Abstract
We have provided evidence for a multifaceted antitumor-function of the Toll-like receptor 7 (TLR7) agonist imiquimod, which rapidly recruits plasmacytoid dendritic cells and possibly other immune cells into tumors by inducing the secretion of CCL2 by dermal cells. Imiquimod induces pDC maturation and their conversion into cytolytic killer cells, which are capable of eliminating tumors independently from the adaptive immune system.
Keywords: imiquimod, pDC, CCL2, Type I IFN, cancer
One of the few success stories in the field of cancer immunotherapy has been the establishment of imiquimod as a standard therapy for the treatment of superficial skin cancers like basal cell carcinoma (BCC), actinic keratosis or preinvasive melanoma (lentigo maligna). The imidazochinoline imiquimod is topically applied to the skin as a 5% cream, and several modes of action against tumors have been suggested. Direct effects on tumor cells can be distinguished from those mediated via the immune system.1 Imiquimod is capable of directly inducing caspase-mediated apoptosis and inhibiting adenosine signaling in transformed cells in a Toll-like receptor 7 (TLR7)-independent manner. Moreover, imiquimod act as an agonist for TLR7, which is mainly expressed by cells of the immune system, leading to activation and recruitment to the site of imiquimod application. Imiquimod-induced tumor regression is characterized by skin erythema and the presence of an inflammatory infiltrate in and around the tumor, mainly consisting—in humans as well as in mice—of different types of dendritic cells (DCs) as well as CD4+ and CD8+ T cells. Interestingly, B and natural killer (NK) cells have rarely been found in imiquimod-induced tumor infiltrates.2 Commonly, CD8+ T cells and NK cells need to acquire special features to kill tumor cells, such as the expression of granzyme B and perforin as well as that of tumor necrosis factor (TNF) family members like TNF-related apoptosis-inducing ligand (TRAIL). Usually, DCs are critically involved in priming NK- and T-cell dependent antitumor immune responses.3 However, more recently DC subtypes like interferon (IFN)-producing killer DCs (IKDCs) have been characterized for their ability to directly kill tumor cells, although their true identity as DCs has recently been challenged.4 In BCC patients receiving imiquimod, skin myeloid DCs and plasmacytoid DCs (pDCs) have been shown to express granzyme B plus perforin and TRAIL, respectively, indicating that imiquimod-stimulated DCs might not only play an important role in regulating but also in executing antitumor immune responses by directly killing malignant cells.5
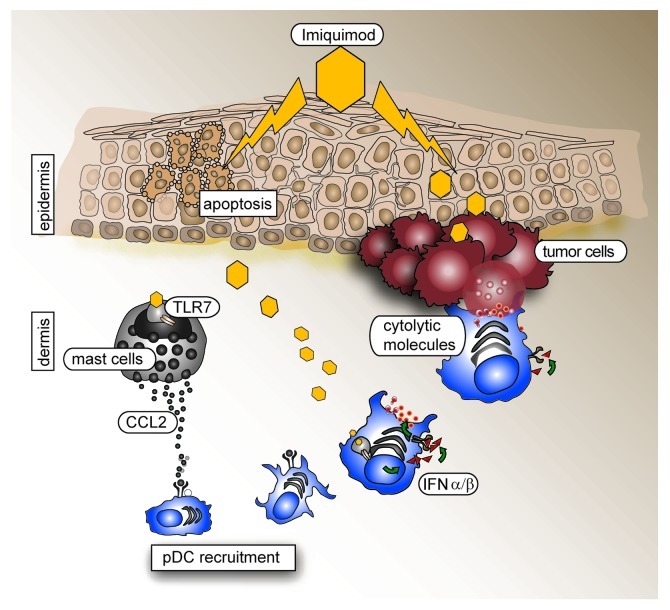
In an orthotopic mouse model of melanoma, we investigated the mechanisms by which imiquimod recruits immune cells into the skin, with the aim to identify the effector cells that are necessary for tumor killing (Fig. 1).6 Since imiquimod is applied topically, we assumed that the first cells responding to imiquimod would be skin-resident cells. By employing bone marrow chimeras we discovered that TLR7 expression by hematopoietic cells, but not other skin cells, is required for the antitumor effect of imiquimod. Mast cells and a few skin-resident pDCs express TLR7 and mast cells have been reported to play a critical role in the induction of adaptive immune responses upon TLR7 ligation.7 When analyzing imiquimod-treated skins, we found increased amounts of CCL2 and this finding correlated with a higher number of activated mast cells in the dermis (Amberg N., unpublished data). Moreover, mast cells produced CCL2 when stimulated with imiquimod (Fig. 1). Since pDCs express CCR2, the receptor for CCL2, it is likely that their recruitment to the skin is induced by CCL2 secreted by mast cells in a TLR7- and MyD88-dependent manner after imiquimod stimulation. Interestingly, CCL2 production by mast cells also relied on Type I IFN signaling (Fig. 1). Thus, it cannot be formally excluded that resident pDCs themselves produce CCL2. Mice lacking mast cells will have to be studied to clarify this issue.
Figure 1. Schematic representation of how topical imiquimod recruits and stimulates plasmacytoid dendritic cells to kill tumor cells. Toll-like receptor 7 (TLR7)-independent effects on keratinocytes are paralleled by the TLR7-dependent activation of mast cells and plasmacytoid dendritic cells (pDCs) resident in the skin. Chemokines emitted, like CCL2, recruit pDCs that secrete Type I interferon (IFN). Paracrine and autocrine IFN signaling induces the upregulation of death receptors on tumor cells and the exposure/secretion of death receptor ligands by pDCs, respectively, resulting in the enhanced killing of tumor cells.
The key role of pDCs in this setting was confirmed by the finding that pDC depletion in BDCA2-DTR mice completely abolished the antitumor effect of imiquimod. The antitumor effects of imiquimod persisted in Rag2−/− and athymic mice, de facto excluding T and B cells as putative effector cell candidates. The same held true for NK cells: imiquimod remained active in mice subjected to NK-cell depletion. Thus, pDCs alone seem to be sufficient for tumor clearance as induced by imiquimod. The recruitment of pDCs into tumors was previously considered as a bad prognostic factor, which correlated for instance with the induction of regulatory T cells (by immature pDCs).8 Imiquimod, however, induces the TLR7- and MyD88-dependent maturation of pDCs, resulting in the upregulation of CD8 and in the production of Type I IFN, in vitro and in vivo. CD8 and the Type I IFN receptor 1 (IFNAR1) seem to be critical for the antitumor activity of imiquimod since this was abolished by the depletion of CD8α+ cells. Similarly, Imiquimod was also ineffective in Ifnar1−/− mice.
Type I IFN may kill tumor cells either directly, in a caspase-dependent manner, or indirectly, by upregulating the expression of death-inducing factors by immune cells and/or of the respective receptors by tumor cells.9 Blocking Type I IFN in in vitro killing assays involving imiquimod-stimulated pDCs and melanoma cells did not reduce the lysis of the latter, thus excluding a direct effect of Type I IFN. Instead, autocrine Type I IFN signaling in pDCs increased TRAIL and granzyme B production and secretion. Blocking granzyme B completely abolished the activitiy of imiquimod in vitro, whereas blocking TRAIL or its receptor DR5 was less effective. A recent study identified a similar mechanism for imiquimod-treated human pDCs.10
Our study confirms that only fully matured pDCs are capable of efficiently fighting tumors. Future studies will not only focus on the aforementioned mechanisms of immune-cell recruitment as induced by imiquimod but also on whether the described stimulation of pDCs is a selective feature of the imiquimod-TLR7 signaling axis or whether other ligand-receptor pairs may provide pDCs with comparable killing activities.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22033
References
- 1.Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190–9. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 2.Palamara F, Meindl S, Holcmann M, Lührs P, Stingl G, Sibilia M. Identification and characterization of pDC-like cells in normal mouse skin and melanomas treated with imiquimod. J Immunol. 2004;173:3051–61. doi: 10.4049/jimmunol.173.5.3051. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Lou Y, Lizée G, Qin H, Liu S, Rabinovich B, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–75. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zitvogel L, Housseau F. IKDCs or B220+ NK cells are pre-mNK cells. Blood. 2012;119:4345–6. doi: 10.1182/blood-2012-03-415026. [DOI] [PubMed] [Google Scholar]
- 5.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–51. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drobits B, Holcmann M, Amberg N, Swiecki M, Grundtner R, Hammer M, et al. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J Clin Invest. 2012;122:575–85. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heib V, Becker M, Warger T, Rechtsteiner G, Tertilt C, Klein M, et al. Mast cells are crucial for early inflammation, migration of Langerhans cells, and CTL responses following topical application of TLR7 ligand in mice. Blood. 2007;110:946–53. doi: 10.1182/blood-2006-07-036889. [DOI] [PubMed] [Google Scholar]
- 8.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 9.Thyrell L, Erickson S, Zhivotovsky B, Pokrovskaja K, Sangfelt O, Castro J, et al. Mechanisms of Interferon-alpha induced apoptosis in malignant cells. Oncogene. 2002;21:1251–62. doi: 10.1038/sj.onc.1205179. [DOI] [PubMed] [Google Scholar]
- 10.Kalb ML, Glaser A, Stary G, Koszik F, Stingl G. TRAIL(+) human plasmacytoid dendritic cells kill tumor cells in vitro: mechanisms of imiquimod- and IFN-α-mediated antitumor reactivity. J Immunol. 2012;188:1583–91. doi: 10.4049/jimmunol.1102437. [DOI] [PubMed] [Google Scholar]