Abstract
Platelet-derived growth factor alpha (PDGFA) is frequently upregulated in various cancers and thought to function as a key player in the development and progression of tumor growth by regulating aspects of cell proliferation, angiogenesis, and metastasis. However, the mechanism by which it is upregulated is not fully understood. Previously, we demonstrated that conditional deletion of two transcription factors that signal for the bone morphogenetic proteins (Smad1 and Smad5) in ovarian granulosa cells causes metastatic granulosa cell tumors in female mice and phenocopies human juvenile granulosa cell tumors (JGCTs). Smad1/5 double conditional knockout tumors, as well as human JGCTs, are highly vascularized, hemorrhagic, and mitotically active. Expression analysis of these tumors and their metastases revealed a significant upregulation of key proliferation and pro-angiogenic factors such as Pdgfa, Pdgfb, and Vegf. We examined whether these genes were direct targets of SMAD1 and SMAD5. Knockdown of SMAD1 and SMAD5 in mouse primary granulosa cells and a human granulosa cell tumor-derived cell line (COV434) resulted in upregulation of PDGFA, but not PDGFB nor VEGF. We identified several putative SMAD1/5 binding sites in the PDGFA promoter, and chromatin immunoprecipitation and reporter assays demonstrated that SMAD1/5 interact with the PDGFA promoter to regulate its activity. Further, SMAD1/5 antagonize the activity of the transcription factor Sp1, a well-known positive regulator of PDGFA, by inhibiting its occupancy at a key regulatory site on the proximal PDGFA promoter. Collectively, our studies establish that loss of SMAD1/5 leads to upregulation of PDGFA in ovarian granulosa cells, and that a novel regulatory interaction exists between the BR-SMADs and Sp1 in controlling PDGFA expression during granulosa cell tumorigenesis.
Keywords: bone morphogenetic protein, platelet-derived growth factor, SMAD1, SMAD5, granulosa cell tumor
Introduction
While the tumor promoting functions of the transforming growth factor beta (TGFB) signaling pathway have been studied extensively (1), the contribution of the bone morphogenetic protein (BMP) family, and their receptor-regulated transcription factors [i.e., Smad1 and Smad5 (herein called BR-SMADs)], to cancer progression is less well characterized. In vitro studies have shown that BMPs can suppress growth of normal cells and human colon, prostate, and breast cancer cell lines when the BMP signaling components are intact (2). In addition, the BMP pathway is inactivated in 70% of colorectal cancers, and germline mutations have been found in the BMP type I receptor, BMPRIA/ALK3, in patients with juvenile polyposis syndrome (3, 4). Deletion of two of the BMP type I receptors, Bmpr1a and Bmpr1b, in granulosa cells of the ovary in mice causes granulosa cell tumor development with evidence of increased TGFB and hedgehog signaling (5). In addition, conditional deletion in granulosa cells of Smad1 and Smad5 [Smad1/5 double conditional knockout (dKO)], which are phosphorylated by these receptors in response to BMP signaling, leads to the formation of highly vascularized granulosa cell tumors with full penetrance and with an increased incidence of peritoneal metastases and hemorrhagic ascites with age (6). Subsequent studies revealed that this mouse model develops a disease profile most similar to human juvenile granulosa cell tumors (JGCTs), a rare form of sex cord stromal tumors (7). Although these studies suggest that disruption of the BMP signaling pathway at the receptor or transcription factor level promotes cancer development, little is known about the mechanism involved in the switch towards malignancy when the BMP signaling pathway is disrupted.
A number of well-known proliferation and pro-angiogenic growth factors including isoforms of platelet-derived growth factors (PDGF) were altered in a microarray analysis of Smad1/5 dKO tumors (6). PDGFs are well known signaling molecules implicated in various developmental process and human diseases, including cancer (8). PDGFs are composed of four polypeptide subunits, designated PDGF-A, -B, -C and -D. These subunits function as homodimers (PDGF-AA, PDGF-BB, PDGF-CC and PDGF-DD) or heterodimers (PDGF-AB) and activate the PDGF receptors, α and β to stimulate various cellular functions including proliferation, differentiation and apoptosis (8). A growing body of literature strongly suggests that autocrine and paracrine activated PDGF signaling is commonly observed in the development of many tumors and inhibition of PDGF/PDGFRs slows tumor cell growth in several experimental models (9). While it has been shown that TGFB positively regulates PDGFB transcription via its transcription factors SMAD2 or SMAD3, and the effect of TGFB on cell proliferation depends on PDGFB/PDGFR signaling (10), a link between SMAD1/5 and PDGF expression has not been reported. Given the importance of PDGF signaling, as well as a proposed tumor suppressor function for the BMP/SMAD pathway in cancer development, we hypothesized that the BR-SMADs are integrally involved in the regulation of PDGFA, an interaction that would facilitate tumorigenesis if either pathway were misregulated. In the current study, we demonstrate that SMAD1/5 are novel DNA-binding repressors of PDGFA, and establish a mechanistic link between BR-SMADs loss and PDGFA upregulation in ovarian granulosa cells tumors.
Results
Angiogenic factors are upregulated in Smad1/5 dKO tumors
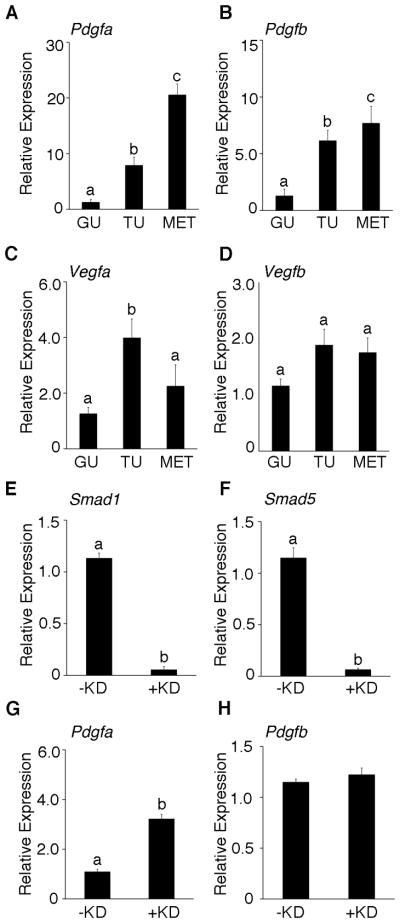
Based on the hemorrhagic and highly vascular phenotype of the Smad1/5 dKO tumors and a preliminary microarray analyses (6), we analyzed the expression level of key angiogenic factors in wild type mouse granulosa cells, Smad1/5 dKO tumors and peritoneal metastases. Quantitative PCR (qPCR) of Smad1/5 dKO tumors and metastasis demonstrated significant upregulation of Pdgfa (Figure 1A), Pdgfb (Figure 1B) and Vegfa (Figure 1C), but not Vegfb (Figure 1D) compared to the wild type mouse granulosa cells, suggesting that the vascular and hemorrhagic phenotype of the tumors from Smad1/5 dKO mice might be due to changes in expression of these growth factors.
Figure 1.
Angiogenic factors are upregulated in Smad1/5 dKO granulosa cell tumors. Real-time quantitative PCR was performed to determine expression levels of Pdgfa (A), Pdgfb (B), Vegfa (C) and Vegfb (D) in wild type mouse granulosa cells (GC), Smad1/5 dKO tumors (TU) and metastasis (MET). siRNA-mediated knockdown (+KD) of both Smad1 and Smad5 in mouse granulosa cells resulted in more than 85% reduction in Smad1 (E) and Smad5 (F) transcript levels compared to the cells transfected cells with a scrambled control siRNA (-KD). A significant increase in Pdgfa (G) expression is observed following Smad1/5 knockdown, without any change in Pdgfb (H) levels. Different letters above the bars indicate statistically different means by ANOVA and post hoc analysis (n=4; P< 0.05).
Loss of Smad1/5 increases Pdgfa expression in mouse granulosa cells
To determine whether any of the increases in Pdgfa, Pdgfb, or Vegfa expression in the Smad1/5 dKO tumors were a direct result of Smad1 and Smad5 loss, we co-transfected siRNA specific to both Smad1 and Smad5 into primary cultures of wild type mouse granulosa cells. We used a double knockdown strategy because single conditional deletion of either gene in mice has no effect, while their double conditional deletion causes granulosa cell tumor development (6). Knockdown of Smad1 and Smad5 (Figure 1E and F) resulted in a significant increase in expression of Pdgfa (Figure 1G), but not Pdgfb (Figure 1H), Vegfa, or Vegfb (Supplementary Figure S1A and B). These data suggest that Pdgfa may be a direct downstream target of Smad1/5, and the upregulation of Pdgfb and Vegfa in Smad1/5 dKO tumors likely occurs secondarily to Smad1/5 loss.
Upregulation of Pdgfa in mouse granulosa cells is independent of BMP signalling
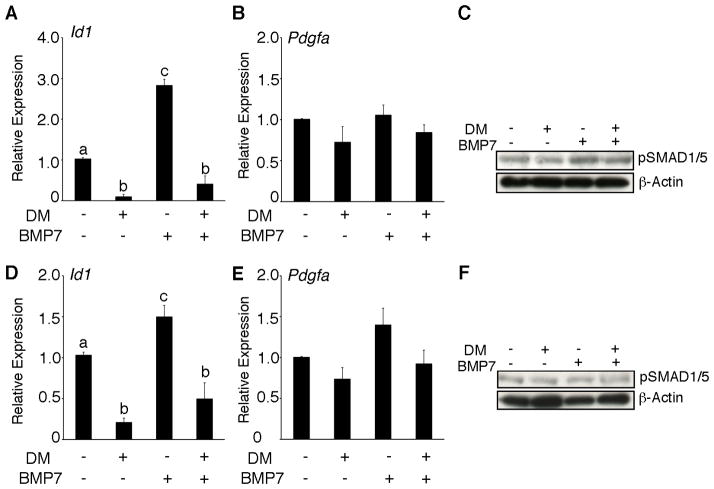
In order to test whether Pdgfa regulation depends upon BMP signaling, wild type mouse granulosa cells were treated with dorsomorphin; (DM) (11), a small molecule inhibitor for ALK1/2/3/6 in combination with BMP7 ligand for 5h and 12h. To validate the efficacy of dorsomorphin treatment and its ability to inhibit BMP7-induced phosphorylation of BMP-responsive SMADs, we evaluated the expression of Id1, the promoter of which is known to be a direct target of BMP signaling through the BR-SMADs (12, 13) and which also has been shown to be downregulated in Smad1/5 dKO tumors (6). Dorsomorphin significantly suppressed BMP7- induced Id1 expression at both time points (Figure 2A and D). In addition, Id1 expression is also significantly downregulated in Smad1/5 knockdown mouse granulosa cells (Supplementary Figure S2A, B and C), indicating that both treatments can effectively block BMP signaling. We expected that dorsomorphin treatment would mimic the siRNA knockdown experiments and lead to an increase in Pdgfa expression. Interestingly, Pdgfa transcript levels were not changed in response to any treatment at both 5h (Figure 2B) and 12h (Figure 2E). Furthermore, in order to determine the activation of major BMP signaling pathway, Western blotting was performed using antiphospho-Smad1/5/8 antibodies to detect phosphorylated (activated) Smad proteins. BMP7 induced phophorylation of SMAD1/5/8 within 5 h of treatment (Figure 2C) and gradually diminished with time (Figure 2F). These data suggest that BR-SMAD regulation of Pdgfa expression in granulosa cells may not be dependent upon phosphorylation of Smad1 or Smad5, but may instead depend on their presence/absence.
Figure 2.
Regulation of Pdgfa is independent of BMP signaling through type I receptor. Cells were the treated with vehicle control medium or with 100 ng/ml BMP7 in the presence or absence of 10 μM dorsomorphin (DM) for 5h and 12h. Cells were harvested for total RNA and protein for real-time PCR and Western blotting. DM markedly reduced Id1 induction by BMP7 at 5h (A) and 12h (D), while no change is observed in Pdgfa expression (B and E). (C and F) Phosphorylation of SMAD1/5/8 in mouse granulosa cells detected by immunoblot after pretreatment with DM for 30 min followed by treatment with BMP7 for 5h and 12h. The membrane was stripped and re-probed for β-actin antibody for loading control. Different letters above the bars indicate statistical significance (n=3; P< 0.05).
Human JGCTs expresses components of PDGF and BMP signaling pathway
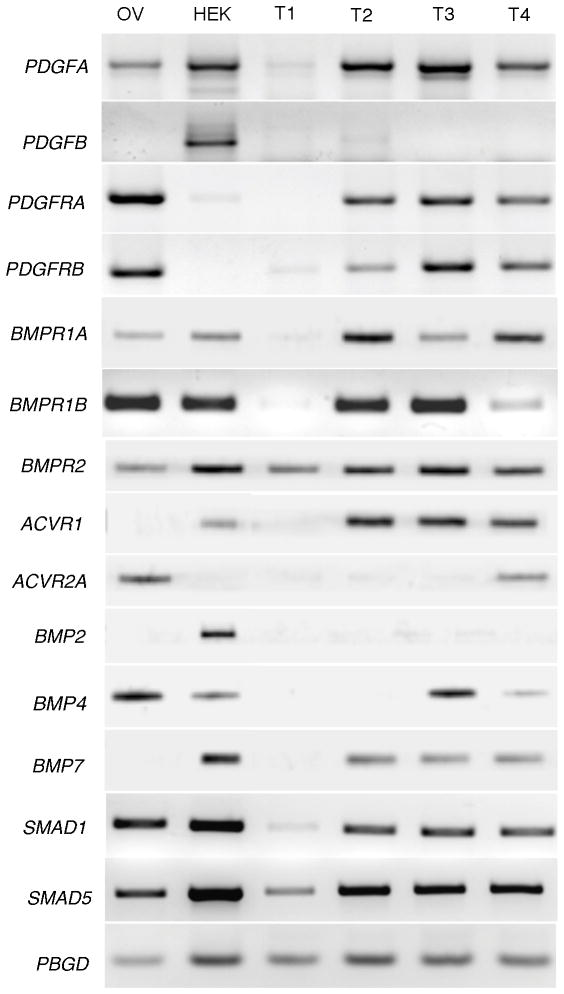
JGCT is a rare disease and expression of PDGF and BMP signaling components in JGCT has not been previously reported. Expression analysis of PDGF pathway signaling components in human JGCTs show that PDGFA and its receptors (PDGFRA and PDGFRB) are expressed in the 75% of the tumors used in this study (Figure 3) indicating that like many other cancers, human JGCTs express PDGF members. Contrary to prior observations of PDGFB expression in the granulosa cells and oocytes in the ovary (14), PDGFB is not expressed in JGCT samples (Figure 3). To address whether human JGCTs possess BMP signaling components, we characterized expression of BMP receptors [BMPR1A/ALK3; BMPR1B/ALK6; BMPR2 and ACVR1/ALK2;] BMP ligands [BMP4 and BMP7] and BR-SMADs [SMAD1 and SMAD5] in the tumors. Although, a majority of the human JGCTs expresses transcripts for most components of BMP signaling pathway (Figure 3), their functional status remains unclear and warrants further investigation.
Figure 3.
Human juvenile granulosa cell tumors express PDGF and BMP receptors and ligands. RT-PCR was performed using RNA from normal human ovary (Ov), human embryonic kidney HEK293T cells (HEK) as a non-JGCT control, or four human JGCT’s (T1-T4) to examine expression of PDGF and BMP signaling components. Porphobilinogen deaminase (PBGD) was used as a loading control for amplification.
Loss of SMAD1/5 in immortalized human granulosa cell tumor-derived cell line (COV434) upregulates PDGFA
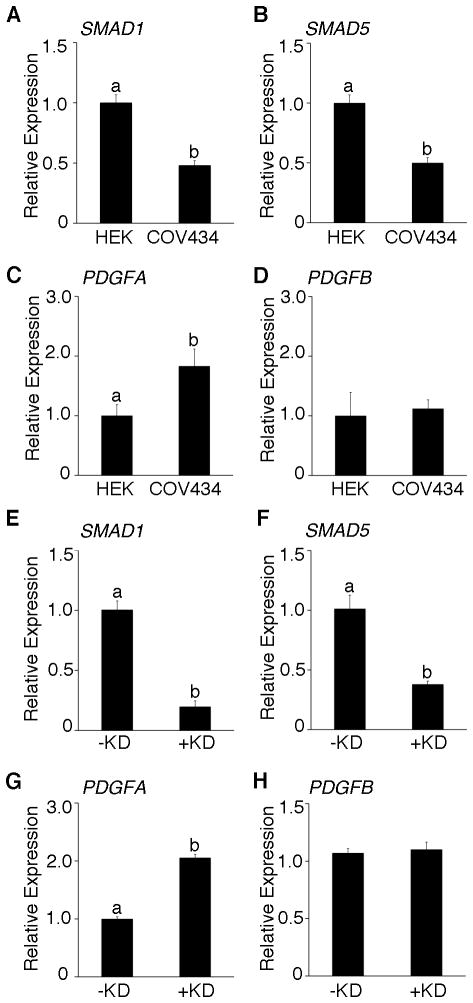
To verify if loss of SMAD1/5 leads to an upregulation of PDGFA in the context of human granulosa cell tumors, we used a well-characterized juvenile human granulosa cell tumor-derived cell line (COV434) (15). COV434 exhibited lower levels of SMAD1/5 (Figure 4A and B) and elevated levels of PDGFA transcripts (Figure 4C), but not PDGFB (Figure 4D), compared to a non-JGCT cell lines (HEK 293T). Further knockdown of residual SMAD1/5 (Figure 4E, F and Supplementary Figure S3) in COV434 cells resulted in a significant upregulation of PDGFA mRNA and protein expression (Figure 4G and Supplementary Figure S3), but not PDGFB (Figure 4H), indicating that BR-SMAD regulation of PDGFA is conserved between mouse and human granulosa cell tumors.
Figure 4.
Loss of SMAD1/5 upregulates PDGFA in a human juvenile granulosa cell tumor-derived cell line (COV434). Real-time PCR was performed to compare the expression levels of SMAD1 (A) and SMAD5 (B), PDGFA (C) and PDGFB (D) in human embryonic kidney cell-line HEK293T (HEK) and human granulosa cell tumor derived cell line (COV434). Transfection of scrambled control siRNA (-KD) or siRNAs specific for SMAD1 and SMAD5 (+KD) resulted in a significant reduction of SMAD1 (E) and SMAD5 (F) in COV434 cells. Concomitantly, a significant 2-fold increase in PDGFA (G) is achieved following SMAD1 and SMAD5 reduction by siRNA, without a measurable change in PDGFB (H). Different letters above the bars indicate statistical significance (n=3; P< 0.05).
BR-SMADs bind to the PDGFA promoter and regulate its activity
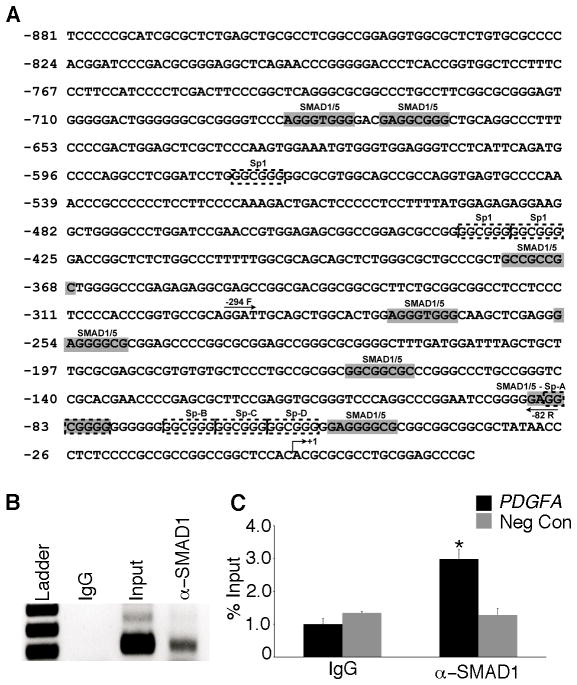
The human PDGFA promoter has been studied extensively, but its regulation by the BR-SMADs has not previously been reported. Putative SMAD1 and SMAD5 interaction sequences have been described previously (16, 17) and using bioinformatics, we identified several putative SMAD1/5 binding sites within the PDGFA proximal promoter (Figure 5A). Importantly, there are two SMAD1/5 downstream binding sites close to the transcription initiation site that overlap a region that is known to contribute 80% of the promoter activity. This region is known to be regulated by Specificity Protein 1 (Sp1), as well as several other transcription factors (18). These findings prompted the hypothesis that SMAD1 and SMAD5 may directly bind to the Pdgfa promoter and mediate its repression by interplay between BR-SMADs and Sp1.
Figure 5.
SMAD1/5 bind to the PDGFA promoter. (A) In silico promoter analysis identified several putative SMAD1/5 binding sites (gray shaded region) in the human PDGFA promoter region. Also shown are binding sites for Sp1 (dashed boxes), a known positive regulator for PDGFA promoter activity. The arrows in the promoter region indicate the sites where forward (-294F) and reverse (-82R) primers are designed for the ChIP assay. (B) Chromatin immunoprecipitation (ChIP) assay with an anti-SMAD1/5 antibody or control IgG was performed on cell extracts from COV434 cells demonstrated the in vivo binding of SMAD1/5 to the PDGFA promoter. (C) Real-time PCR analysis of immunoprecipitated DNA using locus specific primers demonstrated three-fold enrichment (ChIP/Input DNA) of SMAD1/5 occupancy at the PDGFA promoter compared to negative control (no locus-specific primers) and IgG. Asterisks indicate statistical significance (n=3; P< 0.05).
To examine whether SMAD1 and SMAD5 directly interact with the PDGFA promoter, chromatin immunoprecipitation was performed on COV434 cells using an anti-SMAD1 antibody that recognizes both SMAD1 and SMAD5 (16). SMAD1/5 bind to the PDGFA promoter (Figure 5B) and qPCR of immunoprecipitated PDGFA DNA revealed a significant three-fold enrichment of SMAD1/5 at the proximal promoter region (-294F to -82R) relative to IgG and negative control (no-locus specific primers) (Figure 5C). These data clearly demonstrate the specific interaction of BR-SMADs with the PDGFA promoter.
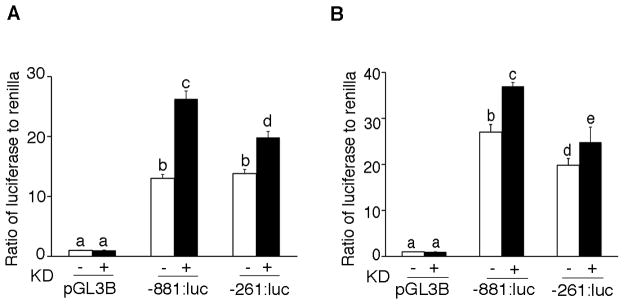
To analyze the ability of SMAD1 and SMAD5 to regulate the PDGFA promoter, we co-transfected luciferase reporter plasmids containing -881 or -261 base pairs upstream of the PDGFA transcriptional start site into wild type mouse granulosa cells and human COV434 cells along with control (scrambled) siRNA or siRNA specific for SMAD1 and SMAD5 (Figure 6A and B). A significant increase in luciferase activity was observed in cells transfected with each luciferase plasmid compared to cells transfected with the promoterless parent vector alone (pGL3-Basic). Cells co-transfected with either the -881:luc or -261:luc in combination with SMAD1 and SMAD5 specific siRNAs exhibited a statistically significant increase in reporter expression, indicating that the BR-SMADs suppress expression of PDGFA. These data also suggest that SMAD binding elements span both of these constructs, though the increase during siRNA knockdown is less in the -261:luc construct than in the longer -881:luc construct.
Figure 6.
Induction of the human PDGFA promoter following knockdown of SMAD1 and SMAD5. Luciferase reporter plasmids containing -881 or -261 base pairs of the human PDGFA promoter were transfected into wild type mouse granulosa cells (A) and human COV434 cells (B) following transfection of control (scrambled) siRNA (-KD) or siRNAs specific for SMAD1 and SMAD5 (+KD) along with the renilla luciferase control plasmid (pRL-TK). Twenty-four hours after transfection, the cells were lysed and assessed for luciferase activity. Luciferase activity from both the constructs was significantly increased in the absence of SMAD1 and SMAD5. Data are presented as the ratio of firefly luciferase to renilla. Different letters above the bars indicate statistically different means by ANOVA and post hoc analysis (n=4; P< 0.05). pGL3B indicates the pGL3-Basic promoterless parent plasmid.
BR-SMADs antagonizes Sp1 activation of the PDGFA promoter
Numerous studies have shown an interaction, either direct or indirect, between the TGFB/activin downstream transcription factor SMAD3 and two well-known positive regulators of PGDFA transcription, Sp1 and early growth response protein 1 (Egr1) (19). In the current study, we did not detect an increase in the expression of Sp1 or Egr1 in Smad1/5 dKO tumor cells compared to wild type mouse granulosa cells, and in fact, Egr1 expression was reduced (Supplementary Figure S4A and B). Additionally, knockdown of Smad1/5 in wild type mouse granulosa cells and COV434 had no effect on Sp1 expression levels (Supplementary Figure S4C and D). Furthermore, mammalian two-hybrid assay experiments revealed that SMAD1/5 did not interact with Sp1 (Supplementary Figure S5). Collectively, these results infer that BR-SMADs likely do not inhibit Sp1 activity by directly binding to Sp1, but instead, compete with Sp1 for binding sites on the promoter.
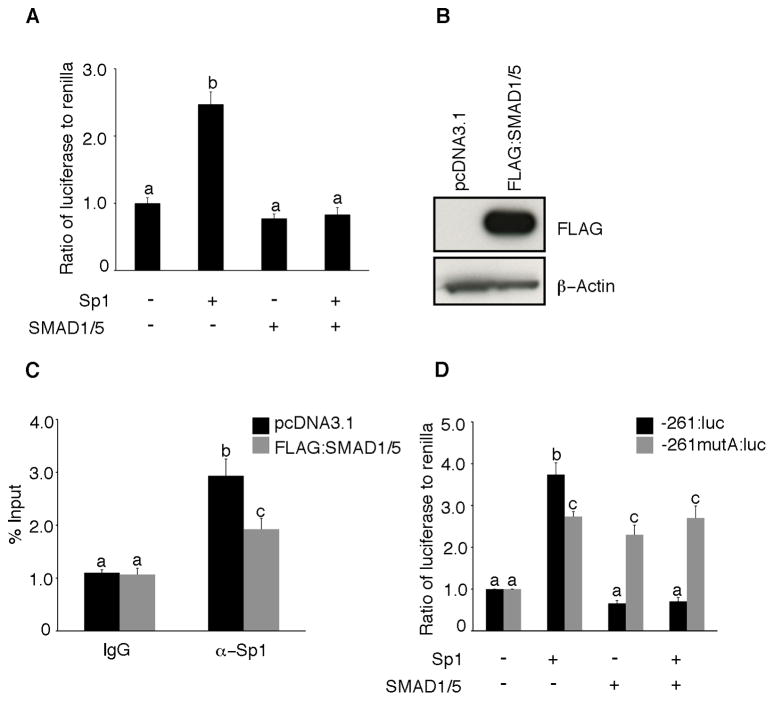
Several transcription factors such as Wilms tumor protein 1 (WT1), GC factor 2 (GCF2), and Nuclear factor I/X (NFI/X) have binding motifs in the PDGFA promoter that overlap with Sp1 binding sites, and overexpression of these factors repressed the activity of the PDGFA promoter by competing with Sp1 for DNA binding (20–22). To determine whether SMAD1/5 has the ability to compete with Sp1, we transiently overexpressed SMAD1/5 and Sp1 in cells expressing the PDGFA luciferase reporter plasmid (-881:luc). As expected, PDGFA promoter-dependent expression was induced more than two-fold upon overexpression of Sp1 alone (Figure 7A). Co-expression of Sp1 with SMAD1/5 resulted in the inhibition of inducible PDGFA promoter activity (Figure 7A), suggesting that SMAD1/5 exerts its repressive activity via a competitive DNA-binding mechanism similar to the other transcriptional repressors of PDGFA.
Figure 7.
SMAD1/5 expression antagonizes Sp1 induction of PDGFA promoter. (A) COV434 cells were transiently co-transfected with 1 μg of -881:luc of the human PDGFA promoter and 0.5 μg of Sp1 and SMAD1/5 along with the renilla luciferase control plasmid (pRL-TK). Forty-eight hours after transfection, the cells were lysed and assessed for luciferase activity. Data are presented as the ratio of firefly luciferase to renilla. (B) Representative Western blot of whole cell lysates from COV434 cells transfected with either pcDNA3.1 or Flag-tagged SMAD1/5 expression plasmids and blotted with mouse anti-FLAG M2 antibody and mouse anti-β-actin antibody (loading control). (C) ChIP analysis in COV434 cells transfected with pcDNA3.1 or Flag-tagged SMAD1/5 expression plasmids. Chromatin cross-linked protein DNA complexes were immunoprecipitated with either anti-Sp1 antibody or with non-specific IgG and the PDGF-A promoter amplified by real-time PCR using locus specific primers. (D) SMAD1/5 represses the wild-type PDGFA promoter (-261:luc) but not the promoter bearing a mutation in the SMAD1/5 binding site (-261mutA: luc). COV434 cells were transiently co-transfected with 1 μg of -261:luc (control) or -261mutA: luc (mutant) and 0.5 μg of Sp1 and SMAD1/5 along with the renilla luciferase control plasmid (pRL-TK). Forty-eight hours after transfection, the cells were lysed and assessed for luciferase activity. Data are presented as the ratio of firefly luciferase to renilla. Different letters above the bars indicate statistically different means by ANOVA and post hoc analysis (n=4; P< 0.05).
To demonstrate whether over expression of BR-SMADs inhibit Sp1 occupancy of the PDGFA promoter, we next performed chromatin immunoprecipitation using Sp1 antibody in COV434 cells transfected with pcDNA3.1 (control) and Flag-tagged SMAD1 and SMAD5. Western blot analysis confirmed the SMAD1/5 overexpression in COV434 cells transfected with Flag-tagged SMAD1/5 expression plasmids compared to control cells transfected with pcDNA3.1 (Figure 7B). Upon overexpression of SMAD1/5, interaction of the SP1 with the PDGFA promoter was significantly decreased compared to the control (Figure 7C). These data suggest that SMAD1/5 compete with Sp1 for binding to the proximal region of the PDGFA promoter.
To gain insight into the potential functional significance of the SMAD1/5 binding site overlapping the 5′-most Sp1 site in the proximal promoter (labeled as SMAD1/5 - Sp-A in Figure 5A), we introduced mutations into site of the PDGFA promoter (-216 mutA: luc). As expected and similar to the previously observed result (Figure 7A), transient co-expression of Sp1 with SMAD1/5 in cells expressing the PDGFA luciferase reporter plasmid (-261: luc; control) resulted in the complete inhibition of inducible PDGFA promoter activity. In contrast, mutation in the SMAD1/5 binding site (GAGGCGGGG to CTGGCGGGG; -261 mutA: luc) failed to repress the promoter activity of PDGFA and Sp1 transactivation upon over-expression of SMAD1/5 (Figure 7D). Collectively, these data and our DNA binding studies suggest that the regulation of PDGFA gene transcription can be governed by interplay between SMAD1/5 and Sp1.
Discussion
Recent advances in elucidating the molecular pathogenetic mechanisms that regulate various signaling pathways in tumorigenesis have allowed for the development of new therapeutic approaches in treating human cancers. In the present study, we demonstrate that Smad1/5 dKO tumors and human JGCTs display elevated levels of PDGFA and we show regulation of PDGFA by BR-SMADs in ovarian granulosa cells and tumors. While conditional deletion of Smad1 and Smad5 in ovarian granulosa cells causes an increase in expression of Pdgfa in tumors, it was unclear if this was a direct or indirect effect of Smad1/5 deletion. However, RNA interference (RNAi), chromatin-immunoprecipitation, and luciferase reporter experiments demonstrate that BR-SMADs directly mediate the expression of PDGFA by binding to conserved SMAD1/5 binding sites in the proximal promoter region of this gene, which have not been previously reported. This novel regulatory mechanism appears to be conserved in both humans and mouse granulosa cells.
The PDGFA promoter is well characterized and regulated by number of transcriptional activators [the (Sp) family and EGR1] and repressors (WT1, GCF2 and NFI/X) (23). Most of these repressors mediate transcriptional repression through competition with the transcriptional activators for common binding sites within the promoter region (18). From our analysis, we observed a SMAD1/5 binding element that overlaps completely with the 5′ most Sp1 site in the PDGFA proximal promoter (labeled as Sp-A in Supplementary Figure S2). Studies in different cell lines have shown that deletion of this region results in a significant decrease of signal in CAT reporter assays, an effect that can be further enhanced when downstream sites (Sp-binding sites B–D) are also deleted (23, 24). Results from the present study demonstrate that the BR-SMADs block Sp1 induction by inhibiting its occupancy of the proximal PDGFA promoter in a similar fashion as the other transcriptional repressors. These findings indicate that under normal conditions in preovulatory granulosa cells, the repression of PDGFA transcription is likely governed by an interplay between BR-SMADs and Sp1 at this site. However, further studies are warranted to understand the functional relevance of the putative SMAD elements on the proximal PDGFA promoter in the context of regulating tumorigenesis.
While the role of BMPs in tumor development is not well described, the dual nature of TGFB to act as both a tumor suppressor or as an oncogene in different cell types is well known. In some human cancers, the TGFB/SMAD2/3 pathway is hyperactivated and in part, TGFB promotes tumor growth and metastasis formation through the induction of various growth factors including PDGFB and VEGF (1). Our published studies show that granulosa cell tumors derived from Smad1/5 dKO mice, as well as JGCT in humans, display active TGFB signaling (6, 7); however, the contribution of dysregulated TGFB signaling to granulosa cell tumor development is not known. Results from the current study demonstrate significant upregulation of Pdgf and Vegf isoforms in the Smad1/5 dKO tumors and metastasis. However, knockdown of Smad1/5 in short-term in vitro cultures of granulosa cells results in the upregulation of Pdgfa, and not Pdgfb or Vegf isoforms. These findings suggest that induction of Pdgfa may acts as an initial/primary event during granulosa cell tumor progression, which may in turn favor these cells to directly or indirectly acquire dysfunctional TGFB-SMAD2/3 signaling and a tumor phenotype. Thus SMAD2/3 signaling in the context of loss of SMAD1/5 could eventually lead to secondary changes such upregulation of Pdgfb and Vegfa that drive additional aspects required for tumor development, such as neovascularization or metastasis.
Overexpression of PDGFA has been reported in several cancers including ovarian clear cell carcinoma (8, 25, 26). In addition, autocrine mechanisms of PDGFA action have been observed in models of glioblastoma that express multiple isoforms of the PDGF ligands and receptors (27). Recent studies exploring the use imatinib mesylate (Gleevec) (a potent tyrosine kinase inhibitor) have shown promising results in the treatment of chronic myeloid leukemia and gastrointestinal stromal tumors, which exhibit activating mutations in PDGFR (28). Granulosa cell tumors (GCTs) are generally considered indolent tumors, but are associated with late recurrences, at which time the survival rate is significantly shortened (29). Previous studies have demonstrated the expression of PDGF receptors in both adult and juvenile forms of GCTs (30, 31), but the function or expression of PDGFA in JGCTs has not been reported. This tumor type is rare, and though our samples size is small, the majority of tumor samples used in the present study exhibited expression of PDGFA, PDGFRA and PDGFRB, suggesting that autocrine PDGF receptor stimulation could contribute to the tumor growth. Our data suggest that the expression of the PDGF pathway may make JGCT or its recurrences a suitable target for treatment with tyrosine kinase inhibitors. This line of treatment is also interesting because of a recent report that imatinib can protect oocytes and follicle development in immature mice that have received the chemotherapeutic agent cisplatin (32).
Collectively, our studies demonstrate that loss or reduced expression of BR-SMADs in granulosa cells thus leads to the upregulation of PDGFA, which may in turn facilitate aspects of tumor growth, angiogenesis and/or metastasis in granulosa cell tumors (Figure 8). These data are relevant not only to granulosa cell tumorigenesis or other tumors that show upregulation of PDGFA, but also have implications for reproductive physiology. Female Smad1/5 dKO mice are subfertile prior to developing granulosa cell tumors (6), though the mechanism underlying their reproductive defect is unknown. While we show that in preovulatory granulosa cells, BR-SMADs suppress expression of PDGFA, the consequence of pathologic expression of PDGFA in Smad1/5 null cells is unknown in part because PDGF function or regulation in the ovary is not well studied. However, our data suggest that BR-SMAD regulation of PDGFA is an initial step in a process that ultimately results in the development of JGCT. Because the PDGF ligands and receptors are also expressed in human JGCT, inhibition of this pathway could thus be exploited as a potential treatment regime.
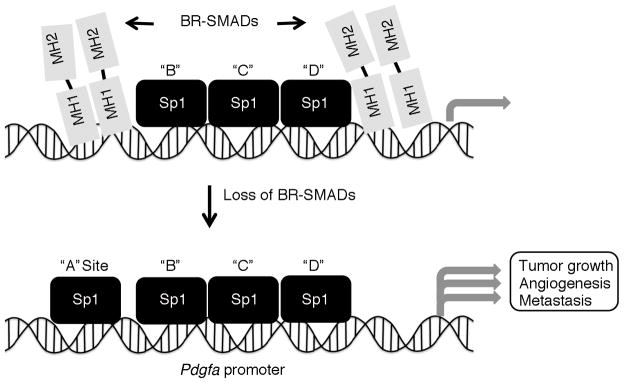
Figure 8.
Model of BR-Smad repression of PDGFA promoter activity. Earlier studies demonstrated that Sp1 mediates the basal transcription of the PDGFA gene through binding to four consensus binding sites in the proximal promoter region (denoted sites “A–D”). Based on ChIP data, we suggest that SMAD1 and SMAD5 bind to the proximal promoter region of PDGFA and block the “A” site for the Sp1 binding, thereby inhibiting gene expression. Loss of the BR-SMADs (SMAD1 and SMAD5) in ovarian granulosa cells thus allows Sp1 to bind to the “A” site and leads to increased PDGFA activation, possibly initiated cell proliferation and contributing to disease onset.
Materials and methods
Granulosa cell culture
All experimental animals were maintained in accordance with the NIH Guide for the Care and Use of Laboratory animals using Institutional Care and Use Committee approved protocols at Baylor College of Medicine. Collection and culture of wild type mouse granulosa cells were performed as previously described (33).
Human samples
Archived, de-identified human JGCT samples from surgical resections were acquired from the Department of Pathology’s Tissue Bank at Texas Children’s Hospital (Houston, TX). Tissues were treated with in accordance with Baylor College of Medicine Institutional Review Board and a waiver of consent was approved (Institutional Review Board no. H-23139). Normal human ovary RNA was purchased from Promega.
COV434 cell culture
The COV434 line of immortalized granulosa cells derived from a primary human granulosa cell tumor was purchased from Health Protection Agency (HPA) culture collections, Salisbury, UK. COV434 cells were maintained in Dulbecco’s modified Eagle’s medium-F12 (DMEM-F12) with GlutaMAX (Invitrogen) and supplemented with 10% FBS (Invitrogen) and 10U/ml penicillin and streptomycin (Invitrogen) at 37°C in a humidified atmosphere of 5% CO2.
SMAD1/5 siRNA transfection
siRNA transfections were performed using the Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer’s instructions. Briefly, cells were plated overnight in medium containing 10% FBS prior to transfection. Three hours before transfection medium was removed and cells were rinsed once with PBS and replaced with fresh medium. Scrambled control (“mock”) siRNA or combined SMAD1 and SMAD5 siRNAs (Ambion) were transfected at a concentration of 50μM. The cells were cultured with siRNA complexes for 12 hours, followed by a change in the medium, and an additional 24 h culture for mouse granulosa cells and 48h culture for COV434 before harvesting for analysis. We did not observe any difference in the knockdown levels of SMAD1 and SMAD5 in COV434 incubated at 24h or 48h. For small molecule inhibitor assay, wild type mouse granulosa cells were treated with vehicle control buffer (0.1% DMSO), 10μM dorsomorphin (Calbiochem) and rhBMP7 (100ng/ml) (R&D biosystems) preincubated with/without dorsomorphin. Cells were harvested for RNA and protein after 5hr and 12 hr of treatment.
RT-PCR and qPCR analysis
Total RNA from cells and tumor tissues were extracted using RNeasy Micro or Mini Kit (Qiagen) according to the manufacturer’s instructions cDNA synthesis from total RNA was performed using High Capacity RNA to cDNA Master Mix (Applied Biosystems) in accordance with the manufactures instructions. Real time quantitative PCR was performed using an Applied Biosystems Prism 7500 or Step-One Plus sequence detection system and data were analyzed according to the ΔΔCT method and expressed relative to the control mean (set to equal “1”) as described previously (6). All primer sequences used for real-time and semi-quantitative PCR are provided in Supplemental Table 1.
Protein extraction and Western Blotting
Primary mouse granulosa cells and human COV434 cells were lysed in RIPA buffer with protease inhibitors. Lystaes were incubated on ice for 30 min and centrifuged at 13,200xg for 10 min at 4 °C and were boiled for 10 min before loading. Protein samples were separated on a 4–12% gradient polyacrylamide gel (Invitrogen) and electroblotted onto a polyvinylindene difluoride (PVDF) membrane (Invitrogen). Following transfer and blocking in 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for one hour, the membrane was then incubated overnight at 4 °C with rabbit anti-phospho-Smad1/5 (Ser463/465) antibody (Cell Signaling, 41D10) diluted at 1:1000 or mouse anti-human SMAD1 antibody (Biomatrix research, BMR 00479) diluted at 1:500 or rabbit anti-human PDGF-A antibody (Santa Cruz, N-30) diluted at 1:500 or mouse anti-FLAG M2 antibody (Sigma- Aldrich, A8592) diluted at 1:1000 or mouse anti-β-actin antibody (Sigma- Aldrich, A5316) diluted at 1:10,000. The membrane is washed for three times 5 min each and then incubated with peroxidase labeled secondary antibody diluted at 1:10,000 in the blocking solution. The membrane was washed again with TBST, followed by detection with SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL).
Promoter analysis rVISTA
software (34) was used to identify putative SMAD1 and SMAD5 transcription factor binding sites within the human PDGFA proximal promoter region.
Plasmid Constructs
The plasmids -881:luc and -261:luc containing 881bp and 261 bp of the human PDGFA-promoter upstream of Firefly luciferase were obtained from Dr. David Kaetzel and Marian Novak (University of Kentucky). Mutant form of -261:luc plasmid, -261mutA:luc was constructed using the GeneArt site-directed mutagenesis kit (Invitrogen) in accordance with the manufacturer’s instructions. The expression plasmids FLAG: SMAD1 was obtained from Xin-Hua Feng (Baylor college of medicine), FLAG: SMAD5, pcDNA3.1: Sp1, pACT: SMAD1, pACT: SMAD5 and pBIND: Sp1 plasmids were generated by PCR-based technique in our laboratory. Primer sequences used for cloning are provided in Supplemental Table 1.
Transient transfection and luciferase assays
Wild type mouse granulosa cells and COV434 cells were seeded in 24-well tissue culture plate for 24 hours prior to transfection. When ~70–80% confluent, the cells were transfected with respective plasmids and incubated for 24h followed by SMAD1/5 siRNA transfection as mentioned above. Transfections also contained the renilla luciferase plasmid (pRL-TK) (Promega) as a control for transfection efficiency. Luciferase plasmids were transfected using FuGENE HD transfection reagent (Roche Molecular Biochemicals) according to the manufacturer’s protocol. Luciferase activity of the cell lysates was assessed 48h after transfection using the Dual-luciferase Reporter Assay System (Promega). For overexpression of Flag tagged SMAD1 and SMAD5, COV434 cells were plated at a density of 6×104 cells per well in 6-well tissue culture plate for 24h prior to transfection. Cells were transfected using FuGENE HD transfection reagent (Roche Molecular Biochemicals) according to the manufacturer’s protocol and incubated for 72h before being harvested for analysis.
Chromatin immunoprecipitation assay (ChIP)
ChIP assays were performed using Imprint Chromatin Immunoprecipitation Kit (Sigma-Aldrich, St. Louis, USA) according to the manufacturer’s protocol using an anti-Smad1/5 antibody (BMR 00479; Bio Matrix Research, Chiba, Japan) and Sp1 antibody (17–601; Millipore). Primer sequences used for ChIP analysis are provided in Supplemental Table 1.
Mammalian Two-Hybrid System
Interaction between BR-SMADs and Sp1 were examined using the CheckMateTM/FlexiH Vector Mammalian Two- Hybrid System (Promega) according to the manufacturers instructions. Briefly, mammalian two-hybrid vectors pACT and pBIND, which express VP16-SMAD1 and SMAD5 and GAL4-Sp1 fusion proteins respectively, were constructed and cotransfected with pGL4Cherry [mCherry/GAL4UAS/Hygro] vector (35) in COV434 cells using FuGENE HD Transfection Reagent (Roche Molecular Biochemicals) according to the manufacturer’s protocol pBIND vector was modified to expresses yellow fluorescent protein (YFP) by separate promoter as a control for transfection efficiency (35). Forty-eight hours later the transfected cells were lysed using cell lysis buffer from Dual-luciferase Reporter Assay system kit (Promega) and red fluorescence (mCherry) and yellow fluorescence (YFP) were measured using a POLAR Star Omega microplate reader (BMG LabTech, Offenburg, Germany). Protein interactions were quantified using the ratio of red fluorescence to yellow fluorescence.
Statistical analysis
Statistical analyses were performed using the GraphPad Prism 5 (GraphPad Software, La Jolla, CA) or SPSS Statistics v.19 (IBM). Single comparisons were carried out using two-tailed, unpaired t-tests and P<0.05 was considered statistically significant. Multiple comparisons were carried out using ANOVA followed by Fisher’s Least Significant Difference (LSD) test. All replicates included four independent experiments unless otherwise indicated in the figure or figure legend.
Supplementary Material
Acknowledgments
This work was supported by a Burroughs Wellcome Career award in Biomedical Sciences and a NIH CA138628 to SAP. The authors would like to thank Rebecca James, Hermann Piard, and Yuxuan Lin for technical assistance and Nadera Mansouri-Attia, Krishna Jagarlamudi for critical reading of manuscript.
Footnotes
Conflict of interest: The authors declare no conflict of interest
References
- 1.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 2.Thawani JP, Wang AC, Than KD, Lin C-Y, La Marca F, Park P. Bone morphogenetic proteins and cancer: review of the literature. Neurosurgery. 2010;66:233–246. doi: 10.1227/01.NEU.0000363722.42097.C2. [DOI] [PubMed] [Google Scholar]
- 3.Howe JR, Bair JL, Sayed MG, Anderson ME, Mitros FA, Petersen GM, et al. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet. 2001;28:184–187. doi: 10.1038/88919. [DOI] [PubMed] [Google Scholar]
- 4.Kodach LL, Wiercinska E, de Miranda NFCC, Bleuming SA, Musler AR, Peppelenbosch MP, et al. The bone morphogenetic protein pathway is inactivated in the majority of sporadic colorectal cancers. Gastroenterology. 2008;134:1332–13341. doi: 10.1053/j.gastro.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 5.Edson MA, Nalam RL, Clementi C, Franco HL, DeMayo FJ, Lyons KM, et al. Granulosa cell-expressed BMPR1A and BMPR1B have unique functions in regulating fertility but act redundantly to suppress ovarian tumor development. Mol Endocrinol. 2010;24:1251–1266. doi: 10.1210/me.2009-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, et al. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol. 2008;28:248–257. doi: 10.1128/MCB.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middlebrook BS, Eldin K, Li X, Shivasankaran S, Pangas SA. Smad1-Smad5 ovarian conditional knockout mice develop a disease profile similar to the juvenile form of human granulosa cell tumors. Endocrinology. 2009;150:5208–5217. doi: 10.1210/en.2009-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhrbom L, Hesselager G, Nistér M, Westermark B. Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res. 1998;58:5275–5279. [PubMed] [Google Scholar]
- 10.Bruna A, Darken RS, Rojo F, Ocaña A, Peñuelas S, Arias A, et al. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11:147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korchynskyi O, Ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 13.López-Rovira T, Chalaux E, Massagué J, Rosa JL, Ventura F. Direct binding of Smad1 and Smad4 to two distinct motifs mediates bone morphogenetic protein-specific transcriptional activation of Id1 gene. J Biol Chem. 2002;277:3176–3185. doi: 10.1074/jbc.M106826200. [DOI] [PubMed] [Google Scholar]
- 14.Pinkas H, Fisch B, Rozansky G, Felz C, Kessler-Icekson G, Krissi H, et al. Platelet-derived growth factors (PDGF-A and -B) and their receptors in human fetal and adult ovaries. Molecular Human Reproduction. 2008;14:199–206. doi: 10.1093/molehr/gan011. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Vollmer M, De Geyter M, Litzistorf Y, Ladewig A, Dürrenberger M, et al. Characterization of an immortalized human granulosa cell line (COV434) Molecular Human Reproduction. 2000;6:146–153. doi: 10.1093/molehr/6.2.146. [DOI] [PubMed] [Google Scholar]
- 16.Morikawa M, Koinuma D, Tsutsumi S, Vasilaki E, Kanki Y, Heldin C-H, et al. ChIP-seq reveals cell type-specific binding patterns of BMP-specific Smads and a novel binding motif. Nucleic Acids Res. 2011;39:8712–8727. doi: 10.1093/nar/gkr572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fei T, Xia K, Li Z, Zhou B, Zhu S, Chen H, et al. Genome-wide mapping of SMAD target genes reveals the role of BMP signaling in embryonic stem cell fate determination. Genome Res. 2010;20:36–44. doi: 10.1101/gr.092114.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaetzel DM. Transcription of the platelet-derived growth factor A-chain gene. Cytokine Growth Factor Rev. 2003;14:427–46. doi: 10.1016/s1359-6101(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 19.Fortin J, Bernard DJ. SMAD3 and EGR1 physically and functionally interact in promoter-specific fashion. Cell Signal. 2010;22:936–43. doi: 10.1016/j.cellsig.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Gashler AL, Bonthron DT, Madden SL, Rauscher FJ, Collins T, Sukhatme VP. Human platelet-derived growth factor A chain is transcriptionally repressed by the Wilms tumor suppressor WT1. Proc Natl Acad Sci USA. 1992;89:10984–10988. doi: 10.1073/pnas.89.22.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khachigian LM, Santiago FS, Rafty LA, Chan OL, Delbridge GJ, Bobik A, et al. GC factor 2 represses platelet-derived growth factor A-chain gene transcription and is itself induced by arterial injury. Circ Res. 1999;84:1258–1267. doi: 10.1161/01.res.84.11.1258. [DOI] [PubMed] [Google Scholar]
- 22.Rafty LA, Santiago FS, Khachigian LM. NF1/X represses PDGF A-chain transcription by interacting with Sp1 and antagonizing Sp1 occupancy of the promoter. EMBO J. 2002;21:334–343. doi: 10.1093/emboj/21.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khachigian LM, Williams AJ, Collins T. Interplay of Sp1 and Egr-1 in the proximal platelet-derived growth factor A-chain promoter in cultured vascular endothelial cells. J Biol Chem. 1995;270:27679–27686. doi: 10.1074/jbc.270.46.27679. [DOI] [PubMed] [Google Scholar]
- 24.Kaetzel DM, Maul RS, Liu B, Bonthron D, Fenstermaker RA, Coyne DW. Platelet-derived growth factor A-chain gene transcription is mediated by positive and negative regulatory regions in the promoter. Biochem J. 1994;301:321–327. doi: 10.1042/bj3010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann A-C, Mori R, Vallbohmer D, Brabender J, Klein E, Drebber U, et al. High expression of HIF1a is a predictor of clinical outcome in patients with pancreatic ductal adenocarcinomas and correlated to PDGFA, VEGF, and bFGF. Neoplasia. 2008;10:674–679. doi: 10.1593/neo.08292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto S, Tsuda H, Takano M, Kita T, Kudoh K, Furuya K, et al. Expression of platelet-derived growth factors and their receptors in ovarian clear-cell carcinoma and its putative precursors. Mod Pathol. 2008;21:115–124. doi: 10.1038/modpathol.3800984. [DOI] [PubMed] [Google Scholar]
- 27.Shih AH, Holland EC. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006;232:139–147. doi: 10.1016/j.canlet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 28.George D. Targeting PDGF receptors in cancer--rationales and proof of concept clinical trials. Adv Exp Med Biol. 2003;532:141–151. doi: 10.1007/978-1-4615-0081-0_12. [DOI] [PubMed] [Google Scholar]
- 29.Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003;21:1180–1189. doi: 10.1200/JCO.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Rocconi RP, Matthews KS, Kimball KJ, Conner MG, Baker AC, Barnes MN. Expression of c-kit and platelet-derived growth factor receptors in ovarian granulosa cell tumors. Reprod Sci. 2008;15:673–677. doi: 10.1177/1933719108317584. [DOI] [PubMed] [Google Scholar]
- 31.Chu S, Alexiadis M, Fuller PJ. Expression, mutational analysis and in vitro response of imatinib mesylate and nilotinib target genes in ovarian granulosa cell tumors. Gynecol Oncol. 2008;108:182–190. doi: 10.1016/j.ygyno.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15:1179–1185. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- 33.Pangas SA, Jorgez CJ, Matzuk MM. Growth differentiation factor 9 regulates expression of the bone morphogenetic protein antagonist gremlin. J Biol Chem. 2004;279:32281–32286. doi: 10.1074/jbc.M403212200. [DOI] [PubMed] [Google Scholar]
- 34.Loots GG, Ovcharenko I, Pachter L, Dubchak I, Rubin EM. rVista for comparative sequence-based discovery of functional transcription factor binding sites. Genome Res. 2002;12:832–839. doi: 10.1101/gr.225502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwamori T, Iwamori N, Ma L, Edson MA, Greenbaum MP, Matzuk MM. TEX14 interacts with CEP55 to block cell abscission. Mol Cell Biol. 2010;30:2280–22892. doi: 10.1128/MCB.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.