Abstract
Short sleep duration and obesity are common occurrence in today's society. An extensive literature from cross-sectional and longitudinal epidemiological studies shows a relationship between short sleep and prevalence of obesity and weight gain. However, causality cannot be inferred from such studies. Clinical intervention studies have examined whether reducing sleep in normal sleepers, typically sleeping 7–9 h/night, can affect energy intake, energy expenditure, and endocrine regulators of energy balance. The aim of this review is to evaluate studies that have assessed food intake, energy expenditure, and leptin and ghrelin levels after periods of restricted and normal sleep. Most studies support the notion that restricting sleep increases food intake, but the effects on energy expenditure are mixed. Differences in methodology and component of energy expenditure analyzed may account for the discrepancies. Studies examining the effects of sleep on leptin and ghrelin have provided conflicting results with increased, reduced, or unchanged leptin and ghrelin levels after restricted sleep compared to normal sleep. Energy balance of study participants and potential sex differences may account for the varied results. Studies should strive for constant energy balance and feeding schedules when assessing the role of sleep on hormonal profile. Although studies suggest that restricting sleep may lead to weight gain via increased food intake, research is needed to examine the impact on energy expenditure and endocrine controls. Also, studies have been of short duration, and there is little knowledge on the reverse question: does increasing sleep duration in short sleepers lead to negative energy balance?
Citation:
St-Onge MP. The role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditure. J Clin Sleep Med 2013;9(1):73–80.
Keywords: Energy balance, energy expenditure, food intake, ghrelin, leptin
There is much epidemiological evidence supporting an association between self-reported short sleep duration and obesity. This has been the topic of several meta-analyses and systematic reviews.1–3 These studies show that the odds of obesity are increased in those who report sleeping short, generally < 7 h/night, compared to those who report sleeping a recommended amount of time, about 7–8 h/night. Since those reviews were published, additional support has been put forth from various population studies.4–9 Although the literature is somewhat consistent, some studies do not show an association in specific population subgroups, such as the elderly.6,10 Nevertheless, it is generally agreed that there is a link between short sleep duration and obesity. However, whether this relationship is causal is debatable. The purpose of this review is to examine the literature to determine if alterations in sleep duration lead to changes in the energy balance equation: Energy intake = Energy output (weight stability). The focus will be on adult clinical studies examining the impact of reductions in sleep duration on energy intake and energy expenditure (EE). If short sleep duration is a causal factor in the path towards obesity, then restricting sleep should produce an imbalance in the energy balance equation favoring increased energy intake relative to expenditure or reduced EE relative to intake (Energy intake > Energy output).
There are various ways to assess the impact of sleep duration on energy balance. This review will provide a overview of the effects of sleep deficiency on each component of the energy balance equation, with subcategories based on the sleep protocol: total sleep deprivation (no sleep at all during ≥ 24-h period), partial sleep restriction (sleep prescription < 7 h per 24-h period), and sleep quality (alterations in sleep architecture or disrupted sleep with little or no reduction in sleep duration).
1. Does sleep deficiency affect appetite and hunger?
a. Total sleep deprivation
Self-reported hunger and appetite ratings, usually obtained on linear scales, as well as measured food intake in the laboratory, have been obtained in various sleep protocols. Schmid et al.11 compared hunger ratings after a night of total sleep deprivation, a night of 4.5 h sleep, and a night of 7 h sleep and noted increased hunger ratings in the restricted sleep conditions. Compared to the night of 7 h sleep, young men reported more than double the hunger rating after total sleep deprivation and almost 50% (not significant) higher hunger rating after short sleep, such that there was an incremental impact on feelings of hunger with increased restriction in sleep. On the other hand, another study conducted in a sample of men and women did not find differences in hunger ratings after one night of total sleep deprivation compared to one night of recovery sleep (8 h in bed).12 Actual food intake was not measured in these studies, and therefore it is unknown whether participants would have acted on their feelings of hunger by actually increasing their food intake during the day of total sleep deprivation compared to the day in which sleep was permitted.
b. Partial sleep restriction
Spiegel and colleagues13 were the first to report a rise in subjective feelings of hunger and appetite after 2 d of restricted sleep to 4 h/night relative to approximately 9 h sleep (habitual sleep). Men reported a 24% higher rating of hunger and 23% higher rating of appetite on a 10-cm visual analog scale; the difference in appetite ratings was most marked for calorie-dense, high-carbohydrate foods. Similar results were not observed in a separate study from Schmid et al. comparing hunger and appetite ratings after 2 nights of either 4 h or 8 h bed times in men14 or after a period of 3 nights of 4 h in bed compared to 3 nights of 9 h in bed in a sample of men and women.15 Also, in a study involving women only, hunger and craving scores were not different after one night of 3 h sleep compared to one night of 10 h sleep opportunity16 or after 4 nights of progressive sleep loss compared to baseline 9 h sleep.17 However, in the study by St-Onge et al.,15 there was a sex by sleep interaction on fullness, such that men had lower subjective ratings of fullness during the period of short sleep compared to habitual sleep that was not observed in women.
c. Sleep quality
Sleep quality has also been associated with eating behaviors. Using the Three-Factor Eating Questionnaire, Kilkus et al.18 observed that poor sleep quality, but not sleep duration, was associated with increased hunger, uncontrolled and emotional eating, as well as cognitive restraint, even after controlling for habitual sleep duration and physical activity. In that study, sleep quality was assessed by questionnaire and sleep duration and physical activity were assessed by actigraphy. Along those lines, it is interesting to note that, in the Québec Family Study, disinhibited eating, but not hunger and restraint, and sleep duration interacted to predict weight gain over a 6-y follow-up period.19 In that study, individuals categorized as short sleepers and disinhibited eaters were more likely to gain weight compared to all other sleep and disinhibited eating combinations (average or long sleep and low disinhibition were other categories). Moreover, the risk of incident overweight/obesity was greatest in that group. Of note, < 5% of the study population fell into the short sleep-high disinhibition group. Nevertheless, the studies by Kilkus et al.18 and Chaput et al.19 suggest that eating behavior traits are related to sleep and may be important in the relationship between sleep and obesity.
Gonnissen et al. examined the impact of sleep fragmentation independent of sleep duration on subjective feelings of appetite.20 In that study, healthy men were randomized to two 24-h sleep periods in which they either slept uninterrupted for 8 h or had their sleep disturbed by wake-up calls every 90 min over 8 h. The wake-up calls did not lead to a significant reduction in total sleep duration, but rather led to a reduction in REM sleep and a corresponding increase in stage 2 sleep. Participants reported feeling less full in the afternoon after the night of fragmented sleep and reported a greater desire to eat after dinner compared to after the night of undisturbed sleep. The authors concluded that sleep quality may be more important than sleep duration for appetite regulation but did not measure food intake in their study.
d. Summary: Sleep deficiency and appetite/hunger
Several study-related differences may account for the discordant results regarding the impact of restricting sleep duration on hunger and appetite ratings. In the original study by Spiegel et al.,13 the impact of sleep may have been amplified by the study conditions: men were on constant intravenous glucose infusion throughout the measurement period. Data by Schmid et al.11 were collected at one time point—morning fasted—whereas others collected hunger ratings before dinner,16 15–30 min before each meal,12 or throughout the day.15 Differences in the degree of sleep restriction may also account for some of the variability in the data, although this is less clear; for example, 2 studies of total sleep deprivation have opposite results.11,12 Interestingly, the 2 studies that report differences in hunger ratings between restricted and habitual sleep include only young, healthy men,11,13 whereas the others also included women.12,15,16 It is possible that sleep deficiency alter subjective feelings of hunger and appetite differently in men and women.
2. Does sleep deficiency affect food intake?
Studies described above suggest that sleep duration or quality may affect feelings of hunger and eating behaviors. Clinical studies have also examined whether alterations in sleep duration affect actual food intake. To date, 5 studies have assessed food intake during periods of restricted and habitual sleep.14,15,17,21,22 Two studies enrolled normal weight men exclusively.14,21 In the study by Brondel et al.,21 men self-recorded afternoon and evening food intake, and breakfast and lunch intakes were assessed in the laboratory after one night of 8 h or 4 h in bed. Energy intakes were 22.5% higher, equivalent to 559 kcal, after a night of restricted sleep compared to habitual sleep. Percent energy from fat was also higher. In the study by Schmid et al.,14 food intake in the lab was measured after 2 nights of either 8 h or 4 h in bed. Participants were given a buffet-style breakfast until 11:00 and a snack buffet from 11:00 onwards; meals were selected from a menu and served in 1,200 kcal quantities upon request. Total energy intake, energy from meals and snacks, and energy from breakfast were not different between sleep phases (2.5% increase, equivalent to 101 kcal), but percent energy from fat was higher after the period of restricted vs habitual sleep. Participants chose more foods from the “fat” category but not from sweet or salty snacks during restricted sleep. These data contrast with those from Nedeltcheva et al.,22 who reported a specific increase in snack food energy intake during a 14-d period of 5.5 h time in bed compared to 7.5 h time in bed in overweight men and women. In that study, total daily energy intake was higher during restricted sleep compared to habitual sleep (8.7%, equivalent to 297 kcal), but the difference became nonsignificant after adjusting for body weight. The increase in snack intake was mostly from high carbohydrate foods and occurred at night (19:00-07:00) rather than during the day (07:00-19:00). It is important to note that in that study, meals were served in excess during specific times during the day (08:00-09:00, 13:00-14:00, and 18:30-19:30), and snack foods were available 24 h.
It is possible that Nedeltcheva et al. were underpowered to detect differences in total energy intakes: a larger study in normal weight men and women found statistically significant results of a similar magnitude (approximately 12% above recorded habitual intake, equivalent to 296 kcal) after 4 nights of restricted sleep relative to habitual sleep.15
Only one study was conducted exclusively in women (8 normal weight, 6 overweight or obese).17 Participants kept food records during 2 d of 9 h sleep, 4 d of 5.5 h sleep, and 2 d of 9.3 h sleep. Energy intakes increased by 20%, equivalent to 415 kcal, during the period of restricted sleep relative to baseline habitual sleep, with no change in macronutrient distribution.
Overall, 4 of the 5 studies measuring food intake with alterations in sleep duration report an increase in energy intake ranging from approximately 300 kcal/d15,22 to 559 kcal/d21 (Table 1). Differences in measurement method—participant self-records17,21 or investigator-measured14,15,22—as well as method of food presentation, buffet style/constant availability,14 fixed meal times,22 or complete control over food selection and timing of eating occasions15 may account for some of the differences in study results. Also, the 2 studies in men excluded restrained eaters,14,21 whereas the other studies did not assess eating behavior trait. In general, however, the clinical data support epidemiological data showing that short sleep is associated with low fruit and vegetable consumption, high fat diet, high frequency of fast food consumption,23 reduced tendency to eat during conventional eating hours, and dominance of snack intakes over meals.24
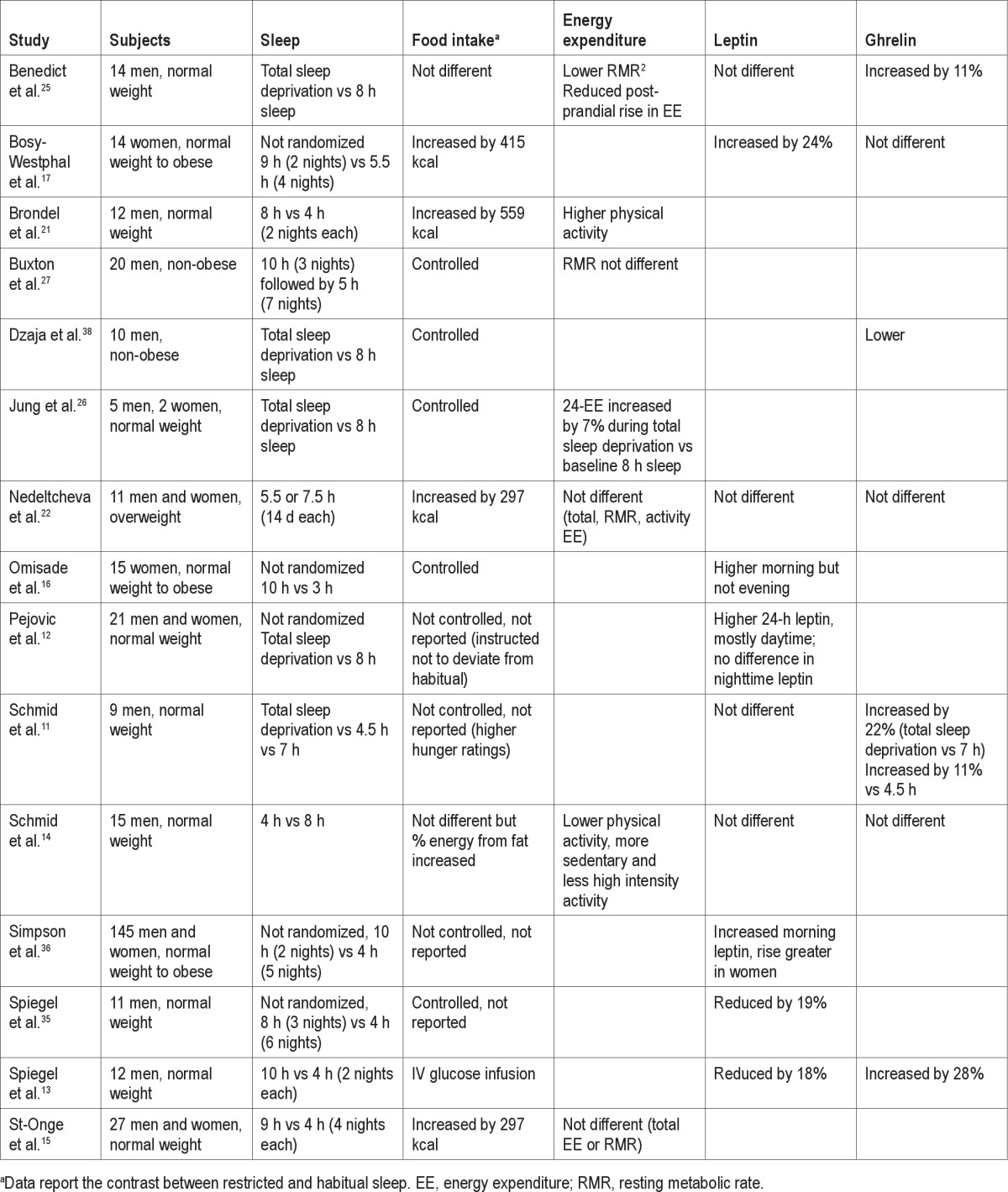
Table 1.
Summary of clinical studies of sleep restriction to assess the role of sleep duration on energy balance
3. Does sleep deficiency affect energy expenditure?
Energy expenditure is a complex component of energy balance. Total daily EE can be divided into resting metabolic rate (RMR), the measurement of EE under resting but wakeful conditions, postprandial EE, the measurement of EE after a meal, activity EE in response to physical activity, non-exercise activity thermogenesis, EE due to fidgeting or other movements not considered exercise, and sleeping metabolic rate, the EE under sleeping conditions. Additionally, each component can be measured using various methods. Total daily EE can be measured using doubly-labeled water for total free-living measurements or using a metabolic chamber, in which a person is confined to a small room while room gases are collected to assess oxygen consumption and carbon dioxide production used in the calculation of EE. Indirect calorimetry using a ventilated hood metabolic cart can be used to assess oxygen consumption and carbon dioxide production over several hours. With that method, the calculated EE can be extrapolated to a 24-h period. Usually, this is the method of choice for measuring RMR or postprandial thermogenesis. Activity EE can be measured in the metabolic chamber, using a metabolic cart, or by actigraphy. These methods will be mentioned in the following sections.
a. Total sleep deprivation
Although studies overwhelmingly support a role of sleep restriction on food intake, studies are mixed with respect of its effects on EE. Two studies of total sleep deprivation report discordant results on various components of EE.25,26 Benedict et al.25 subjected 14 young normal-weight men to 2 intervention periods of 24 h each in which they either slept between 23:00 and 07:00 or remained awake. Men were kept in a supine position between 18:00 on the first night and 13:00 the following day, after which they sat until their 18:00 discharge. Food intake was controlled for dinner, breakfast, and lunch, and EE was measured by indirect calorimetry. RMR was reduced by 5.2% after the night of total sleep deprivation, and the rise in postprandial EE after breakfast was 20% lower than after the night that permitted sleep. In contrast, 7 young normal-weight men (n = 5) and women (n = 2) spent 3 d in a metabolic chamber for measurement of 24-h EE during a day which included 8 h sleep (baseline) followed by a day of total sleep deprivation and another day with 8 h sleep (recovery).26 Twenty-four hour EE was 7% higher during the day of total sleep deprivation compared to baseline and, contrary to results by Benedict et al.,25 post-meal EE was higher after every meal during total sleep deprivation than at baseline. When data were divided into daytime and nighttime EE, daytime EE was similar between conditions but nighttime EE was increased by 32% during total sleep deprivation. In that study, participants remained semi-recumbent during scheduled wakefulness. The authors concluded that sleep was a state of energy conservation.
b. Partial sleep restriction
Other studies measuring EE during periods of restricted sleep and habitual sleep have used various protocols and procedures. Both Nedeltcheva et al.22 and St-Onge et al.15 measured free-living EE over 14 or 6 d, respectively, of restricted sleep (5.5 or 4 h/night) and habitual sleep (approximately 7.5 h/night) using doubly-labeled water and found no difference in total daily EE. These two studies also measured RMR by indirect calorimetry and did not find any difference between sleep periods. Nedeltcheva et al.22 also reported no effect of sleep duration on activity EE, measured by actigraphy, whereas St-Onge et al.15 reported a trend for lower peak activity and significantly lower percent time spent in heavy and very heavy physical activity during the period of restricted relative to habitual sleep. These two studies enrolled equal numbers of men and women, young-to-middle adulthood, and overweight22 or normal weight.15 Similarly, Bosy-Westphal et al.17 reported no difference in RMR (measured by indirect calorimetry) after a period of restricted sleep relative to baseline habitual sleep in women and no difference in total daily EE estimated by heart rate monitoring. However, they found that the change in resting EE after an oral glucose tolerance test was 32% higher after restricted sleep compared to baseline.
Two other studies by Buxton and colleagues27,28 found different effects of sleep restriction on RMR, assessed by indirect calorimetry. In a study including non-obese men, they found that restricting sleep to 5 h/night for 7 nights had no effect on RMR,27 whereas in the other study—in which they both restricted sleep to 6.5 h per 28 h period while extending the day to 28 h—inducing both sleep restriction and circadian disruption, RMR was reduced by 8% compared to the sleep replete baseline period.28 The latter study included both men and women and young and older (average age 23 and 60 years, respectively) participants. It is possible that the shift in circadian rhythm may be a key factor in disturbing RMR in healthy individuals.
Two studies which measured free-living physical activity using actigraphy also found contradictory results.14,21 Brondel et al.21 found that physical activity during one afternoon and evening after a 4-h night was higher than after an 8-h night, whereas Schmid et al.14 found lower free-living physical activity over one day after a night of 4 h sleep compared to a night of 7.5 h sleep. In that study, the proportion of sedentary activity was increased and high intensity activity decreased after short sleep. Both studies enrolled normal-weight men.
c. Sleep quality
Another study aimed to examine the effect of sleep fragmentation on EE was performed on 15 young normal-weight men.29 In that study, participants spent 48 h in a metabolic chamber with allowed time in bed between 23:30 and 07:30. On one occasion sleep was undisturbed, whereas in the other sleep was fragmented by hourly wake-up calls. Although the goal was to study fragmented sleep and not restricted sleep, total sleep time was reduced by approximately 1.5 h in the fragmented sleep arm. In that study, total EE and RMR were not different between sleep conditions, but activity EE was higher during the fragmented sleep phase. Sleep fragmentation also increased respiratory quotient and carbohydrate oxidation and reduced fat oxidation. All components of EE were assessed in the metabolic chamber.
d. Summary: Sleep deficiency and energy expenditure
Thus far, studies examining the impact of sleep duration on EE have produced disparate results. This is in part due to differences in sleep protocols (either total sleep deprivation25,26 or partial sleep restriction14,15,21,22,29) and measurement type (doubly-labeled water,15,22 indirect calorimetry,15,17,22,25 metabolic chamber,26,29 or actigraphy14,15,21). Some studies have used a randomized, multiple-phase design,14,15,21,22,25,29 but others were not randomized and may have an order effect.17,26 Moreover, only one study enrolled more than 15 participants,15 only one enrolled overweight participants,22 and 4 studies enrolled both men and women.15,17,22,26 The majority of studies have therefore enrolled young (mean age < 30 y) normal-weight men.14,21,25,26,29 More research is necessary to determine: (1) if sleep duration affects total EE; (2) if sleep duration affects each component of total EE similarly (sleep, resting, activity, and postprandial EE, non-exercise activity thermogenesis); and (3) whether the effects of sleep duration on EE are similar between men and women, normal weight and overweight/obese, and young and older individuals. One concept that has been overlooked both in epidemiological and clinical research literature is the relationship between sleep duration and physical fitness. Intra-individual variability in sleep onset latency is reduced by regular physical activity,30 and it is possible that improving physical activity improves sleep, which would then impact other components of energy balance regulation described in this review. In fact, Penev has also commented that the potential effects of sleep deficiency in raising RMR may be offset by reductions in total daily activity EE.31
4. What hormonal controls involved in the regulation of energy balance are altered by sleep deficiency? The case of leptin and ghrelin
Leptin and total ghrelin (unless otherwise specified) have been the 2 most extensively studied hormones related to energy balance regulation during periods of restricted and habitual sleep. A few cross-sectional studies have examined these hormones and found lower leptin32,33 and higher ghrelin levels33 in short sleepers than normal sleepers. On the other hand, Hayes et al.34 reported that each hour reduction in sleep duration was associated with a 6% increase in leptin, after adjusting for age, gender, race, and body mass index in participants from the Cleveland Family Study. Interestingly, as described below, discrepant results have also been observed in clinical studies.
Spiegel et al.13 were among the first to report altered leptin and ghrelin levels after short-term sleep restriction in 12 young normal-weight men. They found that average leptin levels measured after 2 nights of 4 h bedtimes were 18% lower and ghrelin 28% higher than after 2 nights of 10 h bedtimes. These results accounted for approximately 70% of the variance in increased hunger ratings with sleep restriction. In a similar study of longer duration, this same group reported mean leptin levels that were 19% lower after 6 nights of 4 h bedtimes compared to 12 h bedtimes.35 In that study, participants were at bed rest throughout the measurement period and received identical meals during both test periods. Whether participants were in energy balance which, as elaborated below can be critical in the role of sleep on leptin and ghrelin, was not mentioned.
Since then, other studies of varying protocols have reported opposite results. For example, Bosy-Westphal et al.17 reported a 24% increase in leptin levels after 2 nights of restricted sleep (approximately 5.5 h) relative to habitual sleep (9 h sleep) in women, with no difference in ghrelin levels between sleep periods. In that study, participants were in positive energy balance, at least during the period of restricted sleep (or in relative positive energy balance compared to habitual sleep). Simpson et al.36 also found a 33% increase in morning leptin levels after 5 nights of 4-h bedtimes compared to baseline levels obtained after 2 nights of 10-h bedtimes. State of energy balance in that study is not known, as participants were permitted to self-select their food intake. Increased morning, but not evening, leptin levels were also reported after one night of 3-h bedtimes relative to a baseline night of 10 h in young women.16 In that study, food intake was controlled and identical on both test days but again, the state of energy balance was not reported. Finally, Pejovic et al.12 reported increased mean 24-h leptin after one night of total sleep deprivation relative to a baseline measurement after 3 nights of habitual sleep. These authors also found that the rise in leptin mostly occurred during the daytime with no differences between sleep conditions in nighttime leptin. Food intake was self-selected, and no information was provided on energy intakes or state of energy balance.
Other studies have, however, failed to observe an effect of sleep duration on leptin levels. In a cross-sectional study of actigraphy-measured sleep, Knutson et al.37 reported no association between sleep duration, efficiency or disturbances and leptin. In intervention studies, Nedeltcheva et al.22 and Schmid et al.14 found no effect of sleep duration on mean 24-h leptin or ghrelin levels in men and women. In the study by Nedeltcheva et al.,22 participants were in positive energy balance, and it can be assumed similarly for the study by Schmid et al.14 since participants consumed approximately 4,000 kcal in both study phases. One night of total sleep deprivation also had no impact on leptin levels in normal weight men,25 although ghrelin levels were increased by approximately 11% relative to habitual (8 h) sleep. State of energy balance could not be determined from that study. A similar pattern of no effect on leptin but significant, albeit greater, rise in ghrelin was observed when comparing total sleep deprivation to 7 h sleep when participants were asked to maintain their regular dietary habits.11 Finally, Gonnissen et al., who assessed the impact of one night of sleep fragmentation on leptin and active ghrelin found no difference in 24-h hormone levels compared to a night of undisturbed sleep in young men.20 Although diet was controlled in that study, state of energy balance was not ascertained.
One study that did not measure leptin but measured ghrelin reported increased ghrelin levels when participants were permitted to sleep for 8 h compared to a night of total sleep deprivation.38 In that study, ghrelin levels rose sharply around midnight in the sleep condition and decreased slowly until morning, whereas the overnight rise was blunted during sleep deprivation such that ghrelin rose slowly to a plateau early morning and decreased after breakfast. In that study, participants were fed an 1,800 kcal diet, an energy intake level likely below requirements for a subject population with a range of body mass index between 20.5 and 29.5 kg/m2.
Only one study has assessed the effects of sleep duration on active acylated ghrelin levels.28 Compared to a baseline period of 3 nights of 10 h in bed, sleep restriction to 6.5 h in bed per 28-h day (combination of circadian disruption and sleep restriction) for 7 nights and 9 recovery nights of 10 h in bed both resulted in slightly higher 24-h ghrelin and slightly lower 24-h leptin profiles. In that study, despite controlled eucaloric diets adjusted when body weight changed by ≥ 1 kg, participants lost weight over the course of the study. Therefore, despite efforts to maintain stable energy balance, participants were in a state of negative energy balance.
The effects of sleep duration on leptin and ghrelin levels are quite mixed. Two studies by Spiegel et al. found reduced leptin13,35 and increased ghrelin13 after a period of restricted sleep relative to habitual sleep under controlled feeding conditions35 or constant intravenous glucose infusion.13 Of 8 other studies assessing the effects of sleep restriction, either partial or total, on leptin levels, half found no change11,14,22,25 and half found increased leptin12,16,17,36 compared to habitual sleep. These studies differed in their feeding protocol, with 2 providing controlled diets16,25 and the others allowing participants to self-select their food intake.11,12,14,17,22 However, no pattern can be identified between controlled and uncontrolled feeding studies. Of note, however, is that studies finding lower or no change in leptin between restricted and habitual sleep have enrolled men exclusively,11,13,14,25,35 whereas those finding increased leptin enrolled exclusively women16,17 or both men and women.12,36
The effects of sleep duration on ghrelin levels are also mixed. Of 7 published studies measuring ghrelin, 3 found no difference,14,17,22 3 found greater,11,13,25 and one found lower38 levels after restricted sleep relative to habitual sleep. The studies reporting no effect of sleep duration on ghrelin levels also reported positive energy balance17,22 or greater percent energy intake from fat14 during the period of restricted sleep. Of the 2 studies that provided fixed energy intakes, one reported increased ghrelin25 and one reported lower ghrelin38 after restricted sleep. Both studies enrolled men exclusively and used a total sleep deprivation paradigm.25,38 It is important to note that although food intake was controlled in several studies, this does not guarantee stable energy balance. Due to inaccuracies in energy requirement estimation equations, and inter-individual variability, participants can be overfed or underfed on a controlled diet. The predictive value of equations to estimate energy requirements has been reviewed by Heymsfield and colleagues.39 The review highlights issues that are often not taken into account by predictive equations, such as race, height (for some equations), weight stability (post-weight loss resting energy expenditure may be different than that of a similar weight/height/sex person who has not lost weight), and environmental factors, which all affect energy requirements. In addition, predictive equations were developed to estimate energy requirements for populations or groups, not specifically for individuals. As a result, it is often difficult to accurately estimate energy requirements for individuals, especially under short periods of time. It is possible that energy balance status may play a role in the effects of sleep duration on ghrelin levels and possibly also leptin levels. Kilkus et al.18 have suggested that sleep loss increases ghrelin levels only in the presence of negative energy balance. This concept has also been highlighted by Penev.31 Unfortunately, studies that report increased ghrelin with restricted sleep relative to habitual sleep have not reported energy intakes.11,13 However, all studies in which we can determine that participants were in a state of positive energy balance showed no effects of sleep deficiency on total ghrelin levels.14,17,22 On the other hand, studies by Dzaja et al.38 and Buxton et al.,28 conducted under states of negative energy balance, found a reduction in total and acylated ghrelin, respectively, with sleep deficiency (although in the case of Buxton et al., this was also observed in the sleep recovery period compared to baseline).
It is possible that genetic variation may play a role in the leptin and ghrelin responses to sleep deficiency. Garaulet et al.40 reported associations between CLOCK3111T/C allele genotype and ghrelin but not leptin levels. Individuals who were carriers of the C allele had higher ghrelin levels than those with TT genotype. They also reported shorter sleep duration and greater evening chronotype preference.
Summary and Conclusions
The relationship between sleep duration and obesity has been extensively reported in the epidemiological literature.1,3 There is now mounting evidence from clinical intervention studies for a causal effect of sleep on obesity risk. However, if sleep duration plays a causal role in the development of obesity, then it must lead to positive energy balance. This can be achieved by increasing food intake relative to EE or reducing EE relative to energy intake or a combination of the two such that: Energy intake > EE. Studies to date show a clear pattern of increased food intake during periods of restricted sleep relative to habitual sleep, mostly in normal-weight normal sleepers. It is interesting here to note that food restriction increases sleep onset latency and reduces total slow wave sleep.41 It is possible that overeating during a period of sleep deficiency is a physiological attempt to restore sleep, as it is known that higher food intake promotes sleep.42 The impact of sleep duration on EE is less clear. This may be due to the multiple components of EE: RMR, sleeping metabolic rate, thermic effect of food, physical activity EE, non-exercise activity thermogenesis. Each of these components requires different measurement tools, and each can be differentially affected by sleep.
How sleep affects food intake, however, remains to be elucidated. There is evidence of altered endocrine control of energy balance, but the effect can also be at the cognitive level. Nedeltcheva et al.22 suggested that hypothalamic orexigenic neurons involved in the modulation of reward and motivation may be altered by restricting sleep duration. This is supported by data from St-Onge et al.43 and Benedict et al.,44 who found increased neuronal activation in the frontal cortex—involved in the reward value of food—in response to food stimuli after restricted sleep. It is possible that restricting sleep increases the rewarding value of food while increasing endocrine drivers of food intake, such as ghrelin, leading to increased food consumption. However, more research is necessary to determine the role of energy balance itself on the impact of sleep on endocrine factors. In addition, studies of sleep extension are necessary to determine if increasing sleep duration in short sleepers has the opposite effect as reducing sleep in normal sleepers.
There remain numerous gaps in the literature surrounding sleep duration and energy balance. The majority of published studies have been conducted on young, normal-weight, healthy men. It is unknown whether the effects are similar in women, and there are suggestions that women may respond differently than men. Also, clinical intervention studies have created large sleep differences when studying sleep restriction compared to sleep sufficiency, usually at least 3 h. Whether less drastic reductions that are sustained for long periods of time achieve similar effects to large reductions over a short period of time remains to be determined. Such reductions in sleep may be more akin to the effects of aging on sleep duration45 and would be consistent with the relationship between aging and energy expenditure.46 Of critical importance is the understanding of the role of energy balance on the effects of sleep on hormonal and metabolic risk profile. Is sleep deficiency more harmful in the context of positive energy balance? Does sleep restriction amplify the counter-regulatory effects of negative energy balance? These questions are important to answer as individuals are in constant energy flux; some days of positive energy balance are counterbalanced with days of negative energy balance to ensure relatively stable body weight for most individuals. Finally, whether increasing sleep duration in habitual short sleepers results in the opposite observations obtained when reducing sleep in habitual good sleepers remains to be determined. However, it will not be long before we start getting some answers to the sleep extension question.47
DISCLOSURE STATEMENT
Dr. St-Onge has indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Work for this study was supported by HL091352 and P30 DK 26687.
REFERENCES
- 1.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–74. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 3.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh SD, Muto T, Murase T, Tsuji H, Arase Y. Association of short sleep duration with obesity, diabetes, fatty liver and behavioral factors in Japanese men. Intern Med. 2011;50:2499–502. doi: 10.2169/internalmedicine.50.5844. [DOI] [PubMed] [Google Scholar]
- 5.Chaput JP, Lambert M, Gray-Donald K, et al. Short sleep duration is independently associated with overweight and obesity in Quebec children. Can J Public Health. 2011;102:369–74. doi: 10.1007/BF03404179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magee CA, Caputi P, Iverson DC. Is sleep duration associated with obesity in older Australian adults? J Aging Health. 2010;22:1235–55. doi: 10.1177/0898264310372780. [DOI] [PubMed] [Google Scholar]
- 7.Anic GM, Titus-Ernstoff L, Newcomb PA, Trentham-Dietz A, Egan KM. Sleep duration and obesity in a population-based study. Sleep Med. 2010;11:447–51. doi: 10.1016/j.sleep.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SE, Kim HM, Kim DH, Kim J, Cha BS, Kim DJ. The association between sleep duration and general and abdominal obesity in Koreans: data from the Korean National Health and Nutrition Examination Survey, 2001 and 2005. Obesity (Silver Spring) 2009;17:767–71. doi: 10.1038/oby.2008.586. [DOI] [PubMed] [Google Scholar]
- 9.St-Onge M-P, Desmond R, Lewis CE, Yan LL, Person SD, Allison DB. Gender differences in the association between sleep duration and body composition: the Cardia Study. Int J Endocrinol. 2010;2010:726071. doi: 10.1155/2010/726071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 11.Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17:331–4. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 12.Pejovic S, Vgontzas AN, Basta M, et al. Leptin and hunger levels in young healthy adults after one night of sleep loss. J Sleep Res. 2010;19:552–8. doi: 10.1111/j.1365-2869.2010.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 14.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–82. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 15.St-Onge M-P, Roberts A, Chen J, Kelleman M, O'Keeffe M, Jones P. Short sleep duration increases energy intakes but does not change expenditure expenditure in normal weight individuals. Am J Clin Nutr. 2011;94:410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–6. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1:266–73. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilkus JM, Booth JN, Bromley LE, Darukhanavala AP, Imperial JG, Penev PD. Sleep and eating behavior in adults at risk for type 2 diabetes. Obesity (Silver Spring) 2012;20:112–7. doi: 10.1038/oby.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between short sleep duration and weight gain is dependent on disinhibited eating behavior in adults. Sleep. 2011;34:1291–7. doi: 10.5665/SLEEP.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr. 2012:1–9. doi: 10.1017/S0007114512001894. [DOI] [PubMed] [Google Scholar]
- 21.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 22.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamatakis KA, Brownson RC. Sleep duration and obesity-related risk factors in the rural Midwest. Prev Med. 2008;46:439–44. doi: 10.1016/j.ypmed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S, DeRoo LA, Sandler DP. Eating patterns and nutritional characteristics associated with sleep duration. Public Health Nutr. 2011;14:889–95. doi: 10.1017/S136898001000296X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedict C, Hallschmid M, Lassen A, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93:1229–36. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- 26.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589(Pt 1):235–44. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–33. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buxton OM, Cain SW, O'Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hursel R, Rutters F, Gonnissen HK, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation in healthy men on energy expenditure, substrate oxidation, physical activity, and exhaustion measured over 48 h in a respiratory chamber. Am J Clin Nutr. 2011;94:804–8. doi: 10.3945/ajcn.111.017632. [DOI] [PubMed] [Google Scholar]
- 30.Buman MP, Hekler EB, Bliwise DL, King AC. Exercise effects on night-to-night fluctuations in self-rated sleep among older adults with sleep complaints. J Sleep Res. 2011;20(1 Pt 1):28–37. doi: 10.1111/j.1365-2869.2010.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab. 2012;97:1792–801. doi: 10.1210/jc.2012-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–61. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 33.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes AL, Xu F, Babineau D, Patel SR. Sleep duration and circulating adipokine levels. Sleep. 2011;34:147–52. doi: 10.1093/sleep/34.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 36.Simpson NS, Banks S, Dinges DF. Sleep restriction is associated with increased morning plasma leptin concentrations, especially in women. Biol Res Nurs. 2010;12:47–53. doi: 10.1177/1099800410366301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knutson KL, Galli G, Zhao X, Mattingly M, Cizza G, Study NSE. No association between leptin levels and sleep duration or quality in obese adults. Obesity (Silver Spring) 2011;19:2433–5. doi: 10.1038/oby.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dzaja A, Dalal MA, Himmerich H, Uhr M, Pollmacher T, Schuld A. Sleep enhances nocturnal plasma ghrelin levels in healthy subjects. Am J Physiol Endocrinol Metab. 2004;286:E963–7. doi: 10.1152/ajpendo.00527.2003. [DOI] [PubMed] [Google Scholar]
- 39.Heymsfield SB, Harp JB, Rowell PN, Nguyen AM, Pietrobelli A. How much may I eat? Calorie estimates based upon energy expenditure prediction equations. Obes Rev. 2006;7:361–70. doi: 10.1111/j.1467-789X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 40.Garaulet M, Sanchez-Moreno C, Smith CE, Lee YC, Nicolas F, Ordovas JM. Ghrelin, sleep reduction and evening preference: relationships to CLOCK 3111 T/C SNP and weight loss. PLoS One. 2011;6:e17435. doi: 10.1371/journal.pone.0017435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karklin A, Driver HS, Buffenstein R. Restricted energy intake affects nocturnal body temperature and sleep patterns. Am J Clin Nutr. 1994;59:346–9. doi: 10.1093/ajcn/59.2.346. [DOI] [PubMed] [Google Scholar]
- 42.Penev PD. Sleep deprivation and energy metabolism: to sleep, perchance to eat? Curr Opin Endocrinol Diabetes Obes. 2007;14:374–81. doi: 10.1097/MED.0b013e3282be9093. [DOI] [PubMed] [Google Scholar]
- 43.St-Onge M-P, McReynolds A, Trivedi Z, Roberts A, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95:818–24. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedict C, Brooks SJ, O'Daly OG, et al. Acute sleep deprivation enhances the brain's response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab. 2012;97:E443–7. doi: 10.1210/jc.2011-2759. [DOI] [PubMed] [Google Scholar]
- 45.Rolls A. Hypothalamic control of sleep in aging. Neuromolecular Med. 2012;14:139–53. doi: 10.1007/s12017-012-8175-0. [DOI] [PubMed] [Google Scholar]
- 46.St-Onge MP, Gallagher D. Body composition changes with aging: The cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010;26:152–5. doi: 10.1016/j.nut.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cizza G, Marincola P, Mattingly M, et al. Treatment of obesity with extension of sleep duration: a randomized, prospective, controlled trial. Clin Trials. 2010;7:274–85. doi: 10.1177/1740774510368298. [DOI] [PMC free article] [PubMed] [Google Scholar]


