Abstract
Background
Targeting higher hemoglobin with erythropoiesis-stimulating agents (ESAs) to treat anemia of chronic kidney disease (CKD) is associated with increased cardiovascular risk.
Study Design
Meta-regression analysis examining the association of ESA dose with adverse outcomes, independent of target or achieved hemoglobin.
Setting and Population
Patients with anemia of CKD, irrespective of dialysis status.
Selection Criteria for Studies
We searched MEDLINE (inception to August 2010) and bibliographies of published meta-analyses and selected randomized controlled trials assessing the efficacy of ESAs for treatment of anemia in adults with CKD, with minimum 3-month duration. Two authors independently screened citations and extracted relevant data. Individual study arms were treated as cohorts and constituted the unit of analysis.
Predictors
ESA dose standardized to a weekly epoetin alfa equivalent, and hemoglobin levels.
Outcomes
All-cause and cardiovascular mortality, cardiovascular events, kidney disease progression or transfusion requirement.
Results
31 trials (12,956 patients) met criteria. All-cause mortality was associated with higher (per epoetin-alfa–equivalent 10,000-U/wk increment) first-3-month mean ESA dose (incidence rate ratio [IRR], 1.42; 95% CI, 1.10–1.83) and higher total-study-period mean ESA dose (IRR, 1.09; 95% CI, 1.02–1.18). First-3-month ESA dose remained significant after adjusting for first-3-month mean hemoglobin (IRR, 1.48; 95% CI, 1.02- 2.14), as did total-study-period mean ESA dose adjusting for target hemoglobin (IRR, 2 1.41; 95% CI, 1.08–1.82). Parameter estimates between ESA dose and cardiovascular mortality were similar in magnitude and direction but not statistically significant. Higher total-study-period mean ESA dose was also associated with increased rate of hypertension, stroke, and thrombotic events including dialysis vascular access-related thrombotic events.
Limitations
use of study-level aggregated data; use of epoetin alfa–equivalent doses; lack of adjustment for confounders.
Conclusions
In patients with CKD, higher ESA dose might be associated with all-cause mortality and cardiovascular complications independent of hemoglobin.
Keywords: erythropoietin, ESA, epoetin, darbepoetin, anemia, CKD, dose, mortality, cardiovascular morbidity, meta-regression
The lack of endogenous erythropoietin production in patients with chronic kidney disease (CKD) results in the development of anemia, which is associated with impaired quality of life 1,2, and increased morbidity and mortality 3–5. The gold standard for managing anemia of CKD is the use of erythropoiesis-stimulating agents (ESAs). Targeting higher hemoglobin levels with ESAs has, however, been associated with an increased risk of cardiovascular disease–related morbidity and mortality 6–8. It is not well established whether the ESA dose itself has an effect on adverse clinical outcomes. Understanding the potential role of the ESA dose, versus the target or achieved hemoglobin level, is of crucial importance as there is no universal consensus on the exact ESA dosage algorithm that should be adopted while minimizing patient exposure to these potential health risks. If such an association exists, high doses of ESAs could result in increased morbidity even at low hemoglobin levels.
Several previously published systematic reviews have established a clear association between target hemoglobin level and adverse outcomes in patients with CKD 2,6,7,9–15, resulting in a change to the ESA label regarding target hemoglobin. However, these analyses did not address whether there is a dose-response gradient of ESAs and potential harm. To examine this question, we performed a systematic review with metaregression of randomized controlled trials (RCTs) of ESAs in patients with CKD to evaluate whether the potential harm associated with their use for the treatment of anemia follows a dose-response gradient, adjusting for the target or achieved hemoglobin level.
METHODS
Data Sources and Selection
We searched MEDLINE for all published RCTs that examined the use of ESAs in CKD (inception-August 2010) and for published systematic reviews (inception-June 2010) (search strategies shown in Item S1, available as online supplementary material). The searches were limited to human studies with no language restrictions. Two authors (MA and IK) screened titles and abstracts to identify potentially relevant articles. We selected parallel-arm RCTs reporting efficacy (i.e., change in hemoglobin level) of ESAs with a minimum 12-week treatment duration that documented doses of ESA, levels of baseline and achieved hemoglobin, and at least one endpoint of interest (as defined below). We included only studies of epoetin alfa, epoetin beta, or darbepoetin alfa. Reports of ESA trials in clinical settings other than anemia of CKD (e.g., cancer or heart failure) were excluded.
Data Extraction and Quality Assessment
Data were independently extracted in duplicate from full-text articles by two authors (MA and IK). Where indicated, the G3 data graph analyzer (version 1.5.3) was used to extract data from graphs 16. Disagreements were resolved through consensus and arbitration by a third author (BLJ). Corresponding authors of four trials were contacted for data clarification.
Data extracted from full-text articles included country of origin, year of publication, study sponsor, study design, sample size in each arm, CKD stage and dialysis status, sex, mean age, mean weight, comorbidities, target hemoglobin in each arm, ESA type (epoetin alfa, epoetin beta, or darbepoetin alfa), prior ESA use, route of ESA administration (subcutaneous vs. intravenous) and trial duration. Due to inconsistent reporting, total and average follow-up times were used interchangeably. For each study 5 arm, according to a pre-specified analysis plan, we extracted the mean ESA dose and mean hemoglobin level at enrollment, during the first 3 months (in an attempt to capture rapid correction of anemia), and throughout the total follow-up period.
In studies reporting only hematocrit values, hemoglobin values were calculated by dividing the hematocrit by 3 17. We converted the darbepoetin alfa dose to an equivalent epoetin alfa dose using a dose conversion ratio of 331 units of epoetin alfa per 1 µg of darbepoetin alfa 18,19. The doses of epoetin alfa and beta were considered equivalent. If reported in units/kg/wk, the recombinant erythropoietin dose was converted to units/week by using the mean weight of the participants in each study arm. If not reported, a weighted average weight was calculated using data from the National Health and Nutrition Examination Survey (NHANES) 20,21 for patients with CKD who were not on dialysis, and the 2008 annual data report from the United States Renal Data System (USRDS) for dialysis patients 22. The weighted average used in these calculations took into consideration the year of publication, mean age, and sex ratio in each study arm.
Our two primary outcomes of interest were all-cause mortality and cardiovascular mortality as defined by the authors. Secondary outcomes included cardiovascular events, acute myocardial infarction, de novo or worsening angina, heart failure, arrhythmias, stroke, de novo or worsening hypertension as defined by the authors, thrombotic events (e.g., deep venous thrombosis, peripheral arterial thrombotic events, and dialysis vascular access thrombosis), any serious adverse event (SAE) as defined in the individual trials, progression to kidney failure (end-stage renal disease), need for blood transfusions, and change in glomerular filtration rate (GFR).
The estimated GFR, measured GFR, and creatinine clearance were assumed to be 6 equivalent. When reported in ml/min, the GFR was transformed to ml/min/1.73 m2 by calculating the mean body surface area of the participants in each arm according to the Mosteller method 23, using the provided mean height and weight. If not reported, we used a weighted mean body surface area based on the NHANES database 20,21. Median values were converted to estimates of means if the study arm included more than 25 participants 24.
All categorical outcomes were expressed as incidence rates (events per personyears of follow-up). The continuous outcome (GFR) was expressed as an annual slope (change from baseline over the length of follow-up in years).
We assessed study methodological quality in terms of randomization adequacy, blinding, and attrition rates using the Jadad scale 25.
Data Synthesis and Analysis
Using individual study arms (cohorts) as the unit of analysis, we performed metaregressions to separately explore the association of the first-3-month and total-studyperiod mean recombinant erythropoietin dose (in units/week) with the outcomes of interest. For the two primary outcomes (all-cause and cardiovascular mortality), we performed three sets of analyses. The first set of analyses used the first-3-month and total-study-period mean recombinant erythropoietin dose (in units/week) as the sole predictor. The second set added the target hemoglobin level to the model. The third set adjusted either for the first-3-month or total-study period achieved hemoglobin level (corresponding to the mean ESA dose time frame).
Models were deemed fit only if there were at least four degrees of freedom more than the number of predictors in each model. We did not report analyses where the 7 covariance matrix of the resulting coefficients indicated presence of collinearity among predictors. For the secondary outcomes, we performed only the first two sets of metaregressions due to a lack of sufficient observations; however, we performed additional analyses adjusting for the mortality rate (per 1000 person-years) in the control group of each trial in an attempt to control for heterogeneity of comorbidities in the study populations.
All analyses of binary outcomes were fit using generalized linear random-effects Poisson regressions with a fixed slope and random intercept, accounting for the clustering of cohorts (or trial arms) by study, and the person-years of exposure as the offset. The respective exposure for the Poisson is in terms of person time; the weighting of each study arm (cohort) is thus naturally accounted for through the Poisson distribution. When exponentiated, the coefficient expresses a change in the incidence rate of an event per unit change in the predictor. We report these results as incidence rate ratios (IRRs) with accompanying 95% confidence intervals (CI). For the annualized change in GFR, we fit random-effects variance-weighted meta-regressions 26. The GFR analysis is reported as an estimate of the annual change in GFR (in ml/min/1.73m2) per unit of change in the predictor.
In sensitivity analyses of all-cause mortality models with statistically significant predictors, we arbitrarily removed two cohorts with the highest mortality rate to evaluate the robustness of the results. In addition, we conducted subgroup analyses for the primary outcome stratified according to dialysis status, baseline hemoglobin level (< vs. ≥ 10.5 g/dL, representing the median value), and ESA type (epoetin vs. darbepoetin). All analyses were performed in Stata SE version 11 (Stata Corp, College Station, TX) and 8 Meta-Analyst 27 version 3 (Tufts Medical Center, Boston, MA). All p-values were two tailed and considered to be statistically significant at the 0.05 level.
RESULTS
Search Yield
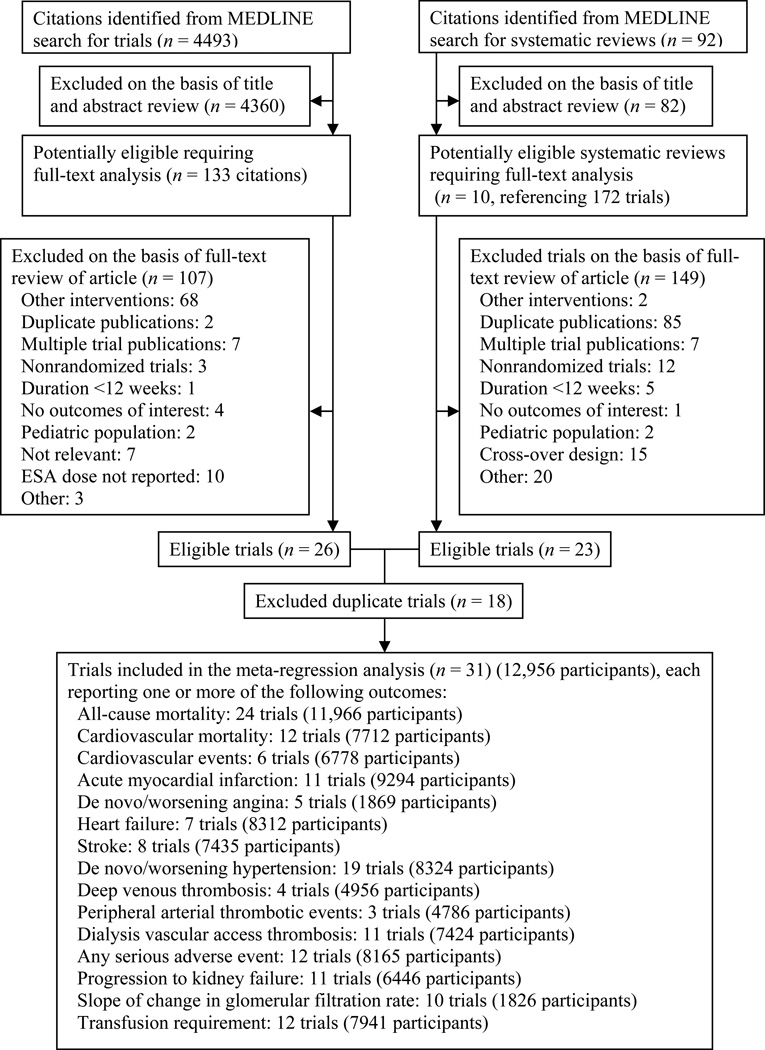
A total of 4493 potentially relevant citations were identified and screened (Figure 1). We retrieved full-text articles of 133 citations for evaluation, of which 26 satisfied the selection criteria. In addition, 92 potentially relevant systematic reviews were identified and screened, 10 of which were evaluated in full-text; 172 potentially relevant citations were identified from the references of these systematic reviews, of which 23 satisfied the selection criteria. After removal of duplicate reports, 31 unique trials with 72 study arms (cohorts) were included 8,28–57. All eligible studies were in English.
Figure 1.
Literature search and selection. ESA denotes erythropoiesis-stimulating agent.
Study Characteristics
Table 1 summarizes the trial characteristics. Published over 20 years, there were 8 placebo-controlled trials and 23 active comparator trials, of which 25 were industry sponsored, 5 provided no sponsorship disclosures, and 1 was funded by a non-industry source. The 31 trials enrolled a total of 12,956 participants. Sample sizes ranged from 42 to 4038 patients. The percentages of men ranged from 35% to 99%, and mean ages from 51 to 71 years. Fifteen trials were restricted to dialysis patients. Mean baseline GFRs (reported in 16 trials) ranged from 9.2 to 45.1 ml/min/1.73 m2. Mean weights ranged from 64.0 to 85.4 kg. Two trials included only patients with diabetes.
Table 1.
Characteristics of trials included in the meta-regression analysis.
| Study | Industry funded |
CKD Stage |
No. Pt |
agea (y) |
Men (%) |
Wta (kg) |
GFRb |
DM (%) |
F/U (wk) |
ESA type |
Hb (g/dL) | ESA dose (U/wk) | Fe use |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target | Baselinea | 1st 3moa | Total- study- perioda |
End- of- studya |
Startinga | 1st 3 moa |
Total- study- perioda |
End-of- studya |
||||||||||||
| Canadian EPO Study Group 28 (1990) |
NR | 5-HD | 118 | 45 | 59 | 74.9* | - | NR | 26 | Placebo | NR | 7.1 | 7.1 | 7.2 | 7.4 | 0 | NR | NR | 0 | Y |
| Epo | 10.3 | 6.9 | 9 | 9.7 | 10.2 | 21,686¶ | NR | NR | 14,746¶ | Y | ||||||||||
| Epo | 10.3 | 7.1 | 9.5 | 10.6 | 11.7 | 23,302¶ | NR | NR | 15,845¶ | Y | ||||||||||
| Abraham 29 (1991) | Y | 5-HD | 151 | 52 | 23 | 70.4* | - | NR | 12 | Epo-β | NR | 7.1 | 7.1 | 9 | 10.9 | 25,613¶ | NR | NR | NR | Y |
| Placebo | NR | 7 | 7 | 7.3 | 7.5 | 0 | NR | NR | NR | Y | ||||||||||
| Epo-β | NR | 7.2 | 7.2 | 8 | 8.8 | 5,468¶ | NR | NR | NR | Y | ||||||||||
| Epo-β | NR | 7 | 7 | 9 | 11 | 21,870¶ | NR | NR | NR | Y | ||||||||||
| Epo-β | NR | 6.9 | 6.9 | 9.3 | 11.6 | 43,740¶ | NR | NR | NR | Y | ||||||||||
| Bahlmann 30 (1991) |
NR | 5-HD | 129 | 57 | 38 | 64 | - | 20 | 26 | Epo | 10.8 | 7.7 | 9.2 | 9.5 | 10.4 | 16,152 | NR | 8,076 | 8,076 | NR |
| Placebo | NR | 7.7 | 7.7 | 7.7 | 8 | 0 | 0 | 0 | 0 | NR | ||||||||||
| Clyne 31 (1992) | N | 4, 5- NDD |
20 | 46 | 55 | 79* | 9.2 | 0 | 13 | Epo-β | NR | 8.6 | NR | NR | 11.7 | 23,505¶ | NR | NR | 18,409¶ | Y |
| Placebo | NR | 9.3 | NR | NR | 9.4 | 0 | 0 | 0 | 0 | Y | ||||||||||
| Muirhead 32 (1992) |
Y | 5-HD | 128 | 58 | 55 | 66 | - | NR | 24 | Epo | 11.5 | 8 | NR | 11.8 | 10.9 | 9,870 | NR | 13,548 | 9,679 | NR |
| Epo | 11.5 | 7.7 | NR | 11.5 | 11.2 | 9,915 | NR | 18,118 | 12,156 | NR | ||||||||||
| Roth 33 (1994) | Y | 4, 5- NDD |
83 | 57 | 33 | 78.1* | 9.2 | NR | 48 | Epo | 11.7 | 8.9 | 10.7 | 10.8 | 10.9 | 8,298¶ | 8,792¶ | 8,657¶ | 9,106¶ | Y |
| Placebo | NR | 8.9 | 8.9 | 8.9 | 9 | 0 | 0 | 0 | 0 | Y | ||||||||||
| Nissenson 34 (1995) |
Y | 5-PD | 152 | 48 | 39 | 71.2 | - | 26 | 12 | Epo-α | 11.7 | 7.9 | 10 | 10 | 11.2 | 12,000 | 8,000 | 8,000 | NR | Y |
| Placebo | NR | 7.9 | 8 | 8 | 8 | 0 | 0 | 0 | NR | Y | ||||||||||
| Virot 35 (1996) | NR | 5-HD | 49 | 63 | 55 | 70.6* | - | NR | 17 | Epo-α | 9.5 | 9.6 | 9.7 | 9.7 | 9.9 | 7,424¶ | 7,696¶ | 7,691¶ | 7,844¶ | NR |
| Epo-α | 9.5 | 9.9 | 9.6 | 9.6 | 9.7 | 6,864¶ | 6,392¶ | 6,303¶ | 5,883¶ | NR | ||||||||||
| Besarab 36 (1998) | Y | 5-HD | 1233 | 65 | 49 | 70.9* | - | 56 | 60 | Epo-α | 14 | 10.2 | 11 | 13 | 13.2 | 11,777¶ | 19,298¶ | 31,067¶ | 30,239¶ | Y |
| Epo-α | 10 | 10.2 | 10.4 | 10.4 | 10 | 11,349¶ | 11,801¶ | 11,179¶ | 9,704¶ | Y | ||||||||||
| Kaufman 37 (1998) | Y | 5-HD | 208 | 60 | 99 | 76.1 | - | NR | 46 | Epo-α | 10.5 | 10.5 | NR | 10.4 | NR | NR | NR | 7,397 | NR | Y |
| 48 | Epo-α | 10.5 | 10.6 | NR | 10.3 | NR | NR | NR | 10,068 | NR | Y | |||||||||
| Berns 38 (1999) | Y | 5-HD | 28 | 61 | 33 | 73.3 | - | 54 | 52 | Epo-α | 14 | 10.2 | 11 | 12.4 | 14 | 13,200 | 21,700 | 31,300 | 32,200 | NR |
| Epo-α | 10 | 10.2 | 10.3 | 10.5 | 10.1 | 10,900 | 11,700 | 9,120 | 6,400 | NR | ||||||||||
| Conlon 39 (2000 | NR | 5-HD | 31 | 55 | 55 | NR | - | NR | 28 | Epo-α | 14 | 9.7 | 10.5 | 12.3 | 13.6 | 14,400 | NR | NR | 43,200 | Y |
| Epo-α | 10 | 9.7 | 9.7 | 9.9 | 10 | 10,095 | NR | NR | 18,300 | Y | ||||||||||
| Foley 40 (2000 | Y | 5-HD | 146 | 62 | 62 | 68 | - | NR | 48 | Epo-α | 13.5 | 10.2 | 11.2 | 12.3 | 12.8 | 8,110 | 15,397 | 20,122 | 21,190 | Y |
| Epo-α | 10 | 10.1 | 10.4 | 10.4 | 10.6 | 9,265 | 9,509 | 8,536 | 6,459 | Y | ||||||||||
| Furuland 41 (2003) | Y | 4, 5- NDD, 5- HD/PD§ |
416 | 63 | 65 | 73 | 16.5 | 19 | 48 | Epo-α | 15.1 | 10.9 | 12 | 13.3 | 13.4 | 6,862 | NR | NR | 15,133 | Y |
| Epo-α | 10.8 | 11 | 11.2 | 11.4 | 11.3 | 6,986 | NR | NR | 8,052 | Y | ||||||||||
| Levin 42 (2005) | Y | 2, 3, 4 | 172 | 57 | 62 | 78 | 26.3 | 34 | 104 | Epo-α | 13 | 11.8 | 12.4 | 12.7 | 12.7 | 2,000 | NR | NR | 3,146 | Y |
| Epo-α | 9.8 | 11.7 | 11.7 | 11.7 | 11.4 | 0 | NR | NR | 3,552 | Y | ||||||||||
| Parfrey 43 (2005) | Y | 5-HD | 596 | 51 | 60 | 74.4 | - | NR | 96 | Epo-α | 14 | 11 | 11.6 | 12.9 | 13 | 7,185 | 8,981 | 10,955 | 13,077 | NR |
| Epo-α | 10.5 | 11 | 11 | 10.9 | 10.9 | 5,910 | 5,682 | 5,728 | 5,634 | NR | ||||||||||
| Provenzano 44(2005) | Y | 4, 5- NDD |
519 | 69 | 51 | 84.2 | 21.1 | NR | 16 | Epo-α | 12 | 11.8 | 12.4 | 12.4 | 12.2 | 10,000 | NR | 9,235 | NR | Y |
| Epo-α | 12 | 11.8 | 12.1 | 12.1 | 11.9 | 10,000 | NR | 9,681 | NR | Y | ||||||||||
| Epo-α | 12 | 11.9 | 11.8 | 11.7 | 11.2 | 10,000 | NR | 9,489 | NR | Y | ||||||||||
| Epo-α | 12 | 11.9 | 11.9 | 11.7 | 11.4 | 10000 | NR | 9,748 | NR | Y | ||||||||||
| Drüeke 45 (2006) | Y | 3, 4 | 603 | 59 | 54 | 73.2 | 22.8 | 26 | 52 | Epo-β | 14 | 11.6 | 12.2 | 13.2 | 13.5 | 2,000 | 2,646 | 5,350 | 5,833 | Y |
| 54 | Epo-β | 11 | 11.6 | 11.6 | 11.5 | 12 | 2,000 | 2,077 | 2,983 | 3,310 | Y | |||||||||
| Rossert 46 (2006) | Y | 3, 4 | 390 | 58 | 60 | 58.3* | 29.3 | 35 | 47 | Epo-α | 14 | 11.5 | 12.5 | 13.3 | 13.5 | 5,333¶ | NR | 4,514 | NR | Y |
| 54 | Epo-α | 11.5 | 11.6 | 11.8 | 11.9 | 11.9 | 5,333¶ | NR | 2,730 | NR | Y | |||||||||
| Singh 47 (2006) | Y | 3, 4 | 1432 | 66 | 45 | 30.4‡ | 36.9 | NR | 69 | Epo-α | 13.5 | 10.1 | 11.5 | 12.7 | 12 | 9,896 | 10,743 | 11,215 | 13,869 | Y |
| Epo-α | 11.3 | 10.1 | 11.2 | 11.4 | 11.1 | 9,357 | 7,052 | 6,276 | 7,248 | Y | ||||||||||
| Macdougall 48 (2007 | Y | 2, 3, 4, 5-NDD |
197 | 55 | 62 | 78 | 21.7 | 22 | 154 | Epo-α | 11 | 10.9 | NR | 11.5 | 11 | 2,000 | NR | 3,761 | 3,219 | Y |
| Epo-α | 9 | 10.8 | NR | 10.9 | 10.5 | 0 | NR | 1,531 | 1,837 | Y | ||||||||||
| Ritz 49 (2007 | Y | 1, 2, 3 | 170 | 58 | 51 | 72.9 | 45.1 | 100 | 64 | Epo-β | 14 | 11.9 | 12.7 | 13.3 | 13.6 | 2,000 | NR | 3,500 | NR | Y |
| Epo-β | 11 | 11.7 | 11.8 | 11.9 | 12.2 | 2,000 | NR | NR | NR | Y | ||||||||||
| Bommer 50 (2008) | Y | 5-HD | 114 | 62 | 46 | 71.3 | - | NR | 48 | Darb† | 11.5 | 11.6 | NR | 11.9 | 11.6 | 10,480 | NR | 10,976 | 10,282 | Y |
| Darb† | 11.5 | 12 | NR | 11.9 | 11.7 | 8,529 | NR | 8,199 | 8,893 | Y | ||||||||||
| Chen 51 (2008) | NR | 4, 5- NDD |
42 | 64 | 36 | 81.8* | 10.4 | 24 | 24 | Epo-α | 10.5 | 8.5 | NR | NR | 10.6 | 7,414¶ | NR | 2,350 | NR | Y |
| Darb† | 10.5 | 8.2 | NR | NR | 10.7 | 7,321¶ | NR | 3,759 | NR | Y | ||||||||||
| Spinowitz 54 (2008) | Y | 2, 3, 4 | 259 | 67 | 41 | 30.6‡ | 30.2 | NR | 16 | Epo-α | 11.5 | 10.3 | 11.2 | 11.3 | 11.5 | 10,000 | NR | 5,943 | NR | Y |
| Epo-α | 11.5 | 10.4 | 11.1 | 11.2 | 11.4 | 10,000 | NR | 7,376 | NR | Y | ||||||||||
| Epo-α | 11.5 | 10.1 | 10.8 | 10.9 | 11.2 | 5,000 | NR | 4,522 | NR | Y | ||||||||||
| Epo-α | 11.5 | 10.2 | 11 | 11.1 | 11.4 | 10,000 | NR | 8,660 | NR | Y | ||||||||||
| Cianciaruso 52 (2008) | Y | 2, 3, 4 | 95 | 58 | 62 | 69.5 | 26.2 | 18 | 52 | Epo-α | 13 | 11.6 | 11.8 | 12.2 | 12.3 | 2,000 | NR | 2,000 | NR | Y |
| Placebo | 9.8 | 11.7 | 11.6 | 11.5 | 11.3 | 0 | 0 | 0 | NR | Y | ||||||||||
| Locatelli 53 (2008) | Y | 5-HD | 287 | 66 | 58 | 66.4 | - | NR | 28 | Epo-α | 12 | 11.6 | 11.5 | 11.5 | 11.3 | 6,210 | 6,516 | 6,344 | 6,239 | NR |
| Epo-α | 12 | 11.6 | 11.1 | 11 | 10.8 | 6,791 | 7,445 | 8,069 | 8,936 | NR | ||||||||||
| Pfeffer 8 (2009) | Y | 3, 4 | 4038 | 68 | 43 | 82.8* | 33.5 | 100 | 125 | Darb† | 13 | 10.5 | 11.5 | 12.5 | 12.8 | 20,473 | NR | 13,577 | NR | Y |
| Placebo | 9 | 10.4 | 10.5 | 10.9 | 11.4 | 0 | NR | 386∥ | NR | Y | ||||||||||
| Pergola 56 (2009) | Y | 3, 4 | 369 | 70 | 35 | 76* | 30 | NR | 22 | Epo-α | 11.5 | 9.6 | 11 | 11.3 | 11.3 | 11,393 | NR | 5,039 | NR | Y |
| Epo-α | 11.5 | 9.7 | 10.8 | 11.1 | 11.3 | 10,000 | NR | 5,035 | NR | Y | ||||||||||
| Epo-α | 11.5 | 9.8 | 10.9 | 11 | 11.1 | 10,000 | NR | 6,662 | NR | Y | ||||||||||
| Chazot 55 (2009) | Y | 5-HD | 154 | 63 | 46 | 84.9* | - | NR | 26 | Darb† | 10 | 11.6 | 11.7 | 11.7 | 11.7 | 12,624 | 12,905 | 12,718 | 12,344 | NR |
| Darb† | 10 | 11.5 | 11.7 | 11.8 | 12 | 12,905 | 13,045 | 12,998 | 12,905 | NR | ||||||||||
| Pergola 57 (2010) | Y | 3, 4 | 428 | 71 | 38 | 85.4 | 28.1 | NR | 36 | Epo-α | 11.5 | 11 | 11.1 | 11 | 11 | 5,503 | NR | 2,967 | NR | Y |
| Epo-α | 11.5 | 11.1 | 11.2 | 11.1 | 11.1 | 6,348 | NR | 4,529 | NR | Y | ||||||||||
| Epo-α | 11.5 | 11.2 | 11.3 | 11.1 | 11.1 | 5,991 | NR | 5,423 | NR | Y | ||||||||||
1st, first; CKD, chronic kidney disease; DM, diabetes mellitus; Fe, iron; F/U, follow-up; GFR, glomerular filtration rate; HD, hemodialysis; PD, peritoneal dialysis; D, dialysis; NDD, non—dialysis-dependent; NR, not reported; EPO, (recombinant) erythropoietin; Epo, epoetin (alfa or beta not specified in individual study); Epo-α, epoetin alfa; Epo-β , epoetin beta; Darb, Darbopoietin; ESA, erthropoietin-stimulating agent; Hb, hemoglobin; Pt, patient; Wt, weight
Weighted average derived from NHANES (for the NDD-CKD subjects) or USRDS (for those receiving dialysis) according to age, sex and year of report.
The ESA dose was converted to an equivalent epoetin alfa dose.
The value represents the mean body mass index (kg/m2).
72 in 4,5-NDD group; 293 in 5-HD group; 46 in 5-PD group.
This was a placebo arm where the protocol necessitated rescue ESA therapy for Hb < 9 gm/dL. Over the course of this trial, 46% of patients in this placebo arm received at least one dose of darbepoetin alfa as rescue therapy.
Denotes conversion of ESA dose from U/kg/wk to U/wk by using the mean weight of the participants in each study arm.
mean
in mL/min/1.73 m2.
The anemia parameters for each trial arm, including the mean hemoglobin and recombinant erythropoietin dose (or dose equivalent) throughout the study period are summarized in Table 1. Epoetin was used in 58 cohorts (43 used alfa, 9 used beta, and 6 9 did not specify); 6 used darbepoetin alfa, and 8 included placebo. Follow-up durations ranged from 3 to 36 months.
The randomization procedure was described in 14 RCTs 8,34,42–50,52,54,55. All but two trials documented blinding, but the procedure was described in only 8, of which 6 were “double blinded.” The blinding procedure was well-documented in only four studies 8,28,34,43. All-cause mortality was ascertained in all trials throughout the follow-up period. The ascertainment of cardiovascular mortality was more heterogeneous since it relied upon arbitrary definitions of composite outcomes. Similarly, secondary outcome definitions, such as hypertension and any serious adverse event, varied widely across studies. Two trials did not report the mean ESA dose but instead described a protocoldriven algorithm of the ESA dosing regimen 30,34. The attrition rates over the full duration of follow-up, reported in 29 trials, ranged from 0% to 80%. Six trials reported drop-out rates of less than 10% 29,31,35,49,56,57 and 7 trials of more than 40% 33,37,38,41,43,46,48. Two trials did not report their drop-out rates 39,53. Among the 29 trials that provided sufficient documentation, the intention-to-treat principle was followed in 21 8,28–31,33–37,41–43,45- 49,52,53,55.
ESA Dose and All-Cause Mortality
In the unadjusted analysis (Table 2, full models provided in Table S1), higher first- 3-month mean ESA dose (per epoetin alfa–equivalent 10,000-U/wk increment) was associated with a higher rate of all-cause mortality (IRR, 1.42; 95% CI, 1.10–1.83).This association persisted after adjustment for the first-3-month achieved mean hemoglobin level (IRR, 1.48; 95% CI, 1.02–2.14). After adjustment for the target hemoglobin level, the association strengthened in magnitude but lost statistical significance (IRR, 1.71; 95% 10 CI, 0.90–3.24).
Table 2.
Metaregression analyses of the association of ESA dose with all-cause and cardiovascular mortality.
| Outcome / predictor | No. patients |
No. trials | IRR (95% CI) | P |
|---|---|---|---|---|
| All-cause mortality | ||||
| First-3-month mean ESA dose | ||||
| Unadjusted | 4565 | 11 | 1.42 (1.10–1.83) | 0.007 |
| Adjusted for target Hb | 4385 | 10 | 1.71 (0.90–3.24) | 0.1 |
| Adjusted for first-3-month achieved mean Hb | 4565 | 11 | 1.48 (1.02–2.14) | 0.04 |
| Total-study-period mean ESA dose | ||||
| Unadjusted | 11,285 | 21 | 1.09 (1.02–1.18) | 0.02 |
| Adjusted for target Hb | 11,105 | 21 | 1.41 (1.08–1.82) | 0.01 |
| Adjusted for total-study-period achieved mean Hb | 11,285 | 21 | 1.27 (0.97–1.65) | 0.08 |
| Cardiovascular mortality | ||||
| First-3-month mean ESA dose | ||||
| Unadjusted | 2085 | 6 | 1.31 (0.92–1.86) | 0.1 |
| Adjusted for target Hb | 1979 | 5 | Not performed* | - |
| Adjusted for first-3-month achieved mean Hb | 2085 | 6 | Not performed* | - |
| Total-study-period mean ESA dose | ||||
| Unadjusted | 7148 | 10 | 1.07 (0.97–1.17) | 0.2 |
| Adjusted for target Hb | 7042 | 10 | Not performed† | - |
| Adjusted for total-study-period achieved mean Hb | 7148 | 10 | 1.38 (0.93–2.03) | 0.1 |
ESA dose is per epoetin alfa--equivalent 10,000-U/wk increment. IRR, incidence rate ratio; CI, confidence interval; Hb, hemoglobin; ESA, erythropoiesis-stimulating agent.
The analysis was not performed due to insufficient observations.
The analysis was not performed due to collinearity.
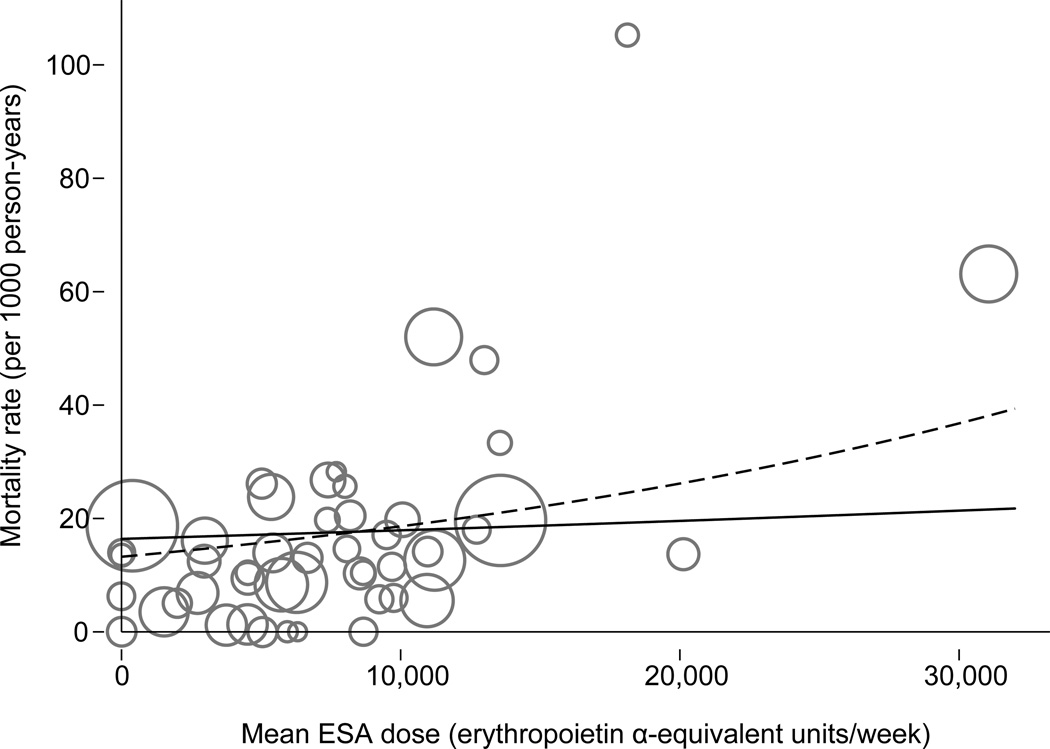
A similar association (Figure 2) was observed in the unadjusted analysis for the association of the total-study-period mean ESA dose and all-cause mortality (IRR, 1.09; 95% CI, 1.02–1.18). This association persisted after adjustment for the trials’ target hemoglobin level (IRR, 1.41; 95% CI, 1.08–1.82); after adjustment for the total-studyperiod mean hemoglobin level, the parameter estimate remained similar but lost statistical significance (IRR, 1.27; 95% CI, 0.97–1.65).. Of note, the target hemoglobin level was associated with a lower rate of all-cause mortality after adjustment for the total-studyperiod mean ESA dose (IRR, 0.91; 95% CI, 0.82–1.00).
Figure 2.
Association of the total-study-period mean weekly ESA dose with all-cause mortality. Continuous line, unadjusted analysis (IRR 1.09; 95% CI 1.02, 1.18; P = 0.02); Dashed line, target hemoglobin-adjusted (fixed at 11 gm/dL) analysis (IRR, 1.41; 95% CI, 1.08–1.82; P = 0.01). Each circle represents a study arm. The radius of a circle corresponds to a study arm’s weight in the metaregression. Here, “erythropoietin α” refers to epoetin alfa.
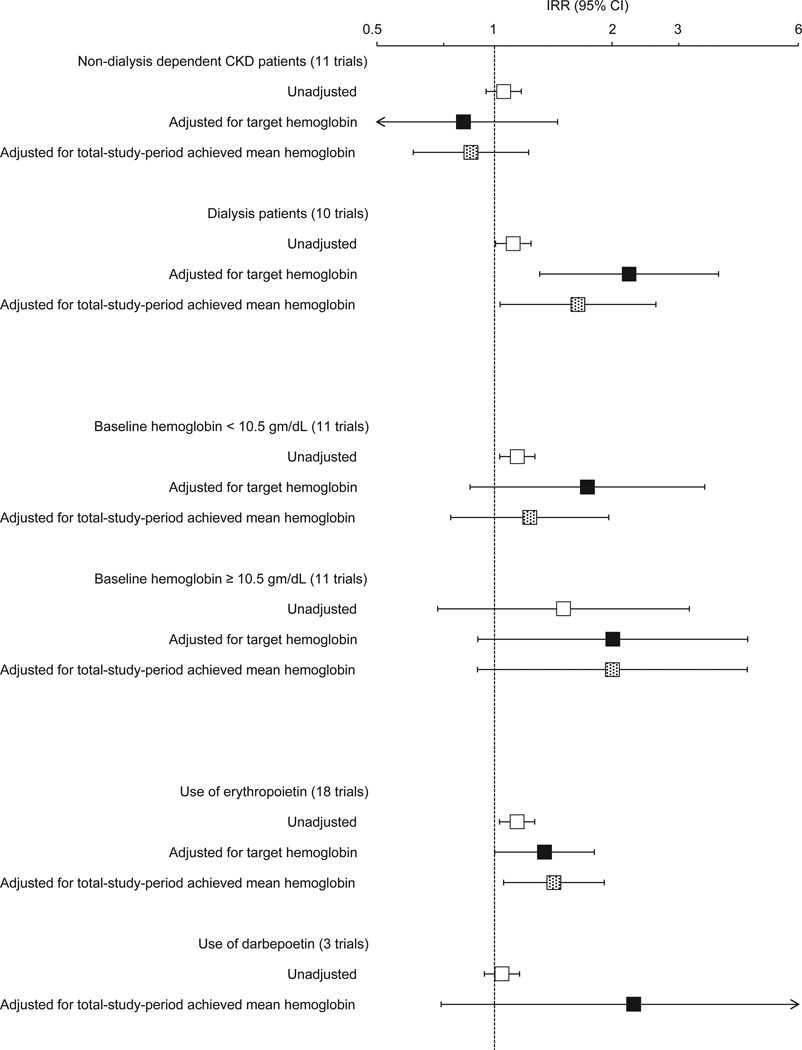
In sensitivity analyses after removing the 2 cohorts with the highest all-cause mortality rates, only the total-study-period mean ESA dose, adjusted for target hemoglobin, remained significantly associated with all-cause mortality (IRR, 1.54; 95% CI, 1.02–2.30). Subgroup analyses are shown in Figure 3. In studies of dialysis patients, higher ESA dose was associated with higher mortality in the unadjusted (IRR, 1.12; 95% CI, 1.01–1.24) as well as the adjusted (IRR, 2.21; 95% CI, 1.30–3.75) analyses for target hemoglobin and achieved mean hemoglobin (IRR, 1.64; 95% CI, 1.03–2.59). In studies that used epoetin, the association of ESA dose with mortality persisted in the unadjusted and adjusted analyses.
Figure 3.
Subgroup meta-regression analyses examining the association of total-study-period ESA dose (per epoetin alfa–equivalent 10,000-U/wk increment) with all-cause mortality. The incidence rate ratio (IRR) and 95% confidence interval (CI) is displayed on a logarithmic scale. Here, “erythropoietin” refers to epoetin (alfa or beta); “darbepoetin” refers to darbepoetin alfa.
ESA Dose and Cardiovascular Mortality
The relationship between mean ESA dose and cardiovascular mortality was in the same direction as with overall mortality, albeit not statistically significant (Table 2, Table S1). In unadjusted analyses, IRRs of the first-3-month and total-study-period mean ESA dose (per epoetin alfa–equivalent 10,000-U/wk increment) were 1.31 (95% CI, 0.92–1.86) 11 and 1.07 (95% CI, 0.97–1.17), respectively. Adjusted analyses were limited due to the insufficient number of observations or collinearity between the predictor variables.
ESA Dose and Other Adverse Outcomes
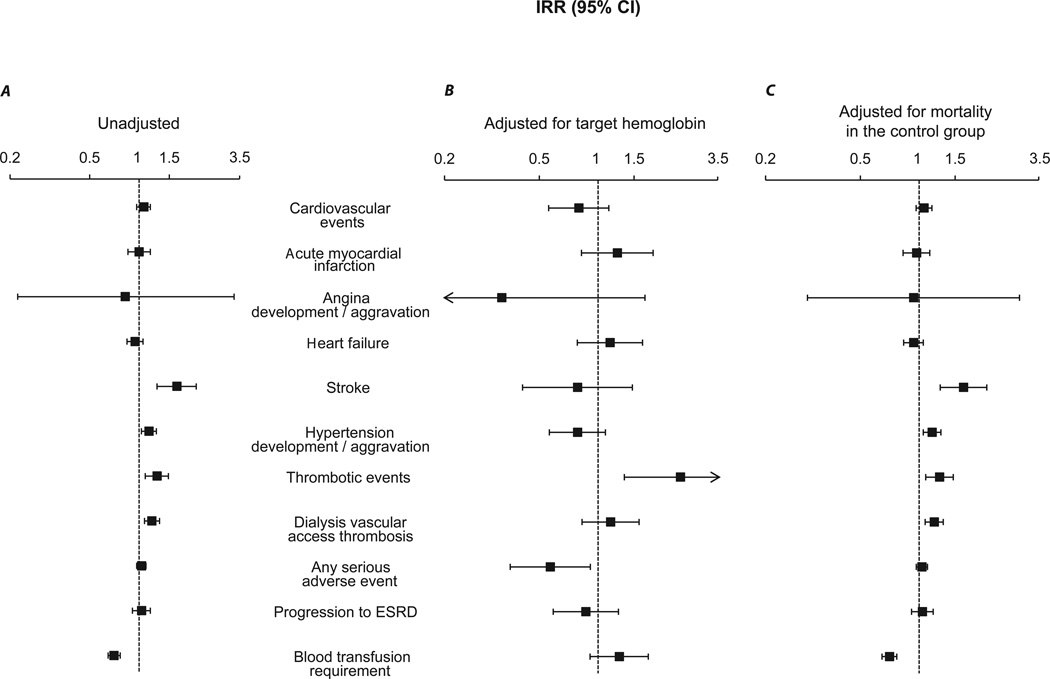
In the unadjusted analyses (Figure 4A), the total-study-period mean ESA dose was associated with a higher rate of stroke (IRR, 1.60; 95% CI, 1.25–2.04), de novo or worsening hypertension (IRR, 1.13; 95% CI, 1.03–1.24), thrombotic events (IRR, 1.25; 95% CI, 1.08–1.44), and dialysis vascular access thrombosis (IRR, 1.17; 95% CI, 1.07–1.29), and with a lower rate of transfusion requirement (IRR, 0.73; 95% CI, 0.68–0.79). Similar associations were observed for the first-3-month mean ESA dose in the unadjusted analyses (data not shown) with the exception of a lower rate of stroke (IRR, 0.43; 95% CI, 0.19–0.93).
Figure 4.
Meta-regression analyses examining the association of total-study-period ESA dose (per epoetin alfa–equivalent 10,000 U/wk increment) with the secondary outcomes [4A, unadjusted; 4B, adjusted for target hemoglobin; and 4C, adjusted for mortality rate (expressed per 1000 person-years) in the control group]. The incidence rate ratio (IRR) and 95% confidence interval (CI) is displayed on a logarithmic scale. ESRD denotes end-stage renal disease.
After adjustment for target hemoglobin (Figure 4B), the association of the totalstudy- period mean ESA dose strengthened only with the outcome of thrombotic events (IRR, 2.37; 95% CI, 1.32–4.27) while a lower rate for any serious adverse event was observed (IRR, 0.61; 95% CI, 0.40–0.92). After adjustment for mortality rate in the control group of each trial (Figure 4C), the results were strikingly similar to the unadjusted analyses, suggesting that the effects of these two predictors are completely orthogonal.
We found no association between the total-study-period mean ESA dose and the annual GFR change (in ml/min/1.73m2 per epoetin alfa–equivalent 10,000-U/wk increment) either in the unadjusted analysis (−0.50; 95% CI, −15.93 to 14.93) or after adjustment for target hemoglobin (−0.42; 95% CI, −22.24 to 21.40).
DISCUSSION
In the present meta-regression analysis, we identify an association between the first-3-month and total-study-period mean ESA dose and all-cause mortality, both in unadjusted models and models adjusting for target hemoglobin. When restricting the analyses to dialysis patients or those treated with epoetin, the association persisted in both the unadjusted and adjusted analyses. Although not significant, a similar relationship was observed for cardiovascular mortality. We also observed an association between total-study- period mean ESA dose and several secondary endpoints including development of hypertension, stroke, and thrombotic events. These findings favor the recent US Food and Drug Administration’s relabeling on ESAs, recommending a more conservative dosing regimen for the treatment of patients with CKD 58.
In a post hoc analysis of the CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) trial, a higher epoetin alfa dose was associated with increased risk for the composite endpoint of mortality, myocardial infarction, stroke, or heart failurerelated hospitalization, independently of randomization to a higher hemoglobin target 59. Another post hoc analysis, of TREAT (Trial to Reduce Cardiovascular Events with Aranesp [darbepoetin alfa] Therapy), demonstrated that escalation of the darbepoetin alfa dose in “poor responders”, attempting to reach the target hemoglobin level, was associated with an increased risk of death or cardiovascular events 60. Treatment-byindication bias might account for this association as the need for a higher ESA dose might be a proxy for comorbidities and inflammation thereby contributing to ESA hyporesponsiveness. More specifically, patients with ESA hypo-responsiveness were more likely to be older, have more comorbidities, and lower GFR levels, driving the association of higher ESA dose with higher mortality. In our analysis, the adjustment for 13 achieved hemoglobin partially controls for ESA hypo-responsiveness. Furthermore, the use of randomized trials minimized comorbidity imbalances among patients assigned to higher vs. lower target hemoglobin levels. Nevertheless, presence of ecological fallacy, especially in light of the heterogeneous dispersion depicted in Figure 2, cannot be ruled out and treatment-by-indication bias towards higher ESA doses among patients with ESA hypo-responsiveness might have influenced our results.
The risk of poorly controlled hypertension in ESA-treated patients targeted to higher target hemoglobin levels has previously been shown 6,61–64 and a drug effect has been theorized 8,45,47. Our unadjusted analysis demonstrated an association between ESA dose and hypertension; the analysis that was adjusted for the mortality rate in the control group confirmed this finding, but the target hemoglobin-adjusted analysis did not. The similar association between ESA dose and increased risk of stroke in our analyses supports the findings of TREAT8, and raises concerns about the use of these agents, particularly in patients with poorly controlled hypertension or in those with a prior history of stroke.
We found strong associations between ESA dose and increased risk of thrombotic events, which had previously been observed in some 8,36,41 but not all trials 45,46.
The unexpected finding of a protective effect of the higher total-study-period mean ESA dose on the incidence of any serious adverse event, after adjustment for target hemoglobin level, is of unclear significance. Significant heterogeneity in the definition of this clinical endpoint raises concerns about its content validity. Similarly, the protective effect of a higher first-3-month mean ESA dose against stroke is of unclear significance as the total-study-period mean ESA dose was not protective. Alternatively, a potential 14 ESA neuroprotective effect might be short-term lived 65.
To our knowledge, there are no published trials explicitly designed to answer the potential harm of ESA dose. A recent retrospective cohort study found that, at higher hematocrit levels, an increased risk of death was associated with greater ESA and iron use 66. Prior systematic reviews on this topic either did not explore the potential effect of ESA dose on mortality or other adverse outcomes 2,6,9–15 or reported that the data were insufficient for this analysis 7.
We observed a non-significant trend between a higher target hemoglobin level and a lower adjusted IRR for all-cause and cardiovascular mortality. This counterintuitive observation might be due to collinearity between predictors, whereby the hemoglobin level may be an intermediate factor between the ESA dose and mortality or a determinant of ESA dose. Adjusting for an intermediate factor typically results in estimates that are biased towards the null 67. This protective effect could hold true but the possibility of collinearity does not allow such inference, especially in light of several large RCTs demonstrating an association between higher target hemoglobin and adverse outcomes 8,36,40,42,43,45,47. The presence of this counterintuitive protective effect suggests that collinearity, ecological fallacy, or treatment-by-indication bias, were not addressed adequately, a problem that is impossible to unwind in the setting of meta-regression without access to patient-level data.
To our knowledge, this is the first meta-regression analysis that formally explores the association of the ESA dose, adjusted for target and achieved hemoglobin level, with several clinically important endpoints in patients with CKD. The inclusion of RCTs, which typically mandate pre-defined outcome assessment and have more complete follow 15 up compared to cohort studies, helped minimize ascertainment bias. We also dissected the differential effect of ESA dose over the first 3 months of therapy vs. the total-study period. If not spurious, our findings are consistent with the notion that rapid correction of anemia with ESAs might be an independent predictor of adverse outcomes 59, a factor commonly overlooked by clinicians that might deserve more attention.
Our major limitation is the use of study-level aggregated data, which are susceptible to ecological fallacy. In addition, numerous assumptions and transformations were required to harmonize results from individual trials and bring them into the same unit and scale, possibly introducing additional biases. Similarly, we were unable to effectively differentiate between mean follow-up time and total duration of individual trials, inserting bias into the ascertainment of our outcomes. We used epoetin alfa– equivalent dose, which is an oversimplification, as ESAs likely have different properties. Finally, we could not adequately control for potential confounding effects of other factors, and heterogeneity among the selected trials.
Our analysis raises concerns as to whether the ESA dose is an independent predictor of mortality and other adverse cardiovascular events in patients with CKD. Our results call for the design of trials that examine the effect of the ESA dose rather than target hemoglobin on cardiovascular endpoints. Such trials, using an absolute dosing protocol rather than a titration protocol, would hopefully advance the field and help revise current anemia treatment guidelines in CKD by incorporating not only the target hemoglobin but also the optimal ESA dose.
In conclusion, after adjusting for target or mean achieved hemoglobin, higher ESA dose for the treatment of anemia in patients with CKD might be associated with a higher 16 mortality risk. Lack of adjustment for comorbidities and inflammatory markers as well as inadequate control for treatment-by-indication bias and ecological fallacy in the setting of meta-regression precludes definitive conclusions.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Paul Rufo for his contribution to the verification of the data extracted from graphs using the G3data graph analyzer.
Support: This work was supported in part by Grant number UL1 RR025752 from the National Center for Research Resources (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented in part at the Kidney Week of the American Society of Nephrology, Philadelphia, PA, November 08–13, 2011.
Financial Disclosure: Dr Jaber is a Scientific Advisor for NxStage Medical, Inc. The other authors declare that they have no relevant financial interests.
REFERENCES
- 1.Leaf DE, Goldfarb DS. Interpretation and review of health-related quality of life data in CKD patients receiving treatment for anemia. Kidney Int. 2009 Jan;75(1):15–24. doi: 10.1038/ki.2008.414. [DOI] [PubMed] [Google Scholar]
- 2.Jones M, Ibels L, Schenkel B, Zagari M. Impact of epoetin alfa on clinical end points in patients with chronic renal failure: a meta-analysis. Kidney Int. 2004 Mar;65(3):757–767. doi: 10.1111/j.1523-1755.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 3.Rao M, Pereira BJ. Optimal anemia management reduces cardiovascular morbidity, mortality, and costs in chronic kidney disease. Kidney Int. 2005 Oct;68(4):1432–1438. doi: 10.1111/j.1523-1755.2005.00554.x. [DOI] [PubMed] [Google Scholar]
- 4.Vlagopoulos PT, Tighiouart H, Weiner DE, et al. Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease. J Am Soc Nephrol. 2005 Nov;16(11):3403–3410. doi: 10.1681/ASN.2005030226. [DOI] [PubMed] [Google Scholar]
- 5.Locatelli F, Pisoni RL, Combe C, et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2004 Jan;19(1):121–132. doi: 10.1093/ndt/gfg458. [DOI] [PubMed] [Google Scholar]
- 6.Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007 Feb 3;369(9559):381–388. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 7.Palmer SC, Navaneethan SD, Craig JC, et al. Meta-analysis: erythropoiesisstimulating agents in patients with chronic kidney disease. Ann Intern Med. 2010 Jul 6;153(1):23–33. doi: 10.7326/0003-4819-153-1-201007060-00252. [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009 Nov 19;361(21):2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 9.Cody J, Daly C, Campbell M, et al. Recombinant human erythropoietin for chronic renal failure anaemia in pre-dialysis patients. Cochrane Database Syst Rev. 2001;(4):CD003266. doi: 10.1002/14651858.CD003266. [DOI] [PubMed] [Google Scholar]
- 10.Besarab A, Reyes CM, Hornberger J. Meta-analysis of subcutaneous versus intravenous epoetin in maintenance treatment of anemia in hemodialysis patients. Am J Kidney Dis. 2002 Sep;40(3):439–446. doi: 10.1053/ajkd.2002.34881. [DOI] [PubMed] [Google Scholar]
- 11.Strippoli GF, Manno C, Schena FP, Craig JC. Haemoglobin and haematocrit targets for the anaemia of chronic renal disease. Cochrane Database Syst Rev. 2003;(1):CD003967. doi: 10.1002/14651858.CD003967. [DOI] [PubMed] [Google Scholar]
- 12.Strippoli GF, Craig JC, Manno C, Schena FP. Hemoglobin targets for the anemia of chronic kidney disease: a meta-analysis of randomized, controlled trials. J Am Soc Nephrol. 2004 Dec;15(12):3154–3165. doi: 10.1097/01.ASN.0000145436.09176.A7. [DOI] [PubMed] [Google Scholar]
- 13.Cody J, Daly C, Campbell M, et al. Recombinant human erythropoietin for chronic renal failure anaemia in pre-dialysis patients. Cochrane Database Syst Rev. 2005;(3):CD003266. doi: 10.1002/14651858.CD003266.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Strippoli GF, Navaneethan SD, Craig JC. Haemoglobin and haematocrit targets for the anaemia of chronic kidney disease. Cochrane Database Syst Rev. 2006;(4):CD003967. doi: 10.1002/14651858.CD003967.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Clement FM, Klarenbach S, Tonelli M, Johnson JA, Manns BJ. The impact of selecting a high hemoglobin target level on health-related quality of life for patients with chronic kidney disease: a systematic review and meta-analysis. Arch Intern Med. 2009 Jun 22;169(12):1104–1112. doi: 10.1001/archinternmed.2009.112. [DOI] [PubMed] [Google Scholar]
- 16.G3data graph analyzer shareware released under the GNU General Public License. URL http://www.frantz.fi/software/g3data.php.
- 17.Billett HH. Hemoglobin and Hematocrit. 1990. [PubMed] [Google Scholar]
- 18.Horowitz J, Agarwal A, Huang F, Gitlin M, Gandra SR, Cangialose CB. Empirical methods to calculate an erythropoiesis-stimulating agent dose conversion ratio in nondialyzed patients with chronic kidney disease. J Manag Care Pharm. 2009 Nov-Dec;15(9):741–750. doi: 10.18553/jmcp.2009.15.9.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma A, Yee J, Gandra SR, Khan I, Petersen J. Estimate of maintenance EPO to darbepoetin alfa dose conversion ratio in a hospital-based dialysis patient population. Curr Med Res Opin. 2010 Nov;26(11):2679–2687. doi: 10.1185/03007995.2010.526598. [DOI] [PubMed] [Google Scholar]
- 20.Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States 1960–2002. Adv Data. 2004 Oct 27;(347):1–17. [PubMed] [Google Scholar]
- 21.Margaret A, McDowell PD, MPH, RD, Cheryl D Fryar, MSPH, Cynthia L Ogden, PhD, Katherine M Flegal., PhD [accessed in 10/12/2010];Anthropometric Reference Data for Children and Adults: United States, 2003–2006. http://www.cdc.gov/nchs/data/nhsr/nhsr010.pdf.
- 22. [accessed in 10/12/2010];2008 data report from the United States Renal Data System (USRDS) for dialysis patients. http://www.usrds.org/reference_2008.htm.
- 23.Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism. 2006 Apr;55(4):515–524. doi: 10.1016/j.metabol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996 Feb;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 26.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003 Sep 15;22(17):2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 27.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for metaanalysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. Canadian Erythropoietin Study Group. BMJ. 1990 Mar 3;300(6724):573–578. doi: 10.1136/bmj.300.6724.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abraham PA, Macres MG. Blood pressure in hemodialysis patients during amelioration of anemia with erythropoietin. J Am Soc Nephrol. 1991 Oct;2(4):927–936. doi: 10.1681/ASN.V24927. [DOI] [PubMed] [Google Scholar]
- 30.Bahlmann J, Schoter KH, Scigalla P, et al. Morbidity and mortality in hemodialysis patients with and without erythropoietin treatment: a controlled study. Contrib Nephrol. 1991;88:90–106. doi: 10.1159/000419519. [DOI] [PubMed] [Google Scholar]
- 31.Clyne N, Jogestrand T. Effect of erythropoietin treatment on physical exercise capacity and on renal function in predialytic uremic patients. Nephron. 1992;60(4):390–396. doi: 10.1159/000186797. [DOI] [PubMed] [Google Scholar]
- 32.Muirhead N, Churchill DN, Goldstein M, et al. Comparison of subcutaneous and intravenous recombinant human erythropoietin for anemia in hemodialysis patients with significant comorbid disease. Am J Nephrol. 1992;12(5):303–310. doi: 10.1159/000168464. [DOI] [PubMed] [Google Scholar]
- 33.Roth D, Smith RD, Schulman G, et al. Effects of recombinant human erythropoietin on renal function in chronic renal failure predialysis patients. Am J Kidney Dis. 1994 Nov;24(5):777–784. doi: 10.1016/s0272-6386(12)80671-8. [DOI] [PubMed] [Google Scholar]
- 34.Nissenson AR, Korbet S, Faber M, et al. Multicenter trial of erythropoietin in patients on peritoneal dialysis. J Am Soc Nephrol. 1995 Jan;5(7):1517–1529. doi: 10.1681/ASN.V571517. [DOI] [PubMed] [Google Scholar]
- 35.Virot JS, Janin G, Guillaumie J, et al. Must erythropoietin be injected by the subcutaneous route for every hemodialyzed patient? Am J Kidney Dis. 1996 Sep;28(3):400–408. doi: 10.1016/s0272-6386(96)90498-9. [DOI] [PubMed] [Google Scholar]
- 36.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998 Aug 27;339(9):584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 37.Kaufman JS, Reda DJ, Fye CL, et al. Subcutaneous compared with intravenous epoetin in patients receiving hemodialysis. Department of Veterans Affairs Cooperative Study Group on Erythropoietin in Hemodialysis Patients. N Engl J Med. 1998 Aug 27;339(9):578–583. doi: 10.1056/NEJM199808273390902. [DOI] [PubMed] [Google Scholar]
- 38.Berns JS, Rudnick MR, Cohen RM, Bower JD, Wood BC. Effects of normal hematocrit on ambulatory blood pressure in epoetin-treated hemodialysis patients with cardiac disease. Kidney Int. 1999 Jul;56(1):253–260. doi: 10.1046/j.1523-1755.1999.00531.x. [DOI] [PubMed] [Google Scholar]
- 39.Conlon PJ, Kovalik E, Schumm D, Minda S, Schwab SJ. Normalization of hematocrit in hemodialysis patients with cardiac disease does not increase blood pressure. Ren Fail. 2000;22(4):435–444. doi: 10.1081/jdi-100100885. [DOI] [PubMed] [Google Scholar]
- 40.Foley RN, Parfrey PS, Morgan J, et al. Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney Int. 2000 Sep;58(3):1325–1335. doi: 10.1046/j.1523-1755.2000.00289.x. [DOI] [PubMed] [Google Scholar]
- 41.Furuland H, Linde T, Ahlmen J, Christensson A, Strombom U, Danielson BG. A randomized controlled trial of haemoglobin normalization with epoetin alfa in pre-dialysis and dialysis patients. Nephrol Dial Transplant. 2003 Feb;18(2):353–361. doi: 10.1093/ndt/18.2.353. [DOI] [PubMed] [Google Scholar]
- 42.Levin A, Djurdjev O, Thompson C, et al. Canadian randomized trial of hemoglobin maintenance to prevent or delay left ventricular mass growth in patients with CKD. Am J Kidney Dis. 2005 Nov;46(5):799–811. doi: 10.1053/j.ajkd.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Doubleblind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol. 2005 Jul;16(7):2180–2189. doi: 10.1681/ASN.2004121039. [DOI] [PubMed] [Google Scholar]
- 44.Provenzano R, Bhaduri S, Singh AK. Extended epoetin alfa dosing as maintenance treatment for the anemia of chronic kidney disease: the PROMPT study. Clin Nephrol. 2005 Aug;64(2):113–123. doi: 10.5414/cnp64113. [DOI] [PubMed] [Google Scholar]
- 45.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006 Nov 16;355(20):2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 46.Rossert J, Levin A, Roger SD, et al. Effect of early correction of anemia on the progression of CKD. Am J Kidney Dis. 2006 May;47(5):738–750. doi: 10.1053/j.ajkd.2006.02.170. [DOI] [PubMed] [Google Scholar]
- 47.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006 Nov 16;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 48.Macdougall IC, Temple RM, Kwan JT. Is early treatment of anaemia with epoetin-alpha beneficial to pre-dialysis chronic kidney disease patients? Results of a multicentre, open-label, prospective, randomized, comparative group trial. Nephrol Dial Transplant. 2007 Mar;22(3):784–793. doi: 10.1093/ndt/gfl483. [DOI] [PubMed] [Google Scholar]
- 49.Ritz E, Laville M, Bilous RW, et al. Target level for hemoglobin correction in patients with diabetes and CKD: primary results of the Anemia Correction in Diabetes (ACORD) Study. Am J Kidney Dis. 2007 Feb;49(2):194–207. doi: 10.1053/j.ajkd.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 50.Bommer J, Asmus G, Wenning M, Bommer G. A comparison of haemoglobin levels and doses in haemodialysis patients treated with subcutaneous or intravenous darbepoetin alfa: a German prospective, randomized, multicentre study. Nephrol Dial Transplant. 2008 Dec;23(12):4002–4008. doi: 10.1093/ndt/gfn416. [DOI] [PubMed] [Google Scholar]
- 51.Chen HH, Tarng DC, Lee KF, Wu CY, Chen YC. Epoetin alfa and darbepoetin alfa: effects on ventricular hypertrophy in patients with chronic kidney disease. J Nephrol. 2008 Jul-Aug;21(4):543–549. [PubMed] [Google Scholar]
- 52.Cianciaruso B, Ravani P, Barrett BJ, Levin A. Italian randomized trial of hemoglobin maintenance to prevent or delay left ventricular hypertrophy in chronic kidney disease. J Nephrol. 2008 Nov-Dec;21(6):861–870. [PubMed] [Google Scholar]
- 53.Locatelli F, Villa G, Messa P, et al. Efficacy and safety of once-weekly intravenous epoetin alfa in maintaining hemoglobin levels in hemodialysis patients. J Nephrol. 2008 May-Jun;21(3):412–420. [PubMed] [Google Scholar]
- 54.Spinowitz B, Germain M, Benz R, et al. A randomized study of extended dosing regimens for initiation of epoetin alfa treatment for anemia of chronic kidney disease. Clin J Am Soc Nephrol. 2008 Jul;3(4):1015–1021. doi: 10.2215/CJN.05681207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chazot C, Terrat JC, Dumoulin A, et al. Randomized equivalence study evaluating the possibility of switching hemodialysis patients receiving subcutaneous human erythropoietin directly to intravenous darbepoetin alfa. Ann Pharmacother. 2009 Feb;43(2):228–234. doi: 10.1345/aph.1K664. [DOI] [PubMed] [Google Scholar]
- 56.Pergola PE, Gartenberg G, Fu M, Wolfson M, Rao S, Bowers P. A randomized controlled study of weekly and biweekly dosing of epoetin alfa in CKD Patients with anemia. Clin J Am Soc Nephrol. 2009 Nov;4(11):1731–1740. doi: 10.2215/CJN.03470509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pergola PE, Gartenberg G, Fu M, Sun S, Wolfson M, Bowers P. A randomized controlled study comparing once-weekly to every-2-week and every-4-week dosing of epoetin alfa in CKD patients with anemia. Clin J Am Soc Nephrol. 2010 Apr;5(4):598–606. doi: 10.2215/CJN.06770909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. [accessed in 8/17/2011];FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of Erythropoiesis-Stimulating Agents (ESAs) in chronic kidney disease. http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm.
- 59.Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008 Sep;74(6):791–798. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010 Sep 16;363(12):1146–1155. doi: 10.1056/NEJMoa1005109. [DOI] [PubMed] [Google Scholar]
- 61.Hand MF, Haynes WG, Johnstone HA, Anderton JL, Webb DJ. Erythropoietin enhances vascular responsiveness to norepinephrine in renal failure. Kidney Int. 1995 Sep;48(3):806–813. doi: 10.1038/ki.1995.354. [DOI] [PubMed] [Google Scholar]
- 62.Vaziri ND. Mechanism of erythropoietin-induced hypertension. Am J Kidney Dis. 1999 May;33(5):821–828. doi: 10.1016/s0272-6386(99)70413-0. [DOI] [PubMed] [Google Scholar]
- 63.Brochu E, Lacasse S, Lariviere R, Kingma I, Grose JH, Lebel M. Differential effects of endothelin-1 antagonists on erythropoietin-induced hypertension in renal failure. J Am Soc Nephrol. 1999 Jul;10(7):1440–1446. doi: 10.1681/ASN.V1071440. [DOI] [PubMed] [Google Scholar]
- 64.Kang DH, Yoon KI, Han DS. Acute effects of recombinant human erythropoietin on plasma levels of proendothelin-1 and endothelin-1 in haemodialysis patients. Nephrol Dial Transplant. 1998 Nov;13(11):2877–2883. doi: 10.1093/ndt/13.11.2877. [DOI] [PubMed] [Google Scholar]
- 65.Ehrenreich H, Weissenborn K, Prange H, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009 Dec;40(12):e647–656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 66.Brookhart MA, Schneeweiss S, Avorn J, Bradbury BD, Liu J, Winkelmayer WC. Comparative mortality risk of anemia management practices in incident hemodialysis patients. JAMA. 2010 Mar 3;303(9):857–864. doi: 10.1001/jama.2010.206. [DOI] [PubMed] [Google Scholar]
- 67.Kenneth J Rothman. Epidemiology: An Introduction. 198 Madison Avenue, New York, NY: Oxford University Press; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.