Abstract
Recombinant vectors based on a non-pathogenic human parvovirus, the adeno-associated virus (AAV), have gained attention as a potentially safe and useful alternative to the more commonly used retroviral and adenoviral vectors. AAV vectors are currently in use in Phase I/II clinical trials for gene therapy of a number of diseases such as cystic fibrosis, α-1 antitrypsin deficiency, muscular dystrophy, Batten’s disease, and Parkinson’s disease, and have shown efficacy in patients with Leber’s congenital amaurosis, and hemophilia B. For patients with hemophilia B, however, relatively large vector doses are needed to achieve therapeutic benefits. Large vector doses also trigger an immune response as significant fraction of the vectors fails to traffic efficiently to the nucleus, and is targeted for degradation by the host cell proteasome machinery. With a better understanding of the various steps in the life cycle of AAV vectors, strategies leading to the development of novel AAV vectors that are capable of high-efficiency transduction at lower doses are needed. In this review, we summarize our strategies to develop novel AAV vectors for the potential gene therapy of both hemophilia B and hemophilia A, based on our recent studies on the basic molecular biology of AAV. These strategies, including the development of novel AAV vectors by site-directed mutagenesis of critical surface-exposed tyrosine residues on AAV2 capsids to circumvent the ubiquitination step and the use of different AAV serotypes and self-complementary (sc) AAV2 vectors, and their use as helper vectors to circumvent the obstacles of second-strand DNA synthesis of single-stranded (ss) AAV, should dramatically accelerate the progress towards the potential gene therapy of both hemophilia A and hemophilia B.
Keywords: AAV vectors, Hemophilia, Gene therapy
Introduction
Adeno-associated virus serotype 2 (AAV2), a human parvovirus, contains a single-stranded DNA genome, and has been studied extensively [1,2]. AAV2 causes no known disease even though ~90% of the human population is seropositive against AAV [3]. AAV2 is dependent on a helper virus, such as adenovirus [4], herpesvirus [5], vaccinia virus [6], or human papillomavirus [7] for its optimal replication [1], but in the absence of a helper virus, AAV establishes a latent infection where the wild-type (WT) viral genome integrates into the human chromosomal DNA site-specifically [8–10]. Indeed, the non-pathogenic nature of the virus prompted the development of recombinant AAV2 vectors [11–18]. Recombinant AAV2 vectors have been successfully used to transduce a variety of genes in a number of cell types in vitro, and the ability of these vectors to mediate persistent transgene expression has been validated in a number of small and large animal models in vivo, including hemophilia [19–24]. Since clotting factors are synthesized in the liver, and since AAV vectors target the hepatocytes well, the use of AAV vectors in the potential gene therapy of hemophilia is well justified [25], and has recently shown clinical efficacy [26].
Although AAV2 vector transduction efficiency varies greatly in different cell and tissue types, the molecular basis of the observed differential transduction is becoming clearer [24]. All recombinant AAV2 vectors utilized in human gene therapy applications thus far are devoid of any of the WT AAV coding sequences. Although cytotoxic T lymphocyte (CTL) response to AAV2 vectors was not observed in pre-clinical studies [27–29], in a gene therapy trial for hemophilia B, such a response was indeed observed, especially at high vector doses [25]. This review briefly summarizes the recent advances that have been made to circumvent, in part, some of the obstacles that have been encountered.
Next generation of recombinant AAV vectors for the potential gene therapy of hemophilia B
As stated above, in a clinical trial with AAV2 vectors, two patients with severe hemophilia B developed a vector dose-dependent transaminitis that limited duration of hepatocyte-derived hF.IX expression to <8 weeks [25]. Subsequent analyses demonstrated presence of memory CD8+ T cells to AAV2 capsids in one of the hemophilia B patients, which mirrored the time course of the transaminitis. It was concluded that this CD8+ T cell response to input capsid eliminated AAV2-transduced hepatocytes was most likely due to the Major Histo-compatibility Class I (MHC-I)-restricted, capsid specific cytotoxic T lymphocyte (CTL) response following proteasome-mediated degradation of AAV2 capsids and peptide presentation by professional antigen-presenting cells (APCs) [30].
Circumvention of ubiquitination, and proteasome-mediated degradation of AAV2 vectors
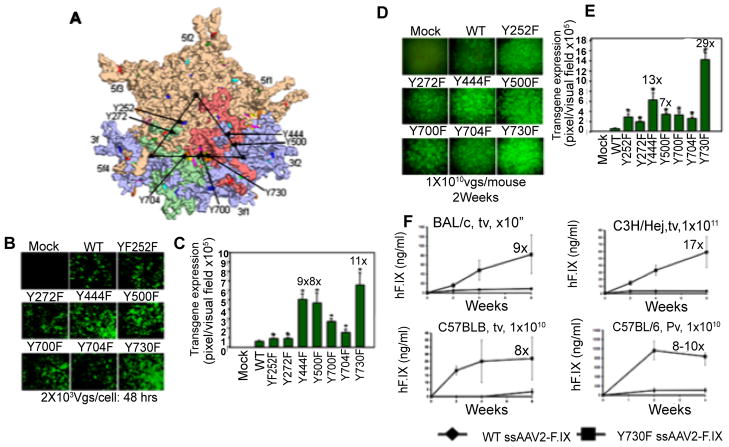
Based on our previous studies, in which we observed that AAV2 capsids become phosphorylated at tyrosine residues by the cellular epidermal growth factor receptor-protein tyrosine kinase (EGFR-PTK) activity [31,32], we hypothesized that tyrosine-phosphorylation of capsid proteins is a pre-requisite for ubiquitination, followed by proteasome-mediated degradation of intact AAV2 capsids, leading to inefficient intracellular trafficking, and thus, unfavorable to viral transduction [31,32]. We reasoned, therefore, that substitution of surface-exposed tyrosine residues on AAV2 capsids might allow the vectors to escape ubiquitination as well as proteasome-mediated degradation. We tested our hypothesis by modifying each of the 7 surface-exposed tyrosine residues (Y252, Y272, Y444, Y500, Y700, Y704, and Y730) on AAV2 capsids (Figure 1A) by site-directed mutagenesis, which were conservatively substituted with phenylalanine residues (tyrosine-phenylalanine, Y-F) [33]. The transduction efficiency of each of the surface-exposed tyrosine-mutant vectors was analyzed and compared with the WT scAAV2-EGFP vector in HeLa cells in vitro under identical conditions [33]. From the results shown in (Figure 1B), it is evident that the transduction efficiency of each of the tyrosine-mutant vectors was significantly higher compared with the WT scAAV2-EGFP vector at 2,000 viral particles/cell. Specifically, the transduction efficiency of Y444F, Y500F, Y730F vectors was ~8–11-fold higher than the WT vector (Figure 1C). The efficacy of WT and tyrosine-mutant scAAV2-EGFP vectors was also evaluated in a mouse model in vivo. Approximately 1×1010 particles of these vectors were injected via the tail vein of normal C57BL/6 mice. Two weeks post-injection, liver tissues were harvested and analyzed for EGFP gene expression using fluorescence microscopy. From the results shown in (Figure 1D), it is evident that the transduction efficiency of each of the tyrosine-mutant vectors was significantly higher compared with the WT scAAV2-EGFP vectors. Specifically, the transduction efficiency of Y444F and Y730F vectors was ~13–29-fold higher than the WT vector (Figure 1E). In subsequent studies, we also documented that tyrosine-mutations prevent ubiquitination of AAV2 capsids, leading to decreased proteasome-mediated degradation and improved nuclear transport of AAV2 vectors. When other tissues, such as heart, lung, kidney, spleen, pancreas, GI tract (jejunum, colon), testis, skeletal muscle, and brain were harvested from mice injected with 1×1010 particles of the tyrosine-mutant vectors and analyzed, no evidence of EGFP gene expression was seen (data not shown). Thus, mutations in the surface-exposed tyrosine residues did not alter the liver-tropism of these vectors in vivo. The use of single-stranded AAV2-Y730F vectors also led to production of therapeutic levels of human Factor IX (F.IX) at an ~10-fold reduced vector dose (Panel F).
Figure 1.
Novel tyrosine-mutant AAV2 vectors. (A) The position of the 7 surface-exposed tyrosine residues on the AAV2 capsid surface, Y252, Y272, Y444, Y500, Y700, Y704, and Y730, are indicated by the arrows. Site-directed mutations of these tyrosine residues to phenylalanine residues (tyrosine-phenylalanine, Y-F) were performed and tyrosine-mutant capsid scAAV2-EGFP vectors were generated. (B) AAV2-mediated transgene expression in HeLa cells following transduction with tyrosine-mutant scAAV2-EGFP vectors. (C) Quantitative analyses of the transduction efficiency*P<0.01 vs. WT scAAV2-EGFP. (D) AAV2-mediated transduction of hepatocytes from normal C57BL/6 mice injected via tail vein with tyrosine-mutant capsid scAAV2-EGFP vectors. (E) Quantitative analyses of AAV2 transduction efficiency. *P < 0.01 vs WT scAAV2-EGFP. (F) Comparative analyses of the WT or Y730F ssAAV2-ApoE/hAAT-hF.IX vector-mediated transduction efficiency in hepatocytes in mice in vivo. Human F.IX (hF.IX) expression in plasma as a function of time after injection of 1×1011 viral particles/animal in BALB/c and C3H/HeJ mice via tail vein (tv), and 1×1010 viral particles/animal in C57BL/6 mice via tail vein (tv) or portal vein (pv). Fold-increase of hF.IX peak levels of Y730F vectors compared to the WT capsid vectors is indicated for each panel [Proc. Natl. Acad. Sci., USA, 105: 7827–7832, 2008].
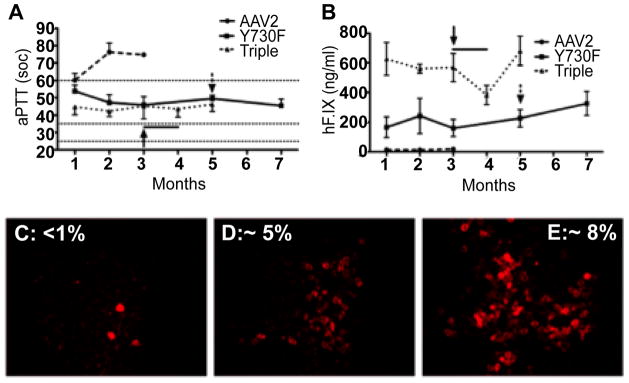
Next, we generated various permutations and combinations of tyrosine-mutations as follows: 7 Double (Y730+252F; Y730+272F; Y730+444F; Y730+500F; Y730+700F; Y730+704F; Y444+500F); 1 Triple (Y730+500+444F); 1 Quadruple (Y730+500+444+272F); 2 Pentuple (Y730+ 704+500+444+272F; Y730+700+500+444+272F); 2 Sextuple (Y730+704+500+444+272+252F; Y730+ 700+500+444+272+252F); and 1 Septuple (Y730+704+700+500+444 +272+252F), each of which was capable of encapsidation of the viral genome to similar titers, and each mutant was biologically active in HeLa cells in vitro, and in murine hepatocytes in vivo. The triple-mutant (Y730+500+500F) was ~3-fold more efficient than the single-mutant (Y730F), but the transduction efficiency of the septuple-mutant (Y730+704+700+500+444+272+252F) was not significantly higher than the single-mutant in HeLa cells in vitro. Similarly, the triple-mutant was ~3-fold more efficient than the single-mutant, and the transduction efficiency of the septuple-mutant was not significantly higher than the single-mutant in murine hepatocytes in vivo, following tail-vein injections. These Y-F mutant vectors were also evaluated for efficacy in hemophilia B mice. In C3H/HeJ F9−/− mice [34,35], hepatic AAV2-F. IX gene transfer resulted in stable systemic expression of ~200 ng/ml (4% of normal) using Y730F capsid and in 3-times higher levels (~600 ng/ml or 12% of normal) with triple-mutant capsid (Y730+500+444F). Expression data correlated with shortened coagulation times. F.IX−/− C3H/HeJ mice (n=4, targeted F.IX gene deletion) were injected into the tail vein using 2×1011 vgs of AAV2-ApoE/hAAT-hF.IX vectors (WT, Y730F, or Y730+500+444F triple-mutant capsid). This dose of WT AAV2 ApoE/hAAT-hF.IX vector results in only sub-therapeutic expression of hF.IX in C3H/HeJ mice [36]. Furthermore, mice of this strain mount CD8+ T cell and inhibitor responses against hF.IX after hepatic gene transfer with AAV2 vector [37–39]. Consistent with our prior data, WT AAV2 capsid vector yielded transient low-level expression with partial thrombloplastin time (aPTT) values of ~60 sec (~1% of normal) at 1 month, which subsequently increased to >70 sec (i.e. no correction; Figure 2A). In contrast, Y730F and Y730+500+444F vectors gave sustained expression of ~4% and ~12% of normal human levels, respectively, and, accordingly, had more robust correction of aPTTs for the duration of the experiment (5–7 months; Figure 2A and Figure 2B). These mice failed to form inhibitors even after challenge with exogenous hF.IX protein. The levels of expression of hF.IX in murine hepatocytes 3–7 months after gene transfer with WT (Figure 2C), Y730F (Figure 2D), and Y730+500+444F (Figure 2E) capsid vectors were also evaluated and quantitated by immunostaining.
Figure 2.
F.IX−/− C3H/HeJ mice (n=4/vector) were injected into the tail vein with 2×1011 vgs of AAV-ApoE/hAAT-hF.IX packaged into WT AAV-2 (dashed line), or AAV2-Y730F (solid line) or AAV2-Y730+500+444F (dotted line) capsids. Resulting systemic hF.IX expression (A) and coagulation times (aPTT) (B) are shown. Each line represents average SD. Horizontal lines in A mark the range of aPTTs for normal mouse plasma (25–35 sec) or untreated hemophilia B mouse plasma (>60 sec). Y-F vector-treated mice were challenged with subcutaneous hF.IX/CFA (arrow) or weekly intravenous hF.IX (arrow + line). C–E. Immunostain for hF.IX expressing hepatocytes 3–7 months after gene transfer with WT (C), Y730F (D), or Y730+500+444F (E) capsid vectors. Average percent of hF.IX positive hepatocytes are indicated [Mol. Ther., 18: 2048–2056, 2010].
We hypothesize that transduction efficiency could be further improved by other combinatorial mutants that are currently being evaluated and by analogous experiments with other serotypes. The availability of such a vast repertoire of novel tyrosine-mutant AAV serotype vectors should allow us to gain a better understanding of the role of tyrosine-phosphorylation of AAV capsids in various steps in the virus life cycle, which is likely to have important implications in the optimal use of recombinant AAV serotype tyrosine-mutant vectors in the potential gene therapy of hemophilia B.
The use of serotype vectors other than AAV2
In addition to the CTL response to AAV2 vectors described above, neutralizing antibodies can and do affect the efficacy of repeat dosing of AAV2 vectors [36,40,41]. An increasing number of investigators are now focusing on the other naturally occurring serotypes of AAV (AAV-1, 3-12, and over 100 variants have also been isolated), which are structurally and functionally different from AAV2 [42–45]. Several strategies have been devised to cross-package an AAV2 vector genome into the capsids of the other AAV serotypes, resulting in a new generation of “pseudotyped” AAV vectors [46]. A number of studies have reported various tissue-tropism of different AAV serotype vectors [47–59].
It is noteworthy that with a few exceptions (Y444 positioned equivalent to a lysine in AAV4 and arginine in AAV5; Y700 positioned equivalent to phenylalanine in AAV4 and AAV5; and Y704 positioned equivalent to a phenylalanine in AAV7), these tyrosine residues are highly conserved in AAV serotypes 1 through 10. Y-F mutants of each of these serotypes have been generated. Petrs-Silva and colleagues have tested the efficacy of two tyrosine-mutants of AAV8 and AAV9 serotype vectors in the mouse retina [60]. Approximately 1×109 vgs of the WT and Y-F mutant vectors were injected intravitreally, and 10-days post-injections, retinas were harvested and examined under a fluorescent microscope. These results documented a dramatic increase in the transduction efficiency by both mutant serotype vectors compared with their respective WT counterparts. Similar results were also recently reported by Pang et al. [61], and Dalkara et. al. [62] with tyrosine-mutant AAV8 and AAV9 vectors, respectively, and Ussher and Taylor have reported optimized transduction of human monocyte-derived dendritic cells by tyrosine-mutant AAV6 vectors [63]. Dr. Xiao Xiao at the University of North Carolina at Chapel Hill, and Dr. Dongsheng Duan at the University of Missouri at Columbia, evaluated the transduction efficiencies of tyrosine-mutants of AAV6, AAV8 and AAV9 serotypes, respectively, in the murine muscles [64,65]. The results documented that mutants AAV6-Y445F and -Y731F vectors were more potent in muscle tissue than WT AAV6 vector. The expression level from 10-fold lower mutant AAV9-Y731F at MOI of 1×1011 vgs/mouse matched that of WT AAV9 vector at MOI of 1×1011 vgs/mouse [66]. Jayandharan and colleagues generated point-mutations in each of the six tyrosine residues (Y252F, Y273F, Y445F, Y701F, Y705F, Y731F) in AAV6 capsid [67]. scAAV6-CBAp-EGFP vectors containing the wild-type (WT) and each one of the six tyrosine-mutant vectors were evaluated for their transduction potential in primary human CD34+ cells at 5×103 vgs/cell. These results have shown that the transduction efficiency of two of the tyrosine-mutant vectors (Y705F>Y731F) was significantly higher than that of the WT AAV6 vectors. Glushakova and colleagues generated a point-mutation in tyrosine residue of 731 in AAV3 (Y731F), and packaged Y731F AAV3-AFPp-EGFP vectors [68]. These data indicate that the transduction efficiency of each Y-F mutant vector is tissue-specific, and establish that these conserved residues are a crucial determinant of transduction efficiency of AAV serotype vectors. Thus, vectors based on serotypes other than AAV2 might be able to circumvent problems associated with AAV2, and some of these serotypes vectors are beginning to prove efficacious in clinical trials in humans [69,70]. However, the search for an ideal serotype of hepatocyte-specific transduction is still on, and in our recent studies, we have identified AAV3 as a potential candidate, and generated optimized AAV3 serotype vectors for high-efficiency transduction of hepatocytes [71–74].
A novel strategy for the potential gene therapy of hemophilia A
With the exception of the currently ongoing clinical trial for hemophilia B with AAV8 serotype vectors, all other trials reported thus far have been conducted using the single-stranded (ss) AAV vectors [75–80]. However, others and we have demonstrated that viral second-strand synthesis is the major rate-limiting step in ssAAV vector-mediated transgene expression due to the transcriptionally inactive nature of single-stranded AAV genome [81–87]. The use of self-complementary AAV (scAAV) vectors that bypass the requirement for viral second-strand DNA synthesis can circumvent this problem [88,89], but their widespread use is limited by their limited packaging capacity (~3.3 kb vs ~6 kb for ssAAV) [90,91]. This case is illustrated in the gene therapy for hemophilia A, where single-stranded vectors alone are used for delivery of the coagulation Factor VIII gene [92,93], as they exceed the packaging capacity of scAAV vectors. Therefore, strategies to improve the transduction efficiency of conventional ssAAV vectors in vivo are needed.
We have previously reported that phosphorylated forms of a 52-kDa cellular chaperone protein, FKBP52, interacts specifically with the D-sequence within the inverted terminal repeat (ITR) of the AAV genome [94,95]. Phosphorylation of FKBP52 at serine/threonine (Ser/r) and tyrosine (Tyr) residues inhibits viral second-strand DNA synthesis by ~40% and ~90%, respectively, leading to inefficient transgene expression [96–99]. However, de-phosphorylation of FKBP52 at Tyr residues by the cellular T-cell protein tyrosine phosphatase (TC-PTP), and at Ser/r residues by protein phosphatase 5 (PP5) prevents FKBP52 binding to the D-sequence, leading to efficient viral second-strand DNA synthesis [84,85]. Augmented transgene expression from ssAAV2 vectors also occurs in transgenic mice over-expressing TC-PTP [84–86], and in mice deficient in FKBP52. Subsequently, we also developed scAAV-TC-PTP and scAAV-PP5 vectors [31,32,86,87,95,96,98–100]. We reasoned that if scAAV-TC-PTP and scAAV-PP5 vectors were admixed with a conventional ssAAV vector prior to transduction, the rapid and simultaneous expression of TC-PTP and PP5 from scAAV vectors, which do not require viral second-strand DNA synthesis, would completely de-phosphorylate FKBP52 at both Tyr and Ser/r residues, respectively. This would lead to a more efficient second-strand DNA synthesis of the ssAAV vector resulting in high-efficiency transgene expression. Indeed, this co-administration strategy led to ~16-fold increase in the transduction efficiency of ssAAV2 vectors in primary murine hepatocytes in vivo [100].
In an effort to augment the transduction efficiency of ssAAV vectors in liver-directed gene therapy, subsequent studies were designed to enhance the helper-functions of scAAV-TC-PTP and/or scAAV-PP5 vectors by optimizing various parameters including the promoter, the AAV packaging serotype, and the helper-virus dosage. In addition, the scAAV2 vector carrying the tyrosine to phenylalanine mutation in codon 730 of VP3 region of AAV2 capsid (AAV2-Y730F), shown to facilitate high-efficiency transduction of hepatocytes in vivo [100] was also tested to examine whether the optimized helper-virus was capable of allowing expression of a therapeutic gene (human F.IX) in the mouse liver at reduced vector doses.
Based on our previous studies in which we reported that co-injection of scAAV2-TC-PTP and scAAV2-PP5 vectors resulted in ~16-fold increase in the transduction efficiency of ssAAV2-EGFP vectors in murine hepatocytes in vivo [100], we extended these studies to include the AAV8 serotype vectors, which are known to transduce mouse hepatocytes efficiently [43]. We evaluated whether the use of scAAV8-TC-PTP and scAAV8-PP5 helper-viruses would lead to a further increase in the transduction efficiency of ssAAV2-EGFP vectors. When normal C57BL/6J mice were injected with 5×1010 vgs each of ssAAV2-EGFP vectors alone, or together with scAAV8-RSV-TC-PTP and/or scAAV8-RSV-PP5 vectors via the tail-vein, consistent with previously published studies, whereas little green fluorescence was detected in hepatocytes two-weeks post-injection of conventional ssAAV2-EGFP vectors alone, co-injection with scAAV8-TC-PTP helper-virus led to ~6-fold increase in the transduction efficiency of ssAAV2-EGFP vectors. Interestingly, scAAV8-PP5 vectors alone led to ~24-fold increase in EGFP expression, which was significantly higher than ~11-fold increase when scAAV8-TC-PTP+scAAV8-PP5 were co-injected. Thus, it became clear from these experiments that co-administration of scAAV-PP5 vectors alone could achieve superior helper function, and therefore, optimization of scAAV-PP5 helper-viruses was envisaged.
We also reasoned that the transduction efficiency mediated by the scAAV2-PP5 helper vector could be further augmented by optimizing either the expression cassette or modifications in the AAV2 capsid, or both. We evaluated the trans-thyretin (TTR) promoter, which is hepatocyte-specific, compared with the RSV promoter, expression from which is known to be ubiquitous. Second, we compared the efficiency of scAAV2-PP5 helper-virus with or without capsid modifications in AAV2, since we have shown recently that an Y730F mutation in AAV2 capsid significantly enhances transgene expression by bypassing the proteasome-mediated degradation. This was evaluated in murine hepatocytes in vivo, at a dose of 5×1010 vgs each of these optimized helper-viruses. These results showed that the RSV-promoter-driven PP5 helper-virus increased the transduction efficiency of ssAAV2-EGFP vectors by ~9-fold, which is similar to the increase reported previously [98]. Interestingly, co-injection of the RSV promoter-driven PP5 vector packaged into an Y730F-AAV2 capsid enhanced the transgene expression by ~17-fold, which was further augmented to ~22-fold by replacing the RSV promoter with the TTR promoter. These data correlate well with our previous studies that showed the transduction efficiency of Y730F-AAV2 vectors to be ~29-fold higher than the wild-type (WT) AAV2 vectors in murine hepatocytes in vivo [98]. These results demonstrate that scAAV2-PP5 helper-viruses under the control of TTR promoter and the Y730F capsid mutation can additively augment PP5 expression, which in turn, significantly enhances the transduction efficiency of ssAAV2-EGFP vectors. Furthermore, the use of AAV8 serotype and a liver-specific TTR promoter was shown to further enhance transgene expression from ssAAV2-EGFP vectors [100].
Finally, it was important to examine whether scAAV-PP5 helper-viruses were capable of allowing expression of a therapeutic gene efficiently at a reduced vector dose in vivo. To this end, 1×1010 vgs of a ssAAV2-hF.IX vector alone or co-administered with 1×1010 vgs of the most efficient helper-virus (scAAV8-TTR-PP5) were evaluated in C57BL/6J mice. Over a period of 8-weeks, the PP5 helper-virus led to expression of ~6–7-fold higher levels of circulating hF.IX compared with ssAAV2-F.IX vectors alone (Figure 3A). At this dose of ssAAV2-F.IX vector (1×1010 vgs/mouse), expression was augmented from sub-therapeutic to therapeutic levels (216±30 ng/ml vs. 30±11 ng/ml, 4–5% vs. <1% of normal human F.IX levels). This enhancement was also observed when 1×109 vgs/animal of helper virus was used confirming our findings with EGFP that scAAV8-TTR-PP5 augments transgene expression even at reduced doses. A 5-fold higher dose of ssAAV2-F.IX vector alone was required to achieve similar therapeutic levels (Figure 3A). When combined with the high-dose ssAAV2-F. IX vector, the helper-virus only marginally increased F.IX levels. To investigate if this enhancement was caused by an increase in hF.IX-producing hepatocytes, liver tissues were collected at 10-weeks from mice receiving a high- or low-dose of ssAAV2-hAAT-F.IX, with or without 1×1010 vgs of scAAV8-TTR-F.IX helper-virus, and immunofluorescence staining for hF.IX was performed. Percent hF.IX-positive hepatocytes were estimated in each group and results are shown in (Figure 3B) along with representative images from each group. The results of fluorescence staining correlated well with the ELISA data in that the helper-virus had the greatest effect in mice receiving the lower dose of ssAAV2-F.IX vectors, augmenting the percent positive hepatocytes from <1% without the helper-virus to 3–5% with the helper-virus, and up to 14% in mice receiving a higher dose of ssAAV2-F.IX vectors (Figure 3B) [100].
Figure 3.
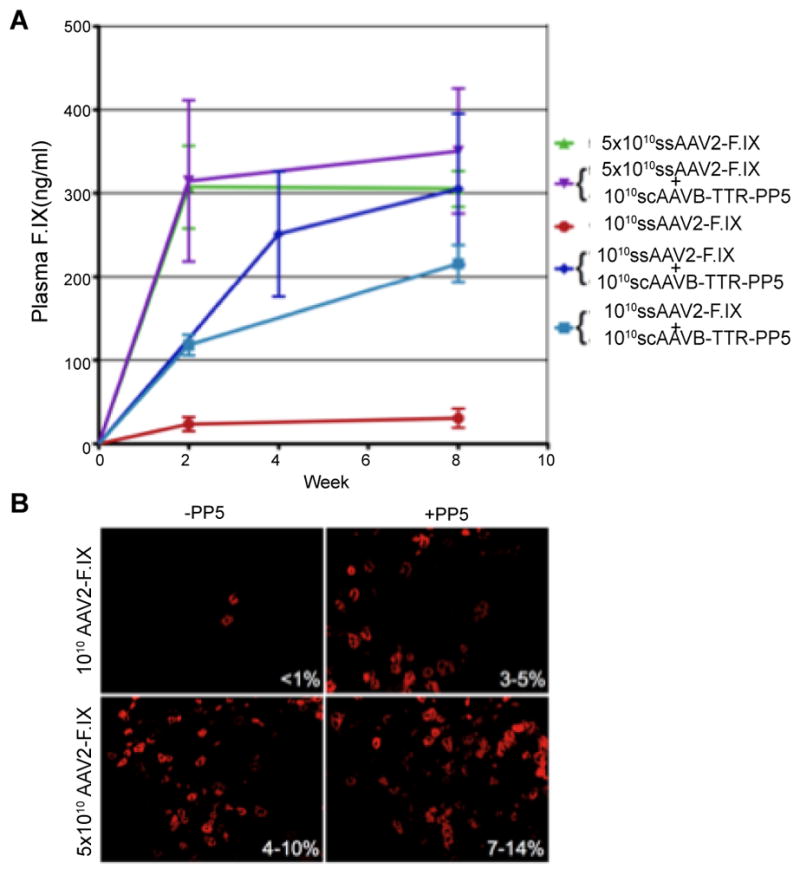
Comparative analyses of the ssAAV2-ApoE/hAAT-hF.IX vector-mediated transduction efficiency in hepatocytes with and without co-administration of scAAV8-PP5 helper-viruses in C57BL/6 mice in vivo. (A) Human F.IX (hF.IX) expression in plasma was determined as a function of time after injection of 1×1010 or 5×1010 vgs of each vector/animal. Data are mean ± SEM (n=4 per experimental group). (B) Representative liver sections obtained 10 weeks following injection of ssAAV2-hAAT-hF.IX vectors (AAV2-F.IX) with or without scAAV8-TTR-PP5 helper-virus (PP5). Sections were immunofluorescently stained for hF.IX and the ranges of percent positive hepatocytes for each group are shown in each panel. Original magnification: 200x [Hum. Gene Ther., 21: 271–283, 2010].
One of the advantages of this helper-virus system is that this approach is applicable to any ssAAV transgene cassette, and can be adapted to any of the current clinical protocols without modifications to the therapeutic vector. This could be achieved by a choice of either a scAAV2 tyrosine-mutant or scAAV8 capsids, with or without cell-specific promoters for the desired cell-tropism. The use of a scAAV8-TTR-PP5 helper-virus was the most efficient in enhancing transgene expression from a ssAAV-F.IX vector in the mouse liver. At least ~6–7-fold higher circulating hF.IX levels in a therapeutic range (>200 ng/ml) were detected in helper-virus-administered mice compared with those injected with the ssAAV2-F.IX vectors alone. This should enable one to attain the same therapeutic level of expression of a therapeutic gene at a log lower dose, thus minimizing immunological responses against the transgene and the viral capsid. Furthermore, enhancement was possible at a dose of 1×109 vgs/mouse of scAAV8-TTR-PP5 helper-virus, implicating that such a low-dose of the helper-virus was sufficient to achieve therapeutic levels of expression. This is important in light of recent evidence suggesting that the level of neutralizing antibody is proportional to the vector dose administered for AAV1 [69], and the loss of hepatic h.FIX expression in the hemophilia B clinical trial was due to the CD8+ T cell response to input capsids in patients who received high vector doses [25,30,69].
The additional optimization steps with the PP5 transgene cassette yielded a further 3-fold increase in transgene expression compared with our previously reported co-administration strategy with scAAV2-TC-PTP and scAAV2-PP5 vectors (36-fold vs 11-fold) [98]. Only low-levels of PP5 are required for complete dephosphorylation of hepatocytes FKBP52, and this in turn pushes transduction through the bottle-neck of second-strand synthesis, thereby yielding a substantial effect on expression from the therapeutic vector, while additional expression has limited effects. Furthermore, PP5 is known to be anti-apoptotic, and the recent development of a viable PP5-deficient mouse model suggests that PP5 is a modulatory, rather than an essential factor, in phosphorylation pathways [101]. This bodes well for the potential use of this strategy in a liver-directed gene therapy application to minimize any vector dose-associated liver toxicity. This is further supported by the fact that toxicological studies performed with FKBP52-dephosphorylating enzymes in normal C57BL/6J mice with a scAAV-PP5 vector showed no evidence of toxicity up to 12-weeks [98], and over-expression of PP5 did not affect cellular growth [87], or hepatocyte characteristics in vivo in C57BL/6 mice [98,100]. Additional studies are warranted to evaluate the safety and efficacy of scAAV-PP5 vector-mediated enhanced transduction by ssAAV-h.FVIII vectors for the potential gene therapy of hemophilia A.
Conclusions
The non-pathogenic nature of AAV, persistence of the proviral genome, and sustained transgene expression make these extremely attractive vectors for the potential gene therapy of hemophilia. With a more complete understanding of the virus life cycle and virus–host cell interactions as well as the development of new techniques, a vast repertoire of novel AAV vectors will most certainly be generated, which promises to lead to further improvements in the production of therapeutic levels of coagulation factors at reduced vector doses. Once all safety and efficacy issues have been dealt with in canine and non-human primate models, the remarkable stability and versatility of AAV vectors are likely to be utilized for their eventual application in the potential gene therapy of both hemophilia A and hemophilia B in humans.
Acknowledgments
This work was supported by NIH grant R01 HL097088.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Berns KI, Bohenzky RA. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–306. doi: 10.1016/s0065-3527(08)60479-0. [DOI] [PubMed] [Google Scholar]
- 2.Muzyczka N, Berns K. In Fields Virology. Philadelphia, PA: Lippincott, Williams and Wilkins; 2001. [Google Scholar]
- 3.Pattison J. Parvovirus and human disease. Boca Raton: CRC Press; 1988. [Google Scholar]
- 4.Hoggan MD, Blacklow NR, Rowe WP. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966;55:1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buller RM, Janik JE, Sebring ED, Rose JA. Herpes simplex virus types 1 and 2 completely help adenovirus-associated virus replication. J Virol. 1981;40:241–247. doi: 10.1128/jvi.40.1.241-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlehofer JR, Ehrbar M, zur Hausen H. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology. 1986;152:110–117. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- 7.Cao M, Zhu H, Bandyopadhyay S, You H, Hermonat PL. HPV-16 E1, E2 and E6 each complement the Ad5 helper gene set, increasing rAAV2 and wt AAV2 production. Gene Ther. 2012 doi: 10.1038/gt.2011.115. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotin RM, Menninger JC, Ward DC, Berns KI. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics. 1991;10:831–834. doi: 10.1016/0888-7543(91)90470-y. [DOI] [PubMed] [Google Scholar]
- 9.Kotin RM, Siniscalco M, Samulski RJ, Zhu XD, Hunter L, et al. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci U S A. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samulski RJ, Zhu X, Xiao X, Brook JD, Housman DE, et al. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. Embo J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahreini P, Woody MJ, Zhou SZ, Srivastava A. Versatile adeno-associated virus 2-based vectors for constructing recombinant virions. Gene. 1993;124:257–262. doi: 10.1016/0378-1119(93)90402-o. [DOI] [PubMed] [Google Scholar]
- 12.Zhou SZ, Broxmeyer HE, Cooper S, Harrington MA, Srivastava A. Adeno-associated virus 2-mediated gene transfer in murine hematopoietic progenitor cells. Exp Hematol. 1993;21:928–933. [PubMed] [Google Scholar]
- 13.Ponnazhagan S, Nallari ML, Srivastava A. Suppression of human alpha-globin gene expression mediated by the recombinant adeno-associated virus 2-based antisense vectors. J Exp Med. 1994;179:733–738. doi: 10.1084/jem.179.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou SZ, Cooper S, Kang LY, Ruggieri L, Heimfeld S, et al. Adeno-associated virus 2-mediated high efficiency gene transfer into immature and mature subsets of hematopoietic progenitor cells in human umbilical cord blood. J Exp Med. 1994;179:1867–1875. doi: 10.1084/jem.179.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo F, Zhou SZ, Cooper S, Munshi NC, Boswell HS, et al. Adeno-associated virus 2-mediated gene transfer and functional expression of the human granulocyte-macrophage colony-stimulating factor. Exp Hematol. 1995;23:1261–1267. [PubMed] [Google Scholar]
- 16.Lebkowski JS, McNally MM, Okarma TB, Lerch LB. Adeno-associated virus: a vector system for efficient introduction and integration of DNA into a variety of mammalian cell types. Mol Cell Biol. 1988;8:3988–3996. doi: 10.1128/mcb.8.10.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 18.Tratschin JD, Miller IL, Smith MG, Carter BJ. Adeno-associated virus vector for high-frequency integration, expression, and rescue of genes in mammalian cells. Mol Cell Biol. 1985;5:3251–3260. doi: 10.1128/mcb.5.11.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermonat PL, Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 21.Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flotte TR, Afione SA, Conrad C, McGrath SA, Solow R, et al. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci U S A. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanlioglu S, Monick MM, Luleci G, Hunninghake GW, Engelhardt JF. Rate limiting steps of AAV transduction and implications for human gene therapy. Curr Gene Ther. 2001;1:137–147. doi: 10.2174/1566523013348788. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava A. Obstacles to human hematopoietic stem cell transduction by recombinant adeno-associated virus 2 vectors. J Cell Biochem Suppl. 2002;38:39–45. doi: 10.1002/jcb.10053. [DOI] [PubMed] [Google Scholar]
- 25.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 26.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, et al. Adenovirus-Associated Virus Vector-Mediated Gene Transfer in Hemophilia B. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monahan PE, Jooss K, Sands MS. Safety of adeno-associated virus gene therapy vectors: a current evaluation. Expert Opin Drug Saf. 2002;1:79–91. doi: 10.1517/14740338.1.1.79. [DOI] [PubMed] [Google Scholar]
- 28.Sun JY, Chatterjee S, Wong KK., Jr Immunogenic issues concerning recombinant adeno-associated virus vectors for gene therapy. Curr Gene Ther. 2002;2:485–500. doi: 10.2174/1566523023347616. [DOI] [PubMed] [Google Scholar]
- 29.Sun JY, Anand-Jawa V, Chatterjee S, Wong KK. Immune responses to adeno-associated virus and its recombinant vectors. Gene Ther. 2003;10:964–976. doi: 10.1038/sj.gt.3302039. [DOI] [PubMed] [Google Scholar]
- 30.Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 31.Zhong L, Zhao W, Wu J, Li B, Zolotukhin S, et al. A Dual Role of EGFR Protein Tyrosine Kinase Signaling in Ubiquitination of AAV2 Capsids and Viral Second-strand DNA Synthesis. Mol Ther. 2007;15:1323–1330. doi: 10.1038/sj.mt.6300170. [DOI] [PubMed] [Google Scholar]
- 32.Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381:194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markusic DM, Herzog RW, Aslanidi GV, Hoffman BE, Li B, et al. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther. 2010;18:2048–2056. doi: 10.1038/mt.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markusic D, Cooper M, Zolotukhin I, Zhong L, Srivastava A, et al. Novel AAV2 tyrosine mutants provide long-term therapeutic F.IX expression in a difficult to tolerize murine hemophilia model. Mol Ther. 2009;17:S292. [Google Scholar]
- 36.Cao O, Hoffman BE, Moghimi B, Nayak S, Cooper M, et al. Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol Ther. 2009;17:1733–1742. doi: 10.1038/mt.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper M, Nayak S, Hoffman BE, Terhorst C, Cao O, et al. Improved Induction of Immune Tolerance to Factor IX by Hepatic AAV-8 Gene Transfer. Hum Gene Ther. 2009;20:767–776. doi: 10.1089/hum.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao O, Armstrong E, Schlachterman A, Wang L, Okita DK, et al. Immune deviation by mucosal antigen administration suppresses gene-transfer-induced inhibitor formation to factor IX. Blood. 2006;108:480–486. doi: 10.1182/blood-2005-11-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rapti K, Louis-Jeune V, Kohlbrenner E, Ishikawa K, Ladage D, et al. Neutralizing Antibodies Against AAV Serotypes 1, 2, 6, and 9 in Sera of Commonly Used Animal Models. Mol Ther. 2011;20:73–83. doi: 10.1038/mt.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Calcedo R, Bell P, Lin J, Grant RL, et al. Impact of Pre-Existing Immunity on Gene Transfer to Nonhuman Primate Liver with Adeno-Associated Virus 8 Vectors. Hum Gene Ther. 2011;22:1389–1401. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- 44.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1988;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chao H, Monahan PE, Liu Y, Samulski RJ, Walsh CE. Sustained and complete phenotype correction of hemophilia B mice following intramuscular injection of AAV1 serotype vectors. Mol Ther. 2001;4:217–222. doi: 10.1006/mthe.2001.0449. [DOI] [PubMed] [Google Scholar]
- 48.Duan D, Yan Z, Yue Y, Ding W, Engelhardt JF. Enhancement of muscle gene delivery with pseudotyped adeno-associated virus type 5 correlates with myoblast differentiation. J Virol. 2001;75:7662–7671. doi: 10.1128/JVI.75.16.7662-7671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grimm D, Kay MA. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther. 2003;3:281–304. doi: 10.2174/1566523034578285. [DOI] [PubMed] [Google Scholar]
- 50.Weber M, Rabinowitz J, Provost N, Conrath H, Folliot S, et al. Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol Ther. 2003;7:774–781. doi: 10.1016/s1525-0016(03)00098-4. [DOI] [PubMed] [Google Scholar]
- 51.Arruda VR, Schuettrumpf J, Herzog RW, Nichols TC, Robinson N, et al. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood. 2004;103:85–92. doi: 10.1182/blood-2003-05-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Du L, Kido M, Lee DV, Rabinowitz JE, Samulski RJ, et al. Differential myocardial gene delivery by recombinant serotype-specific adeno-associated viral vectors. Mol Ther. 2004;10:604–608. doi: 10.1016/j.ymthe.2004.06.110. [DOI] [PubMed] [Google Scholar]
- 53.Mori S, Wang L, Takeuchi T, Kanda T. Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology. 2004;330:375–383. doi: 10.1016/j.virol.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y, Okada T, Sheykholeslami K, Shimazaki K, Nomoto T, et al. Specific and efficient transduction of cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol Ther. 2005;12:725–733. doi: 10.1016/j.ymthe.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Riviere C, Danos O, Douar AM. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–1308. doi: 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- 56.Hauck B, Xiao W. Characterization of tissue tropism determinants of adeno-associated virus type 1. J Virol. 2003;77:2768–2774. doi: 10.1128/JVI.77.4.2768-2774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci U S A. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin D, Fantz CR, Levy B, Rafi MA, Vogler C, et al. AAV2/5 vector expressing galactocerebrosidase ameliorates CNS disease in the murine model of globoid-cell leukodystrophy more efficiently than AAV2. Mol Ther. 2005;12:422–430. doi: 10.1016/j.ymthe.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Zhu T, Qiao C, Zhou L, Wang B, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 60.Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17:463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pang JJ, Dai X, Boye SE, Barone I, Boye SL, et al. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol Ther. 2011;19:234–242. doi: 10.1038/mt.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dalkara D, Byrne LC, Lee T, Hoffmann NV, Schaffer DV, et al. Enhanced gene delivery to the neonatal retina through systemic administration of tyrosine-mutated AAV9. Gene Ther. 2012 doi: 10.1038/gt.2011.163. in press. [DOI] [PubMed] [Google Scholar]
- 63.Ussher JE, Taylor JA. Optimized transduction of human monocyte-derived dendritic cells by recombinant adeno-associated virus serotype 6. Hum Gene Ther. 2010;21:1675–1686. doi: 10.1089/hum.2010.087. [DOI] [PubMed] [Google Scholar]
- 64.Qiao C, Zheng H, Yuan Z, Li J, Zhong L, et al. Comparison of transduction efficiency of tyrosine-mutant AAV vectors in muscle. Mol Ther. 2009;17:S175–S6. [Google Scholar]
- 65.Qiao C, Zhang W, Yuan Z, Shin JH, Li J, et al. Adeno-associated virus serotype 6 capsid tyrosine-to-phenylalanine mutations improve gene transfer to skeletal muscle. Hum Gene Ther. 2010;21:1343–1348. doi: 10.1089/hum.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang W, Yue Y, Ghosh A, Jayandharan G, Zhong L, et al. Novel tyrosine mutant vectors expand the utilities of AAV-mediated muscle gene therapy. Mol Ther. 2009;17:S176. [Google Scholar]
- 67.Jayandharan G, Rivers AE, Ling C, Andino L, Zhong L, et al. Human hematopoietic stem cell transduction by AAV vectors: Identification of AAV6 as the most efficient serotype, and further augmentation in transduction efficiency with point-mutations at tyrosine residues 705 and 731 in the viral capsid. Mol Ther. 2009;17:S145. [Google Scholar]
- 68.Glushakova L, Lisankie M, Eruslanov E, Ojano-Dirain C, Zolotukhin I, et al. High-efficiency transduction of human hepatoblastoma and hepatocellular carcinoma cells by wild-type and tyrosine-mutant AAV3 serotype vectors. Mol Ther. 2009;17:S179. [Google Scholar]
- 69.Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ponder KP. Hemophilia gene therapy: a Holy Grail found. Mol Ther. 2011;19:427–428. doi: 10.1038/mt.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ling C, Lu Y, Cheng B, McGoogan KE, Gee SW, et al. High-efficiency transduction of liver cancer cells by recombinant adeno-associated virus serotype 3 vectors. J Vis Exp. 2012;22(49) doi: 10.3791/2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng B, Ling C, Dai Y, Lu Y, Glushakova LG, et al. Development of optimized AAV3 serotype vectors: mechanism of high-efficiency transduction of human liver cancer cells. Gene Ther. 2012 doi: 10.1038/gt.2011.105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glushakova LG, Lisankie MJ, Eruslanov EB, Ojano-Dirain C, Zolotukhin I, et al. AAV3-mediated transfer and expression of the pyruvate dehydrogenase E1 alpha subunit gene causes metabolic remodeling and apoptosis of human liver cancer cells. Mol Genet Metab. 2009;98:289–299. doi: 10.1016/j.ymgme.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ling C, Lu Y, Kalsi JK, Jayandharan GR, Li B, et al. Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum Gene Ther. 2010;21:1741–1747. doi: 10.1089/hum.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, et al. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum Gene Ther. 1996;7:1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- 76.Flotte TR, Brantly ML, Spencer LT, Byrne BJ, Spencer CT, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults. Hum Gene Ther. 2004;15:93–128. doi: 10.1089/10430340460732490. [DOI] [PubMed] [Google Scholar]
- 77.Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 78.Aitken ML, Moss RB, Waltz DA, Dovey ME, Tonelli MR, et al. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum Gene Ther. 2001;12:1907–1916. doi: 10.1089/104303401753153956. [DOI] [PubMed] [Google Scholar]
- 79.Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 80.Wagner JA, Nepomuceno IB, Messner AH, Moran ML, Batson EP, et al. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther. 2002;13:1349–1359. doi: 10.1089/104303402760128577. [DOI] [PubMed] [Google Scholar]
- 81.Fisher KJ, Gao GP, Weitzman MD, DeMatteo R, Burda JF, et al. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrari FK, Samulski T, Shenk T, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mah C, Qing K, Khuntirat B, Ponnazhagan S, Wang XS, et al. Adeno-associated virus type 2-mediated gene transfer: role of epidermal growth factor receptor protein tyrosine kinase in transgene expression. J Virol. 1998;72:9835–9843. doi: 10.1128/jvi.72.12.9835-9843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qing K, Khuntirat B, Mah C, Kube DM, Wang XS, et al. Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J Virol. 1998;72:1593–1599. doi: 10.1128/jvi.72.2.1593-1599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qing K, Wang XS, Kube DM, Ponnazhagan S, Bajpai A, et al. Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc Natl Acad Sci U S A. 1997;94:10879–10884. doi: 10.1073/pnas.94.20.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhong L, Chen L, Li Y, Qing K, Weigel-Kelley KA, et al. Self-complementary adeno-associated virus 2 (AAV)-T cell protein tyrosine phosphatase vectors as helper viruses to improve transduction efficiency of conventional single-stranded AAV vectors in vitro and in vivo. Mol Ther. 2004;10:950–957. doi: 10.1016/j.ymthe.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 87.Zhao W, Wu J, Zhong L, Srivastava A. Adeno-associated virus 2-mediated gene transfer: role of a cellular serine/threonine protein phosphatase in augmenting transduction efficiency. Gene Ther. 2007;14:545–550. doi: 10.1038/sj.gt.3302886. [DOI] [PubMed] [Google Scholar]
- 88.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 89.Wang Z, Ma HI, Li J, Sun L, Zhang J, et al. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- 90.Grieger JC, Samulski RJ. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol. 2005;79:9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu J, Zhao W, Zhong L, Han Z, Li B, et al. Self-complementary recombinant adeno-associated viral vectors: packaging capacity and the role of rep proteins in vector purity. Hum Gene Ther. 2007;18:171–182. doi: 10.1089/hum.2006.088. [DOI] [PubMed] [Google Scholar]
- 92.Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108:107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- 93.Sarkar R, Xiao W, Kazazian HH., Jr A single adeno-associated virus (AAV)-murine factor VIII vector partially corrects the hemophilia A phenotype. J Thromb Haemost. 2003;1:220–226. doi: 10.1046/j.1538-7836.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 94.Qing K, Hansen J, Weigel-Kelley KA, Tan M, Zhou S, et al. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. J Virol. 2001;75:8968–8976. doi: 10.1128/JVI.75.19.8968-8976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qing K, Li W, Zhong L, Tan M, Hansen J, et al. Adeno-associated virus type 2-mediated gene transfer: role of cellular T-cell protein tyrosine phosphatase in transgene expression in established cell lines in vitro and transgenic mice in vivo. J Virol. 2003;77:2741–2746. doi: 10.1128/JVI.77.4.2741-2746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhong L, Li W, Yang Z, Chen L, Li Y, et al. Improved transduction of primary murine hepatocytes by recombinant adeno-associated virus 2 vectors in vivo. Gene Ther. 2004;11:1165–1169. doi: 10.1038/sj.gt.3302283. [DOI] [PubMed] [Google Scholar]
- 97.Zhong L, Li W, Yang Z, Qing K, Tan M, et al. Impaired nuclear transport and uncoating limit recombinant adeno-associated virus 2 vector-mediated transduction of primary murine hematopoietic cells. Hum Gene Ther. 2004;15:1207–1218. doi: 10.1089/hum.2004.15.1207. [DOI] [PubMed] [Google Scholar]
- 98.Jayandharan GR, Zhong L, Li B, Kachniarz B, Srivastava A. Strategies for improving the transduction efficiency of single-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2008;15:1287–1293. doi: 10.1038/gt.2008.89. [DOI] [PubMed] [Google Scholar]
- 99.Zhao W, Zhong L, Wu J, Chen L, Qing K, et al. Role of cellular FKBP52 protein in intracellular trafficking of recombinant adeno-associated virus 2 vectors. Virology. 2006;353:283–293. doi: 10.1016/j.virol.2006.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jayandharan GR, Zhong L, Sack BK, Rivers AE, Li M, et al. Optimized adeno-associated virus (AAV)-protein phosphatase-5 helper viruses for efficient liver transduction by single-stranded AAV vectors: therapeutic expression of factor IX at reduced vector doses. Hum Gene Ther. 2010;21:271–283. doi: 10.1089/hum.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hinds TD, Jr, Sanchez ER. Protein phosphatase 5. Int J Biochem Cell Biol. 2008;40:2358–2362. doi: 10.1016/j.biocel.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]