Abstract
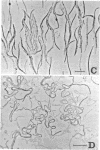
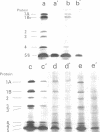
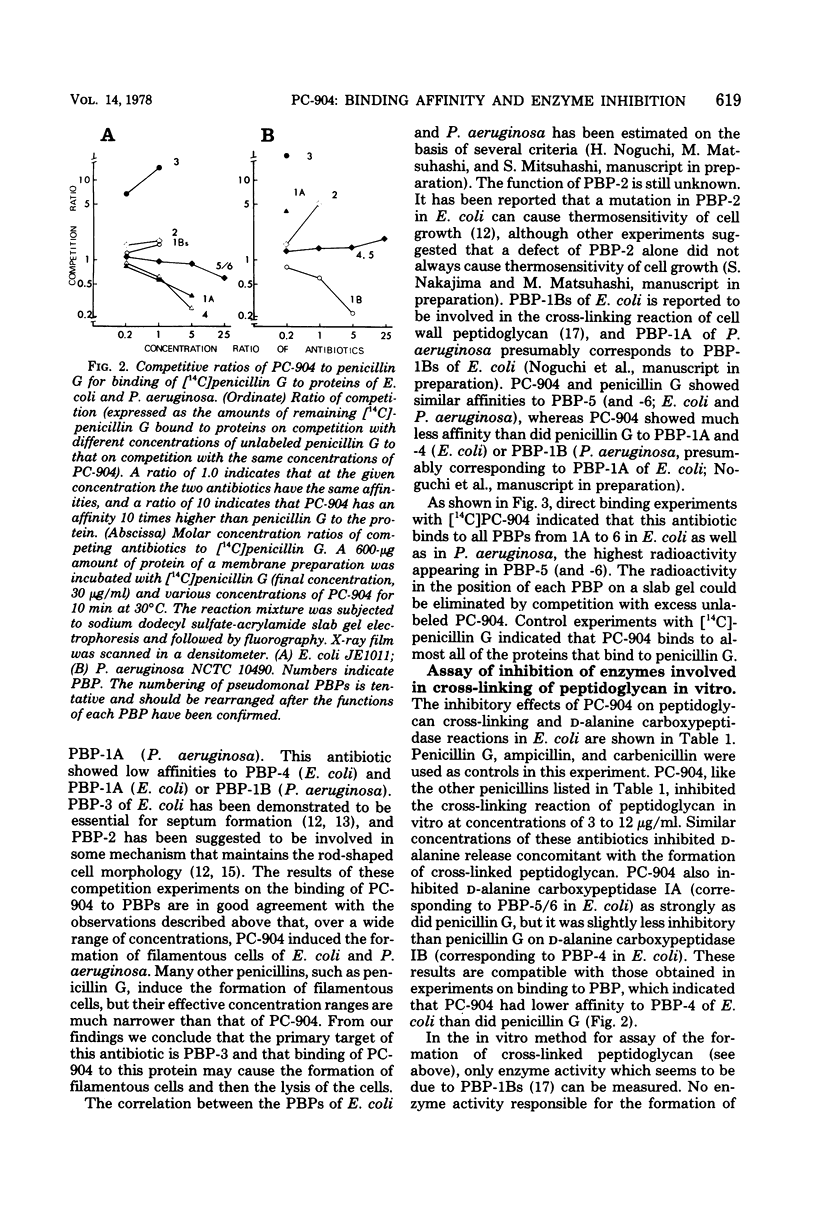
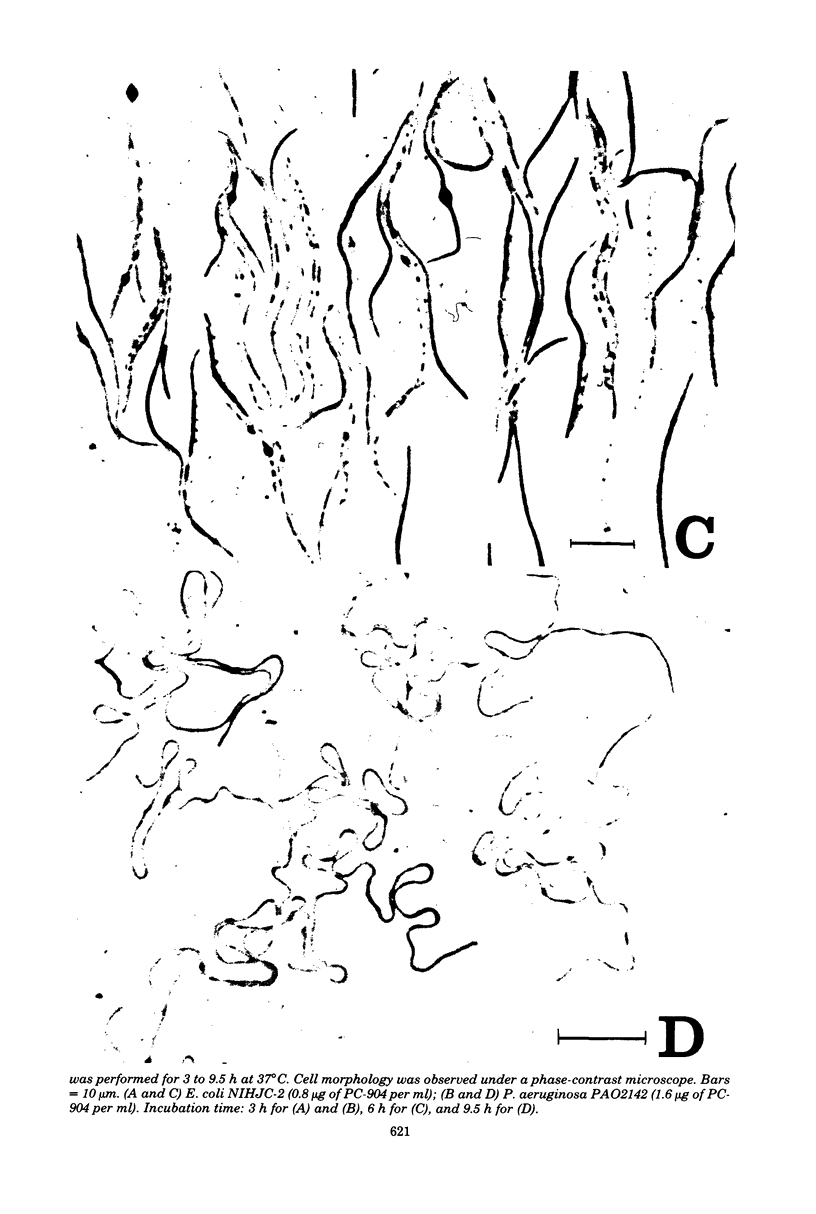
The mechanism of action of a new antipseudomonal penicillin, PC-904, was studied with respect to its binding affinities to penicillin-binding proteins (PBPs) and its inhibitory activities on cross-linking enzymes of peptidoglycan synthesis in vitro. PC-904 showed especially high affinity (compared with that of penicillin G) to Escherichia coli PBP-3. It also had high affinities to PBP-2 and -1Bs and low affinities to PBP-1A, -4, -5, and -6. Similar results were obtained with Pseudomonas aeruginosa, in which this antibiotic showed very high affinity (compared with that of penicillin G) to PBP-3, -1A (presumably corresponding to E. coli PBP-1Bs), and -2; there was especially high affinity to PBP-3 and much less affinity to PBP-1B (presumably corresponding to E. coli PBP-1A). These results are compatible with morphological observations that at concentrations near its minimal inhibitory concentration or less, this antibiotic induced the formation of filamentous cells of E. coli and P. aeruginosa. At higher concentrations or after prolonged incubation, it induced lysis of the cells. The remarkably high affinity of PC-904 to pseudomonal PBP-3, -1A, and -2 may partly explain the potent antipseudomonal activity of this antibiotic. In E. coli, the concentration of PC-904 required to inhibit the cross-linking reaction in enzymatic peptidoglycan synthesis, presumably carried out by PBP-1Bs, was as low as the inhibitory concentrations of penicillin G, ampicillin, and carbenicillin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altemeier W. A., Hummel R. P., Hill E. O., Lewis S. Changing patterns in surgical infections. Ann Surg. 1973 Oct;178(4):436–445. doi: 10.1097/00000658-197310000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finland M. Changing ecology of bacterial infections as related to antibacterial therapy. J Infect Dis. 1970 Nov;122(5):419–431. doi: 10.1093/infdis/122.5.419. [DOI] [PubMed] [Google Scholar]
- Iwaya M., Strominger J. L. Simultaneous deletion of D-alanine carboxypeptidase IB-C and penicillin-binding component IV in a mutant of Escherichia coli K12. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2980–2984. doi: 10.1073/pnas.74.7.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Glycopeptide transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):656–663. doi: 10.1073/pnas.55.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Maruyama I. N., Takagaki Y., Tamaki S., Nishimura Y., Hirota Y. Isolation of a mutant of Escherichia coli lacking penicillin-sensitive D-alanine carboxypeptidase IA. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2631–2635. doi: 10.1073/pnas.75.6.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Takagaki Y., Maruyama I. N., Tamaki S., Nishimura Y., Suzuki H., Ogino U., Hirota Y. Mutants of Escherichia coli lacking in highly penicillin-sensitive D-alanine carboxypeptidase activity. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2976–2979. doi: 10.1073/pnas.74.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T., Matsuzawa H., Matsuhashi M., Sugino Y. Cell wall peptidoglycan mutants of Escherichia coli K-12: existence of two clusters of genes, mra and mrb, for cell wall peptidoglycan biosynthesis. J Bacteriol. 1972 Nov;112(2):950–958. doi: 10.1128/jb.112.2.950-958.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Eda Y., Tobiki H., Nakagome T., Komatsu T. PC-904, a novel broad-spectrum semisynthetic penicillin with marked antipseudomonal activity: microbiological evaluation. Antimicrob Agents Chemother. 1976 Feb;9(2):262–273. doi: 10.1128/aac.9.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Kubo M., Kurashige S., Mitsuhashi S. Antibacterial activity of apalcillin (PC-904) against gram-negative bacilli, especially ampicillin-, carbenicillin-, and gentamicin-resistant clinical isolates. Antimicrob Agents Chemother. 1978 May;13(5):745–752. doi: 10.1128/aac.13.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Jobanputra V. Mutants of Escherichia coli which lack a component of penicillin-binding protein 1 are viable. FEBS Lett. 1977 Jul 15;79(2):374–378. doi: 10.1016/0014-5793(77)80824-7. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Strominger J. L. Identification of the major penicillin-binding proteins of Escherichia coli as D-alanine carboxypeptidase IA. J Bacteriol. 1976 Jul;127(1):660–663. doi: 10.1128/jb.127.1.660-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]