Abstract
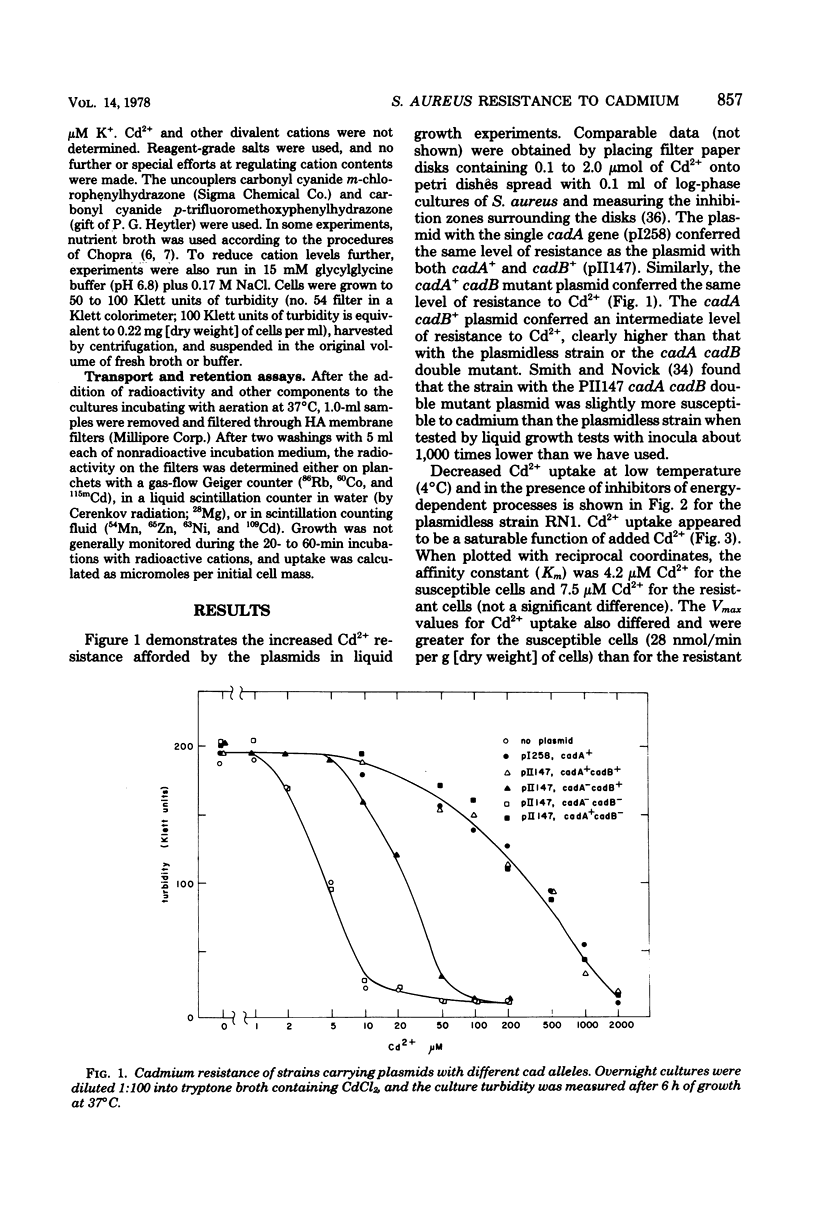
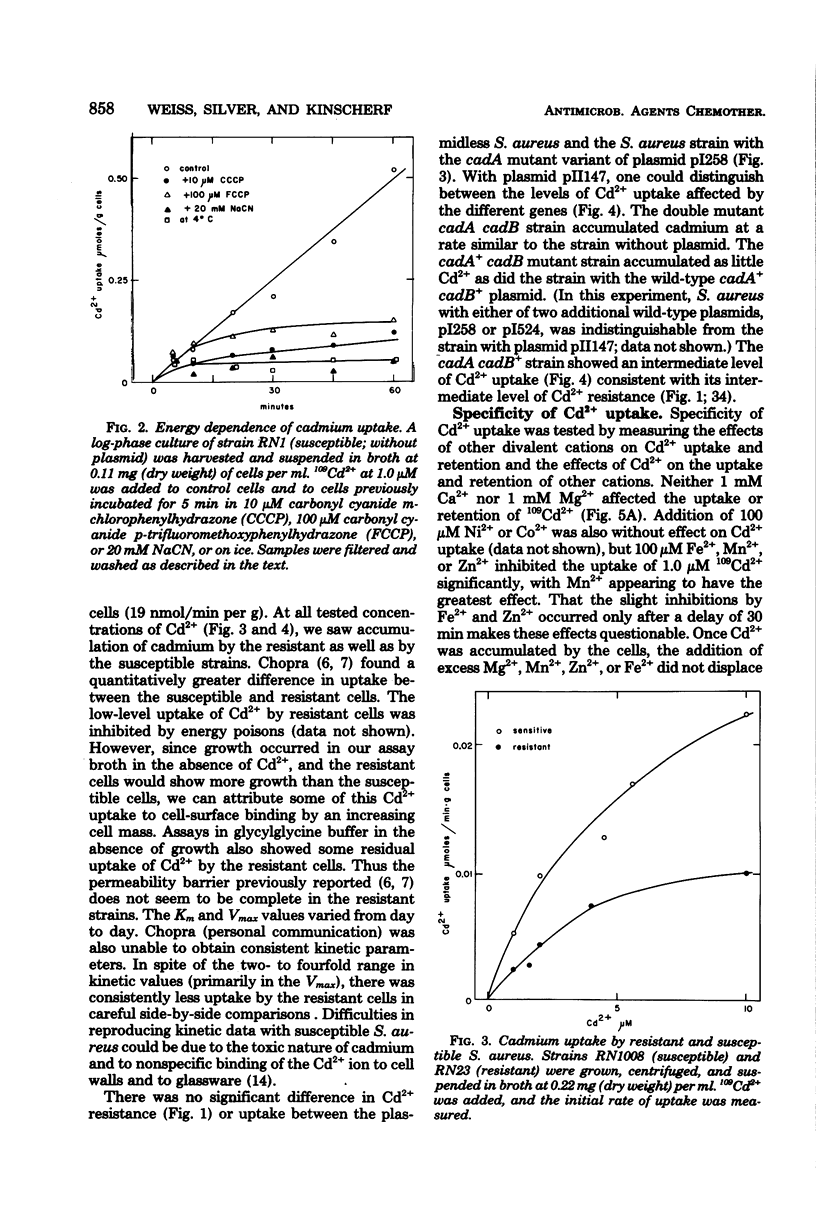
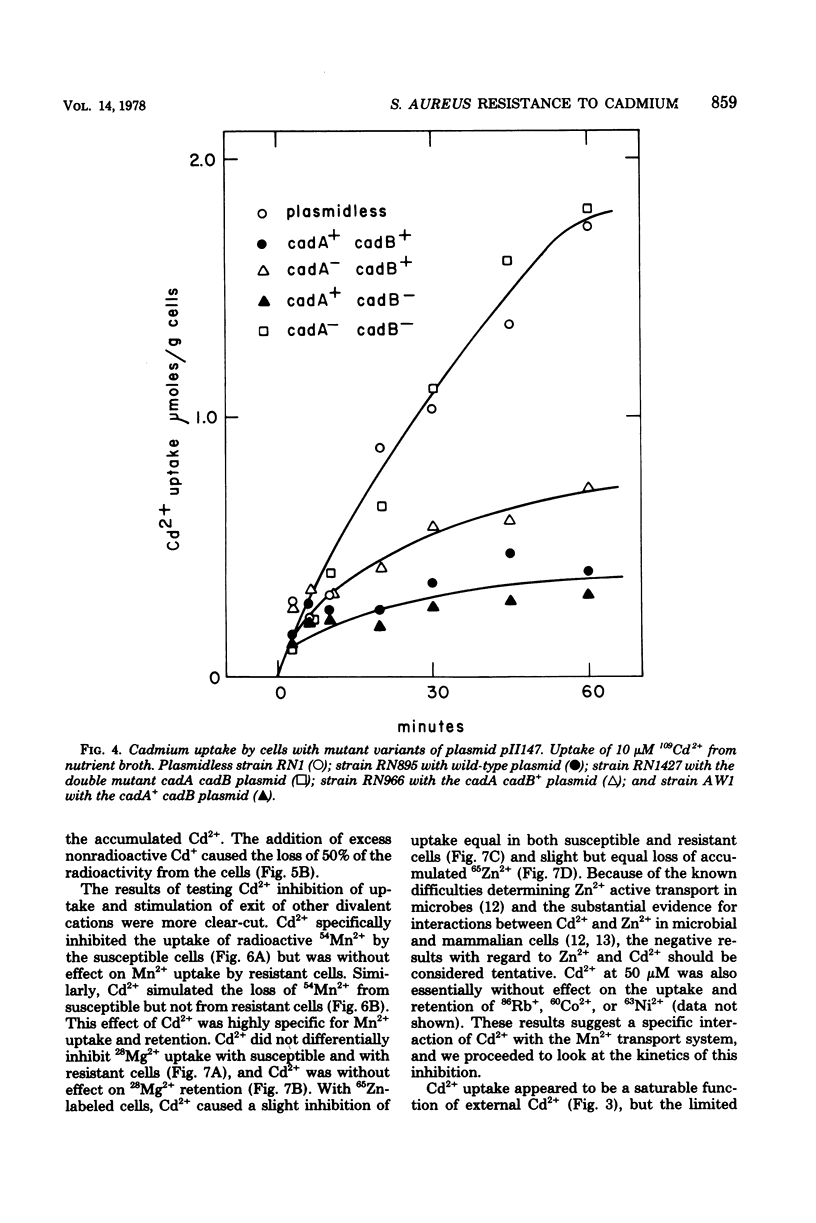
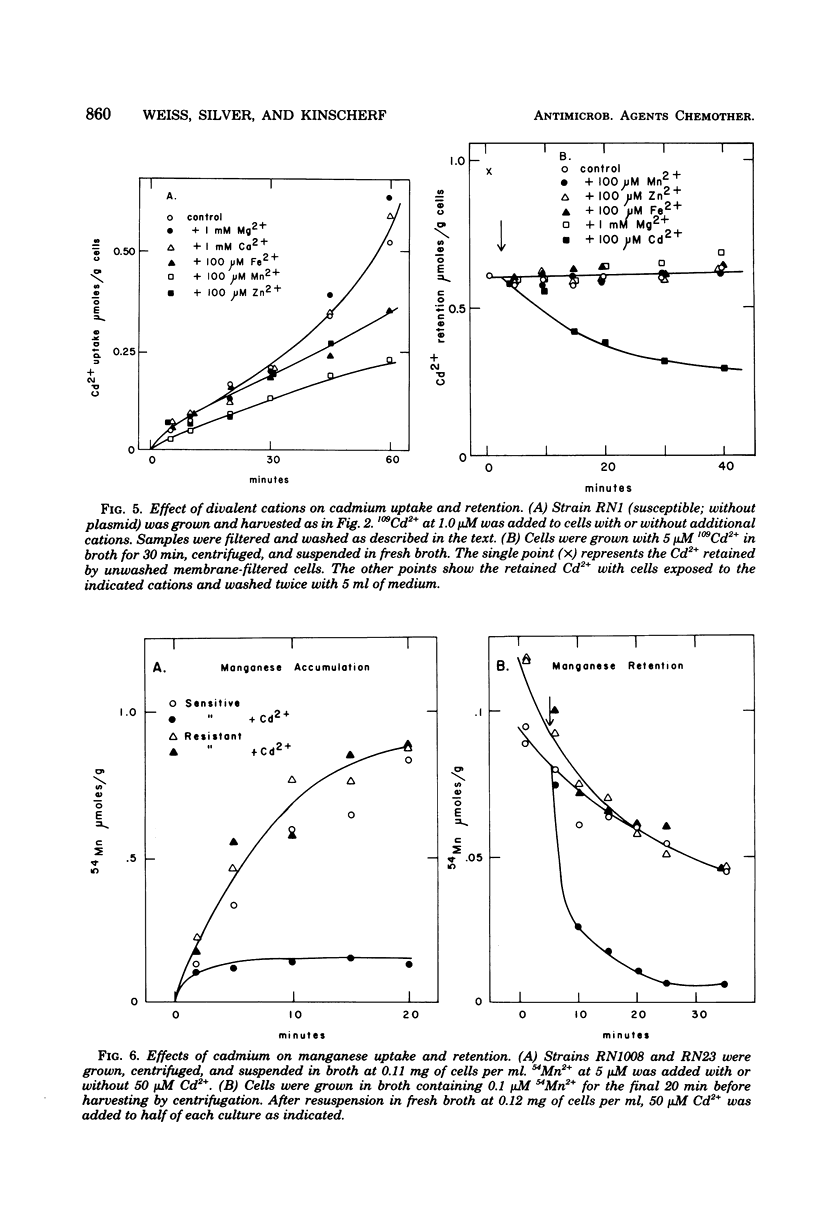
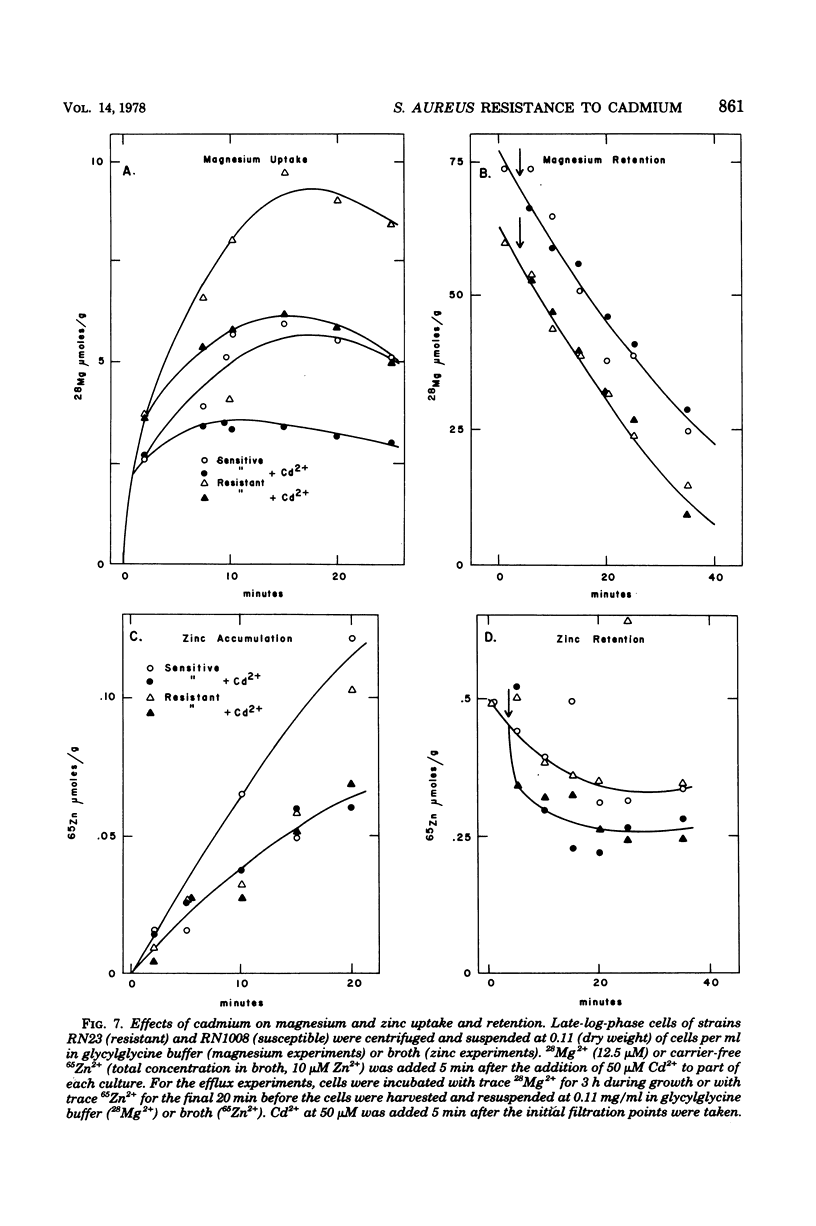
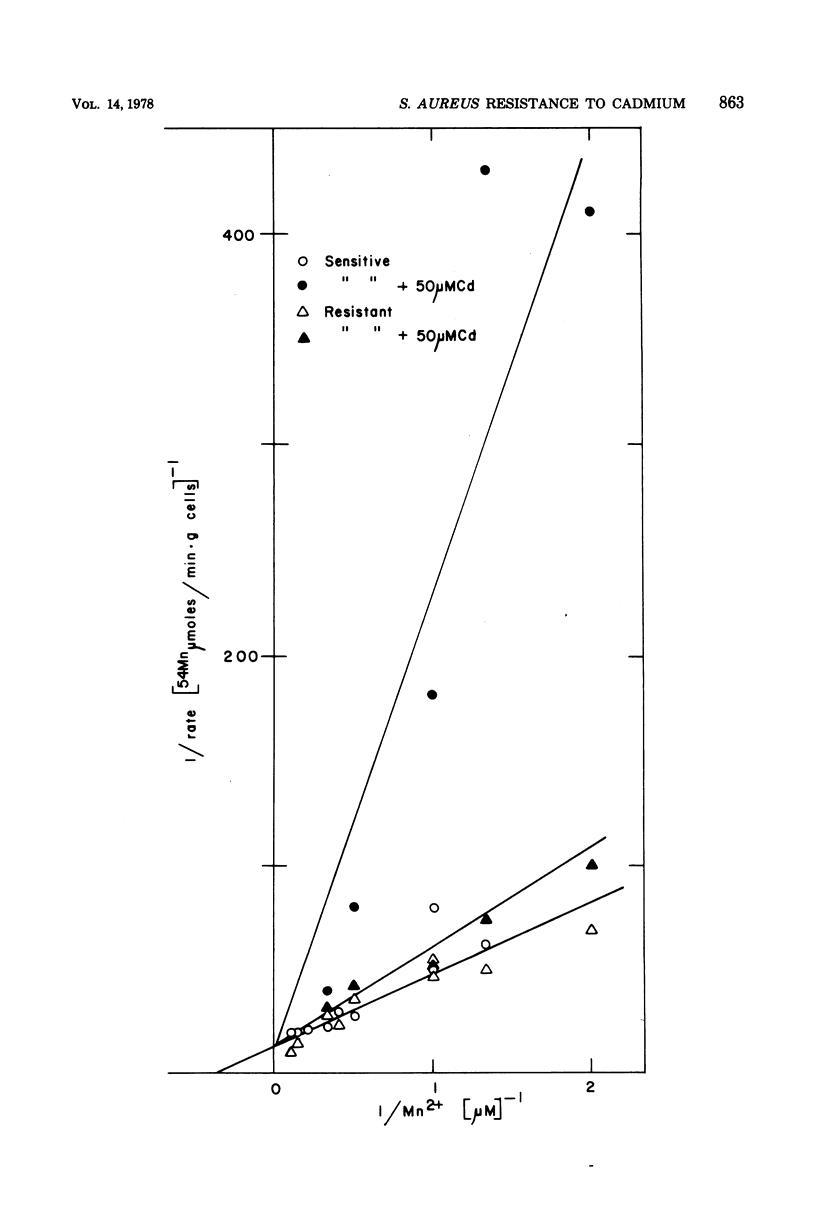
Plasmid-determined resistance to cadmium has only been found with plasmids from Staphylococcus aureus. Resistance to cadmium was associated with a lower accumulation of Cd2+ ions by the plasmid-bearing resistant cells. Cadmium accumulation by susceptible cells was energy dependent and had those characteristics usually associated with a transmembrane active transport system. There was a specific interrelationship between cadmium accumulation and manganese accumulation and retention. Cd2+ inhibited the uptake of Mn2+ and accelerated the loss of intracellular Mn2+ by the susceptible cells, but was without effect on Mn2+ transport in resistant S. aureus cells. Under similar conditions, there was no differential effect of Cd2+ on Mg2+, Zn2+, Co2+, Ni2+, or Rb+ accumulation or exchange between the susceptible and the resistant strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya P. Active Transport of Manganese in Isolated Membranes of Escherichia coli. J Bacteriol. 1970 Dec;104(3):1307–1311. doi: 10.1128/jb.104.3.1307-1311.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya P. Active transport of manganese in isolated membrane vesicles of Bacillus subtilis. J Bacteriol. 1975 Jul;123(1):123–127. doi: 10.1128/jb.123.1.123-127.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheder F., Broda E. Energy-dependent zinc transport by escherichia coli. Eur J Biochem. 1974 Jun 15;45(2):555–559. doi: 10.1111/j.1432-1033.1974.tb03581.x. [DOI] [PubMed] [Google Scholar]
- Cherian M. G. Isolation and purification of cadmium binding proteins from rat liver. Biochem Biophys Res Commun. 1974 Dec 11;61(3):920–926. doi: 10.1016/0006-291x(74)90243-5. [DOI] [PubMed] [Google Scholar]
- Chopra I. Decreased uptake of cadmium by a resistant strain of Staphylococcus aureus. J Gen Microbiol. 1970 Oct;63(2):265–267. doi: 10.1099/00221287-63-2-265. [DOI] [PubMed] [Google Scholar]
- Chopra I., Lacey R. W., Connolly J. Biochemical and genetic basis of tetracycline resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1974 Oct;6(4):397–404. doi: 10.1128/aac.6.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. Mechanism of plasmic-mediated resistance to cadmium in Staphylococcus aureus. Antimicrob Agents Chemother. 1975 Jan;7(1):8–14. doi: 10.1128/aac.7.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J. J., Marshall R. T., Pfander W. H. Effects of cadmium on the growth and uptake of cadmium by microorganisms. Appl Microbiol. 1975 Apr;29(4):562–564. doi: 10.1128/am.29.4.562-564.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke K. G., Parker M. T., Richmond M. H. Penicillinase production and metal-ion resistance in Staphylococcus aureus cultures isolated from hospital patients. J Med Microbiol. 1970 Feb;3(1):125–136. doi: 10.1099/00222615-3-1-125. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E., Fisher S., Der C. L., Silver S. Manganese transport in Bacillus subtilis W23 during growth and sporulation. J Bacteriol. 1973 Mar;113(3):1363–1372. doi: 10.1128/jb.113.3.1363-1372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failla M. L., Cousins R. J. Zinc uptake by isolated rat liver parenchymal cells. Biochim Biophys Acta. 1978 Feb 1;538(3):435–444. doi: 10.1016/0304-4165(78)90405-1. [DOI] [PubMed] [Google Scholar]
- Kondo I., Ishikawa T., Nakahara H. Mercury and cadmium resistances mediated by the penicillinase plasmid in Staphylococcus aureus. J Bacteriol. 1974 Jan;117(1):1–7. doi: 10.1128/jb.117.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R. S., Gray R. H., Chin B., Bernstein I. A. Molecular mechanisms of accommodation in Escherichia coli to toxic levels of Cd2+. J Bacteriol. 1975 Mar;121(3):1180–1188. doi: 10.1128/jb.121.3.1180-1188.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Nakahara H., Ishikawa T., Sarai Y., Kondo I. Frequency of heavy-metal resistance in bacteria from inpatients in Japan. Nature. 1977 Mar 10;266(5598):165–167. doi: 10.1038/266165a0. [DOI] [PubMed] [Google Scholar]
- Nakahara H., Ishikawa T., Sarai Y., Kondo I., Kozukue H., Silver S. Linkage of mercury, cadmium, and arsenate and drug resistance in clinical isolates of Pseudomonas aeruginosa. Appl Environ Microbiol. 1977 Apr;33(4):975–976. doi: 10.1128/aem.33.4.975-976.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Kennedy E. P. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J Biol Chem. 1971 May 10;246(9):3042–3049. [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Park M. H., Wong B. B., Lusk J. E. Mutants in three genes affecting transport of magnesium in Escherichia coli: genetics and physiology. J Bacteriol. 1976 Jun;126(3):1096–1103. doi: 10.1128/jb.126.3.1096-1103.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. Further studies on amino acid transport in Staphylococcus aureus membrane vesicles. J Biol Chem. 1974 Jul 10;249(13):4275–4281. [PubMed] [Google Scholar]
- Silver S., Johnseine P., King K. Manganese Active Transport in Escherichia coli. J Bacteriol. 1970 Dec;104(3):1299–1306. doi: 10.1128/jb.104.3.1299-1306.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K., Novick R. P. Genetic studies on plasmid-linked cadmium resistance in Staphylococcus aureus. J Bacteriol. 1972 Nov;112(2):761–772. doi: 10.1128/jb.112.2.761-772.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Zajac J., Goś Z. Plasmid dependent impermeability barrier to cadmium ions in Staphylococcus aureus. Acta Microbiol Pol A. 1975;7(1):11–20. [PubMed] [Google Scholar]
- Weiss A. A., Murphy S. D., Silver S. Mercury and organomercurial resistances determined by plasmids in Staphylococcus aureus. J Bacteriol. 1977 Oct;132(1):197–208. doi: 10.1128/jb.132.1.197-208.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Levy S. B. Synthesis of an R plasmid protein associated with tetracycline resistance is negatively regulated. Proc Natl Acad Sci U S A. 1976 May;73(5):1509–1512. doi: 10.1073/pnas.73.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]


