We demonstrate that Pneumocystis reaches a >90% prevalence peak at 3–5 months of age and associates with increased mucus (MUC5AC), suggesting airway epithelium stimulation in infants during this age range. Host ability to clear mucus would determine pathogenic expression.
Keywords: immunocompetent, non-specific immune response, autopsy, MUC5AC, Sudden Infant Death Syndrome (SIDS)
Abstract
Background. Pneumocystis without obvious accompanying pathology is occasionally reported in autopsied infant lungs. Its prevalence and significance are unknown. Interestingly, this mild infection induces a strong activation of mucus secretion–related genes in young immunocompetent rodents that has not been explored in infants. Excess mucus is induced by multiple airway offenders through nonspecific pathways and would explain a cofactor role of Pneumocystis in respiratory disease. We undertook characterization of the prevalence of Pneumocystis and associated mucus in infant lungs.
Methods. Samples from 128 infants (mean age, 101 days) who died suddenly and unexpectedly in Santiago during 1999–2004 were examined for Pneumocystis using nested polymerase chain reaction (nPCR) amplification of the P. jirovecii mtLSU ribosomal RNA gene and immunofluorescence microscopy (IF). Pneumocystis-negative infants 28 days and older and their age-closest positives were studied for MUC5AC expression and Pneumocystis burden by Western blot and quantitative PCR, respectively.
Results. Pneumocystis DNA was detected by nPCR in 105 of the 128 infants (82.0%) and Pneumocystis organisms were visualized by IF in 99 (94.3%) of the DNA-positive infants. The infection was commonest at 3–4 months with 40 of 41 (97.6%) infants of that age testing positive. MUC5AC was significantly increased in Pneumocystis-positive tissue specimens (P = .013). Death was unexplained in 113 (88.3%) infants; Pneumocystis was detected in 95 (84.0%) of them vs 10 of 15 (66.7%) with explained death (P = .28).
Conclusions. A highly focal Pneumocystis infection associated to increased mucus expression is almost universally present in the lungs of infants dying unexpectedly in the community regardless of autopsy diagnosis.
(See the Editorial Commentary by Eddens and Kolls, on pages 180–1.)
Most humans experience their first contact with Pneumocystis (ie, primary infection) shortly after birth [1–4]. This infection is rarely diagnosed because it is asymptomatic or may present as a mild upper respiratory infection [4–6]. Autopsy reports of Pneumocystis in infants have been available for many years [7]. However, characterization of this infection has been hampered by the lack of a microbiological culture method for Pneumocystis, by the low sensitivity of any method used to diagnose Pneumocystis pneumonia in the immunocompromised to detect the smaller quantities of this fungus in immunocompetent individuals, and because Pneumocystis cysts do not stain with the standard hematoxylin-and-eosin stain routinely used in most autopsies. Recent autopsy studies describe the focal (patchy) histological distribution [8–10] and that this infection is more frequent between the ages of 2 and 5 months [4, 5, 9–11]. This age range coincides with the most frequent age for sudden unexpected infant death (SUID) and bronchiolitis [12, 13]. However, the coverage extent of this age overlap and whether it carries any pathogenic significance for Pneumocystis are unknown. Increasing evidence shows that Pneumocystis induces a potent immune response in young immunocompetent rodents [3, 14–17], including a strong gene activation of ClCa3, a member of the calcium-activated chloride channel family of genes expressed in the goblet airway epithelial cell that relates to mucus secretion [14]. Mucus is produced constitutively by goblet cells and binds virtually all particles that land in the airway epithelium as an essential component of the mucociliary clearance system aimed to clean the airways from inhaled particles. This system comprises secretory and ciliated cells, a periciliary liquid (PCL) layer where the cilia move to impulse the mucus, and the propelled overlaying mucus [18]. The heights of the PCL and of the mucus layers need fine tuning to secure airway patency while maintaining clearance efficiency [18–20]. Excess PCL will raise the floating mucus layer, making it unreachable to cilia for propulsion, and accumulating mucus could occlude narrow, developing, and distal airways [18–21]. Mucus release is an airway defense reaction stimulated through nonspecific pathways by multiple airway offenders [19–21]. A Pneumocystis-related increase in mucus would then suggest a cofactor role for Pneumocystis in lung disease of the immunocompetent host that is nearly undetectable with current autopsy procedures. Therefore, we undertook this cross-sectional study to describe the prevalence, age distribution, and mucus-associated response to the primary infection by Pneumocystis in autopsied infant lungs.
MATERIALS AND METHODS
Ethics Review
This study was approved by the Ethics Commissions of the North Metropolitan Area of Health, and of the University of Chile School of Medicine in Santiago.
Study Population and Data Collection
The Servicio Médico Legal in Santiago is the coroners' office institution for the Metropolitan Area of Chile. A medico-legal autopsy is required for infants who have died in the community in Chile. Infant autopsies performed during calls of a thanatology specialist physician (M.G.) between 1 May 1999 and 6 July 2004 were selected for the study. Inclusion criteria were unexpected death at home, no hospital admission, no immunocompromising conditions, and normal macroscopic examination. The forensic protocol considered clinical history, macroscopic examination and dissection with histological sampling of major organs, plus laboratory tests including toxicology determinations. No bacterial or viral cultures were considered. Medical information including age, date of death, findings including lung histology report, and autopsy diagnoses were collected from the coroner's report prior to Pneumocystis analyses. Autopsy diagnoses were categorized for the purpose of this study as (1) unexplained death (no abnormal findings at autopsy, sudden infant death syndrome); (2) unexplained death with autopsy findings whose contributory role to death was uncertain; and (3) explained death, when a definitive cause of death was established. (Groups 1, 2, and 3 would correspond to SUID or sudden unexpected death in infancy [12, 13]).
Autopsy Samples
The complete right lung was carefully removed, placed in a sterile plastic bag, and transported to the investigatoŕs laboratory in an ice-pack container after obtaining legally required samples using sterile equipment. Each lung was processed at arrival, one at a time; lobes were dissected inside a biosafety cabinet using new sterile equipment as described [22]. The pleura was carefully removed to access untouched tissue using separate sterile equipment. Small samples were obtained from deep lung tissue through 2-cm-deep multiple incisions in the decorticated surface of each lobe. Specimens were cut into small pieces and distributed for nested polymerase chain reaction (nPCR) and microscopy. Lobes were processed and analyzed separately.
Samples for Pneumocystis Categorization
DNA was extracted and purified from a median of 0.172 g (mean, 0.168 g [range, 0.099–0.226 g]) of pulmonary tissue using the QIAamp DNA Mini Kit (Qiagen, Valencia, California) monitoring for cross-contamination [22]. Pneumocystis jirovecii DNA was identified by nPCR using human β-globin internal controls [22]. Standard cleaning and sterilization procedures using DNA breaking fluids (DNA Away, VWR Scientific Products) were applied to the biosafety cabinet and hood units between each lung.
Infants were categorized as Pneumocystis positive when the P. jirovecii DNA–specific 267 bp band was visualized in 1 or more specimens, and as Pneumocystis negative if no P. jirovecii DNA was documented in the 3 lobes. Pneumocystis-negative lobes were analyzed twice, starting from tissue.
Microscopy Analyses
A median of 0.396 g (mean, 0.399 g [range, 0.319–0.498 g]) of lung tissue was homogenized by magnetic stirrer agitation in sterile phosphate-buffered saline (PBS) pH 7.2 at 4°C for 30 minutes, sterile gauze filtered, centrifuged at 2900g, 10 minutes at 4°C, and the pellet was reconstituted in 700 µL of sterile PBS pH 7.2. Five-microliter drops were used for microscopy slides. Forms of Pneumocystis were identified using immunofluorescence stain (MeriFluo Kit Biosciences, Cincinnati, Ohio) in the 128 infants. Each sample was analyzed separately and blinded to nPCR results. The 3 lobes per infant were analyzed in duplicate for each lobe.
Additional Microscopy Methods
The first 36 of the 128 infant samples were additionally studied using Gomori-Grocott methenamine silver and Rapid Giemsa (Diff-Quick) staining of lung section imprints. For either microscopy technique, infants were considered “positive” when typical Pneumocystis forms were identified and agreed on by 2 observers (R.B. and C.P. or S.L.V.) in 1 or more lobes and “negative” if the 3 lobes contained no Pneumocystis. Interpretation was performed blinded to the results obtained using other techniques. Microscopy reading took up to 45 minutes per patient.
Samples for P. jirovecii and MUC5AC Quantifications
Additional lung samples (1 g) were obtained from 59 infants comprising all 20 Pneumocystis-negative infants older than 28 days, and 39 Pneumocystis-positive infants of closest possible age. Samples were flash-frozen, pulverized in liquid nitrogen using a mortar and pestle, homogenized, and frozen until quantitative PCR (qPCR) and Western blot analysis.
Pneumocystis jirovecii Quantification
DNA was extracted from a 0.4-g aliquot. The multicopy msg gene was selected as target using primers PC MSG Forward (5′-CAA AAA TAA CAY TSA CAT CAA CRA GG-3′) and PC MSG Reverse (5′-AAA TCA TGA ACG AAA TAA CCA TTG C-3′) generating a fragment of 156 bp [23] that was cloned in pGEM-T Easy vector (Promega), and used for generating a calibration curve (range of 1 × 101 to 1 × 106 copies/μL). Amplified product was detected using SYBR Green I (Quantace, Bioscan). Quantitative PCR was done in triplicate using the LightCycler 2.0 (Roche) with preincubation period of 10 minutes at 95°C and 46 cycles of 10 seconds at 95°C, 10 seconds at 53°C, and 20 seconds at 72°C each, ending with 7 minutes at 72°C. Each run included negative (ultrapure H2O) and positive (DNA from a patient with Pneumocystis pneumonia) controls and 3 different plasmid standards used in the calibration curve. The specificity of amplified products was verified by melting-curve analysis. Human β-globin gene was used as internal control and for normalization of results as described [5, 24].
Mucin Determinations
Each aliquot (0.6 g) and a gastric tissue sample (control) were disrupted using a Tissue Tearor (Biospec) in chilled RIPA-modified lysis buffer. Total protein was quantified in supernatant by Bradford (Bio-Rad). Thirty-microgram aliquots were subject to sodium dodecyl sulfate polyacrylamide gel electrophoresis (4% stacking and 8% resolving Tris-Glycine gels). Proteins were transferred to polyvinylidene difluoride membranes and blocked. Mouse anti-MUC5AC immunoglobulin G (IgG) antibody (1:500, 45M1, SCBT) and goat antimouse IgG horseradish peroxidase (HRP)–conjugated antibody (1:2000, SCBT) were used for MUC5AC detection. Membranes were stripped, blocked, and reprobed using standard antiactin antibodies (goat antiactin IgG, 1:2000, SCBT and donkey antigoat IgG HRP, 1:3000, SCBT). Enhanced chemiluminescence reagent was used for membrane development (Pierce ECL WB Substrate, Thermo Scientific). Films were analyzed with Image J software (National Institutes of Health).
Statistical Analysis
GraphPad Prism 5 software (San Diego, California) was used to compare prevalence of Pneumocystis in explained vs unexplained deaths using χ2 with Yates’ correction, Pneumocystis (MSG copies) at sequential age intervals using analysis of variance, MUC5AC expression according to Pneumocystis presence using unpaired t test with Welch's correction, and to analyze the correlation between expression of MUC5AC and Pneumocystis MSG copies using Pearson test. A P value of <.05 was considered significant.
RESULTS
Infants and Lung Sample Characteristics
A total of 669 infants (aged 3 days to 12 months) underwent a legally required autopsy at Servicio Médico Legal during the enrollment period. M.G. conducted 134 infant autopsies that fulfilled entry criteria and in which the right lung was submitted for analysis. Six newborn infants (mean age, 14.8 days; median, 17 days; range, 2–22 days) were excluded because of recent hospitalization, and 128 infants with a median age of 2 months 29 days (mean, 3 months 11 days [range, 7 days to 11 months 27 days]), 70 (54.7%) male, were considered for this study. Infants were assigned to specific diagnostic categories after autopsy completion (Table 1). Complete right lungs were obtained in 111 infants, 2 lobes in 3 and, 1 lobe in 14, respectively.
Table 1.
Detection of Pneumocystis by Nested Polymerase Chain Reaction in Homogenized Lung-Tissue Autopsy Specimens of Different Pulmonary Lobes from 128 Infants Dying Suddenly and Unexpectedly in the Community
|
Pneumocystis DNA |
||||
|---|---|---|---|---|
| Contribution to Diagnosis—Any
Lobec | ||||
| Autopsy Result | No.a | RUL | RML or RLL | Total |
| Unexplained death | 85 | 61 | 10 | 71 (83.5%) |
| Unexplained death with mild autopsy findings | 28 | 18 | 6 | 24 (85.7%) |
| Nonspecific lung inflammation | 15 | |||
| Congenital malformation (compatible with life) | 4 | |||
| Metabolic defect (hypoglycemia) | 1 | |||
| Signs of infection (mild and outside the lung) | 8 | |||
| Explained death | 15 | 6 | 4 | 10 (66.7%) |
| Bronchopneumonia | 4 | |||
| Congenital malformation (cardiac or brain) | 2 | |||
| Traumatic death | 2 | |||
| Asphyxia (immersion or food) | 2 | |||
| Systemic signs of infection (DIVC, meningitis, other) | 5 | |||
| Total | 128 | 85 (80.9%)b | 20 (19.1%)b | 105 (82.0%) |
Abbreviations: DIVC, disseminated intravascular coagulopathy; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
a Age: mean, 3 mo 11 d; median, 2 mo 29 d; range, 7 d to 11 mo 27 d.
b Percentage relative to the 105 Pneumocystis DNA–positive infants to indicate that 80.9% of positives was detected by analyzing the RUL and 19.1% additional positives by analyzing the RML or RLL specimens. For the purpose of this study, infants were considered to be negative for Pneumocystis DNA after analysis of 2 samples in each lobe.
c Prevalence of Pneumocystis DNA among unexplained vs explained deaths, P = .28.
Sensitivity of Diagnostic Techniques
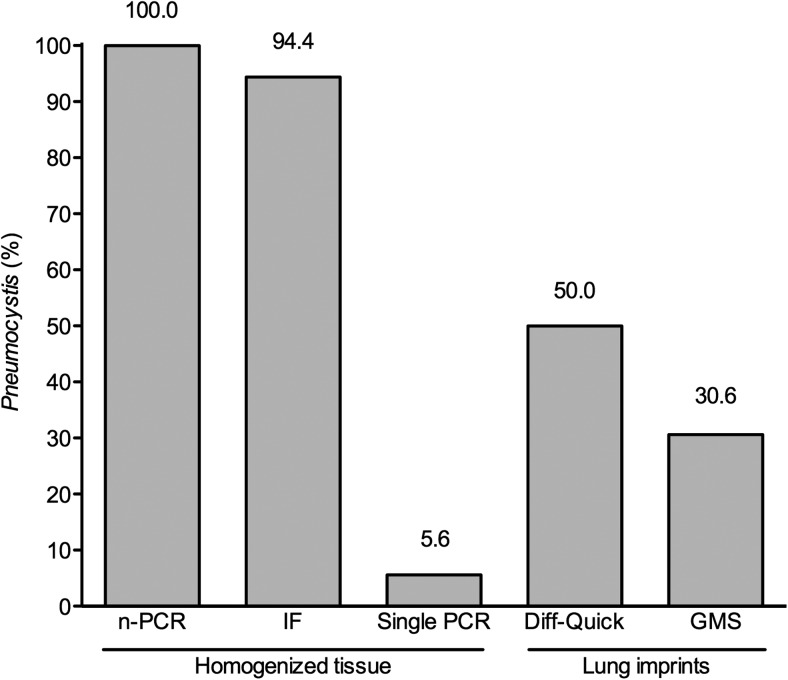
Pneumocystis jirovecii DNA was detected by nPCR in the first 36 infants studied; 34 (94.4%) of them tested positive by immunofluorescence microscopy, and 2 (5.6%) by single PCR in the same homogenized tissue aliquot. Diff-Quick and Gomori-Grocott methenamine silver stains detected Pneumocystis trophic forms in 18 (50.0%) and cyst forms in 11 (30.6%), of lung tissue imprints (Figures 1 and 2).
Figure 1.
Diagnosis of Pneumocystis in infant biopsy specimens requires sensitive techniques applied to homogenized tissue: Percentage of Pneumocystis detection as relative to nested polymerase chain reaction (n-PCR), of immunofluorescence microscopy (IF), and single-round PCR in homogenized lung tissue specimens of 36 infants. Results of microscopy readings using rapid Giemsa (Diff-Quick) and Gomori-Grocott methenamine silver (GMS) stains in imprints of cruent-cut-surface lung tissue adjacent to the sections analyzed by n-PCR and IF are also presented. Abbreviations: IF, immunofluorescence microscopy; GMS, Gomori-Grocott methenamine silver; n-PCR, nested polymerase chain reaction; PCR, polymerase chain reaction.
Figure 2.
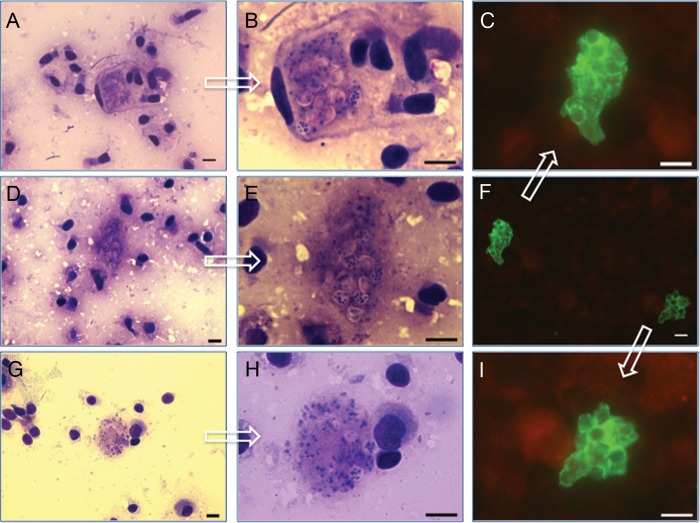
Detection of this highly focal Pneumocystis infection by microscopy examination in homogenized preparations or imprints from lung tissue specimens. Pneumocystis forms as visualized by microscopy using immunofluorescence stain in aliquots of homogenized lung biopsy specimens (F = ×400; C and I = ×1000), or by rapid Giemsa stain (Diff-Quick) in imprints from fresh lung infant autopsy specimen sections (A, D, and G = ×400; B, F, and H = ×1000). Arrows on each ×400 picture point to their ×1000 magnifications. Bar = 10μ.
DNA Amplification
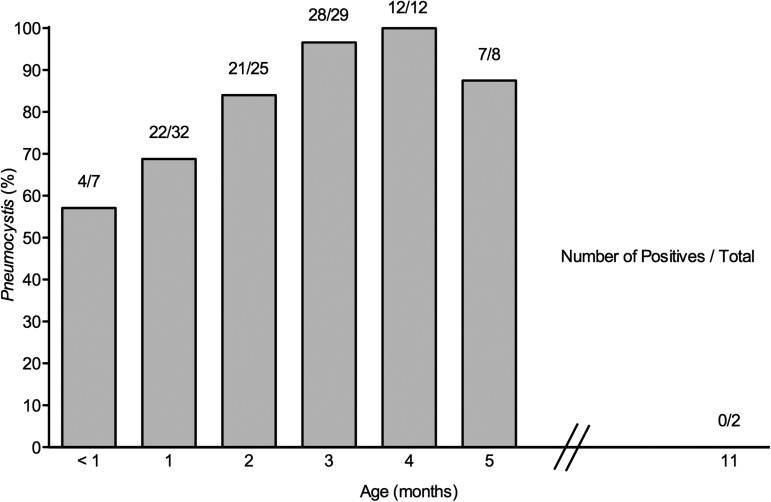
Nested PCR detected P. jirovecii DNA in 105 (82.0%) of the 128 infants: 60 (85.7%) of 70 male and 45 (77.6%) of 58 female infants. Pneumocystic jirovecii DNA was detected in 88 (79.3%) of 111 infants having their 3 lobes analyzed; of them, 35 (39.8%), 21 (23.9%), and 32 (36.3%) had detectable P. jirovecii DNA in 3, 2, or 1 lobes, respectively. The first analysis detected 80 (94%) of the 85 infants whose right upper lobe (RUL) was P. jirovecii DNA positive (Table 1). Pneumocystis jirovecii DNA was detected in 4 of 7 infants < 1 month of age (Figure 3). All amplification reactions of controls for contamination of DNA extraction and purification were negative.
Figure 3.
Pneumocystis jirovecii infection in autopsied infant lungs peaks at 3–5 months. Lung autopsy specimens from 128 infants dying in the community were analyzed for P. jirovecii using nested polymerase chain reaction (nPCR) and immunofluorescence microscopy (IF). P. jirovecii DNA was detected in 105 (82.0%), and Pneumocystis forms were confirmed by IF in 99 (94.2%) of those found positive for P. jirovecii DNA by nPCR and in 0 of 23 infants who tested negative. Each bar represents a minimum of 5 infants. Pneumocystis was additionally detected in 4 of 4, 2 of 2, 2 of 3, 2 of 3, 1 of 1, and 0 of 2 infants dying at 6, 7, 8, 9, 10, and 11 months of age, respectively.
Microscopy Analyses
Lung homogenate specimens from the 128 infants were analyzed by immunofluorescence microscopy in addition to nPCR, and cystic plus smaller trophic Pneumocystis forms were detected in 99 (94.3%) of 105 infants testing positive by nPCR. Immunofluorescence was negative in all 23 infants who were Pneumocystis DNA negative by nPCR (Table 1; Figure 2).
Pneumocystis Quantification
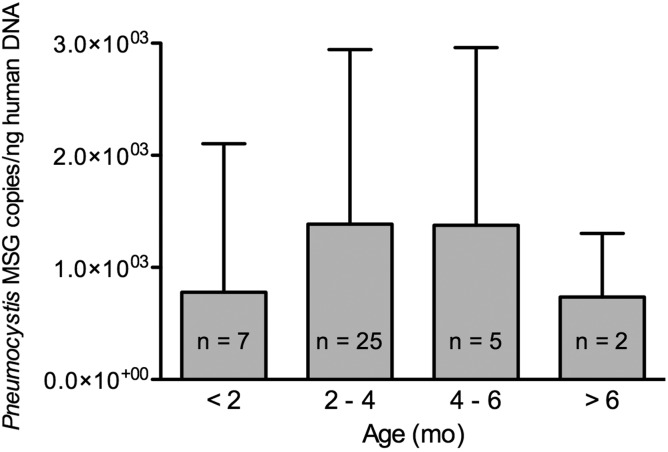
Pneumocystis normalized counts (MSG copies per nanogram of human DNA) were higher between 2 and 5 months and declined thereafter (P = .7630) (Figure 4).
Figure 4.
Pneumocystis organisms burden increases up to 3–5 months of infant age and declines thereafter. Age progression of Pneumocystis organisms load in autopsy lung samples from 39 infants dying suddenly in the community is shown. Pneumocystis MSG quantitative polymerase chain reaction results were normalized to nanograms of human β-globin DNA for comparisons and expressed as the normalized mean of Pneumocystis MSG copies ± SD.
MUC5AC Determinations
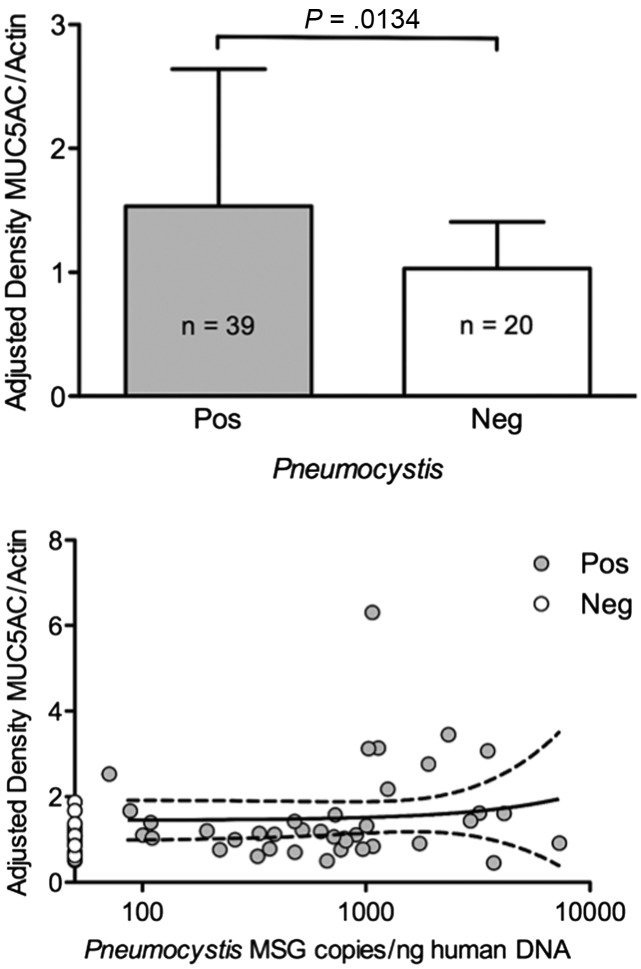
Normalized levels of MUC5AC were significantly increased (P = .0134) in association with the presence of Pneumocystis (Figure 5). This increase was consistent at all age intervals (data not shown), and independent of Pneumocystis burden (Pearson r = 0.0908; P = .5822). MUC5AC determination values were normalized by human actin protein expression, and Pneumocystis MSG determinations by human β-globin levels (mean ± SD).
Figure 5.
Mucus (MUC5AC) expression is increased by Pneumocystis presence and not influenced by organism load. Top: MUC5AC protein expression according to Pneumocystis status in lung tissue specimens from 39 P. jirovecii–positive and 20 P. jirovecii–negative infants (mean ± SD). Bottom: Correlation between normalized MUC5AC protein expression and normalized quantification values of P. jirovecii MSG in the same lung sample specimen for each infant (Pearson r = 0.0908, P = .5822). MUC5AC level values were normalized by human actin protein expression, and Pneumocystis MSG determinations by human β-globin levels (mean ± SD). Abbreviation: MUC5AC, mucus.
DISCUSSION
This study confirms Pneumocystis as the most prevalent microorganism in autopsied infant lungs identified to date, and that Pneumocystis presence associates to increased mucus (MUC5AC) expression, suggesting that it increases the mucociliary clearance workload and upregulates innate immune responses in the airway epithelium [19–21].
Pneumocystis cells and P. jirovecii–specific DNA were identified in the lungs of nearly all infants in this study using immunofluorescence microscopy and nPCR, respectively. This high prevalence is consistent with previous evidence that Pneumocystis is common in infant lungs [9, 10] including a study documenting Pneumocystis DNA by nPCR in all of 58 infants of undisclosed age [25]. Furthermore, the structural forms of the fungus were all recognized using Giemsa and GMS stains, suggesting active replication [26].
The comprehensive diagnostic approach utilized in this study, including examination of up to 6 fresh homogenized tissue samples per infant, increased the sensitivity of detection and underlines the focal distribution of Pneumocystis in the nonimmunocompromised host [8, 9]. This approach detects smaller burdens of Pneumocystis than present in immunocompromised patients with Pneumocystis pneumonia, where the fungus is readily diagnosable by microscopy or single-round PCR. Pneumocystis burden in these infant lungs, although mild, was greater than in immunocompetent adults where diagnosis additionally requires of tissue-concentration techniques [22].
In addition, results show that the age peak with approximately 90% of infants having detectable Pneumocystis, and the higher normalized burden of organisms, coincide at 2–5 months. This age predominance was suggested in previous studies [9–11] and matches the age of onset of severe Pneumocystis pneumonia in immunosuppressed or debilitated infants prior to anti-Pneumocystis prophylaxis [27, 28]. Importantly, young age is by itself a risk factor for Pneumocystis severity exemplified by the worse prognosis of HIV-related Pneumocystis pneumonia in infants whose mortality is 60% vs 10% in adults [27, 28].
This study also documents that Pneumocystis is associated with increased mucus production. Mucus is a gel composed by water (97%) and solids including mucins (3%) [19, 20]. MUC5AC, the gel-forming mucin used as a marker of mucus in this study, is the predominant solid component of mucus in infant airways [29]. Increased normalized levels of MUC5AC have been similarly documented in association with many other well-recognized, less prevalent airway offenders like respiratory viruses, bacteria, acetyl choline, cytokines, prostaglandins, lipopolysaccharides, nitric oxide, and other potential activators of nonspecific airway signaling pathways as the ErbB receptor epidermal growth factor receptor (EGFR) [20, 21]. Additional airway offenders were not studied. MU5AC was consistently increased in Pneumocystis-positive infants at all age intervals, suggesting that Pneumocystis predisposes the host to augmented mucus responses during this age period [19–21], and was unaffected by Pneumocystis burden in agreement with the concept that pathogenesis for Pneumocystis is mostly host dependent [6, 27, 30].
Pathogenically, mucins are heavily glycosylated proteins stored in packaging intracellular granules [31]. Their release in response to airway insults is followed by immediate mucin hydration leading to several hundred-fold intraluminal volume increase in milliseconds [19–21, 31]. This mechanism could represent a risk for narrow, developing infant airways because minor height volume changes in the airway surface liquid can lead to small airway closure in times as short as a breathing cycle [32]. The clinical outcome of increased mucus depends on several factors affecting clearance including airway surface tension, geometry, size, and effective cough [32, 33]. Infants have airways of small diameter, with greater elasticity and compliance, fewer collateral airway channels, and a reduced functional residual capacity, compared with older children or adults [34]. In addition, mucins in infants are more acidic that may reflect greater viscosity [29, 34]. The presence of Pneumocystis could therefore favor airway collapse suggested as a mechanism in current hypotheses for SUID [35, 36]. This may occur with few clinical manifestations until most of the peripheral airways are occluded [21]. Airway collapse would be challenging to diagnose at autopsy as it may immediately resolve with postmortem airway relaxation. In addition, gravitational orientation of the lungs and the release of transpulmonary pressure upon opening the thorax may mobilize airway secretions and further decrease autopsy evidence.
Pneumocystis is common in the general population at any age. Therefore, Pneumocystis-associated mucus increase may also be relevant for chronic respiratory diseases such as chronic obstructive pulmonary disease and cystic fibrosis in which the coexistence of mucus excess and Pneumocystis is described [19, 37, 38].
Other pathways increase mucin in addition to the EGFR in the ErbB family of receptors, and include tumor necrosis factor α, STAT6, interleukin 1β, interleukin 13, and NF-κB and may be activated by Pneumocystis [16, 17, 30, 39]. In addition, Pneumocystis may induce collateral sensitization to a nonspecific antigen in immunocompetent mice, increasing the number of CD45+CD11c+ antigen-presenting cells that explain an hyper-reactive response upon a later challenge [16]. An airway hyperreactive response can explain airway collapse as documented in sensitized mice [40]. This type of response may be relevant to SUID and infant bronchiolitis whose peak incidences coincide with the age peak of Pneumocystis [12, 13, 35, 41].
This autopsy study was conducted in sudden unexpected infant deaths. This is the most frequent form of death in apparently healthy, nonimmunocompromised infants [12]. Pneumocystis prevalence was not different in infants with unexplained vs explained deaths in this study, in agreement with a previous study documenting a similar incidence of Pneumocystis in infants with unexplained deaths vs in those of similar age dying of accidental causes, confirming that Pneumocystis is not sufficient to cause SUID [11]. The high prevalence of Pneumocystis in SUID, however, raises the possibility that Pneumocystis may be a “necessary but not sufficient” cause of SUID as coadjuvant to diverse nonspecific triggers acting on top of Pneumocystis.
Pathology reports in this study showed that inflammation was absent or too mild to explain infant deaths through inflammatory mechanisms, as in previous autopsy series [9]. Autopsy signs of a mild respiratory infection that per se does not explain death are present in approximately half of SUID cases [12]. The lack of evident inflammation in these infants can be explained by death occurring before inflammation develops, or by other reasons including focality of the infection [14]. Animal models demonstrate that the sequence of events leading to lymphocytic response is well demarcated [14, 42], and delayed during low-burden infections such as this one, until Pneumocystis multiplies and is able to induce the transient inflammation that eliminates the pathogen in the immunocompetent host [30].
Airway collapse may be favored by increased mucus and could explain death in a proportion of these infants [35, 36], suggesting that prevention of Pneumocystis-associated mucus increase until the airway is more developed could reduce vulnerability to SUID and, eventually, to bronchiolitis.
Pneumocystis is the most prevalent microorganism in the lungs of small infants. Pneumocystis-associated mucus increase may also be relevant to older children or adults with respiratory conditions associated with Pneumocystis and increased mucus.
Notes
Acknowledgments. We recognize the dedicated work of Isabella Eyzaguirre Valderas and Maria Antonieta Perez Concha as part of their DVM graduate thesis, and thank Drs Jaime Fergie and Mauricio Henríquez for critical review of the manuscript. We are especially grateful to Dr Walter T. Hughes for his constant advice and for his critical review of the manuscript.
Author contributions. S. L. V. was responsible for the hypothesis, literature search, and writing the manuscript; S. L. V., C. A. P., F. P., J.-F. A., R. B., M. C., I. D.-J., P. I., E.-M. A., and E. D.-C. designed the categorization of lung specimens part of the study; S. L. V., C. A. P., F. P., J.-F. A., and R. B. designed the mucus part of the study; M. G. performed the autopsies; C. A. P., M. G., F. P., J.-F. A., R. B., and S. L. V. performed the determinations; S. L. V., C. A. P., F. P., R. F. M., and P. I. analyzed and interpreted the data; R. F. M., C. A. P., F. P., J.-F. A., R. B., M. C., M. G., I. D.-J., P. I., E.-M. A., and E. D. C. critically revised the paper. S. L. V. is the guarantor of the study.
Financial support. This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT-Chile) (grant numbers 1060750 and 1100225 to S. L. V.). Collaboration between French and Chilean groups was supported by the French cooperation Ministry and by ECOS-CONICYT (travel grant C05S02 to E. D.-C. and S. L. V.). Collaboration with R. F. M. was supported by Visiting Professorship funds included in FONDECYT-Chile (grant number 1100225 to S. L. V.) and by travel funds from University College London (to R. F. M.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Vestereng VH, Bishop LR, Hernandez B, Kutty G, Larsen HH, Kovacs JA. Quantitative real-time polymerase chain-reaction assay allows characterization of Pneumocystis infection in immunocompetent mice. J Infect Dis. 2004;189:1540–4. doi: 10.1086/382486. [DOI] [PubMed] [Google Scholar]
- 2.Icenhour CR, Rebholz SL, Collins MS, Cushion MT. Early acquisition of Pneumocystis carinii in neonatal rats as evidenced by PCR and oral swabs. Eukaryot Cell. 2002;1:414–9. doi: 10.1128/EC.1.3.414-419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soulez B, Dei-Cas E, Charet P, Mougeot G, Caillaux M, Camus D. The young rabbit: a nonimmunosuppressed model for Pneumocystis carinii pneumonia. J Infect Dis. 1989;160:355–6. doi: 10.1093/infdis/160.2.355. [DOI] [PubMed] [Google Scholar]
- 4.Vargas SL, Hughes WT, Santolaya ME, et al. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin Infect Dis. 2001;32:855–61. doi: 10.1086/319340. [DOI] [PubMed] [Google Scholar]
- 5.Larsen HH, von Linstow ML, Lundgren B, Hogh B, Westh H, Lundgren JD. Primary Pneumocystis infection in infants hospitalized with acute respiratory tract infection. Emerg Infect Dis. 2007;13:66–72. doi: 10.3201/eid1301.060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas CF, Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–98. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 7.Sheldon WH. Subclinical Pneumocystis pneumonitis. AMA J Dis Child. 1959;97:287–97. doi: 10.1001/archpedi.1959.02070010289005. [DOI] [PubMed] [Google Scholar]
- 8.Morgan DJ, Vargas SL, Reyes-Mugica M, Walterspiel JN, Carver W, Gigliotti F. Identification of Pneumocystis carinii in the lungs of infants dying of sudden infant death syndrome. Pediatr Infect Dis J. 2001;20:306–9. doi: 10.1097/00006454-200103000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Vargas SL, Ponce CA, Hughes WT, et al. Association of primary Pneumocystis carinii infection and sudden infant death syndrome. Clin Infect Dis. 1999;29:1489–93. doi: 10.1086/313521. [DOI] [PubMed] [Google Scholar]
- 10.Vargas SL, Ponce CA, Luchsinger V, et al. Detection of Pneumocystis carinii f. sp. hominis and viruses in presumably immunocompetent infants who died in the hospital or in the community. J Infect Dis. 2005;191:122–6. doi: 10.1086/426451. [DOI] [PubMed] [Google Scholar]
- 11.Vargas SL, Ponce CA, Galvez P, et al. Pneumocystis is not a direct cause of sudden infant death syndrome. Pediatr Infect Dis J. 2007;26:81–3. doi: 10.1097/01.inf.0000247071.40739.fd. [DOI] [PubMed] [Google Scholar]
- 12.Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber MA, Klein NJ, Hartley JC, Lock PE, Malone M, Sebire NJ. Infection and sudden unexpected death in infancy: a systematic retrospective case review. Lancet. 2008;371:1848–53. doi: 10.1016/S0140-6736(08)60798-9. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Novoa B, Bishop L, Logun C, et al. Immune responses to Pneumocystis murina are robust in healthy mice but largely absent in CD40 ligand-deficient mice. J Leukoc Biol. 2008;84:420–30. doi: 10.1189/jlb.1207816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livingston RS, Besch-Williford CL, Myles MH, Franklin CL, Crim MJ, Riley LK. Pneumocystis carinii infection causes lung lesions historically attributed to rat respiratory virus. Comp Med. 2011;61:45–59. [PMC free article] [PubMed] [Google Scholar]
- 16.Swain SD, Meissner N, Han S, Harmsen A. Pneumocystis infection in an immunocompetent host can promote collateral sensitization to respiratory antigens. Infect Immun. 2011;79:1905–14. doi: 10.1128/IAI.01273-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swain SD, Meissner NN, Siemsen DW, McInnerney K, Harmsen AG. Pneumocystis elicits a STAT6-dependent, strain-specific innate immune response and airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2012;46:290–8. doi: 10.1165/rcmb.2011-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–7. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–47. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers DF. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir Care. 2007;52:1134–46. discussion 1146–9. [PubMed] [Google Scholar]
- 21.Burgel PR, Nadel JA. Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. Eur Respir J. 2008;32:1068–81. doi: 10.1183/09031936.00172007. [DOI] [PubMed] [Google Scholar]
- 22.Ponce CA, Gallo M, Bustamante R, Vargas SL. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population. Clin Infect Dis. 2010;50:347–53. doi: 10.1086/649868. [DOI] [PubMed] [Google Scholar]
- 23.Larsen HH, Masur H, Kovacs JA, et al. Development and evaluation of a quantitative, touch-down, real-time PCR assay for diagnosing Pneumocystis carinii pneumonia. J Clin Microbiol. 2002;40:490–4. doi: 10.1128/JCM.40.2.490-494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandt D, Monecke S. Development and evaluation of a real-time PCR assay for detection of Pneumocystis jiroveci. Transpl Infect Dis. 2007;9:196–202. doi: 10.1111/j.1399-3062.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 25.Beard CB, Fox MR, Lawrence GG, et al. Genetic differences in Pneumocystis isolates recovered from immunocompetent infants and from adults with AIDS: epidemiological Implications. J Infect Dis. 2005;192:1815–8. doi: 10.1086/497381. [DOI] [PubMed] [Google Scholar]
- 26.Chabe M, Vargas SL, Eyzaguirre I, et al. Molecular typing of Pneumocystis jirovecii found in formalin-fixed paraffin-embedded lung tissue sections from sudden infant death victims. Microbiology. 2004;150:1167–72. doi: 10.1099/mic.0.26895-0. [DOI] [PubMed] [Google Scholar]
- 27.Leibovitz E, Rigaud M, Pollack H, et al. Pneumocystis carinii pneumonia in infants infected with the human immunodeficiency virus with more than 450 CD4 T lymphocytes per cubic millimeter. N Engl J Med. 1990;323:531–3. doi: 10.1056/NEJM199008233230807. [DOI] [PubMed] [Google Scholar]
- 28.Simonds RJ, Oxtoby MJ, Caldwell MB, Gwinn ML, Rogers MF. Pneumocystis carinii pneumonia among US children with perinatally acquired HIV infection. JAMA. 1993;270:470–3. [PubMed] [Google Scholar]
- 29.Rogers DF. Pulmonary mucus: pediatric perspective. Pediatr Pulmonol. 2003;36:178–88. doi: 10.1002/ppul.10322. [DOI] [PubMed] [Google Scholar]
- 30.Gigliotti F, Wright TW. Immunopathogenesis of Pneumocystis carinii pneumonia. Expert Rev Mol Med. 2005;7:1–16. doi: 10.1017/S1462399405010203. [DOI] [PubMed] [Google Scholar]
- 31.Verdugo P. Mucin exocytosis. Am Rev Respir Dis. 1991;144:S33–7. doi: 10.1164/ajrccm/144.3_pt_2.S33. [DOI] [PubMed] [Google Scholar]
- 32.Heil M, Hazel AL, Smith JA. The mechanics of airway closure. Respir Physiol Neurobiol. 2008;163:214–21. doi: 10.1016/j.resp.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Widdicombe JG. Neurophysiology of the cough reflex. Eur Respir J. 1995;8:1193–202. doi: 10.1183/09031936.95.08071193. [DOI] [PubMed] [Google Scholar]
- 34.Schechter MS. Airway clearance applications in infants and children. Respir Care. 2007;52:1382–90. discussion 1390–1. [PubMed] [Google Scholar]
- 35.Martinez FD. Sudden infant death syndrome and small airway occlusion: facts and a hypothesis. Pediatrics. 1991;87:190–8. [PubMed] [Google Scholar]
- 36.Poets CF, Samuels MP, Southall DP. Potential role of intrapulmonary shunting in the genesis of hypoxemic episodes in infants and young children. Pediatrics. 1992;90:385–91. [PubMed] [Google Scholar]
- 37.Morris A, Sciurba FC, Lebedeva IP, et al. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am J Respir Crit Care Med. 2004;170:408–13. doi: 10.1164/rccm.200401-094OC. [DOI] [PubMed] [Google Scholar]
- 38.Morris A, Wei K, Afshar K, Huang L. Epidemiology and clinical significance of Pneumocystis colonization. J Infect Dis. 2008;197:10–7. doi: 10.1086/523814. [DOI] [PubMed] [Google Scholar]
- 39.Lai H, Rogers DF. New pharmacotherapy for airway mucus hypersecretion in asthma and COPD: targeting intracellular signaling pathways. J Aerosol Med Pulm Drug Deliv. 2010;23:219–31. doi: 10.1089/jamp.2009.0802. [DOI] [PubMed] [Google Scholar]
- 40.Lundblad LK, Thompson-Figueroa J, Allen GB, et al. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med. 2007;175:768–74. doi: 10.1164/rccm.200610-1410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nevez G, Totet A, Pautard JC, Raccurt C. Pneumocystis carinii detection using nested-PCR in nasopharyngeal aspirates of immunocompetent infants with bronchiolitis. J Eukaryot Microbiol. 2001;48(Suppl 1):122S–3S. doi: 10.1111/j.1550-7408.2001.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 42.Chen W, Mills JW, Harmsen AG. Development and resolution of Pneumocystis carinii pneumonia in severe combined immunodeficient mice: a morphological study of host inflammatory responses. Int J Exp Pathol. 1992;73:709–20. [PMC free article] [PubMed] [Google Scholar]