The incidence of obesity and insulin resistance is growing, and the increase in type 2 diabetes mellitus (DM2) constitutes one of the biggest challenges for our healthcare systems. Many theories are proposed for the induction of insulin resistance in glucose and lipid metabolism and its metabolic sequelae. One of these mechanisms is lipotoxicity (1–4): excess lipid supply and subsequent lipid accumulation in insulin-sensitive tissues such as skeletal muscle interfere with insulin-responsive metabolic pathways. Various lipid intermediates, like ceramides, gangliosides, diacylglycerol, and other metabolites, have been held responsible for insulin resistance (2,3,5–10). These intermediates can exert such effects because they are signaling molecules and building blocks of cellular membranes, which harbor the insulin receptor. In addition, lipids play an important role in energy homeostasis. Fatty acids (FA) can be metabolized via mitochondrial FA oxidation (FAO), which yields energy (11). As such, FAO competes with glucose oxidation in a process known as the glucose-FA, or Randle, cycle (12).
Muoio and colleagues (1,13,14) proposed an alternative mechanism in which FAO rate outpaces the tricarboxylic acid cycle (TCA), thereby leading to the accumulation of intermediary metabolites such as acylcarnitines that may interfere with insulin sensitivity. This accumulation of acylcarnitines corroborates with some human studies showing that acylcarnitines are associated with insulin resistance (15–17). In addition, acylcarnitines have a long history in the diagnosis and neonatal screening of FAO defects and other inborn errors of metabolism (18). This knowledge may aid to understand the interaction between FAO and insulin resistance and fuel future research. In this review, we discuss the role of acylcarnitines in FAO and insulin resistance as emerging from animal and human studies.
PHYSIOLOGICAL ROLE OF ACYLCARNITINES
Carnitine biosynthesis and regulation of tissue carnitine content.
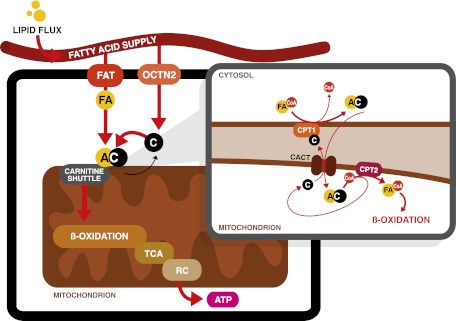
To guarantee continuous energy supply, the human body oxidizes considerable amounts of fat besides glucose. L-carnitine transports activated long-chain FAs from the cytosol into the mitochondrion and is therefore essential for FAO. Carnitine is mainly absorbed from the diet, but can be formed through biosynthesis (19). In several proteins, lysine residues are methylated to trimethyllysine (19). Four enzymes convert trimethyllysine into carnitine (19), of which the last step is the hydroxylation of butyrobetaine into carnitine by γ-butyrobetaine dioxygenase (BBD). BBD is only present in human liver, kidney, and brain, which are the sites where actual carnitine biosynthesis takes place (19). Other tissues such as skeletal muscle must acquire carnitine from the blood. Treatment with a synthetic peroxisome proliferator–activated receptor α (PPARα) agonist increased BBD activity and carnitine levels in liver (20). This suggests that the nuclear receptor PPARα, which plays a crucial role in the adaptive response to fasting, is a regulator of (acyl)carnitine metabolism (20).
The plasmalemmal carrier OCTN2 is responsible for cellular carnitine uptake in various organs, including reabsorption from urine in the kidney. As is the case for BBD, OCTN2 expression in liver is regulated by PPARα. A synthetic PPARα agonist increased OCTN2 expression in wild-type mice and caused a rise in carnitine levels in plasma, liver, kidney, and heart (20). In PPARα−/− mice, low OCTN2 expression contributed to decreased tissue and plasma carnitine levels (20).
The carnitine shuttle.
Once inside the cell, FAs are activated by esterification to CoA. Then, the carnitine shuttle transports long-chain acyl-CoAs into mitochondria via their corresponding carnitine ester (Fig. 1) (21). Long-chain acyl-CoAs are converted to acylcarnitines by carnitine palmitoyltransferase 1 (CPT1), which exchanges the CoA moiety for carnitine. CPT1 is located at the outer mitochondrial membrane, and three isoforms are known: CPT1a, 1b, and 1c are encoded by separate genes (21). CPT1a is expressed in liver and most other abdominal organs, as well as human fibroblasts. CPT1b is selectively expressed in heart, skeletal muscle, adipose tissue, and testes (11). CPT1c is only expressed in the endoplasmic reticulum (and not the mitochondria) of neurons in the brain (22).
FIG. 1.
The carnitine shuttle. After transportation into the cell by FA transporters (FAT), FA are activated by esterification to CoA. Subsequently, CPT1 exchanges the CoA moiety for carnitine (C). The resulting acylcarnitine (AC) is transported across the inner mitochondrial membrane into the mitochondrion by CACT. Once inside, CPT2 reconverts the acylcarnitine back into free carnitine and a long-chain acyl-CoA that can undergo FAO for ATP production via the TCA and respiratory chain (RC).
CPT1 is an important regulator of FAO flux. Glucose oxidation after a meal leads to inhibition of CPT1 activity via the FA-biosynthetic intermediate malonyl-CoA (23), which is produced by acetyl-CoA carboxylase (ACC) (24). There are two ACC isoforms. ACC1 plays a role in FA biosynthesis. ACC2 has been implicated in the regulation of FAO mainly because of its localization to the outer mitochondrial membrane (25). Conversely, in the fasting state, activated AMP-activated protein kinase inhibits ACC resulting in falling malonyl-CoA levels, thereby permitting CPT1 activity and thus FAO. CPT1a is limiting for hepatic FAO and ketogenesis (26). Although the inhibition of malonyl-CoA on CPT1b is more potent than on CPT1a, no unequivocal evidence exists showing its control over muscle FAO (27).
FAO is also regulated at the transcriptional level. PPARα, but also PPARβ/δ, regulates the transcription of many enzymes involved in FAO. There is ample evidence that both PPARs participate in the transcriptional regulation of CPT1b (28–30). Regulation of CPT1a by PPAR is less prominent (21).
After production of acylcarnitines by CPT1, the mitochondrial inner membrane transporter carnitine acylcarnitine translocase (CACT, or SLC25A20) transports the acylcarnitines into the mitochondrial matrix. The FA transporter CD36 possibly facilitates transfer of acylcarnitines from CPT1 to CACT (31). Finally, the enzyme CPT2 reconverts acylcarnitines back into free carnitine and long-chain acyl-CoAs, which can then be oxidized (21) (Fig. 1).
Analysis of acylcarnitines.
With the introduction of tandem mass spectrometry (MS) in clinical chemistry in the 1990s, it became relatively easy to measure acylcarnitine profiles. In these profiles, the mass-to-charge ratio reflects the length and composition of the acyl chain (32). This technique rapidly became the preferred screening test to diagnose inherited disorders in FAO, which lead to prominent changes in the acylcarnitine profile, with a pattern specific for the deficient enzyme. More recently, acylcarnitine analysis is used to investigate more common metabolic derangements such as insulin resistance.
Although most acylcarnitines are derived from FAO, they can be formed from almost any CoA ester (18). Other intermediates that yield acylcarnitines are ketone bodies [C4-3OH-carnitine (33)], degradation products of lysine, tryptophan, valine, leucine, and isoleucine (C3- and C5-carnitine and others), and carbon atoms from glucose (acetylcarnitine) (18).
The standard acylcarnitine analysis using tandem MS cannot discriminate between stereoisomers and other isobaric compounds, which have the same nominal mass but a different molecular structure. These compounds can be separated using liquid chromatography-tandem MS (34). This is illustrated by C4-OH-carnitine, which can be derived from the CoA ester of the ketone body D-3-hydroxybutyrate, (D-C4-OH-carnitine), the FAO intermediate L-3-hydroxybutyryl-CoA (L-C4-OH-carnitine), and L-3-hydroxyisobutyryl-CoA, an intermediate in the degradation of valine (L-isoC4-OH-carnitine) (33).
The origin of plasma acylcarnitines.
The fact that acylcarnitines can be measured in plasma illustrates that they are transported across cell membranes. Two transporters have been implicated in the export of acylcarnitines. In addition to import, OCTN2 can export (acyl)carnitines (35). Also, the monocarboxylate transporter 9 (SLC16A9) may play a role in carnitine efflux (36). Although these putative transporters have been identified, the exact nature of this transport is unknown, but seems largely dependent on the intracellular acylcarnitine concentration (35). Early studies in rodent heart, liver, and brain mitochondria proved mitochondrial efflux of acylcarnitines and suggested this to be dependent on the substrate and tissue as well as the availability of alternative acyl-CoA–utilizing reactions (37). In humans, acylcarnitine efflux is exceptionally well-evidenced by the acylcarnitine profiles of patients with an FAO defect (18). From a more physiological view, diets and fasting modulate the plasma acylcarnitine profile, which reflects changes in flux through the FAO pathway (13,16,38,39). However, exact rates of acylcarnitine production in relation to the FAO flux under different conditions remain to be determined.
It is expected that muscle or liver contribute largely to acylcarnitine turnover. Early studies showed that liver acylcarnitines correlated with plasma acylcarnitines in fasted macaques, but the individual chain lengths were not studied (40). A liver–plasma relation is plausible, considering that the liver accounts for most of the FAO activity during fasting. Human data are lacking, but muscle acylcarnitines did not correlate with plasma acylcarnitines during short-term fasting (16).
The physiological role of acylcarnitine efflux to the plasma compartment is unknown, but several scenarios are likely. Acylcarnitine formation prevents CoA trapping, allowing continuation of CoA-dependent metabolic processes (21,41). In addition to plasma, acylcarnitines are found also in bile and urine (42,43), suggesting that acylcarnitine efflux may serve as a detoxification process. Combined, the total daily bile and urine production of acylcarnitine is <200 μmol. This can be calculated to be <0.01% of daily energy requirements, which is a negligible amount in terms of potential energy loss. Moreover, intestinal reuptake of bile acylcarnitines is possible. Alternatively, plasma acylcarnitines may serve as a means of transportation between cells or organs or sink for cellular/tissue acylcarnitine sequestration. Questions that remain are the contribution of specific tissues and organs to plasma acylcarnitine levels and the turnover rates of the individual acylcarnitine species in plasma.
ACYLCARNITINE METABOLISM IN RELATION TO INSULIN RESISTANCE
Current views on lipid metabolism in insulin resistance.
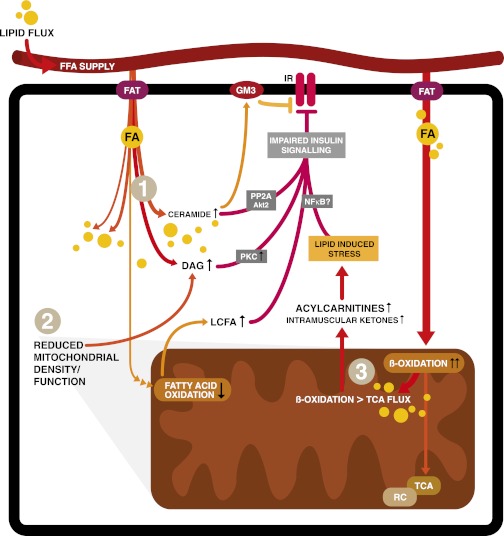
FAO may be quantitatively and qualitatively different in insulin-resistant subjects compared with healthy subjects, but a more pertinent conundrum is if increased FAO is either capable to limit insulin resistance via decreasing lipid accumulation or increasing insulin resistance via accumulation of incomplete FAO products such as acylcarnitines (1–3,13,14). Several theories describe mechanisms within the cytosol that can cause insulin resistance (Fig. 2). It has generally been accepted that chronic overnutrition leads to increased cytosolic lipid content of insulin-responsive tissues (such as liver and skeletal muscle). This negatively affects the insulin sensitivity of these tissues by inhibiting insulin signaling via intermediates as ceramide, diacylglycerol, gangliosides, and possible other long-chain FA-derived metabolites (1,3,5–8,44). Although contested now, cytosolic lipid accumulation was also suggested to arise from mitochondrial dysfunction and, as a consequence, decreased FAO rate (2,9,14,45,46). Likewise, increased levels of malonyl-CoA were suggested to limit the mitochondrial entrance of long-chain FAs by blocking CPT1, thus resulting in accumulating cytosolic long-chain FAs and decreasing FAO rate (10).
FIG. 2.
Mechanisms of lipid-induced insulin resistance. After transportation into the cell, FA can be stored, oxidized, or used as building blocks and signaling molecules (not all shown). Excess lipid supply and subsequent accumulation in insulin-sensitive tissues such as skeletal muscle is proposed to interfere with different insulin-responsive metabolic pathways via various mechanisms. Firstly (1), increased intracellular lipid content inhibits insulin signaling via lipid intermediates such as ceramides, diacylglycerol (DAG), or gangliosides (GM3) via effects on protein phosphatase A2 (PPA2) and protein kinase B (Akt), protein kinase C (PKC), or effects on the insulin receptor in the cell membrane (1,3,5–8,44). Effects of lipid intermediates on inhibitors of nuclear factor-κβ (NFκB) kinase subunit β and c-Jun N-terminal kinase 1 are not depicted. The second mechanism (2) is a decreased number of functional mitochondria resulting in lower FAO rates and increased accumulation of cytosolic lipid, again interfering with insulin sensitivity (2,9). Finally (3), metabolic overload of mitochondria leads to incomplete β-oxidation. In this figure, oxidation of FA outpaces the TCA and respiratory chain (RC), resulting in intramitochondrial accumulation of FAO intermediates like acylcarnitines. These subsequently impinge on insulin signaling (1,48,50–56). In this figure, only the direct effects of acylcarnitines on nuclear factor-κβ have been proposed (70).
Alternatively, more recent mechanistic (13,47,48) and metabolomic (49–54) studies associated obesity-induced insulin resistance with intramitochondrial disturbances. In this model, lipid overload leads to increased rather than decreased FAO in skeletal muscle. This coincides with accumulating acylcarnitines, an inability to switch to carbohydrate substrate, and a depletion of TCA intermediates, suggesting that FAO flux does not match TCA flux, leading to incomplete FAO (13,47,48). In vitro interfering with FA uptake in L6 myocytes or a coordinate induction of FAO and TCA enzymes by exercise or PPARγ coactivator 1α overexpression prevented insulin resistance (13,48). Moreover, using carnitine to stimulate FAO without affecting the TCA in these myocytes was dose-dependently associated with insulin resistance (13). Zucker Diabetic Fatty rats, a model for more severe insulin resistance, had higher acylcarnitines but lower TCA intermediates (such as citrate, malate, and succinate) in skeletal muscle, again suggesting that increased FAO induces insulin resistance when not followed by proportionally increased TCA activity (13). Additionally, the malonyl-CoA decarboxylase−/− mouse that had decreased FAO due to higher malonyl-CoA concentrations resisted diet-induced insulin resistance, which further implicated FAO in the pathogenesis of insulin resistance (13). The available studies on acylcarnitine metabolism and the relationship with insulin resistance will be discussed in the next sections with a focus on human studies.
The effect of increased lipid flux on mitochondrial FA uptake and oxidation: implications for insulin sensitivity.
Insulin-dependent DM2 patients had lower (∼25%) carnitine concentrations, especially with longer-standing or complicated disease (55,56). Interestingly, carnitine infusions increased FAO in lean healthy subjects, but only when high-dose insulin was coadministered (57,58), which may be explained by an increased muscle OCTN2 expression under these conditions (59). The importance of insulin for cellular carnitine uptake is underscored by the finding that insulin and carnitine administration lowered muscle malonyl-CoA and lactate concentrations, whereas muscle glycogen increased (58). These findings are supported by animal studies, which demonstrated that carnitine levels were diminished in skeletal muscle of multiple insulin-resistant rat models. A high-fat diet (HFD) exacerbated the age-related decrease of tissue carnitine content in these rats (primarily skeletal muscle, liver, and kidney) (60). Moreover, carnitine supplementation of HFD animals decreased plasma glucose levels and homeostasis model assessment indices (60,61). Likewise, carnitine supplementation improved insulin-stimulated glucose disposal in mouse models of diet-induced obesity and genetic diabetes (62). Recently, it was shown that 6 months of carnitine supplementation improved glucose homeostasis in insulin-resistant humans (14).
Although supplementation of carnitine possibly augments FAO and insulin sensitivity, the lower carnitine levels in diabetes patients are unexplained. On the one hand, carnitine uptake is insulin-dependent and therefore the absence of or resistance to insulin may be the cause of lower carnitine levels. On the other hand, higher lipid load may lead to higher acylcarnitine concentrations and thus lower free carnitine.
In addition, several studies reported on the carnitine shuttle and its effects on the rate of FAO in the development of insulin resistance. Obese subjects had lower CPT1 and citrate synthase content in muscle and lower FAO, suggesting that lesions at CPT1 and post-CPT1 events (i.e., mitochondrial content) may lower FAO in obesity (63). Although short-term inhibition of CPT1 with etomoxir in humans did not impede insulin sensitivity despite increased intramyocellular lipid accumulation (64), prolonged inhibition in rats resulted in the accumulation of intramyocellular lipid and increased insulin resistance while doubling adiposity despite feeding a low-fat diet (65). These results all led to the assumption that low FAO rates due to decreased function of CPT1 were associated with insulin resistance, possibly caused by an accumulation of intramyocellular lipid intermediates and their interference with insulin signaling. Indeed, CPT1 activity increased after an endurance training program in obese subjects, coinciding with increased FAO, improved glucose tolerance, and insulin sensitivity (66). However, this may also be explained by the stimulatory effect of endurance training on mitochondrial function (i.e., TCA and respiratory chain activity), thereby relieving the heavy lipid burden on mitochondria (48,67). In contrast to the model in which excess FAO induces insulin resistance, these data suggest that decreasing mitochondrial FA uptake results in elevated intramuscular lipid levels and subsequent insulin resistance. However, increasing FAO by carnitine treatment in animals and humans permits mitochondrial FA uptake and oxidation that benefits insulin sensitivity. These observations will have to be reconciled with other studies that implicated incomplete FAO and acylcarnitine accumulation in the pathogenesis of insulin resistance.
Short-chain acylcarnitines in insulin resistance.
Older work reported elevated acylcarnitine levels in obese insulin-resistant subjects (15), but acylcarnitines were not suggested to be implicated in insulin resistance at that time. The shortest acylcarnitine, acetylcarnitine, is of particular interest because it may illustrate the controlling role of acetyl-CoA on substrate switching and thus metabolic flexibility. The mitochondrial enzyme carnitine acetyl-CoA transferase (CrAT) converts acetyl-CoA to the membrane-permeable acetylcarnitine and permits mitochondrial efflux of excess acetyl-CoA that otherwise could inhibit pyruvate dehydrogenase (68). Infusing intralipid decreased insulin sensitivity while increasing muscle acetylcarnitine (69). The same was true for plasma and muscle acetylcarnitine levels under high FAO conditions (starving), suggesting upregulation of CrAT to traffic acetyl-moieties (16). In contrast to lower CrAT expression in diabetic subjects (68), plasma acetylcarnitine levels showed significant positive correlation with HbA1c levels over a wide range of insulin sensitivity, suggesting upregulation of CrAT in insulin-resistant states (70).
There is some complexity, as both lipid and glucose oxidation funnel into acetylcarnitine as supported by different findings (68,71). First, the insulin-mediated suppression of muscle acetylcarnitine occurred under high FAO conditions, but not postabsorptively (i.e., higher glucose availability) (16). Also, muscle acetylcarnitine correlated negatively with FAO in the postabsorptive state (71), whereas plasma acetylcarnitine correlated with plasma glucose levels in the postprandial state (72). In light of these data, the question is interesting if CrAT really favors FA-derived acetyl-CoA over glucose-derived acetyl-CoA because this might imply intracellular compartmentalization of acetyl-CoA (68). Moreover, glucose-derived acetyl-CoA can be carboxylated by ACC, producing the CPT1 inhibitor malonyl-CoA. Direct effects of FAO-derived acetyl-CoA on insulin action are unknown.
C4-OH-carnitine (i.e., the carnitine ester of 3-hydroxybutyrate) has been proposed to cause insulin resistance: hepatic overexpression of malonyl-CoA decarboxylase in rats on an HFD reversed whole-body, liver, and muscle insulin resistance while only decreasing C4-OH-carnitine within the acylcarnitine profile (47). In fasted humans, plasma and muscle C4-OH-carnitine increased (33). The increase in C4-OH-carnitine in these animal and human studies is quantitatively much more pronounced then the increase in acetylcarnitine; thus, C4-OH-carnitine production may exert greater demands on cellular carnitine stores. Moreover, ketone bodies yield acetyl-CoA, which stimulates PDK4 and thus inhibits glucose oxidation (73). In summary, under conditions characterized by higher FAO, elevated short-chain acylcarnitines may reflect higher lipid fluxes, but a direct relation to insulin resistance remains to be established.
Amino acid–derived acylcarnitines in insulin resistance.
Metabolomics showed that branched-chain and aromatic amino acids (isoleucine, leucine, valine, tyrosine, and phenylalanine) (74) significantly correlated with present or future diabetes (54,74,75). In line with this, the branched-chain amino acid–derived C3- and C5-carnitine, together with FA-derived C6- and C8-carnitine, were higher in obese and DM2 subjects compared with lean controls (17,54). In the same study, C4-dicarboxylcarnitine (C4DC-carnitine), also derived from branched-chain amino acid metabolism, showed a positive correlation with basal glucose levels and HbA1c (17). In comparison with obese non–insulin-resistant subjects, DM2 subjects also had higher C3- and C5-carnitine levels compared with controls during insulin administration. In this study, C3- but not C5-carnitine correlated negatively with glucose disposal (17).
At first glance, correlations of acylcarnitines to surrogate markers of insulin resistance fit with mitochondrial overload and incomplete FAO. Acylcarnitines, however, also directly reflect the oxidation rate of FA and amino acids, which is supported by human nutritional intervention studies (16,33,38,39). The uncertainty regarding the direct interference of short-chain acylcarnitines and their metabolism with insulin-signaling processes and insulin sensitivity warrants care when attributing a primary role for amino acid–derived acylcarnitines in the induction of insulin resistance.
Medium- and long-chain acylcarnitines: more evidence for insulin-resistant effects?
Long-chain FA such as palmitic acid were associated with insulin resistance, making a role for long-chain acylcarnitines such as C16 in insulin resistance conceivable (3,44). In 1980, Hoppel et al. (15) showed that the fasting-induced increase in plasma acylcarnitines was restored upon refeeding in lean subjects within 24 h opposed to 4 days in obese subjects, suggesting an impaired metabolic flexibility in the latter.
The hypothesis that obesity-induced alteration in the acylcarnitine profile are caused by incomplete FAO was based largely on two animal studies by the same group showing that long-chain acylcarnitine species (C16, C18:2, C18:1, and C18) were persistently increased in diet-induced obese rats, in both the fed and fasted state (13,48). As reported for humans, most acylcarnitine species decreased upon refeeding in the chow-fed control group, but not in the obese animals, suggesting they were incapable of adjusting their metabolism in response to refeeding. Although excessive and incomplete FAO can be responsible for insulin resistance, it can be argued that FAO probably must be in relative excess to oxidation in TCA and respiratory chain in order to guarantee continuous energy supply.
Obese and insulin-resistant humans had higher plasma long-chain acylcarnitine levels compared with lean controls (17). Upon insulin infusion, long-chain acylcarnitines decreased overall, but to a lesser degree in the diabetic subjects. This was in agreement with lower resting energy expenditure, indicating ongoing FAO or lipid flux (metabolic inflexibility) (17). Moderate correlations between acylcarnitine profiles and various clinical characteristics (i.e., higher BMI, basal free FA levels, insulin sensitivity) point at a causal relationship. The DM2 subjects were unable to suppress acylcarnitines during insulin infusion in contrast to matched obese controls; therefore, elevated long-chain acylcarnitines in the diabetic group likely reflect increased lipid flux and illustrate the tight connection of acylcarnitines with FAO flux (17).
Postprandially, plasma long-chain acylcarnitines did decrease in obese insulin-resistant subjects, but the magnitude of this decrease correlated with both premeal insulin-mediated glucose disposal rates and FAO and has been largely explained by nadir levels of C12:1, C14, and C14:1-carnitine (72). This showed that the more insulin-sensitive subjects are, the more capable they are at metabolizing FAs. Metabolomics in healthy, overweight, calorie-restricted subjects yielded comparable results; in this study, acylcarnitines correlated significantly with plasma insulin and free FA levels, albeit with low correlation coefficient (49).
All in all, acylcarnitines with longer chain lengths are associated with insulin resistance, which seems logic in the light of known effects of long-chain FAs on insulin signaling. Indeed, acylcarnitines can reside in cell membranes because they are amphipathic molecules. Increasing chain length favors partitioning into the membrane phase (e.g., C16- and 18-carnitine) (76). It is interesting to speculate that long-chain acylcarnitines can interfere with insulin signaling directly within the cell membrane (3). In contrast, acylcarnitines seem to track with higher lipid flux and as such may only indicate higher FAO.
ACYLCARNITINES: REFLECTING OR INFLICTING INSULIN RESISTANCE?
The concept of lipotoxicity is generally accepted in the field of obesity-induced impairment of insulin sensitivity, and more and more attention has attributed to intramitochondrial alterations and impairments in FAO, thereby focusing on acylcarnitines (1). Collected evidence shows that acylcarnitines have distinct functions in mitochondrial lipid metabolism. The transmembrane export of acylcarnitines suggests that they not only prevent the accumulation of noxious acyl-CoAs, but also reduce CoA trapping, which is crucial for many metabolic pathways (21,41). Additionally, the metabolism of short-chain acylcarnitines and the interaction of acetyl-CoA and acetylcarnitine via CrAT may regulate the pyruvate dehydrogenase complex, thereby affecting glucose oxidation (68). Besides mitochondrial need to liberate CoA and export acetyl-CoA, acylcarnitines may simply reflect the FAO flux.
The concept of increased, though incomplete, FAO by disproportional regulation of FAO, TCA, and respiratory chain is attractive to explain insulin resistance. However, there remains doubt about this mechanism, and there is no proof that acylcarnitines play a role in the induction of insulin resistance itself. Acylcarnitines are present under physiological conditions, and their levels vary according to dietary circumstances (13,16,38,39). The acylcarnitine fluxes are unknown but probably much lower than FAO flux. Moreover, it can be argued that flux of FAO probably will be in relative excess to downstream oxidation in TCA and respiratory chain to guarantee continuous substrate supply and allow fine tuning and anticipation for metabolic changes (e.g., activity). Otherwise, the organism’s response to increased energy demands will be attenuated, leading to more severe impairment of mitochondrial function as evidenced by the inherited FAO disorders.
Observational studies associating different acylcarnitines to a variety of end points may yield new hypotheses but are unlikely to move the field forward from a mechanistic perspective. Many questions are unanswered, and some issues deserve particular attention. Tracer studies can quantify FAO flux and acylcarnitine production in different insulin-resistant models on the cellular, tissue, and whole-organism level. Multiple animal and human models can help to investigate the effect of carnitine availability on insulin sensitivity. Mouse models for and humans with primary carnitine deficiency can be used to investigate the effect of carnitine availability on substrate switching and insulin sensitivity. In vitro work in muscle or liver cell lines is still important to dissect the influence of acylcarnitines on conventional insulin signaling or mechanisms of nutrient-induced mitochondrial stress. In this respect, different animal and human FAO disorders that accumulate acylcarnitines may undergo insulin sensitivity testing. The contribution of different organs to plasma acylcarnitines can be investigated using transorgan arteriovenous balance isotope-dilution techniques under different conditions. Finally, we may set foot in new areas in which acylcarnitines may have unexpected roles, like interaction with the insulin receptor in the plasma membrane or signaling in the gut when cosecreted with bile. Recently, magnetic resonance spectroscopy was shown to image tissue acetylcarnitine in humans enabling noninvasive techniques to assay tissue acetylcarnitine (77). All of these studies and more are necessary to decide to what extent acylcarnitines are reflecting or inflicting insulin resistance.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
M.G.S. and M.R.S. wrote the first draft of the manuscript. M.G.S., F.M.V., S.M.H., and M.R.S. contributed to the editing of the manuscript. M.G.S. provided the original artwork. M.R.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Muoio DM, Koves TR. Lipid-induced metabolic dysfunction in skeletal muscle. Novartis Found Symp 2007;286:24–38; discussion 38–46, 162–163, 196–203 [DOI] [PubMed] [Google Scholar]
- 2.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 2006;55(Suppl. 2):S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev 2007;65:S39–S46 [DOI] [PubMed] [Google Scholar]
- 4.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 2000;106:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 1999;42:113–116 [DOI] [PubMed] [Google Scholar]
- 6.Gray RE, Tanner CJ, Pories WJ, MacDonald KG, Houmard JA. Effect of weight loss on muscle lipid content in morbidly obese subjects. Am J Physiol Endocrinol Metab 2003;284:E726–E732 [DOI] [PubMed] [Google Scholar]
- 7.Pan DA, Lillioja S, Kriketos AD, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 1997;46:983–988 [DOI] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 2000;49:467–472 [DOI] [PubMed] [Google Scholar]
- 9.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–273 [DOI] [PubMed] [Google Scholar]
- 10.Ruderman NB, Saha AK, Vavvas D, Witters LA. Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol 1999;276:E1–E18 [DOI] [PubMed] [Google Scholar]
- 11.Eaton S. Control of mitochondrial beta-oxidation flux. Prog Lipid Res 2002;41:197–239 [DOI] [PubMed] [Google Scholar]
- 12.Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 2009;297:E578–E591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 14.Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 2012;15:595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoppel CL, Genuth SM. Carnitine metabolism in normal-weight and obese human subjects during fasting. Am J Physiol 1980;238:E409–E415 [DOI] [PubMed] [Google Scholar]
- 16.Soeters MR, Sauerwein HP, Duran M, et al. Muscle acylcarnitines during short-term fasting in lean healthy men. Clin Sci (Lond) 2009;116:585–592 [DOI] [PubMed] [Google Scholar]
- 17.Mihalik SJ, Goodpaster BH, Kelley DE, et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 2010;18:1695–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinaldo P, Cowan TM, Matern D. Acylcarnitine profile analysis. Genet Med 2008;10:151–156 [DOI] [PubMed] [Google Scholar]
- 19.Vaz FM, Wanders RJA. Carnitine biosynthesis in mammals. Biochem J 2002;361:417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Vlies N, Ferdinandusse S, Wanders RJA, Vaz FM. PPARalfa-activation results in enhanced carnitine biosynthesis and OCTN2 expression. Biochim Biophys Acta 1767;11:2007. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay RR, Gandour RD, van der Leij FR. Molecular enzymology of carnitine transfer and transport. Biochim Biophys Acta 2001;1546:21–43 [DOI] [PubMed] [Google Scholar]
- 22.Sierra AY, Gratacós E, Carrasco P, et al. CPT1c is localized in endoplasmic reticulum of neurons and has carnitine palmitoyltransferase activity. J Biol Chem 2008;283:6878–6885 [DOI] [PubMed] [Google Scholar]
- 23.McGarry JD, Mannaerts GP, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest 1977;60:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zammit VA. The malonyl-CoA-long-chain acyl-CoA axis in the maintenance of mammalian cell function. Biochem J 1999;343:505–515 [PMC free article] [PubMed] [Google Scholar]
- 25.Castle JC, Hara Y, Raymond CK, et al. ACC2 is expressed at high levels in human white adipose and has an isoform with a novel N-terminus [corrected]. PLoS ONE 2009;4:e4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drynan L, Quant PA, Zammit VA. Flux control exerted by mitochondrial outer membrane carnitine palmitoyltransferase over beta-oxidation, ketogenesis and tricarboxylic acid cycle activity in hepatocytes isolated from rats in different metabolic states. Biochem J 1996;317:791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eaton S, Fukumoto K, Paladio Duran N, et al. Carnitine palmitoyl transferase I and the control of myocardial beta-oxidation flux. Biochem Soc Trans 2001;29:245–250 [DOI] [PubMed] [Google Scholar]
- 28.Yu GS, Lu YC, Gulick T. Co-regulation of tissue-specific alternative human carnitine palmitoyltransferase Ibeta gene promoters by fatty acid enzyme substrate. J Biol Chem 1998;273:32901–32909 [DOI] [PubMed] [Google Scholar]
- 29.Brandt JM, Djouadi F, Kelly DP. Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor alpha. J Biol Chem 1998;273:23786–23792 [DOI] [PubMed] [Google Scholar]
- 30.Mascaró C, Acosta E, Ortiz JA, Marrero PF, Hegardt FG, Haro D. Control of human muscle-type carnitine palmitoyltransferase I gene transcription by peroxisome proliferator-activated receptor. J Biol Chem 1998;273:8560–8563 [DOI] [PubMed] [Google Scholar]
- 31.Bezaire V, Bruce CR, Heigenhauser GJF, et al. Identification of fatty acid translocase on human skeletal muscle mitochondrial membranes: essential role in fatty acid oxidation. Am J Physiol Endocrinol Metab 2006;290:E509–E515 [DOI] [PubMed] [Google Scholar]
- 32.Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem 2003;49:1797–1817 [DOI] [PubMed] [Google Scholar]
- 33.Soeters MR, Serlie MJ, Sauerwein HP, et al. Characterization of D-3-hydroxybutyrylcarnitine (ketocarnitine): an identified ketosis-induced metabolite. Metabolism 2012;61:966–973 [DOI] [PubMed] [Google Scholar]
- 34.Minkler PE, Stoll MSK, Ingalls ST, Yang S, Kerner J, Hoppel CL. Quantification of carnitine and acylcarnitines in biological matrices by HPLC electrospray ionization-mass spectrometry. Clin Chem 2008;54:1451–1462 [DOI] [PubMed] [Google Scholar]
- 35.Pochini L, Oppedisano F, Indiveri C. Reconstitution into liposomes and functional characterization of the carnitine transporter from renal cell plasma membrane. Biochim Biophys Acta 2004;1661:78–86 [DOI] [PubMed] [Google Scholar]
- 36.Suhre K, Shin SY, Petersen AK, et al. CARDIoGRAM Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011;477:54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lysiak W, Toth PP, Suelter CH, Bieber LL. Quantitation of the efflux of acylcarnitines from rat heart, brain, and liver mitochondria. J Biol Chem 1986;261:13698–13703 [PubMed] [Google Scholar]
- 38.Kien CL, Everingham KI, D Stevens R, Fukagawa NK, Muoio DM. Short-term effects of dietary fatty acids on muscle lipid composition and serum acylcarnitine profile in human subjects. Obesity (Silver Spring) 2011;19:305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa CC, de Almeida IT, Jakobs C, Poll-The BT, Duran M. Dynamic changes of plasma acylcarnitine levels induced by fasting and sunflower oil challenge test in children. Pediatr Res 1999;46:440–444 [DOI] [PubMed] [Google Scholar]
- 40.Bell FP, DeLucia A, Bryant LR, Patt CS, Greenberg HS. Carnitine metabolism in Macaca arctoides: the effects of dietary change and fasting on serum triglycerides, unesterified carnitine, esterified (acyl) carnitine, and beta-hydroxybutyrate. Am J Clin Nutr 1982;36:115–121 [DOI] [PubMed] [Google Scholar]
- 41.Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schönekess BO. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta 1994;1213:263–276 [DOI] [PubMed] [Google Scholar]
- 42.Mueller P, Schulze A, Schindler I, Ethofer T, Buehrdel P, Ceglarek U. Validation of an ESI-MS/MS screening method for acylcarnitine profiling in urine specimens of neonates, children, adolescents and adults. Clin Chim Acta 2003;327:47–57 [DOI] [PubMed] [Google Scholar]
- 43.Chalmers RA, Roe CR, Stacey TE, Hoppel CL. Urinary excretion of l-carnitine and acylcarnitines by patients with disorders of organic acid metabolism: evidence for secondary insufficiency of l-carnitine. Pediatr Res 1984;18:1325–1328 [DOI] [PubMed] [Google Scholar]
- 44.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 2012;148:852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 2003;100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004;350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An J, Muoio DM, Shiota M, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 2004;10:268–274 [DOI] [PubMed] [Google Scholar]
- 48.Koves TR, Li P, An J, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 2005;280:33588–33598 [DOI] [PubMed] [Google Scholar]
- 49.Redman LM, Huffman KM, Landerman LR, et al. Effect of caloric restriction with and without exercise on metabolic intermediates in nonobese men and women. J Clin Endocrinol Metab 2011;96:E312–E321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah SH, Hauser ER, Bain JR, et al. High heritability of metabolomic profiles in families burdened with premature cardiovascular disease. Mol Syst Biol 2009;5:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huffman KM, Shah SH, Stevens RD, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009;32:1678–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huffman KM, Slentz CA, Bateman LA, et al. Exercise-induced changes in metabolic intermediates, hormones, and inflammatory markers associated with improvements in insulin sensitivity. Diabetes Care 2011;34:174–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. Metabolomics applied to diabetes research: moving from information to knowledge. Diabetes 2009;58:2429–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamamoğullari N, Siliğ Y, Içağasioğlu S, Atalay A. Carnitine deficiency in diabetes mellitus complications. J Diabetes Complications 1999;13:251–253 [DOI] [PubMed] [Google Scholar]
- 56.Poorabbas A, Fallah F, Bagdadchi J, et al. Determination of free L-carnitine levels in type II diabetic women with and without complications. Eur J Clin Nutr 2007;61:892–895 [DOI] [PubMed] [Google Scholar]
- 57.Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. A threshold exists for the stimulatory effect of insulin on plasma L-carnitine clearance in humans. Am J Physiol Endocrinol Metab 2007;292:E637–E641 [DOI] [PubMed] [Google Scholar]
- 58.Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. An acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscle. J Clin Endocrinol Metab 2006;91:5013–5018 [DOI] [PubMed] [Google Scholar]
- 59.Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. Insulin stimulates L-carnitine accumulation in human skeletal muscle. FASEB J 2006;20:377–379 [DOI] [PubMed] [Google Scholar]
- 60.Noland RC, Koves TR, Seiler SE, et al. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem 2009;284:22840–22852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van den Broek NMA, Ciapaite J, De Feyter HMML, et al. Increased mitochondrial content rescues in vivo muscle oxidative capacity in long-term high-fat-diet-fed rats. FASEB J 2010;24:1354–1364 [DOI] [PubMed] [Google Scholar]
- 62.Power RA, Hulver MW, Zhang JY, et al. Carnitine revisited: potential use as adjunctive treatment in diabetes. Diabetologia 2007;50:824–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 2000;279:E1039–E1044 [DOI] [PubMed] [Google Scholar]
- 64.Timmers S, Nabben M, Bosma M, et al. Augmenting muscle diacylglycerol and triacylglycerol content by blocking fatty acid oxidation does not impede insulin sensitivity. PNAS 2012;109:11711–11716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD. Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes 2001;50:123–130 [DOI] [PubMed] [Google Scholar]
- 66.Bruce CR, Thrush AB, Mertz VA, et al. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab 2006;291:E99–E107 [DOI] [PubMed] [Google Scholar]
- 67.Meex RCR, Schrauwen-Hinderling VB, Moonen-Kornips E, et al. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 2010;59:572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muoio DM, Noland RC, Kovalik JP, et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab 2012;15:764–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsintzas K, Chokkalingam K, Jewell K, Norton L, Macdonald IA, Constantin-Teodosiu D. Elevated free fatty acids attenuate the insulin-induced suppression of PDK4 gene expression in human skeletal muscle: potential role of intramuscular long-chain acyl-coenzyme A. J Clin Endocrinol Metab 2007;92:3967–3972 [DOI] [PubMed] [Google Scholar]
- 70.Adams SH, Hoppel CL, Lok KH, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 2009;139:1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ebeling P, Tuominen JA, Arenas J, Garcia Benayas C, Koivisto VA. The association of acetyl-L-carnitine with glucose and lipid metabolism in human muscle in vivo: the effect of hyperinsulinemia. Metabolism 1997;46:1454–1457 [DOI] [PubMed] [Google Scholar]
- 72.Ramos-Roman MA, Sweetman L, Valdez MJ, Parks EJ. Postprandial changes in plasma acylcarnitine concentrations as markers of fatty acid flux in overweight and obesity. Metabolism 2012;61:202–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kerbey AL, Randle PJ, Cooper RH, Whitehouse S, Pask HT, Denton RM. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J 1976;154:327–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS ONE 2010;5:e15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ho JK, Duclos RI, Jr, Hamilton JA. Interactions of acyl carnitines with model membranes: a (13)C-NMR study. J Lipid Res 2002;43:1429–1439 [DOI] [PubMed] [Google Scholar]
- 77.Ren J, Lakoski S, Haller RG, Sherry AD, Malloy CR. Dynamic monitoring of carnitine and acetylcarnitine in the trimethylamine signal after exercise in human skeletal muscle by 7T 1H-MRS. Magn Reson Med. 3 April 2012 [Epub ahead of print] [DOI] [PMC free article] [PubMed]