannan CI, Horsthemke B, Bachellerie JP, Brosius J, Huttenhofer A. Identification of brain-specific and imprinted small nucleolar RNA genes exhibiting an unusual genomic organization. Proc Natl AcadSci U S A. 2000;97:14311–6. doi: 10.1073/pnas.250426397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaille J, Seitz H, Paulsen M, Ferguson-Smith AC, Bachellerie JP. Identification of tandemly-repeated C/D snoRNA genes at the imprinted human 14q32 domain reminiscent of those at the Prader-Willi/Angelman syndrome region. Hum Mol Genet. 2002;11:1527–38. doi: 10.1093/hmg/11.13.1527. [DOI] [PubMed] [Google Scholar]
- Chang S, Wen S, Chen D, Jin P. Small regulatory RNAs in neurodevelopmental disorders. Hum Mol Genet. 2009;18:R18–26. doi: 10.1093/hmg/ddp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol. 2009;21:452–60. doi: 10.1016/j.ceb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Chen WL, Lin JW, Huang HJ, Wang SM, Su MT, Lee-Chen GJ, Chen CM, Hsieh-Li HM. SCA8 mRNA expression suggests an antisense regulation of KLHL1 and correlates to SCA8 pathology. Brain Res. 2008;1233:176–84. doi: 10.1016/j.brainres.2008.07.096. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S, Obrietan K. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–29. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Tapscott SJ. Myotonic dystrophy: emerging mechanisms for DM1 and DM2. Biochim Biophys Acta. 2007;1772:195–204. doi: 10.1016/j.bbadis.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Chuang SC, Bianchi R, Wong RK. Group I mGluR activation turns on a voltage-gated inward current in hippocampal pyramidal cells. J Neurophysiol. 2000;83:2844–53. doi: 10.1152/jn.2000.83.5.2844. [DOI] [PubMed] [Google Scholar]
- Chuang SC, Bianchi R, Kim D, Shin HS, Wong RK. Group I metabotropic glutamate receptors elicit epileptiform discharges in the hippocampus through PLCbeta1 signaling. J Neurosci. 2001;21:6387–94. doi: 10.1523/JNEUROSCI.21-16-06387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–68. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Consortium, I.M.G.S.o.A. Further characterization of the autism susceptibility locus AUTS1 on chromosome 7q. Hum Mol Genet. 2001;10:973–82. doi: 10.1093/hmg/10.9.973. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Lindgren V, Leventhal BL, Courchesne R, Lincoln A, Shulman C, Lord C, Courchesne E. Autism or atypical autism in maternally but not paternally derived proximal 15q duplication. Am J Hum Genet. 1997;60:928–34. [PMC free article] [PubMed] [Google Scholar]
- Costa FF. Non-coding RNAs: new players in eukaryotic biology. Gene. 2005;357:83–94. doi: 10.1016/j.gene.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Costa FF. Non-coding RNAs: lost in translation? Gene. 2007;386:1–10. doi: 10.1016/j.gene.2006.09.028. [DOI] [PubMed] [Google Scholar]
- da Rocha ST, Edwards CA, Ito M, Ogata T, Ferguson-Smith AC. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet. 2008;24:306–16. doi: 10.1016/j.tig.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Daughters RS, Tuttle DL, Gao W, Ikeda Y, Moseley ML, Ebner TJ, Swanson MS, Ranum LP. RNA gain-of-function in spinocerebellar ataxia type 8. PLoS Genet. 2009;5:e1000600. doi: 10.1371/journal.pgen.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smith AJ, Purmann C, Walters RG, Ellis RJ, Holder SE, Van Haelst MM, Brady AF, Fairbrother UL, Dattani M, Keogh JM, Henning E, Yeo GS, O'Rahilly S, Froguel P, Farooqi IS, Blakemore AI. A deletion of the HBII-85 class of small nucleolar RNAs (snoRNAs) is associated with hyperphagia, obesity and hypogonadism. Hum Mol Genet. 2009;18:3257–65. doi: 10.1093/hmg/ddp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara TM, Brosius J. Neural BC1 RNA: cDNA clones reveal nonrepetitive sequence content. Proc Natl Acad Sci U S A. 1987;84:2624–8. doi: 10.1073/pnas.84.9.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descheemaeker MJ, Govers V, Vermeulen P, Fryns JP. Pervasive developmental disorders in Prader-Willi syndrome: the Leuven experience in 59 subjects and controls. Am J Med Genet A. 2006;140:1136–42. doi: 10.1002/ajmg.a.31235. [DOI] [PubMed] [Google Scholar]
- Dick KA, Margolis JM, Day JW, Ranum LP. Dominant non-coding repeat expansions in human disease. Genome Dyn. 2006;1:67–83. doi: 10.1159/000092501. [DOI] [PubMed] [Google Scholar]
- Ding F, Li HH, Zhang S, Solomon NM, Camper SA, Cohen P, Francke U. SnoRNA Snord116 (Pwcr1/MBII-85) deletion causes growth deficiency and hyperphagia in mice. PLoS One. 2008a;3:e1709. doi: 10.1371/journal.pone.0001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, Sharma S, Chalasani P, Demidov VV, Broude NE, Dokholyan NV. Ab initio RNA folding by discrete molecular dynamics: from structure prediction to folding mechanisms. RNA. 2008b;14:1164–73. doi: 10.1261/rna.894608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CM, Relkovic D, Garfield AS, Dalley JW, Theobald DE, Humby T, Wilkinson LS, Isles AR. Loss of the imprinted snoRNA mbii-52 leads to increased 5htr2c pre-RNA editing and altered 5HT2CR-mediated behaviour. Hum Mol Genet. 2009;18:2140–8. doi: 10.1093/hmg/ddp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacker SM, Dawson TM, Dawson VL. Understanding microRNAs in neurodegeneration. Nat Rev Neurosci. 2009;10:837–41. doi: 10.1038/nrn2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy SR. Non-coding RNA genes and the modern RNA world. Nat Rev Genet. 2001;2:919–29. doi: 10.1038/35103511. [DOI] [PubMed] [Google Scholar]
- Erdmann VA, Szymanski M, Hochberg A, Groot N, Barciszewski J. Non-coding, mRNA-like RNAs database Y2K. Nucleic Acids Res. 2000;28:197–200. doi: 10.1093/nar/28.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann VA, Barciszewska MZ, Hochberg A, de Groot N, Barciszewski J. Regulatory RNAs. Cell Mol Life Sci. 2001;58:960–77. doi: 10.1007/PL00000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, Finch CE, St Laurent G, 3rd, Kenny PJ, Wahlestedt C. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14:723–30. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardaei M, Larkin K, Brook JD, Hamshere MG. In vivo co-localisation of MBNL protein with DMPK expanded-repeat transcripts. Nucleic Acids Res. 2001;29:2766–71. doi: 10.1093/nar/29.13.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Bi C, Clark BS, Mady R, Shah P, Kohtz JD. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20:1470–84. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg SK, Kosik KS, Davidson BL. MicroRNAs potentiate neural development. Neuron. 2009;64:303–9. doi: 10.1016/j.neuron.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Frellsen J, Moltke I, Thiim M, Mardia KV, Ferkinghoff-Borg J, Hamelryck T. A probabilistic model of RNA conformational space. PLoS Comput Biol. 2009;5:e1000406. doi: 10.1371/journal.pcbi.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French PJ, Bliss TV, O'Connor V. Ntab, a novel non-coding RNA abundantly expressed in rat brain. Neuroscience. 2001;108:207–15. doi: 10.1016/s0306-4522(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Fridman C, Koiffmann CP. Origin of uniparental disomy 15 in patients with Prader-Willi or Angelman syndrome. Am J Med Genet. 2000;94:249–53. doi: 10.1002/1096-8628(20000918)94:3<249::aid-ajmg12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Fu YH, Pizzuti A, Fenwick RG, Jr, King J, Rajnarayan S, Dunne PW, Dubel J, Nasser GA, Ashizawa T, de Jong P, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255:1256–8. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- Fu YH, Friedman DL, Richards S, Pearlman JA, Gibbs RA, Pizzuti A, Ashizawa T, Perryman MB, Scarlato G, Fenwick RG, Jr, et al. Decreased expression of myotonin-protein kinase messenger RNA and protein in adult form of myotonic dystrophy. Science. 1993;260:235–8. doi: 10.1126/science.8469976. [DOI] [PubMed] [Google Scholar]
- Gardner PP, Daub J, Tate JG, Nawrocki EP, Kolbe DL, Lindgreen S, Wilkinson AC, Finn RD, Griffiths-Jones S, Eddy SR, Bateman A. Rfam: updates to the RNA families database. Nucleic Acids Res. 2009;37:D136–40. doi: 10.1093/nar/gkn766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg C. Chromosomal disorders and autism. J Autism Dev Disord. 1998;28:415–25. doi: 10.1023/a:1026004505764. [DOI] [PubMed] [Google Scholar]
- Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–71. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, Ploegh HL, Chowdhury S, Worley PF, Steen J, Greenberg ME. The Angelman Syndrome Protein Ube3A Regulates Synapse Development by Ubiquitinating Arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S. Annotating noncoding RNA genes. Annu Rev Genomics Hum Genet. 2007;8:279–98. doi: 10.1146/annurev.genom.8.080706.092419. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–8. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–7. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JP, O'Neill BL, Stewart CL, Kozlov SV, Croce CM. At least ten genes define the imprinted Dlk1-Dio3 cluster on mouse chromosome 12qF1. PLoS One. 2009;4:e4352. doi: 10.1371/journal.pone.0004352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. The fragile-X premutation: a maturing perspective. Am J Hum Genet. 2004;74:805–16. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–30. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- Hashem V, Galloway JN, Mori M, Willemsen R, Oostra BA, Paylor R, Nelson DL. Ectopic expression of CGG containing mRNA is neurotoxic in mammals. Hum Mol Genet. 2009;18:2443–51. doi: 10.1093/hmg/ddp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzing LB, Kim SJ, Cook EH, Jr, Ledbetter DH. The human aminophospholipid-transporting ATPase gene ATP10C maps adjacent to UBE3A and exhibits similar imprinted expression. Am J Hum Genet. 2001;68:1501–5. doi: 10.1086/320616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann-Radvanyi H, Lavedan C, Rabes JP, Savoy D, Duros C, Johnson K, Junien C. Myotonic dystrophy: absence of CTG enlarged transcript in congenital forms, and low expression of the normal allele. Hum Mol Genet. 1993;2:1263–6. doi: 10.1093/hmg/2.8.1263. [DOI] [PubMed] [Google Scholar]
- Hu JF, Oruganti H, Vu TH, Hoffman AR. Tissue-specific imprinting of the mouse insulin-like growth factor II receptor gene correlates with differential allele-specific DNA methylation. Mol Endocrinol. 1998;12:220–32. doi: 10.1210/mend.12.2.0062. [DOI] [PubMed] [Google Scholar]
- Ito H, Kawakami H, Wate R, Matsumoto S, Imai T, Hirano A, Kusaka H. Clinicopathologic investigation of a family with expanded SCA8 CTA/CTG repeats. Neurology. 2006;67:1479–81. doi: 10.1212/01.wnl.0000240256.13633.7b. [DOI] [PubMed] [Google Scholar]
- Iwahashi CK, Yasui DH, An HJ, Greco CM, Tassone F, Nannen K, Babineau B, Lebrilla CB, Hagerman RJ, Hagerman PJ. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006;129:256–71. doi: 10.1093/brain/awh650. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, Sweatt JD, Beaudet AL. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- Jin P, Duan R, Qurashi A, Qin Y, Tian D, Rosser TC, Liu H, Feng Y, Warren ST. Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile × tremor/ataxia syndrome. Neuron. 2007;55:556–64. doi: 10.1016/j.neuron.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami M, Nishimura G, Okuyama T, Hayashidani M, Takeuchi T, Tanaka S, Ishino F, Kurosawa K, Ogata T. Segmental and full paternal isodisomy for chromosome 14 in three patients: narrowing the critical region and implication for the clinical features. Am J Med Genet A. 2005;138A:127–32. doi: 10.1002/ajmg.a.30941. [DOI] [PubMed] [Google Scholar]
- Kagami M, Sekita Y, Nishimura G, Irie M, Kato F, Okada M, Yamamori S, Kishimoto H, Nakayama M, Tanaka Y, Matsuoka K, Takahashi T, Noguchi M, Masumoto K, Utsunomiya T, Kouzan H, Komatsu Y, Ohashi H, Kurosawa K, Kosaki K, Ferguson-Smith AC, Ishino F, Ogata T. Deletions and epimutations affecting the human 14q32.2 imprinted region in individuals with paternal and maternal upd(14)-like phenotypes. Nat Genet. 2008;40:237–42. doi: 10.1038/ng.2007.56. [DOI] [PubMed] [Google Scholar]
- Kaliman P, Llagostera E. Myotonic dystrophy protein kinase (DMPK) and its role in the pathogenesis of myotonic dystrophy 1. Cell Signal. 2008;20:1935–41. doi: 10.1016/j.cellsig.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Kanadia RN, Johnstone KA, Mankodi A, Lungu C, Thornton CA, Esson D, Timmers AM, Hauswirth WW, Swanson MS. A muscleblind knockout model for myotonic dystrophy. Science. 2003;302:1978–80. doi: 10.1126/science.1088583. [DOI] [PubMed] [Google Scholar]
- Kanber D, Giltay J, Wieczorek D, Zogel C, Hochstenbach R, Caliebe A, Kuechler A, Horsthemke B, Buiting K. A paternal deletion of MKRN3, MAGEL2 and NDN does not result in Prader-Willi syndrome. Eur J Hum Genet. 2009;17:582–90. doi: 10.1038/ejhg.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor B, Shemer R, Razin A. The Prader-Willi/Angelman imprinted domain and its control center. Cytogenet Genome Res. 2006;113:300–5. doi: 10.1159/000090845. [DOI] [PubMed] [Google Scholar]
- Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermuller J, Hofacker IL, Bell I, Cheung E, Drenkow J, Dumais E, Patel S, Helt G, Ganesh M, Ghosh S, Piccolboni A, Sementchenko V, Tammana H, Gingeras TR. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–8. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- Kavanaugh LA, Dietrich FS. Non-coding RNA prediction and verification in Saccharomyces cerevisiae. PLoS Genet. 2009;5:e1000321. doi: 10.1371/journal.pgen.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, Regev A, Lander ES, Rinn JL. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–72. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997;15:70–3. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- Kishore S, Stamm S. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science. 2006;311:230–2. doi: 10.1126/science.1118265. [DOI] [PubMed] [Google Scholar]
- Kloc A, Zaratiegui M, Nora E, Martienssen R. RNA interference guides histone modification during the S phase of chromosomal replication. Curr Biol. 2008;18:490–5. doi: 10.1016/j.cub.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocerha J, Faghihi MA, Lopez-Toledano MA, Huang J, Ramsey AJ, Caron MG, Sales N, Willoughby D, Elmen J, Hansen HF, Orum H, Kauppinen S, Kenny PJ, Wahlestedt C. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc Natl Acad Sci U S A. 2009a;106:3507–12. doi: 10.1073/pnas.0805854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocerha J, Kauppinen S, Wahlestedt C. microRNAs in CNS Disorders. Neuromolecular Med. 2009b doi: 10.1007/s12017-009-8066-1. [DOI] [PubMed] [Google Scholar]
- Koerner MV, Pauler FM, Huang R, Barlow DP. The function of non-coding RNAs in genomic imprinting. Development. 2009;136:1771–83. doi: 10.1242/dev.030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtz JD, Baker DP, Corte G, Fishell G. Regionalization within the mammalian telencephalon is mediated by changes in responsiveness to Sonic Hedgehog. Development. 1998;125:5079–89. doi: 10.1242/dev.125.24.5079. [DOI] [PubMed] [Google Scholar]
- Kohtz JD, Fishell G. Developmental regulation of EVF-1, a novel non-coding RNA transcribed upstream of the mouse Dlx6 gene. Gene Expr Patterns. 2004;4:407–12. doi: 10.1016/j.modgep.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Kondrashov AV, Kiefmann M, Ebnet K, Khanam T, Muddashetty RS, Brosius J. Inhibitory effect of naked neural BC1 RNA or BC200 RNA on eukaryotic in vitro translation systems is reversed by poly(A)-binding protein (PABP) J Mol Biol. 2005;353:88–103. doi: 10.1016/j.jmb.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Koob MD, Moseley ML, Schut LJ, Benzow KA, Bird TD, Day JW, Ranum LP. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8) Nat Genet. 1999;21:379–84. doi: 10.1038/7710. [DOI] [PubMed] [Google Scholar]
- Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–20. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9:1274–81. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky AM, Sonntag KC, Isacson O, Kosik KS. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006;24:857–64. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers M, Calciano MA, Colosi D, Glatt-Deeley H, Wagstaff J, Lalande M. Maternal disruption of Ube3a leads to increased expression of Ube3a-ATS in trans. Nucleic Acids Res. 2005;33:3976–84. doi: 10.1093/nar/gki705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latos PA, Barlow DP. Regulation of imprinted expression by macro non-coding RNAs. RNA Biol. 2009;6 doi: 10.4161/rna.6.2.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur E, Watrin F, Landers M, Sturny R, Lalande M, Muscatelli F. Dynamic developmental regulation of the large non-coding RNA associated with the mouse 7Cimprinted chromosomal region. Dev Biol. 2005;286:587–600. doi: 10.1016/j.ydbio.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–4. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- Leehey MA, Berry-Kravis E, Goetz CG, Zhang L, Hall DA, Li L, Rice CD, Lara R, Cogswell J, Reynolds A, Gane L, Jacquemont S, Tassone F, Grigsby J, Hagerman RJ, Hagerman PJ. FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology. 2008;70:1397–402. doi: 10.1212/01.wnl.0000281692.98200.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lin D, Pestova TV, Hellen CU, Tiedge H. Translational control by a small RNA: dendritic BC1 RNA targets the eukaryotic initiation factor 4A helicase mechanism. Molecular and Cellular Biology. 2008;28:3008–19. doi: 10.1128/MCB.01800-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liquori CL, Ricker K, Moseley ML, Jacobsen JF, Kress W, Naylor SL, Day JW, Ranum LP. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293:864–7. doi: 10.1126/science.1062125. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer's disease hippocampus. Neuroreport. 2007;18:297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]
- Lyle R, Watanabe D, te Vruchte D, Lerchner W, Smrzka OW, Wutz A, Schageman J, Hahner L, Davies C, Barlow DP. The imprinted antisense RNA at the Igf2r locus overlaps but does not imprint Mas1. Nat Genet. 2000;25:19–21. doi: 10.1038/75546. [DOI] [PubMed] [Google Scholar]
- Mathews DH, Turner DH. Prediction of RNA secondary structure by free energy minimization. Curr Opin Struct Biol. 2006;16:270–8. doi: 10.1016/j.sbi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Sutcliffe JS, Fang P, Galjaard RJ, Jiang YH, Benton CS, Rommens JM, Beaudet AL. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat Genet. 1997;15:74–7. doi: 10.1038/ng0197-74. [DOI] [PubMed] [Google Scholar]
- Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15 Spec No 1:R17–29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- Mattick JS. The genetic signatures of noncoding RNAs. PLoS Genet. 2009;5:e1000459. doi: 10.1371/journal.pgen.1000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro M, Kashiwagi A, Mitsuya K, Nakao M, Kondo I, Saitoh S, Oshimura M. A novel maternally expressed gene, ATP10C, encodes a putative aminophospholipid translocase associated with Angelman syndrome. Nat Genet. 2001;28:19–20. doi: 10.1038/ng0501-19. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Mattick JS. Non-coding RNAs in the nervous system. J Physiol. 2006;575:333–41. doi: 10.1113/jphysiol.2006.113191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF, Mattick JS. Noncoding RNAs and RNA editing in brain development, functional diversification, and neurological disease. Physiol Rev. 2007;87:799–823. doi: 10.1152/physrev.00036.2006. [DOI] [PubMed] [Google Scholar]
- Mercer TR, Dinger ME, Mariani J, Kosik KS, Mehler MF, Mattick JS. Noncoding RNAs in Long-Term Memory Formation. Neuroscientist. 2008a;14:434–45. doi: 10.1177/1073858408319187. [DOI] [PubMed] [Google Scholar]
- Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 2008b;105:716–21. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin LR, Wong RK. Role of group I metabotropic glutamate receptors in the patterning of epileptiform activities in vitro. J Neurophysiol. 1997;78:539–44. doi: 10.1152/jn.1997.78.1.539. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Bergold PJ, Wong RK. Requirement of protein synthesis for group I mGluR-mediated induction of epileptiform discharges. J Neurophysiol. 1998;80:989–93. doi: 10.1152/jn.1998.80.2.989. [DOI] [PubMed] [Google Scholar]
- Miller JW, Urbinati CR, Teng-Umnuay P, Stenberg MG, Byrne BJ, Thornton CA, Swanson MS. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J. 2000;19:4439–48. doi: 10.1093/emboj/19.17.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner RJ, Bloom FE, Lai C, Lerner RA, Sutcliffe JG. Brain-specific genes have identifier sequences in their introns. Proc Natl Acad Sci U S A. 1984;81:713–7. doi: 10.1073/pnas.81.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslimov IA, Santi E, Homel P, Perini S, Higgins D, Tiedge H. RNA transport in dendrites: a cis-acting targeting element is contained within neuronal BC1 RNA. J Neurosci. 1997;17:4722–33. doi: 10.1523/JNEUROSCI.17-12-04722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsuddi M, Marshall CM, Benzow KA, Koob MD, Rebay I. The spinocerebellar ataxia 8 noncoding RNA causes neurodegeneration and associates with staufen in Drosophila. Curr Biol. 2004;14:302–8. doi: 10.1016/j.cub.2004.01.034. [DOI] [PubMed] [Google Scholar]
- Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, Tomonaga S, Watanabe Y, Chung YJ, Banerjee R, Iwamoto K, Kato T, Okazawa M, Yamauchi K, Tanda K, Takao K, Miyakawa T, Bradley A, Takumi T. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–46. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napierala M, Krzyzosiak WJ. CUG repeats present in myotonin kinase RNA formmetastable “slippery” hairpins. J Biol Chem. 1997;272:31079–85. doi: 10.1074/jbc.272.49.31079. [DOI] [PubMed] [Google Scholar]
- Nemes JP, Benzow KA, Moseley ML, Ranum LP, Koob MD. The SCA8 transcript is an antisense RNA to a brain-specific transcript encoding a novel actin-binding protein (KLHL1) Hum Mol Genet. 2000;9:1543–51. doi: 10.1093/hmg/9.10.1543. [DOI] [PubMed] [Google Scholar]
- O'Neill MJ. The influence of non-coding RNAs on allele-specific gene expression in mammals. Hum Mol Genet. 2005;14 Spec No 1:R113–20. doi: 10.1093/hmg/ddi108. [DOI] [PubMed] [Google Scholar]
- Onoa B, Tinoco I., Jr RNA folding and unfolding. Curr Opin Struct Biol. 2004;14:374–9. doi: 10.1016/j.sbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Pauler FM, Koerner MV, Barlow DP. Silencing by imprinted noncoding RNAs: is transcription the answer? Trends Genet. 2007;23:284–92. doi: 10.1016/j.tig.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DO, Jeffries C, Sullivan P. Expanding the ‘central dogma’: the regulatory role of nonprotein coding genes and implications for the genetic liability to schizophrenia. Mol Psychiatry. 2005;10:69–78. doi: 10.1038/sj.mp.4001577. [DOI] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, Parker JS, Jin J, Hammond SM. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8:R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KS, Salama SR, Lambert N, Lambot MA, Coppens S, Pedersen JS, Katzman S, King B, Onodera C, Siepel A, Kern AD, Dehay C, Igel H, Ares M, Jr, Vanderhaeghen P, Haussler D. An RNA gene expressed during cortical development evolved rapidly in humans. Nature. 2006;443:167–72. doi: 10.1038/nature05113. [DOI] [PubMed] [Google Scholar]
- Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–41. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Regulation of non-coding RNA networks in the nervous system-What's the REST of the story? Neurosci Lett. 2009 doi: 10.1016/j.neulet.2009.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranum LP, Cooper TA. RNA-mediated neuromuscular disorders. Annu Rev Neurosci. 2006;29:259–77. doi: 10.1146/annurev.neuro.29.051605.113014. [DOI] [PubMed] [Google Scholar]
- Royo H, Cavaille J. Non-coding RNAs in imprinted gene clusters. Biol Cell. 2008;100:149–66. doi: 10.1042/BC20070126. [DOI] [PubMed] [Google Scholar]
- Runte M, Huttenhofer A, Gross S, Kiefmann M, Horsthemke B, Buiting K. The IC-SNURF-SNRPN transcript serves as a host for multiple small nucleolar RNA species and as an antisense RNA for UBE3A. Hum Mol Genet. 2001;10:2687–700. doi: 10.1093/hmg/10.23.2687. [DOI] [PubMed] [Google Scholar]
- Sahoo T, del Gaudio D, German JR, Shinawi M, Peters SU, Person RE, Garnica A, Cheung SW, Beaudet AL. Prader-Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nat Genet. 2008;40:719–21. doi: 10.1038/ng.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterlee JS, Barbee S, Jin P, Krichevsky A, Salama S, Schratt G, Wu DY. Noncoding RNAs in the brain. J Neurosci. 2007;27:11856–9. doi: 10.1523/JNEUROSCI.3624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt G. microRNAs at the synapse. Nat Rev Neurosci. 2009;10:842–9. doi: 10.1038/nrn2763. [DOI] [PubMed] [Google Scholar]
- Shamovsky I, Nudler E. Gene control by large noncoding RNAs. Sci STKE. 2006;2006:pe40. doi: 10.1126/stke.3552006pe40. [DOI] [PubMed] [Google Scholar]
- Sharma S, Ding F, Dokholyan NV. iFoldRNA: three-dimensional RNA structure prediction and folding. Bioinformatics. 2008;24:1951–2. doi: 10.1093/bioinformatics/btn328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skryabin BV, Gubar LV, Seeger B, Pfeiffer J, Handel S, Robeck T, Karpova E, Rozhdestvensky TS, Brosius J. Deletion of the MBII-85 snoRNA gene cluster in mice results in postnatal growth retardation. PLoS Genet. 2007;3:e235. doi: 10.1371/journal.pgen.0030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleutels F, Barlow DP. Investigation of elements sufficient to imprint the mouse Air promoter. Mol Cell Biol. 2001;21:5008–17. doi: 10.1128/MCB.21.15.5008-5017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong BW, Paulson HL. Spinocerebellar ataxias: an update. Curr Opin Neurol. 2007;20:438–46. doi: 10.1097/WCO.0b013e3281fbd3dd. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop P, Haass C. Regulatory RNA goes awry in Alzheimer's disease. Nat Med. 2008;14:711–2. doi: 10.1038/nm0708-711. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, Milner RJ, Bloom FE, Lerner RA. Common 82-nucleotide sequence unique to brain RNA. Proc Natl Acad Sci U S A. 1982;79:4942–6. doi: 10.1073/pnas.79.16.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, Milner RJ, Gottesfeld JM, Lerner RA. Identifier sequences are transcribed specifically in brain. Nature. 1984;308:237–41. doi: 10.1038/308237a0. [DOI] [PubMed] [Google Scholar]
- Szell M, Bata-Csorgo Z, Kemeny L. The enigmatic world of mRNA-like ncRNAs: their role in human evolution and in human diseases. Semin Cancer Biol. 2008;18:141–8. doi: 10.1016/j.semcancer.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Okamoto A, Kobayashi R, Shirai M, Obata Y, Ogawa H, Sotomaru Y, Kono T. Deletion of Gtl2, imprinted non-coding RNA, with its differentially methylated region induces lethal parent-origin-dependent defects in mice. Hum Mol Genet. 2009;18:1879–88. doi: 10.1093/hmg/ddp108. [DOI] [PubMed] [Google Scholar]
- Taniura H, Sng JC, Yoneda Y. Histone modifications in the brain. Neurochem Int. 2007;51:85–91. doi: 10.1016/j.neuint.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Tiedge H, Fremeau RT, Jr, Weinstock PH, Arancio O, Brosius J. Dendritic location of neural BC1 RNA. Proc Natl Acad Sci U S A. 1991;88:2093–7. doi: 10.1073/pnas.88.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedge H, Chen W, Brosius J. Primary structure, neural-specific expression, and dendritic location of human BC200 RNA. Journal of Neuroscience. 1993;13:2382–2390. doi: 10.1523/JNEUROSCI.13-06-02382.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko LT, Miller JW, Timchenko NA, DeVore DR, Datar KV, Lin L, Roberts R, Caskey CT, Swanson MS. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 1996;24:4407–14. doi: 10.1093/nar/24.22.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DH, Satou K, Ho TB. Finding microRNA regulatory modules in human genome using rule induction. BMC Bioinformatics. 2008;9(12):S5. doi: 10.1186/1471-2105-9-S12-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–24. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- Veltman MW, Thompson RJ, Roberts SE, Thomas NS, Whittington J, Bolton PF. Prader-Willi syndrome--a study comparing deletion and uniparental disomy cases with reference to autism spectrum disorders. Eur Child Adolesc Psychiatry. 2004;13:42–50. doi: 10.1007/s00787-004-0354-6. [DOI] [PubMed] [Google Scholar]
- Verona RI, Mann MR, Bartolomei MS. Genomic imprinting: intricacies of epigenetic regulation in clusters. Annu Rev Cell Dev Biol. 2003;19:237–59. doi: 10.1146/annurev.cellbio.19.111401.092717. [DOI] [PubMed] [Google Scholar]
- Vitali P, Basyuk E, Le Meur E, Bertrand E, Muscatelli F, Cavaille J, Huttenhofer A. ADAR2-mediated editing of RNA substrates in the nucleolus is inhibited by C/D small nucleolar RNAs. J Cell Biol. 2005;169:745–53. doi: 10.1083/jcb.200411129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss B, Georg J, Schon V, Ude S, Hess WR. Biocomputational prediction of non-coding RNAs in model cyanobacteria. BMC Genomics. 2009;10:123. doi: 10.1186/1471-2164-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Iacoangeli A, Popp S, Muslimov IA, Imataka H, Sonenberg N, Lomakin IB, Tiedge H. Dendritic BC1 RNA: functional role in regulation of translation initiation. Journal of Neuroscience. 2002;22:10232–10241. doi: 10.1523/JNEUROSCI.22-23-10232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Iacoangeli A, Lin D, Williams K, Denman RB, Hellen CUT, Tiedge H. Dendritic BC1 RNA in translational control mechanisms. Journal of Cell Biology. 2005;171:811–21. doi: 10.1083/jcb.200506006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassink TH, Piven J. The molecular genetics of autism. Curr Psychiatry Rep. 2000;2:170–5. doi: 10.1007/s11920-000-0063-x. [DOI] [PubMed] [Google Scholar]
- Weinberg MS, Wood MJ. Short non-coding RNA biology and neurodegenerative disorders: novel disease targets and therapeutics. Hum Mol Genet. 2009;18:R27–39. doi: 10.1093/hmg/ddp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler TM, Krym MC, Thornton CA. Ribonuclear foci at the neuromuscular junction in myotonic dystrophy type 1. Neuromuscul Disord. 2007;17:242–7. doi: 10.1016/j.nmd.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RK, Bianchi R, Taylor GW, Merlin LR. Role of metabotropic glutamate receptors in epilepsy. Adv Neurol. 1999;79:685–98. [PubMed] [Google Scholar]
- Wutz A, Gribnau J. X inactivation Xplained. Curr Opin Genet Dev. 2007;17:387–93. doi: 10.1016/j.gde.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Xu X, Ji Y, Stormo GD. RNA Sampler: a new sampling based algorithm for common RNA secondary structure prediction and structural alignment. Bioinformatics. 2007;23:1883–91. doi: 10.1093/bioinformatics/btm272. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Kayashima T, Soejima H, Kinoshita A, Yoshiura K, Matsumoto N, Ohta T, Urano T, Masuzaki H, Ishimaru T, Mukai T, Niikawa N, Kishino T. Neuron-specific relaxation of Igf2r imprinting is associated with neuron-specific histone modifications and lack of its antisense transcript Air. Hum Mol Genet. 2005;14:2511–20. doi: 10.1093/hmg/ddi255. [DOI] [PubMed] [Google Scholar]
- Yoon S, De Micheli G. Prediction of regulatory modules comprising microRNAs and target genes. Bioinformatics. 2005a;21(2):ii93–100. doi: 10.1093/bioinformatics/bti1116. [DOI] [PubMed] [Google Scholar]
- Yoon S, De Micheli G. Prediction and Analysis of Human microRNA Regulatory Modules. Conf Proc IEEE Eng Med Biol Soc. 2005b;5:4799–802. doi: 10.1109/IEMBS.2005.1615545. [DOI] [PubMed] [Google Scholar]
- Zhao W, Bianchi R, Wang M, Wong RK. Extracellular signal-regulated kinase 1/2 is required for the induction of group I metabotropic glutamate receptor-mediated epileptiform discharges. J Neurosci. 2004;24:76–84. doi: 10.1523/JNEUROSCI.4515-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Chuang SC, Bianchi R, Zhao W, Lee H, Fenton AA, Wong RK, Tiedge H. BC1 regulation of metabotropic glutamate receptor-mediated neuronal excitability. J Neurosci. 2009;29:9977–86. doi: 10.1523/JNEUROSCI.3893-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]