Abstract
Much is known about the endocrine hormonal mechanisms controlling ovarian development. More recently, attention has been focused on identifying regulatory pathways that, operating within the ovarian microenvironment, contribute to the acquisition of ovarian reproductive competence. Within this framework, the concept has been developed that neurotrophins (NTs) and their Trk tyrosine kinase receptors, long thought to be exclusively required for the development of the nervous system, are also involved in the control of ovarian maturation. The ovary of several species, including rodents, sheep, cows, nonhuman primates and humans, produce NTs and express both the high-affinity receptors and the common p75NTR receptor required for signaling. Studies in humans and rodents have shown that this expression is initiated during fetal life, before the formation of primordial follicles. Gene targeting approaches have identified trkB, the high-affinity receptor for neurotrophin-4/5 (NT-4/5) and brain-derived neurotrophic factor (BDNF), as a signaling module required for follicular assembly, early follicular growth and oocyte survival. A similar approach has shown that nerve growth factor (NGF) contributes independently to the growth of primordial follicles into gonadotropin-responsive structures. Altogether, these observations indicate that NTs are important contributors to the gonadotropin-independent process underlying the formation and initiation of ovarian follicular growth.
Keywords: neurotrophins, ovarian folliculogenesis, oocyte survival, early follicle growth
Introduction
Acquisition of female reproductive capacity requires the extrusion of a mature oocyte from the ovary at ovulation. Oocytes grow surrounded by somatic cells of both epithelial (granulosa) and mesenchymal (theca) origin that together form a functional unit known as the follicle.1 Assembly of germ and somatic cells into these follicular structures, and the growth of newly formed follicles, are complex processes that require a precise coordination between germ cells and somatic cells. Not surprisingly, both developmental events are governed by different, though functionally connected, genes encoding intraovarian factors (reviewed in1–4). In addition, ovarian follicles are assembled and begin to grow independent of pituitary gonadotropin hormone support.5–7 In rodents, follicular organization is initiated and completed within the first week of postnatal life.8,9 Studies in the rat have revealed that this structural organization has an explosive time course: whereas no follicles are detected at birth, about 500 develop 12 h later, and this number doubles in the next 12 h.8,9 Follicular assembly in the mouse ovary also occurs postnatally,10–12 although in some strains of mice it is already initiated on the day of birth.10,13
Morphological Correlates of Early Follicular Growth
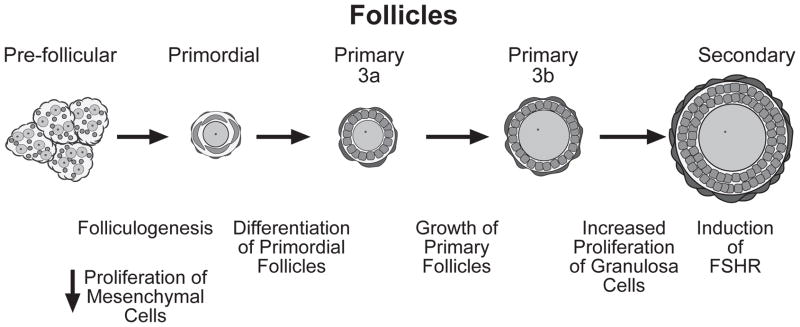
The basic morphological characteristics of growing follicles are well-known (Fig. 1). Primordial follicles, the initial result of follicular assembly, contain an oocyte surrounded by a single layer of flattened pregranulosa cells. They can be further classified as type 1 and type 2, based on the number of granulosa cells surrounding the oocyte.14 Primordial follicles become primary follicles (type 3a)14 by a process that results in the differentiation of the flattened granulosa cells into a cuboidal morphology (Fig. 1).8,14 Granulosa cell proliferation and oocyte growth begin at this point resulting in the formation of larger (type 3b) primary follicles first, and subsequently, secondary follicles with two (type 4) or more layers of granulosa cells (type 5 and larger) (Fig. 1).
Figure 1.
Folliculogenesis and development of ovarian follicles. Before follicular assembly, oogonia are surrounded by somatic cells of epithelium origin forming “nests” of germ cells and pregranulosa cells. These nests are, in turn, surrounded by mesenchymal cells that proliferate and gradually infiltrate the nests, separating clusters of pregranulosa cells into a more discrete configuration, which allows these cells to surround individual oocytes and form primordial follicles. At the time of follicular assembly, mesenchymal cell proliferation subsides. Primordial follicles consist of a non-growing oocyte with a few associated flattened pregranulosa cells. Primary follicles are those in which granulosa cells have acquired a cuboidal shape and are completely enveloping the oocyte. These follicles can be subdivided into two subtypes: type 3a, which contain a nongrowing oocyte surrounded by no more than 20 granulosa cells, and type 3b in which the oocyte has began to grow and is surrounded by 20–60 granulosa cells.14 Secondary follicles contain a growing oocyte surrounded by two layers of cuboidal granulosa cells. It is at this stage that granulosa cells begin to acquire responsiveness to FSH.5,6 FSHR = FSH receptor.
The Control of Follicular Assembly and Early Follicular Growth
In recent years substantial progress has been made towards the identification of genes controlling the assembly and initial growth of ovarian follicles. Three genes have been shown to play critical roles in specifying the fate of germ cells: Bone Morphogenetic Protein 4 (BMP4), required for the generation of germ cells in the primitive epiblast,15 Stem Cell Factor (SCF) necessary for germ cell survival during migration towards the genital ridge,16 and wingless-related MMTV integration site-4 (Wnt-4), a member of the family of locally acting cell signals, required for intragonadal survival of newly formed oocytes.17 It is also clear that formation of primordial follicles requires a transcription factor termed Factor in the Germline alpha (FIGα),18 and that subsequent differentiation and growth of primordial follicles is regulated by several factors produced locally by either granulosa cells or the oocyte itself. Factors that facilitate follicle growth and are produced by granulosa cells include the c-kit ligand KL,19, 20 basic fibroblast growth factor (bFGF),21 leukemia inhibitory factor (LIF),22 and a few others.23 Oocyte factors controlling follicular growth include growth differentiation factor-9 (GDF-9),24 the homeobox gene Nobox (newborn ovary homeobox-encoding gene),25, 26 and the transcription factor Foxo3a, which appears to inhibit the differentiation step underlying conversion of primordial follicles into primary follicles.27
Evidence now exists indicating that a family of polypeptide growth factors, termed neurotrophins (NTs), and their cell membrane-spanning receptors contribute to controlling both the differentiation process that leads to the conversion of primordial follicles into primary follicles, and the proliferative events underlying the growth of primary follicles into larger follicles with more layers of granulosa cells.28–30 Although NTs are required for the development and maintenance of the ovarian innervation,31 it is now well established that they can directly regulate the function of ovarian somatic and germ cells via their transmembrane receptors, without the intermediacy of the ovarian nerves.
The Neurotrophins and their Receptors
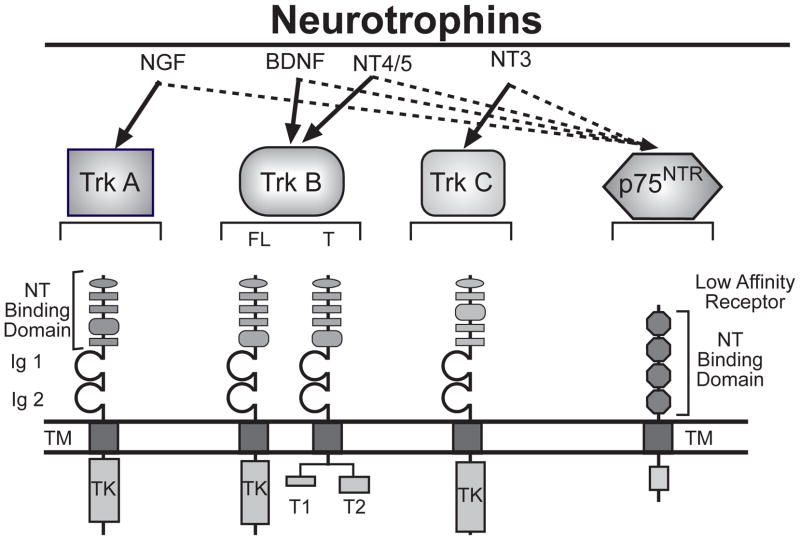
NTs are a family of target-derived trophic factors required for the survival and differentiation of defined neuronal populations in the central and peripheral nervous system.32,33 Four mammalian NTs have been identified to date (Fig. 2), including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5) [reviewed in34,35]. One way the NTs initiate their biological actions is by binding to high-affinity transmembrane tyrosine kinase receptors encoded by members of the trk proto-oncogene family.36 There are three members of the trk receptor family: trkA that binds NGF, trkB that binds BDNF and NT-4/5, and trkC that binds NT-3 (Fig. 2). A second signaling system used by NTs is provided by a more abundantly expressed recognition molecule, which binds all NTs with similar low affinity. This protein is a member of the tumor necrosis receptor family, and is known as the low-affinity neurotrophin receptor (NTR) or p75NTR37,38 (Fig. 2).
Figure 2.
The neurotrophins and their receptors. There are four mammalian neurotrophins (NGF, BDNF, NT-3 ant NT-4/5), three tyrosine kinase receptors (TrkA, TrkB and TrkC) and one pan-NT receptor (p75NTR). The NTs bind to Trk receptors with high affinity (bold arrows) and to p75NTR with low affinity (hatched arrows). FL = full-length; T = truncated; Ig = immunoglobulin-like domain; TM = transmembrane domain region; TK = tyrosine kinase domain.
The Pleiotropic Functions of Neurotrophins
NTs are essential for the differentiation and survival of various neuronal populations in the central and peripheral nervous systems.32,33 Although it was once believed that NTs were required only within the nervous system, the presence of their high-affinity Trk receptors in several nonneuronal tissues has led to the conclusion that NTs are also required for the development and function of organs as diverse as those comprising the cardiovascular, immune, endocrine and reproductive systems (reviewed in39). The critical importance of NGF, BDNF, NT-4/5, and neurotrophin-3 (NT-3), and their respective TrkA, TrkB, and TrkC receptors in the morphogenesis of such organs was recently demonstrated by the severe defects in thymus, heart and ovarian development detected in mice lacking the NT receptors TrkA,40 TrkB,29 TrkC,41 or their ligands NGF,30 BDNF/NT-4/529 and NT-342. NTs also appear to play a role in vascular development since the complete ablation of the pan NT receptor p75NTR results in defects of blood vessel formation.43
Role of NTs in Early Ovarian Development
A role for NTs in the control of ovarian maturation was initially suggested by the finding that the developing ovary not only contains all four of the known NTs (NGF, BDNF, NT-3 and NT-4/544–49), but also expresses the receptors for each of them (p75NTR and the tyrosine kinase high affinity receptors TrkA, TrkB and TrkC29,48–53). It is now clear that the NTs and their respective receptors are expressed in feto-neonatal rodent ovaries and fetal human ovaries before the time of follicular assembly.28,49,53–55 At this early developmental stage and throughout folliculogenesis and differentiation of primordial follicles into secondary follicles, NTs are in general most abundantly expressed in somatic cells of the ovary including granulosa and mesenchymal cells,30,49,53,54 with their trk receptors being relatively more abundant in oocytes.28,30,53,54 However, this distribution is not absolute, because NT4 mRNA and trkB mRNA can be detected in both granulosa cells and oocytes,29,49,54 depending of the stage of development of the ovary. A note of caution should be introduced here with regard to the immunohistochemical localization of ligands in the ovary, because there is a precedent for NGF immunoreaction in granulosa cells in the absence of mRNA.56 Keeping this caveat in mind, these localization studies suggest that depending on the developmental phase both somatic cells and oocytes may produce NTs and be responsive to NT actions via Trk or p75NTR receptor-mediated signaling. Consistent with this rather broad conclusion, mechanistic studies from different groups, including ours, have identified NGF, acting via TrkA receptors,30,57 and BDNF/NT-4/5, recognized by TrkB receptors,28,29 as two NT signaling modules involved in the control of early follicular growth.
The NGF-TrkA signaling module
The contribution of NGF to follicular formation and early follicular development has been demonstrated using Ngf-null mice.30 The ovaries of these animals (analyzed on postnatal day 7) not only have more oocytes that fail to be encapsulated by somatic cells into a follicular structure, but also exhibit a reduced number of primary and secondary follicles, indicating that NGF supports the sequential processes of granulosa cell differentiation and proliferation underlying the conversion of primordial into secondary follicles. The biological mechanisms underlying this supportive action of NGF have not been identified, but they appear to involve a proliferative signal provided by the neurotrophin to ovarian mesenchymal cells, as proliferation of these cells is much reduced in the ovaries of newborn NGF-deficient mice.30 In wild-type ovaries this reduction occurs around the time of follicular assembly,58 probably reflecting a switch from proliferation to differentiation required for follicular assembly. A plausible interpretation of these observations is that the absence of NGF causes a premature decline in mesenchymal cell mitogenesis (Fig. 3), which is required for the build-up of an adequate number of cells for follicular assembly. A stimulatory effect of NGF on cell proliferation has been reported in cells of mesenchymal origin, including fibroblasts59 and differentiated ovarian thecal cells.56
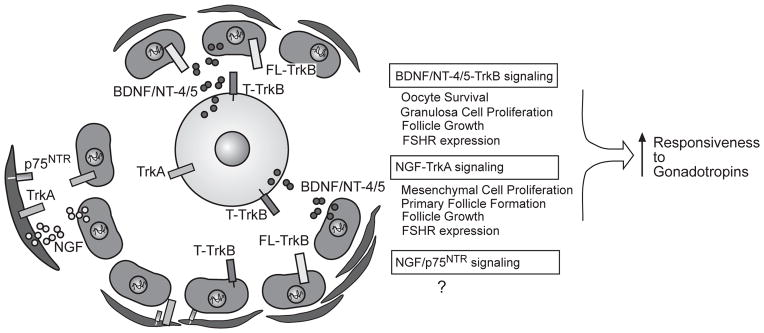
Figure 3.
Proposed NT-dependent pathways involved in the control of early follicle development. Current evidence suggest that NGF is produced by both mesenchymal (pre-thecal) and granulosa cells, and acts on TrkA receptors located in both of these cell types to promote the differentiation of primordial follicles into primary follicles, the subsequent growth of primary follicles, and the acquisition of FSH receptors. BDNF and NT-4/5 are produced by granulosa cells (and perhaps, also temporally produced by oocytes), and act on both granulosa cells and the oocyte to promote proliferation of the former, and survival of the latter. They exert these effects by activating FL-TrkB receptors expressed in granulosa cells and T-TrkB receptors expressed in oocytes. Like NGF, BDNF/NT-4/5 also promotes the formation of FSH receptors. Both NT-dependent systems facilitate the biochemical differentiation of growing follicles, prompting them they become responsive to gonadotropins. For details see text. ↑=stimulation; ? = function suspected, but not yet identified.
No changes in the number of primordial follicles were observed in Ngf KO ovaries collected on postnatal day 7,30 implying that follicular assembly does not require NGF. However, in recent experiments we have revisited this issue by examining Ngf-and trkA KO ovaries on postnatal day 2 and 4 (Kerr, B., Garcia-Rudaz, C, Dissen, G.A., and Ojeda, S.R., unpublished). The results showed that in both cases, the number of primordial follicles was reduced, indicating that in addition to sustaining follicular growth, an NGF signal mediated by its high-affinity trkA receptor contributes to supporting follicular assembly. These findings considered in conjunction with those previously reported using ovaries collected on postnatal day 730 suggest that the absence of trkA-mediated signaling delays, but does not impair, follicular assembly.
Because the ovaries of trkA KOs collected on the day of birth do not show signs of oocyte apoptosis (Kerr, B et al., unpublished), it also appears that the reduced number of primordial follicles observed on postnatal day 2 and 4 is not due to a reduction in the available number of oocytes, but instead is caused by an inability of somatic cells and oocytes to become organized into primordial follicles. In these experiments, we also determined that in vitro treatment of NGF-deficient ovaries with NGF restored follicular formation and growth to wild-type levels, whereas the same treatment of trkA−/− ovaries failed to rescue these defects. These results make it clear that NGF supports follicular assembly and early follicle growth via activation of trkA receptors (Fig. 3).
In the absence of FSH receptors, follicular development proceeds unabated until the follicles reach the secondary stage,6 indicating that at this time they become gonadotropin-dependent. NGF appears to be one of the intraovarian factors that promote this biochemical differentiation because in the absence of NGF, FSH receptor expression decreases.57 Conversely, exposure of ovaries from neonatal wild-type mice to NGF increases FSH receptor expression.57 Thus, NGF not only promotes the early stages of follicle growth, but also induces the initial biochemical differentiation of these follicles into gonadotropin-responsive structures (Fig. 3). The cell to cell mechanisms underlying these actions of NGF are not known. Because in the perinatal rat ovary, trkA receptors are located in both mesenchymal and granulosa cells,30 and the mesenchymal cell localization persists after the development of antral follicles,48 it appears plausible that part of the mechanism used by the neurotrophin to facilitate folliculogenesis and early follicular growth involves the activation of a directional mesenchymal to granulosa cell/oocyte communication pathway (Fig. 3), postulated previously by others.60 The nature of the cell to cell signaling molecules involved remains to be established.
The BDNF/NT-4/5 -TrkB signaling module
Alternative splicing of the TrkB pre-mRNA generates both a full-length (FL) receptor that uses an intracellular tyrosine kinase domain for signaling, and truncated isoforms (known as T1 and T2) lacking the intracellular kinase domain.61,62 Though lacking canonical signaling motifs, these truncated forms, and in particular the T1 isoform, are also able to initiate intracellular signaling.63–65 Full-length, kinase domain-containing immunoreactiveTrkB receptors are expressed at low, and seemingly unchanging levels not only in oogonia and oocytes,28,29 but also in granulosa cells of primordial and growing follicles.29 In contrast, the T1 truncated receptor is remarkably abundant, and predominantly expressed in oocytes. In fact, recent RT-PCR examination of denuded oocytes from infantile mice revealed that oocytes only express TrkB-T1 receptors (Garcia-Rudaz, C. Kerr, B., Dissen, G.A., and Ojeda, S.R., unpublished), suggesting that all BDNF and/or NT4/5 actions on oocytes are mediated by truncated, instead of FL-TrkB receptors.
Using trkB-null mice, two groups recently demonstrated that TrkB signaling is required for oocyte survival and preantral follicular development.28,29 Both studies concluded that TrkB receptors are required for oocyte survival and follicle growth. Employing conventional trkB-null mice (i.e., lacking the intracellular domain of the full-length receptor), one of these groups found a significant loss of oocytes before the formation of primordial follicles, and concluded that TrkB signaling is required for oocyte survival at the time of follicular assembly.28 Surprisingly, K252a, an inhibitor of Trk receptor kinase activity, reduced oocyte survival in newly formed primordial follicles, despite the very low prevalence of FL-TrkB receptors detected in the ovary at this time. This led to the conclusion that FL-TrkB is the receptor isoform required for germ cell survival. Because these conventional trkB KOs express the extracellular-transmembrane domains of the receptor – which can exert undesirable dominant-negative effects – the other group employed newly developed mutant mice lacking all TrkB isoforms, and found that the ovaries of these mice – and those lacking both BDNF and NT-4/5 – suffer a stage-selective deficiency in early follicular development that compromises the ability of follicles to grow beyond the type 3b primary stage29 i.e., when granulosa cells begin to proliferate and oocyte growth is initiated.66 Proliferation of granulosa cells – required for this transition – and expression of FSH receptors, which reflects the degree of biochemical differentiation of growing follicles, are reduced in these “complete” trkB-null mice. Because T-TrkB receptors are abundant in oocytes, and they appear to be targeted to the cell membrane when follicles reach the primary stage,29 it was suggested that T-TrkB receptors may play a role in initiating primary follicle growth.
TrkB-null mice are in poor health, making it difficult to study the development of the ovary after the first week of postnatal life. To overcome this limitation, the ovaries from 4–5-day-old KO animals were grafted under the kidney capsule of wild-type adult female mice and examined two weeks later. The outcome was surprising: the oocytes failed to grow and instead died, resulting in complete loss of follicular organization.29 These observations led to the conclusion that TrkB receptors not only facilitate the early growth of ovarian follicles, but that TrkB-mediated signaling is critical for oocyte survival after follicular assembly is complete (Fig. 3). Because T-TrkB receptors are so abundant in oocytes and appear to be targeted to the oocyte’s cell membrane at the primary stage of follicular growth, it would appear that the survival of oocytes already encapsulated into a follicle requires the presence of intact T1-TrkB receptors instead of TrkB receptors with tyrosine kinase-mediated signaling capabilities (Fig. 3).
Despite the particular differences discerned between the two aforementioned reports,28,29 it is clear that together these studies have not only unveiled an unexpected role for NTs in the control of ovarian development, but have also provided the basis for the novel concept that TrkB signaling is required for oocyte survival. As such, they add a new dimension to the concept that oocytes play an essential role in directing both follicle formation and subsequent follicular development.1,67
In addition, these studies28,29 raise an entirely new set of questions: are FL-TrkB or T-TrkB required for oocyte survival before follicular formation? Which receptor isoform supports follicle growth and oocyte survival after follicular assembly? Which cell type, germ cell/oocytes or granulosa cells, is the primary site of TrkB action? Which are the downstream molecules and cellular mechanisms underlying these novel functions of TrkB signaling in oocyte survival and oocyte-somatic cell reciprocal communication? Future investigation using conditional KO mice in which TrkB receptors are removed in a cell-specific manner will be required to resolve this issue.
Another unresolved issue is the role that the p75NTR plays in early ovarian development. This receptor is prominently expressed in mesenchymal cells long before the initiation of follicle assembly,49 and remains highly expressed in thecal cells of the rat ovary throughout the natural history of follicle growth.52 Because loss of p75NTR expression is incomplete in existing p75NTR KOs,43,68 this issue will have to be resolved using conditional KO mice in which the cell-specific loss of the receptor is complete.
In closing, we should stress the view that none of these molecules can act in a vacuum – and that different degrees of interactions and inter-dependencies must occur between NTs and the growing list of intraovarian factors controlling folliculogenesis and early follicular growth. We believe that implementation of systems biology approaches will be necessary to generate an integrative view of these relationships.
Acknowledgments
This work was supported by NIH grants HD24870 (SRO), Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD18185 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (SRO), and RR00163 for the operation of the Oregon Regional Primate Research Center (GAD, SRO). CG-R was a visiting scientist supported by a fellowship from NICHD TW/HD00668 Fogarty International Training & Research in Population & Health grant.
Contributor Information
Gregory A. Dissen, Email: disseng@ohsu.edu.
Cecilia Garcia-Rudaz, Email: garciaru@ohsu.edu.
Sergio R. Ojeda, Email: ojedas@ohsu.edu.
References
- 1.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: Oocytes carry the conversation. Science. 2002;296:2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 2.Epifano O, Dean J. Genetic control of early folliculogenesis in mice. Trends Endocrinol Metab. 2002;13:169–173. doi: 10.1016/s1043-2760(02)00576-3. [DOI] [PubMed] [Google Scholar]
- 3.Kezel P, Nilsson E, Skinner MK. Cell-cell interactions in primordial follicle assembly and development. Front Biosci. 2002;7:d1990–d1996. doi: 10.2741/kezele. [DOI] [PubMed] [Google Scholar]
- 4.Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005 Sep;11(5):461–471. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- 5.Kumar TR, Wang Y, Lu N, Matsuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 6.Dierich A, Sairam MR, Monaco L, et al. Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang F-P, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172–183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- 8.Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 9.Malamed S, Gibney JA, Ojeda SR. Ovarian innervation develops before initiation of folliculogenesis in the rat. Cell Tissue Res. 1992;270:87–93. doi: 10.1007/BF00381883. [DOI] [PubMed] [Google Scholar]
- 10.Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 11.Durlinger ALL, Gruijters MJG, Kramer P, et al. Anti-müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 12.de la Chesnaye E, Kerr B, Paredes A, Merchant-Larios H, Mendez JP, Ojeda SR. Fbxw15/Fbxo12J is an F-box protein-encoding gene selectively expressed in oocytes of the mouse ovary. Biol Reprod. 2008;78:714–725. doi: 10.1095/biolreprod.107.063826. [DOI] [PubMed] [Google Scholar]
- 13.Canning J, Takai Y, Tilly JL. Evidence for genetic modifiers of ovarian follicular endowment and development from studies of five inbred mouse strains. Endocrinology. 2003 Jan;144(1):9–12. doi: 10.1210/en.2002-220988. [DOI] [PubMed] [Google Scholar]
- 14.Peters H. The development of the mouse ovary from birth to maturity. Acta Endocrinol. 1969;62:98–116. doi: 10.1530/acta.0.0620098. [DOI] [PubMed] [Google Scholar]
- 15.Lawson KA, Dunn NR, Roelen BAJ, et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godin I, Deed R, Cooke J, Zsebo K, Dexter M, Wylie CC. Effects of the steel gene product on mouse primordial germ cells in culture. Nature. 1991;352:807–809. doi: 10.1038/352807a0. [DOI] [PubMed] [Google Scholar]
- 17.Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- 18.Liang L, Soyal SM, Dean J. FIGalpha, a germ cell specific transcription factor involved in the coordinate expression of the zona pellucida genes. Development. 1997;124:4939–4947. doi: 10.1242/dev.124.24.4939. [DOI] [PubMed] [Google Scholar]
- 19.Huang EJ, Manova K, Packer AI, Sanchez S, Bachvarova RF, Besmer P. The murine steel panda mutation affects kit ligand expression and growth of early ovarian follicles. Dev Biol. 1993;157:100–109. doi: 10.1006/dbio.1993.1115. [DOI] [PubMed] [Google Scholar]
- 20.Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–4271. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol. 2001 Apr 25;175(1–2):123–130. doi: 10.1016/s0303-7207(01)00391-4. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol. 2002 Feb 25;188(1–2):65–73. doi: 10.1016/s0303-7207(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 23.Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005 Sep;11(5):461–471. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- 24.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 25.Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- 26.Suzumori N, Yan C, Matzuk MM, Rajkovic A. Nobox is a homeobox-encoding gene preferentially expressed in primordial and growing oocytes. Mech Dev. 2002;111:137–141. doi: 10.1016/s0925-4773(01)00620-7. [DOI] [PubMed] [Google Scholar]
- 27.Brenkman AB, Burgering BM. FoxO3a eggs on fertility and aging. Trends Mol Med. 2003 Nov;9(11):464–467. doi: 10.1016/j.molmed.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Spears N, Molinek MD, Robinson LL, et al. The role of neurotrophin receptors in female germ-cell survival in mouse and human. Development. 2003 Nov;130(22):5481–5491. doi: 10.1242/dev.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paredes A, Romero C, Dissen GA, et al. TrkB receptors are required for follicular growth and oocyte survival in the mammalian ovary. Dev Biol. 2004;267:430–449. doi: 10.1016/j.ydbio.2003.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dissen GA, Romero C, Newman Hirshfield A, Ojeda SR. Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology. 2001;142:2078–2086. doi: 10.1210/endo.142.5.8126. [DOI] [PubMed] [Google Scholar]
- 31.Dissen GA, Paredes A, Romero C, Dees WL, Ojeda SR. Neural and neurotrophic control of ovarian development. In: Leung P, Adashi E, editors. The Ovary. 2. San Diego, CA: Academic Press; 2004. pp. 3–23. [Google Scholar]
- 32.Snider WD. Functions of the neurotrophins during nervous system development: What the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 33.Davies AM. Neurotrophins: Neurotrophic modulation of neurite growth. Curr Biology. 2000;10:R198–R200. doi: 10.1016/s0960-9822(00)00351-1. [DOI] [PubMed] [Google Scholar]
- 34.Bibel M, Barde Y-A. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 35.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005 Aug;6(8):603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 36.Patapoutain A, Reichardt LF. Trk receptors: Mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 37.Dechant G, Barde YA. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002 Nov;5(11):1131–1136. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- 38.Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004 May 27;42(4):529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Tessarollo L. Pleiotrophic functions of neurotrophins in development. Cytokine Growth Factor Rev. 1998;9:125–137. doi: 10.1016/s1359-6101(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 40.García-Suárez O, Germanà A, Hannestad J, et al. TrkA is necessary for the normal development of the murine thymus. J Neuroimmunol. 2000;108:11–21. doi: 10.1016/s0165-5728(00)00251-4. [DOI] [PubMed] [Google Scholar]
- 41.Tessarollo L, Tsoulfas P, Donovan MJ, et al. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc Natl Acad Sci USA. 1997;94:14776–14781. doi: 10.1073/pnas.94.26.14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donovan MJ, Hahn R, Tessarollo L, Hempstead BL. Identification of an essential nonneuronal function of neurotrophin 3 in mammalian cardiac development. Nat Genet. 1996;14:210–213. doi: 10.1038/ng1096-210. [DOI] [PubMed] [Google Scholar]
- 43.von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat Neurosci. 2001;4:977–978. doi: 10.1038/nn730. [DOI] [PubMed] [Google Scholar]
- 44.Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- 45.Hallböök F, Ibañez CF, Persson H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron. 1991;6:845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- 46.Berkemeier LR, Winslow JW, Kaplan DR, Nikolics K, Goeddel DV, Rosenthal A. Neurotrophin-5: A novel neurotrophic factor that activates trk and trkB. Neuron. 1991;7:857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- 47.Lara HE, Hill DF, Katz KH, Ojeda SR. The gene encoding nerve growth factor is expressed in the immature rat ovary: Effect of denervation and hormonal treatment. Endocrinology. 1990;126:357–363. doi: 10.1210/endo-126-1-357. [DOI] [PubMed] [Google Scholar]
- 48.Dissen GA, Hill DF, Costa ME, Dees WL, Lara HE, Ojeda SR. A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinology. 1996;137:198–209. doi: 10.1210/endo.137.1.8536613. [DOI] [PubMed] [Google Scholar]
- 49.Dissen GA, Newman Hirshfield A, Malamed S, Ojeda SR. Expression of neurotrophins and their receptors in the mammalian ovary is developmentally regulated: Changes at the time of folliculogenesis. Endocrinology. 1995;136:4681–4692. doi: 10.1210/endo.136.10.7664689. [DOI] [PubMed] [Google Scholar]
- 50.Klein R, Parada LF, Coulier F, Barbacid M. trkB a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 1989;8:3701–3709. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamballe F, Klein R, Barbacid M. trkC a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991;66:967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- 52.Dissen GA, Hill DF, Costa ME, Ma YJ, Ojeda SR. Nerve growth factor receptors in the peripubertal rat ovary. Mol Endocrinol. 1991;5:1642–1650. doi: 10.1210/mend-5-11-1642. [DOI] [PubMed] [Google Scholar]
- 53.Abir R, Fisch B, Jin G, et al. Presence of NGF and its receptors in ovaries from human fetuses and adults. Mol Hum Reprod. 2005;11:229–236. doi: 10.1093/molehr/gah164. [DOI] [PubMed] [Google Scholar]
- 54.Anderson RA, Robnson LLL, Brooks J, Spears N. Neurotropins and their receptors are expressed in the human fetal ovary. J Clin Endocrinol Metab. 2002;87:890–897. doi: 10.1210/jcem.87.2.8221. [DOI] [PubMed] [Google Scholar]
- 55.Anesetti G, Lombide P, D’Albora H, Ojeda SR. Intrinsic neurons in the human ovary. Cell Tissue Res. 2001;306:231–237. doi: 10.1007/s004410100451. [DOI] [PubMed] [Google Scholar]
- 56.Dissen GA, Parrott JA, Skinner MK, Hill DF, Costa ME, Ojeda SR. Direct effects of nerve growth factor on thecal cells from antral ovarian follicles. Endocrinology. 2000;141:4736–4750. doi: 10.1210/endo.141.12.7850. [DOI] [PubMed] [Google Scholar]
- 57.Romero C, Paredes A, Dissen GA, Ojeda SR. Nerve growth factor induces the expression of functional FSH receptors in newly formed follicles of the rat ovary. Endocrinology. 2002;143:1485–1494. doi: 10.1210/endo.143.4.8711. [DOI] [PubMed] [Google Scholar]
- 58.Hirshfield AN, DeSanti AM. Patterns of ovarian cell proliferation in rats during the embryonic period and the first three weeks postpartum. Biol Reprod. 1995;53:1208–1221. doi: 10.1095/biolreprod53.5.1208. [DOI] [PubMed] [Google Scholar]
- 59.Cordon-Cardo C, Tapley P, Jing S, et al. The trk tyrosine protein kinase mediates the mitogenic properties of nerve growth factor and neurotrophin-3. Cell. 1991;66:173–183. doi: 10.1016/0092-8674(91)90149-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajah R, Glaser EM, Hirshfield AN. The changing architecture of the neonatal rat ovary during histogenesis. Dev Dyn. 1992;194:177–192. doi: 10.1002/aja.1001940303. [DOI] [PubMed] [Google Scholar]
- 61.Middlemas DS, Lindberg RA, Hunter T. trkB, a neural receptor protein kinase: Evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991;11:143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klein R, Conway D, Parada LF, Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61:647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- 63.Baxter GT, Radeke MJ, Kuo RC, et al. Signal transduction mediated by the truncated trkB receptor isoforms, trkB.T1 and trkB. T2 J Neurosci. 1997;17:2683–2690. doi: 10.1523/JNEUROSCI.17-08-02683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rose CR, Blum R, Pichler B, Lepler A, Kafitz KW, Konnerth A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74–78. doi: 10.1038/nature01983. [DOI] [PubMed] [Google Scholar]
- 65.Ohira K, Kumanogoh H, Sahara Y, et al. A truncated tropomyosin-related kinase B receptor, T1, regulates glial cell morphology via Rho GDP dissociation inhibitor 1. J Neurosci. 2005 Feb 9;25(6):1343–1353. doi: 10.1523/JNEUROSCI.4436-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pedersen T. Follicle growth in the immature mouse ovary. Acta Endocrinol (Copenh) 1969;62:117–132. doi: 10.1530/acta.0.0620117. [DOI] [PubMed] [Google Scholar]
- 67.Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci U S A. 2002;99:2890–2894. doi: 10.1073/pnas.052658699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paul CE, Vereker E, Dickson KM, Barker PA. A pro-apoptotic fragment of the p75 neurotrophin receptor is expressed in p75NTRExonIV null mice. J Neurosci. 2004 Feb 25;24(8):1917–1923. doi: 10.1523/JNEUROSCI.5397-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]