Abstract
Rationale and Objectives
T1ρ, dGEMRIC and T2-mapping have shown sensitivity toward different osteoarthritic-associated compositional changes after joint injury, but have not been studied concomitantly in vivo. We hypothesized that these MRI sequences can be used to measure early glycosaminoglycan (GAG) losses and collagen disruption in cartilage of ACL rupture patients.
Materials and Methods
Thirteen acute ACL rupture patients were each imaged during a four hour pre-surgery work-up to acquire a fast-spin-echo-based T1ρ sequence, a multi-echo spin-echo T2 sequence, and a T1-weighted inversion recovery sequence with a gadolinium contrast agent (dGEMRIC) an average of 55.7 days post-injury. After acquisition, the three sequences’ relaxation times were analytically compared.
Results
Site-specific differences were evinced, but non-significant differences in mean relaxation time between layers of the same region and sequence were observed (ANOVA, p<0.05). Spearman’s correlation coefficients of 0.542 (T1ρ vs. T2, p<0.05), −0.026 (T1ρ vs. dGEMRIC, p=0.585) and −0.095 (T2 vs. dGEMRIC, p<0.05), were found.
Conclusion
No appreciable focal GAG loss was detected by dGEMRIC, and T2 was generally elevated in the early acute phase of blunt trauma injury. In contrast, both general and focal elevations in T1ρ relaxation times were identified, indicating an acute increase in unbound water in the matrix after blunt trauma, and show that patient-specific cartilage changes occur within otherwise healthy, young patients. Further investigation into each sequence’s long-term significance is warranted to help clinicians decide which sequence(s) will be the most useful for osteoarthritis prognosis given the challenge of concomitantly acquiring all three in a busy clinical setting.
Keywords: Quantitative MRI, Cartilage, ACL-rupture, Injury assessment
INTRODUCTION
Articular cartilage injuries that lead to post-traumatic osteoarthritis (PTOA) include injury-induced cell death, matrix degradation, cartilage fissures, and alterations in cartilage material and mechanical properties.(1–3) One of the most common joint injuries with damage to articular surfaces in the absence of osteochondral fractures or overt articular surface injury is in the knee with an acute ACL tear. At the time of knee arthroscopy, visible evidence of cartilage injury is not observed in the majority of cases.(4, 5) Morphologic changes in cartilage are less likely to be observed in the first year after ACL reconstruction, compared to imaging sessions observed years after injury, when a patient is closer to OA development.(2, 6) However, within 15 years of injury a large percentage of patients show evidence of structural abnormalities associated with knee osteoarthritis on conventional radiographs, morphologic magnetic resonance imaging (MRI), or arthroscopy.(3, 7, 8) The ability to detect these changes early in the process of developing post-traumatic osteoarthritis (i.e. imaging biomarkers) would be a significant advance as it increases the possibility of intervention before significant joint deterioration.
Emerging quantitative cartilage imaging techniques including T1ρ, T2 mapping and delayed gadolinium enhanced MRI of cartilage (dGEMRIC) are more promising than morphological sequences in acute injury assessment. Evidence in the literature indicates that each of these imaging sequences probes different biologic markers of cartilage degeneration. Researchers have been working to define what each of these quantitative sequences measures. T2 mapping correlates predominately with cartilage hydration and collagen content.(9, 10) T1ρ is a relaxation measurement that probes the rate of exchange between protons of free water and those from water associated with macromolecules in the cartilage’s extra-cellular matrix, giving rise to longer relaxation times where components of the extra-cellular matrix, especially proteoglycans, are disrupted.(11–13) dGEMRIC measures T1 relaxation changes after an intravenous injection of a charged gadolinium-based contrast agent to directly measure the fixed charge density arising from glycosaminoglycan chains of proteoglycan.(14)
Direct comparisons of T1ρ and T2 results have recently been reported, and suggest that T1ρ is more sensitive in detecting cartilage changes associated with osteoarthritis. T1ρ relaxation has been shown to correlate to proteoglycan content in explant samples and differentiate between various grades of OA,(12, 15, 16) whereas T2 relaxation times are less sensitive to proteoglycan-associated osteoarthritic changes in the knee.(12, 17–19) In one case study, a structural lesion discovered during arthroscopy was not identified by morphometric sequences, yet was present as a “lesion” on T1ρ.(20) This observation agrees with other studies in which a known deficiency in cartilage structure and GAG content resulted in elevated T1ρ relaxation times, but T2 values were within “normal” range.(18, 21) In the in vivo study of ACL patients by Li et al.(22), patients’ T1ρ relaxation times varied over the course of one year and differed significantly from those of normal subjects, whereas T2-mapping of the same individuals showed no significant differences between groups. Nishioka et al.(23) also reported that T1ρ imaging (in vivo, prior to resection) had a stronger correlation than T2 to Osteoarthritis Research Society International (OARSI) grades(24) and extracellular matrix components of resected tibial plateaus from primary TKA patients (95% of whom had Grade 4 degeneration on the Kellgren-Lawrence(25) scale). T1ρ and dGEMRIC have also been studied previously in explants for their underlying relationship by Taylor et al., but a relatively weak correlation was found.(9)
This study compares early cartilage changes measured by all three quantitative T2, dGEMRIC, and T1ρ MRI sequences on the day of surgery workup prior to ACL reconstruction. The relative abilities of each sequence to detect early changes in cartilage after ACL injury, and to demonstrate sensitivity to local compositional changes are presented as potentially clinically feasible biomarkers of cartilage health in the early stages after joint injury. While no current technique has been validated to reduce the incidence and severity of post-traumatic osteoarthritis, presumably the earlier such a treatment could be applied, the more likely the treatment would result in positive patient outcomes; thus an early imaging-detection technique would be desirable for such potential interventional treatment.
MATERIALS AND METHODS
Inclusion Criteria and Subjects
Patients between the ages of 18 and 35 years who had a first-time ACL tear confirmed by standard morphologic MRI and physical examination by a sports fellowship trained orthopaedic surgeon were recruited within 72 hours of confirmed ACL injury. Potential subjects were excluded if MRI was contraindicated (i.e. presence of certain metal implants/shrapnel, claustrophobia, anxiety and/or panic disorders). If a subject’s estimated glomerular filtration rate (eGFR, for determining serum creatinine clearance levels) was calculated as below 60 ml/min, the subject was excluded from receiving the dGEMRIC series as per recommendation of the IRB’s Pharmacy and Therapeutics Subcommittee. This recommendation is based on FDA-recognized risks for Nephrogenic Systemic Fibrosis or Nephrogenic Fibrosing Dermopathy (NSF/NFD)(26). This study was approved by the University’s Human Subjects Research Biomedical Institutional Review Board. Written informed consent was obtained prior to enrollment and testing after the nature of the study had been explained.
Thirteen subjects participated in this same-day quantitative imaging study (7 men and 6 women) an average of 55.7 days after injury (range: 7–136 days). Mean age was 23.2 years at time of injury (range: 18–29) and the average body mass index (BMI) was 27.9 (range: 22.4–41.5). Twelve of the patients underwent ACL reconstruction surgery an average of 52.9 days after injury (range: 11 to 150 days); one subject opted out of reconstruction (Table 1). During arthroscopic assisted ACL reconstruction, no visual cartilage changes were observed even with the majority (11 of 13) of patients having bone contusions present near at least one of the knee articular surfaces on their pre-operative MRIs.
Table 1.
Acute ACL-rupture Patients’ Demographics
| ACL Patient | Gender | Age | Height (m) | Weight (kg) | BMI* | Knee Imaged | BC† | Injury to Pre-Op Scan (weeks) |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 19 | 1.89 | 84.0 | 24.4 | Left | Yes | 1.6 |
| 2 | F | 20 | 1.70 | 71.2 | 24.9 | Right | No | 1.3 |
| 3 | M | 28 | 1.73 | 101.0 | 33.7 | Left | Yes | 4.4 |
| 4 | M | 25 | 1.98 | 122. 5 | 30.4 | Right | Yes | 19.0 |
| 5 | F | 22 | 1.70 | 64.0 | 22.5 | Left | Yes | 8.3 |
| 6 | F | 18 | 1.78 | 77.1 | 24.3 | Left | Yes | 5.9 |
| 7 | M | 29 | 1.73 | 66.0 | 22.4 | Left | Yes | 11.1 |
| 8 | M | 24 | 1.78 | 92.4 | 27.5 | Left | Yes | 4.1 |
| 9 | M | 22 | 1.78 | 74.0 | 25.8 | Right | No | 1.3 |
| 10 | F | 23 | 1.70 | 120.00 | 41.50 | Left | Yes | 19.4 |
| 11 | F | 20 | 1.72 | 70.00 | 23.70 | Left | Yes | 1.0 |
| 12 | M | 25 | 1.78 | 95.90 | 30.30 | Left | Yes | 17.1 |
| 13 | M | 22 | 1.78 | 100.00 | 31.60 | Left | Yes | 8.9 |
|
| ||||||||
| Average | - | 23.2 | 1.76 | 84.6 | 27.9 | - | - | 8.0 |
= body mass index
= presence of a bone contusion
Imaging
Thirteen knee imaging studies were performed on a 3 Tesla Siemens TIM Trio scanner (Siemens Medical Solutions, Erlangen, Germany) using a single channel transmit-receive extremity coil. A complete quantitative cartilage imaging study (T1ρ, T2 and dGEMRIC) was collected in two sessions within a four hour period (Figure 1).
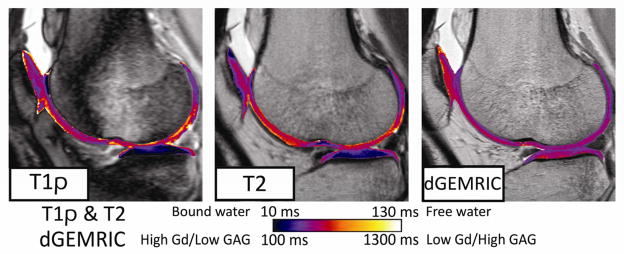
Figure 1.
Patient 6’s pre-surgery, same-day imaging study of cartilage-specific T1ρ, dGEMRIC and T2 MRI 3.1 weeks after the initial ACL injury. A bone contusion and more focal elevations are present in the T1ρ panel at left. T2 relaxation times are globally elevated compared to the T1ρ map, re-affirming that increased fluid content (T2 relaxation times increase) shortly after injury is occurring on a whole-joint level, whereas T1ρ is also affected by PG content and thus displays different relaxation patterns.
The first imaging session combined T1ρ and T2 imaging to assess the same sagittal plane concurrently. This imaging session lasted approximately one hour. For T1ρ imaging, a magnetization preparation preceded a standard fast spin-echo (FSE) imaging protocol (TR=3000ms, TE=9.5ms, in-plane resolution=0.55mm, FOV=140mm, slice thickness=4mm, spacing between slice centers=8mm). The T1ρ magnetization preparation step consisted of a +90°x tip-down, 400 Hz self-compensating spin-lock RF of variable duration (5, 10, 20, 40, 60, 80ms) for T1ρ weighting, and a −90°x tip-up and final crusher gradient.(27) Quantitative T2 spin-echo images were obtained at seven different TE times with a multi-echo spin-echo pulse sequence, similar to one used by the Osteoarthritis Initiative study on Siemens 3T Trio platforms (TE=13.8, 27.6, 41.4, 55.2, 69.0, 82.8 and 96.6ms, TR=2000ms, in-plane resolution=0.55mm, FOV=140mm, slice thickness=3mm, spacing between slice centers=6mm).(28)
Immediately following the first (non-contrast) imaging session and after verification of normal glomerular filtration rate, subjects received an IV injection of Gd-DTPA2– (Magnevist®; Bayer Healthcare, Wayne, NJ, USA) at 0.15 mmol/kg body weight. To ensure proper infiltration of the contrast agent into the cartilage, 30 minutes of walking and stair-climbing (when possible) was completed by the patients. The second imaging session was performed two hours after contrast agent injection. This second imaging session was focused on acquiring the dGEMRIC images and lasted approximately 30 minutes. The dGEMRIC images were based on a standard inversion recovery FSE pulse sequence, at 7 slice locations from the trochlea to the edge of the lateral condyle, encompassing locations of the T1ρ and T2 images (TI=30, 100, 250, 500, 1000, 1500ms, TR=4000ms, in-plane resolution=0.3mm, FOV=140mm, slice thickness=3mm, spacing between slices=6mm).
Image Post-Processing
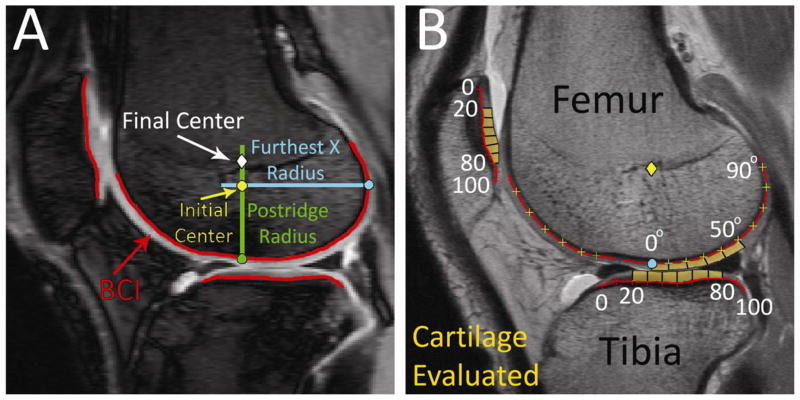
After collection and registration of images to single slice locations at the midline of the lateral femoral condyle, relaxation times were calculated on a voxel-by-voxel basis for each patient’s dataset.(9, 29) An in-house iterative non-linear exponential curve-fitting algorithm (MATLAB, MathWorks Inc., Natick, MA) generated relaxation maps for all three techniques. Direct geometric alignment of MRI images was achieved using a feature-based image co-registration strategy (Figure 2A). This technique objectively identified the bony ridge between the sulcus and the posterior condyle, and co-registered the bone-cartilage boundary within voxel accuracy for different sequences from separate imaging sessions.(30) The posterior condyle radius provided an anthropometric scalar for inter-subject knee size. Sampling at one-degree increments generated geometrically normalized voxel data for inter-subject comparisons, augmented by cartilage zone depth profiles of adjacent sagittal cartilage locations.
Figure 2.
A) Automated image co-registration is achieved by feature-based sulcus location along an objective bone-cartilage-interface (BCI) with anthropometric scaling to posterior condyle radius for inter-knee comparisons in the 20ms T1ρ image of ACL Patient 6. B) Each location of the cartilage examined (orange) was subdivided into smaller regions, as shown in the same patient’s dGEMRIC image. Relaxation times within each subdivision’s radial and transitional cartilage zones were averaged and reported for each patient’s dataset.
Identification and tracking of small but significant focal areas of cartilage is challenging in sequential imaging studies due to random variations associated with single-voxel relaxation measurements and the effective resolution reduction inherent in averaging relaxation times over subjectively identified regions. To address these challenges, the three cartilage imaging sequences were analyzed 1.0mm (cartilage radial zone) and 1.5mm (transitional zone) superficial to the bone-cartilage interface (cartilage deep zone) to avoid voxel partial-volume effects, and to avoid deep contrast infiltration variability so as not to skew dGEMRIC relaxation times for inter-sequence comparisons, and to investigate each sequence’s ability to differentiate zonal layers (lamination in Li et.al’s study of T1ρ and T2(22)). Values in the posterior femoral condyle were recorded from 0–50° from the central reference point (approximate center of rotation in the femur) encompassing weight-bearing cartilage (Figure 2B).(31) Cartilage relaxation times on the terminal sulcus (anterior to 0° in Figure 2B) were not inclu ded in calculations due to potential partial volume averaging between the cartilage and the joint fluid signals. Superior and inferior limits of the patellar cartilage and the anterior and posterior limits of the tibial cartilage were identified. To eliminate artifacts at the margins of the cartilage, only values from 20% to 80% of the region lengths (60 locations) were considered in comparative analyses (Figure 2B). Next, the raw relaxation times from each region were averaged together for every 10 values. In the femur, this yielded five final relaxation samples per cartilage zone in each patient’s scan, since every 10° along the femur was averaged together. Similarly, in the patella and tibial plateau, every 10 raw relaxation times along the normalized length were averaged together to produce six final relaxation values per patient’s scan. This approach to data selection and management yields robust signal-to-noise and location-specific results. Averaging relaxation times belonging to small regions reduced the potential effect of noise-contaminated single-voxel calculations of relaxation times while preserving spatial variability that would be lost by defining whole regions (i.e. one value for the entire tibial plateau, a common approach when examining data from quantitative relaxation maps).(21, 23, 31)
Data Analysis
The values of each quantitative sequence were examined for differences between layers, knee regions, and overall focal sensitivity. Average relaxation times for each patient’s region and cartilage layer were determined to observe overall variability between subjects. Next, all patients’ data from each region and layer were combined into similar groups for each MRI sequence. A one-way ANOVA test was performed to compare these groups of data against the null hypothesis that all samples were drawn from populations with the same mean (followed by Tukey’s honestly significant criterion multiple comparison procedure to determine which sets of data were significantly different). The level of significance was set at α = 0.05. The resulting p-value revealed whether there were differences between the layers’ mean values within each region, and also if there were differences in relaxation times between regions. Focal sensitivity was determined for each sequence by combining each patient’s relaxation times along the multiple subregions within the knee. Finally, relationships between T1ρ, T2, and dGEMRIC relaxation times were assessed by Spearman’s rank correlation coefficient with significance set at p<0.05. The University’s Human Subjects Research Biomedical Institutional Review Board approved this study.
RESULTS
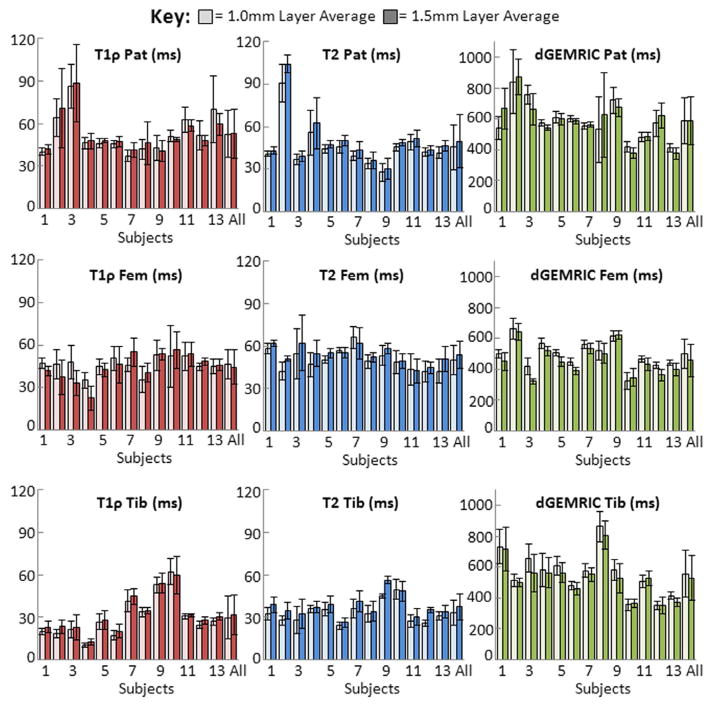
Patients’ relaxation times varied between sites (patella, posterior femur, tibial plateau), and between subjects, as shown by the mean and standard deviation of each region (Figure 3). Additional analysis revealed that these sequences are sensitive to both global and subtle spatial compositional changes (mean and standard deviation, Figure 4), and thus provide important focal information lost through whole-region averaging. The ANOVA test revealed that the means of the two zones were not significantly different within each region for any of the sequences. However, there were significantly different means (p<0.05) when comparing the same layer across regions. dGEMRIC had significantly different means for both 1.0mm and 1.5mm layers between the patella and femur, and between the femur and tibia in the 1.5mm (transitional) layer. T1ρ was significantly different in all same-layer regional comparisons, except between the patellar and femoral 1.0mm (radial) layer. T2 was significantly different in both layers between the patella and tibia, as well as between the femur and tibia. These significant differences in each sequence’s mean relaxation times can be observed visually in Figure 4.
Figure 3.
Whole region average (and standard deviation) for each patient’s (as well as all patients combined) 1.0mm (approximately radial) and 1.5mm (approximately transitional) cartilage layers for T1ρ, T2, and dGEMRIC relaxation times by each region examined (averaged 20–80% in the patella and tibial plateau, 0–50° in the posterior portion of the late ral femoral condyle as defined in Figure 2B).
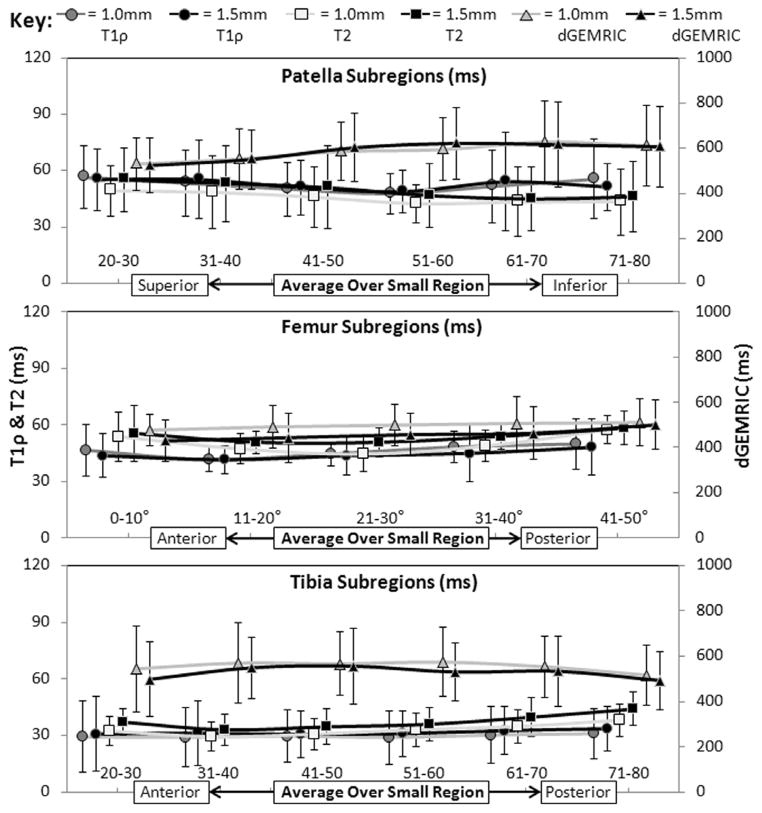
Figure 4.
Relaxation times incremented into subregions and averaged across all patients at both cartilage depths demonstrate differing degrees of site-specific spatial variation across all three sequences.
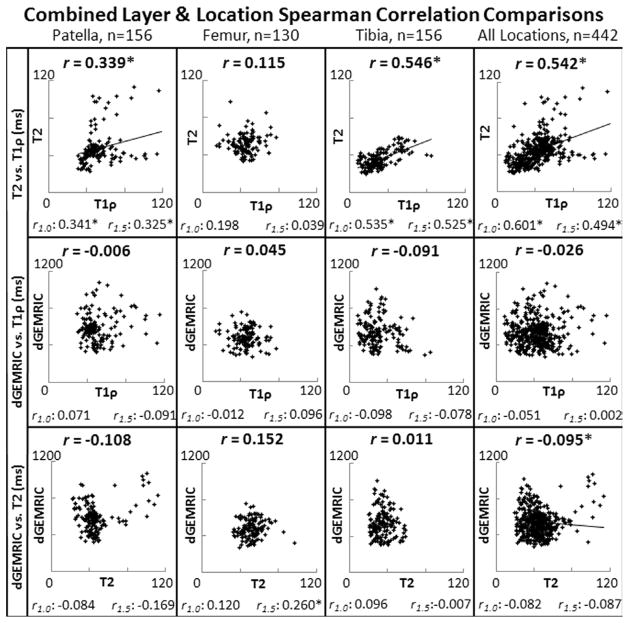
Inter-sequence correlation values varied between knee regions (Figure 5). Overall, T1ρ vs. T2 was the most consistent relationship and displayed the strongest correlations across subgroups, whereas T1ρ vs. dGEMRIC was the weakest and most inconsistent. The strongest correlation (closest to −1.0 or 1.0) was seen in the combined regional samples from the deeper 1.0mm (radial) layer T1ρ vs. T2 comparison (r = 0.601 p≪0.001). Non-significant correlations were observed for T1ρ vs. dGEMRIC, and for most of the T2 vs. dGEMRIC comparisons.
Figure 5.
Spearman’s correlation coefficients (r) are shown for T1ρ compared to T2, T1ρ compared to dGEMRIC, and T2 compared to dGEMRIC relaxation times (*=significant correlation, p<0.05). Correlation values were computed by combining both layers’ subregion (averaged every 10° or 10%) data by patella, femur, tibia, and overall combined regions. The correlation values for each layer’s comparisons (r1.0=correlation value for 1.0mm layer, r1.5=correlation value for 1.5mm layer) are also shown in the lower portion of each box.
DISCUSSION
This study examined the relationships between three quantitative in-vivo MRI sequences concurrently acquired during the acute recovery phase in young, active patients after ACL injury. This information is novel in the literature and an important contribution because T1ρ, T2, and dGEMRIC imaging sequences are being considered as “imaging biomarkers” of osteoarthritis progression by different research groups. In most quantitative imaging studies, a single technique is used, and the relationships between all three approaches have remained unclear. Whole region and sub-region analyses here reported have examined each sequence to elucidate which may possess the greatest potential as a sensitive, clinically feasible biomarker early after injury.
Each sequence showed different responses during the acute phase after ACL rupture between patients and sites (ANOVA results, Figure 3). Variability was not surprising to find in this whole region averaging, since there were differences in acute ACL rupture injury events and biological variability in patient’s response, as well as differences between knee regions’ tissue orientation/structure with respect to the main B0 magnetic field. While whole-region analysis shows some patient-specific differences, patient care would be enhanced if the specific MRI-detected location of cartilage injury could be identified during arthroscopy and subsequent outcomes analysis; therefore the applicability and relevance of a sequence’s clinical use as an effective screening procedure and diagnostic tool is directly related to its focal sensitivity.
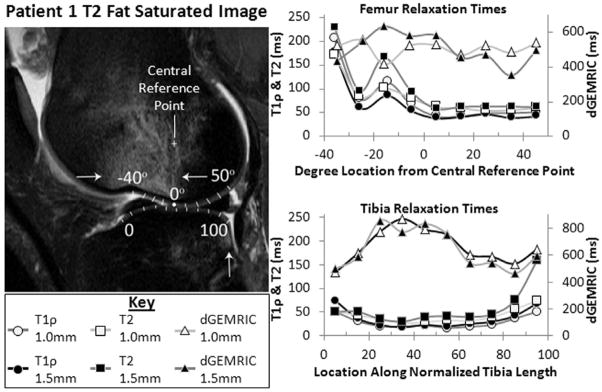
Sub-regional sequence specificity can be observed across the averages of all subjects in Figure 4, along with a closer examination of each sequence’s spatial and focal sensitivity within a single subject (Figure 6). While some of the examined portions (−40° to 0° in the femur, 0 to 20 and 80 to 100 in the tibia) were excluded from the previous analysis due to fluid-dominated signals, we wanted to demonstrate the relationships between these sequences in this clear injury pattern (bone contusions act as indicators of microfracture during injury events such as acute ACL rupture(5)). With this pattern, some differences in relaxation times between cartilage layers within the same knee region can clearly be seen. For example, the T1ρ relaxation times in the 1.0mm (deeper) layer are higher than the 1.5mm layer in the femur. The focal specificity in Figure 6 (i.e. the peaks in T1ρ and T2 values near the contusion) is also demonstrative of what would be lost when whole joint averaging is performed. Therefore, a balance between noise compensation and gross averaging must be struck to provide relevant information for subsequent patient analysis.
Figure 6.
Closer examination of one patient’s subregion relaxation times demonstrates how each sequence’s relaxation times vary across regions. Note in the T2 Fat-Saturated image that increased signal in the femur (superior to the sulcus, between the two arrows) and the posterior aspect of the tibial plateau (single arrow) indicate bone contusions; there are significant elevations in T1ρ and T2 relaxation times near these bone contusions (0° to −25° femur, 80 to 100 tibia displayed in graphs at right). Fluid signal (near the −40° reference) trapped between the meniscus and the femoral condyle precludes overlying sulcus cartilage from meaningful analysis.
Lastly, the Spearman’s correlation coefficient probed the sub-regional relaxation time relationships between T1ρ, T2, and dGEMRIC (Figure 5). Highly correlated sequences, manifested in similar relaxation time trends along the knee’s sagittal profile, may suggest that similar underlying compositional changes were being measured. In Figure 6, the T1ρ and T2 sequences’ relaxation times followed a very similar trend of increased values as proximity to bone contusions increased, which exemplifies their higher correlation (all locations combined were r = 0.542, p<0.05, Figure 5). Therefore, this suggests that T1ρ and T2 are measuring somewhat related processes in the acute phase after ACL reconstruction (elevated fluid content). A significant positive correlation between T1ρ and T2 was also found in other studies spanning osteoarthritis progression (during the first-year after ACL injury(22) to end-stage osteoarthritis patients’ osteochondral specimens(9)).
Elevated relaxation times were also recorded for dGEMRIC (Figure 6), but these showed little change focally. Normally, longer relaxation times would indicate decreased contrast infusion due to greater amounts of GAG side-chains (directly from fixed charge density field of increased proteoglycan presence). Similarly, lower relaxation times would indicate more contrast infiltration (less GAG) in dGEMRIC imaging. In the study by Neuman et al.(32) using a 1.5T scanner, the average dGEMRIC relaxation times in the lateral femoral condyle were 445 ± 41ms for healthy control subjects and 396 ± 48ms for ACL patients. These values are lower than those observed in the present study (538 ± 144ms). We attribute these differences to the likelihood of higher GAG content in our young patients, lower contrast dosage (0.15mmol/kg versus 0.3mmol/kg), and different magnetic field strengths (1.5T versus 3.0T).(33) Besides these imaging environment differences, we also hypothesize that variability in pH levels during inflammatory response and recovery may combine to alter normal gadolinium contrast agent distribution within the cartilage matrix, thereby creating higher global elevation in dGEMRIC values. These may account for the discrepancies between T1ρ and dGEMRIC and for their lack of correlation (all locations combined were r = −0.026, p=0.585) in this study and weak correlation in others.(11, 12, 14) Overall, while some significant relationships existed between sequences, the associations were weak-to-moderate in strength (Figure 5, Spearman rank correlations for all locations were T1ρ vs. T2: 0.542 (p<0.05), T1ρ vs. dGEMRIC: −0.026 (p=0.585), T2 vs. dGEMRIC:−0.095 (p<0.05)), suggesting each provides somewhat different information related to cartilage injury and recovery. This supports the work of Taylor, et al., whose study found similar relationships between these imaging sequences in vitro (Spearman rank correlations were T1ρ vs. T2: 0.24 (p<0.05), T1ρ vs. dGEMRIC: 0.01, T2 vs. dGEMRIC:−0.19)(9).
Absolute relaxation times differ between quantitative sequence results reported from different sites; various factors affecting acquisition and subsequent relaxation times include differences in scanner platforms, magnetic field strengths and software implementations. This should be considered when interpreting results from different institutions. Beside the differences in our absolute dGEMRIC values compared to Neuman et al.(32), Stahl et al. reported absolute T2 values (control subjects: 30.1±3.7 ms, mild OA patients: 31.2±3.0ms) and T1ρ values (control subjects: 38.0±2.6ms) from the lateral femoral condyle that were lower than the corresponding average values in our acute ACL patients (T2: 50.3±10.3ms, T1ρ: 46.1±10.3ms in 1.0mm layer) and values from a “normal” subject cohort (45±7ms).(15, 30) Different sequence implementations on different 3 Tesla platforms (GE versus Siemens), and/or a direct effect of acute injury inflammation in our young patient group may account for these differences. Interestingly, both studies showed the tibial plateau had the lowest T2 relaxation times, which may relate to collagen’s relative structure measured (at the same increment) in the comparatively thicker tibial plateau cartilage.(10, 17, 34) Because such institutional differences can yield different quantitative results, biofidelic cartilage phantoms are recommended and have been used(35) to calibrate scanners between sites.
This study had limitations. Subjects’ willingness to enroll (and return for follow-up exams) was hindered by contiguous imaging duration (4 hours of data collection) and the IRB’s requirement for checking eGFR prior to dGEMRIC approach (based on Burstein et al.(29)). Attempting to keep each sequence’s scan session time to a reasonable duration (≤ 1 hour) for patient comfort, we chose to focus our initial studies on the lateral femoral condyle because this is a region where blunt impact and a majority of bone contusions occur in ACL injury(6). Another limitation was the use of a single channel CP extremity coil. This was the only coil that was available at the beginning of data collection, and improvements in coil technology, such as multi-channel array coils, have been made to create higher quality images. We do not feel that this hinders sequence interpretation, since this coil was used consistently throughout the study and that the selected study parameters yielded a good compromise between image quality, resolution, and scan time for all three sequences collected. Clinical availability of improved coils will no doubt improve the clinical translation of this study. Nevertheless, our presentation of data from concurrent, same-day imaging using the three most promising imaging biomarkers of cartilage health status in ACL patients is novel in part because achieving the aims is very challenging.
Conclusion
This study documented early in vivo cartilage compositional changes in young ACL injury patients using T1ρ, T2, and dGEMRIC imaging during the same-day. We propose that swelling and soft-tissue fluid infusion associated with the inflammatory response to injury (i.e. blunt impact injury as evinced by bone contusions causing such a response) and its eventual acquiescence were responsible for some of the differences observed among pre-operative imaging markers of cartilage composition. No appreciable focal glycosaminoglycan loss was detected by dGEMRIC. This suggests that general and focal elevations in T1ρ were not related only to PG, but perhaps more aptly the ratio of water-to-PG content. This theory is reinforced by the elevated T2 relaxation times, which were frequently longer than the T1ρ relaxation times among these subjects. During the acute phase after injury, the collagen state and fluid content may change significantly, and thus could have led to the higher elevation in the T2 values compared to the T1ρ. However, other investigators have observed trends in T2 relaxation times which were longer than T1ρ relaxation times at 3.0T, even in asymptomatic subjects and particularly in lateral femoral cartilage, thus demonstrating the large effect of the imaging environment on the absolute relaxation times(36). In the end, clinical translation of any of these three sequences will depend primarily on the ability of an imaging sequence to provide clinically meaningful information that is prognostic of future joint health. The FDA recommendation for checking an eGFR and the scan time required represent significant challenges in translating dGEMRIC as an imaging biomarker of cartilage health in busy clinical settings. The relative ease of collecting T1ρ and T2-maps make these approaches more clinically feasible. However, T2-mapping is susceptible to the magic angle effect due to its collagen fiber orientation sensitivity, whereas the T1ρ sequence’s spin-lock pulse may eliminate the collagen’s residual dipolar interaction (responsible for the magic angle effect)(21), and thus may give more consistent insight into cartilage health in a clinical setting. Although additional research is needed to fully define the mechanisms underlying these MRI-detected changes and their prognostic value regarding post-traumatic osteoarthritis, T1ρ’s sensitivity to early cartilage changes found in this study enhance the appeal of T1ρ as a clinically feasible imaging biomarker of cartilage health.
Acknowledgments
Sources of Support: NIH grant P50 AR055533 and the AOSSM provided funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wheaton AJ, Dodge GR, Elliott DM, et al. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med. 2005;54(5):1087–93. doi: 10.1002/mrm.20678. [DOI] [PubMed] [Google Scholar]
- 2.Potter HG, Jain SK, Ma Y, et al. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012;40(2):276–85. doi: 10.1177/0363546511423380. [DOI] [PubMed] [Google Scholar]
- 3.Lohmander LS, Englund PM, Dahl LL, et al. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 4.Borchers JR, Kaeding CC, Pedroza AD, et al. Intra-articular findings in primary and revision anterior cruciate ligament reconstruction surgery: a comparison of the MOON and MARS study groups. Am J Sports Med. 2011;39(9):1889–93. doi: 10.1177/0363546511406871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frobell RB, Le Graverand MP, Buck R, et al. The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. Osteoarthritis Cartilage. 2009;17(2):161–7. doi: 10.1016/j.joca.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Theologis AA, Kuo D, Cheng J, et al. Evaluation of bone bruises and associated cartilage in anterior cruciate ligament-injured and -reconstructed knees using quantitative t(1rho) magnetic resonance imaging: 1-year cohort study. Arthroscopy. 2011;27(1):65–76. doi: 10.1016/j.arthro.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oiestad BE, Engebretsen L, Storheim K, et al. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37(7):1434–43. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 8.Struewer J, Frangen TM, Ishaque B, et al. Knee function and prevalence of osteoarthritis after isolated anterior cruciate ligament reconstruction using bone-patellar tendon-bone graft: long- term follow-up. Int Orthop. 2011 doi: 10.1007/s00264-011-1345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor C, Carballido-Gamio J, Majumdar S, et al. Comparison of quantitative imaging of cartilage for osteoarthritis: T2, T1rho, dGEMRIC and contrast-enhanced computed tomography. Magn Reson Imaging. 2009;27(6):779–84. doi: 10.1016/j.mri.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burstein D, Gray M, Mosher T, et al. Measures of molecular composition and structure in osteoarthritis. Radiol Clin North Am. 2009;47(4):675–86. doi: 10.1016/j.rcl.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Duvvuri U, Goldberg AD, Kranz JK, et al. Water magnetic relaxation dispersion in biological systems: the contribution of proton exchange and implications for the noninvasive detection of cartilage degradation. Proc Natl Acad Sci U S A. 2001;98(22):12479–84. doi: 10.1073/pnas.221471898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duvvuri U, Kudchodkar S, Reddy R, et al. T(1rho) relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthritis Cartilage. 2002;10(11):838–44. doi: 10.1053/joca.2002.0826. [DOI] [PubMed] [Google Scholar]
- 13.Regatte RR, Akella SV, Wheaton AJ, et al. 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol. 2004;11(7):741–9. doi: 10.1016/j.acra.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 14.Gray ML, Burstein D, Kim YJ, et al. 2007 Elizabeth Winston Lanier Award Winner. Magnetic resonance imaging of cartilage glycosaminoglycan: basic principles, imaging technique, and clinical applications. J Orthop Res. 2008;26(3):281–91. doi: 10.1002/jor.20482. [DOI] [PubMed] [Google Scholar]
- 15.Stahl R, Luke A, Li X, et al. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients--a 3.0-Tesla MRI study. Eur Radiol. 2009;19(1):132–43. doi: 10.1007/s00330-008-1107-6. [DOI] [PubMed] [Google Scholar]
- 16.Wheaton AJ, Casey FL, Gougoutas AJ, et al. Correlation of T1rho with fixed charge density in cartilage. J Magn Reson Imaging. 2004;20(3):519–25. doi: 10.1002/jmri.20148. [DOI] [PubMed] [Google Scholar]
- 17.Burstein D. MRI for development of disease-modifying osteoarthritis drugs. NMR Biomed. 2006;19(6):669–80. doi: 10.1002/nbm.1071. [DOI] [PubMed] [Google Scholar]
- 18.Keenan KE, Besier TF, Pauly JM, et al. Prediction of glycosaminoglycan content in human cartilage by age, T1rho and T2 MRI. Osteoarthritis Cartilage. 19(2):171–9. doi: 10.1016/j.joca.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Ma CB, Link TM, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15(7):789–97. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lozano J, Li X, Link TM, et al. Detection of posttraumatic cartilage injury using quantitative T1rho magnetic resonance imaging. A report of two cases with arthroscopic findings. J Bone Joint Surg Am. 2006;88(6):1349–52. doi: 10.2106/JBJS.E.01051. [DOI] [PubMed] [Google Scholar]
- 21.Regatte RR, Akella SV, Lonner JH, et al. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23(4):547–53. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Kuo D, Theologis A, et al. Cartilage in Anterior Cruciate Ligament-Reconstructed Knees: MR Imaging T1(22) and T2--Initial Experience with 1-year Follow-up. Radiology. 258(2):505–14. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishioka H, Hirose J, Nakamura E, et al. T(1rho) and T(2) mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging. 2011 doi: 10.1002/jmri.22811. [DOI] [PubMed] [Google Scholar]
- 24.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Advisory PH FDA. Book Update on Magnetic Resonance Imaging (MRI) Contrast Agents Containing Gadolinium and Nephrogenic Fibrosing Dermopathy. 2006. Update on Magnetic Resonance Imaging (MRI) Contrast Agents Containing Gadolinium and Nephrogenic Fibrosing Dermopathy. [Google Scholar]
- 27.Charagundla SR, Borthakur A, Leigh JS, et al. Artifacts in T(1rho)-weighted imaging: correction with a self-compensating spin-locking pulse. J Magn Reson. 2003;162(1):113–21. doi: 10.1016/s1090-7807(02)00197-0. [DOI] [PubMed] [Google Scholar]
- 28.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–41. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burstein D, Velyvis J, Scott KT, et al. Protocol issues for delayed Gd(DTPA)(2−)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45(1):36–41. doi: 10.1002/1522-2594(200101)45:1<36::aid-mrm1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen DR, Klocke NF, Thedens DR, et al. Integrating Cartilage-Specific T1rho MRI into Knee Clinic Diagnostic Imaging. Iowa Orthop J. 2011:31. [PMC free article] [PubMed] [Google Scholar]
- 31.Stahl R, Blumenkrantz G, Carballido-Gamio J, et al. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthritis Cartilage. 2007;15(11):1225–34. doi: 10.1016/j.joca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Neuman P, Tjornstrand J, Svensson J, et al. Longitudinal assessment of femoral knee cartilage quality using contrast enhanced MRI (dGEMRIC) in patients with anterior cruciate ligament injury--comparison with asymptomatic volunteers. Osteoarthritis Cartilage. 2011;19(8):977–83. doi: 10.1016/j.joca.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Gold GE, Chen CA, Koo S, et al. Recent advances in MRI of articular cartilage. AJR Am J Roentgenol. 2009;193(3):628–38. doi: 10.2214/AJR.09.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shepherd DE, Seedhom BB. Thickness of human articular cartilage in joints of the lower limb. Ann Rheum Dis. 1999;58(1):27–34. doi: 10.1136/ard.58.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thedens DR, Klocke NF, Martin JA, et al. Consistency of T1rho Measurements: A Phantom Study. Annual meeting of the International Society of Magnetic Resonance in Medicine; 2011. p. 2787. [Google Scholar]
- 36.Goto H, Iwama Y, Fujii M, et al. A preliminary study of the T1rho values of normal knee cartilage using 3T-MRI. Eur J Radiol. 2012;81(7):e796–803. doi: 10.1016/j.ejrad.2012.03.022. [DOI] [PubMed] [Google Scholar]