SUMMARY
Background
A 57 years old male had been diagnosed with grade III/IV glioblastoma multiforme. The patient had then enrolled in an adoptive cellular immunotherapy trial. The trial involved infusion of ex vivo expanded autologous cytolytic CD8+ T cells (CTLs), genetically engineered to express the interleukin-13 zetakine gene (therapeutic gene, encoding a receptor protein that targets the T cells to the tumor cells), and the Herpes Simplex virus 1 thymidine kinase (HSV1-tk) suicide gene/positron emission tomography (PET) imaging reporter gene.
Investigations
Whole-body and brain PET scan with 9-[4-[18F]Fluoro-3-(hydroxymethyl)butyl]guanine ([18F]FHBG) to detect HSV1-tk expressing CTLs, and safety monitoring following injection of [18F]FHBG.
Diagnosis
Magnetic resonance imaging detection of grade III/IV glioblastoma multiforme plus recurrence of two tumors after resection of the initial tumor.
Management
Surgical resection of original glioblastoma tumor, enrollment in CTL therapy trial, re-resection of glioma recurrence, infusion of approximately 1 X 109 CTL into the site of tumor re-resection, and [18F]FHBG PET scan to detect infused CTLs.
THE CASE
A 57 years old caucasian male had been diagnosed with grade IV glioblastoma multiforme (GBM) on March 2005. The patient was enrolled in an FDA authorized (BB-IND 10109) adoptive cellular gene immunotherapy (ACGT) trial at City of Hope National Medical Center (COHNMC IRB#01020, See Inclusion and Exclusion Criteria in Supplementary Information). Leukapheresis was initiated after obtaining informed consent and following completion of the primary therapy. The leukapheresis product was transferred to COHNMC’s T cell production facility to initiate T cell cultures.
Nine months after initial diagnosis of GBM a recurrent tumor adjacent to the resection cavity was detected by MRI. The recurrent tumor was resected and a Rickham reservoir was inserted to allow infusion of genetically engineered autologous CD8+ cytolytic T cells (CTL). T cells were isolated from the patient’s peripheral blood mononuclear cells and electroporated, delivering a plasmid DNA construct encoding IL-13 zetakine and Hygromycin/Herpes Simplex virus 1 thymidine kinase (HSV1-tk) genes under the transcriptional control of a modified human Elongation Factor-1α (EF-1α) promoter and the cytomegalovirus (CMV) immediate/early promoter, respectively in a cell production facility at COHNMC. Hygromycin resistant CTLs were cloned in limiting dilution than expanded using the REM method to numbers in excess of 109 and cryopreserved. Following diagnosis of relapse cryopreserved cells were thawed, expanded and formulated for intracranial infusion in 2cc of preservative-free normal saline (PFNS). These cells were infused over a period of 5 weeks on Mondays, Wednesdays and Fridays, with a break on week 3 (Refer to supplementary information). The patient started with a cell dose of 1 X 107. Since he tolerated that dose well, his cell infusion increased to 1 X 108 per day. By the end of the CTL infusions the patient had received approximately 1 X 109 genetically engineered autologous CTLs (Refer to supplementary data for quality assurance analysis of infused CTLs). During the initial course of therapy an enhancing lesion evolved in the posterior corpus callosum in the contralateral hemisphere. This lesion was biopsy proven GBM and the patient received additional focal radiation therapy, avastin and BCNU. Upon further progression the patient received a series of intralesional T cell doses. 14 weeks thereafter MRI revealed a major tumor regression. The patient survived 14-months from the time of initial recurrence. During the T-cell therapy no serious unexpected adverse events were encountered and the major complaint was expected intermittent headache.
Three days after completion of 5-week CTL infusions the patient had an investigational positron emission tomography (PET) scan to detect the CTLs within his body. The CTLs were imaged with the PET reporter probe 9-[4-[18F]Fluoro-3-(hydroxymethyl)butyl]guanine ([18F]FHBG), because they constitutively express the PET reporter gene (PRG) HSV1-tk.1 [18F]FHBG is approved by the FDA as an investigational new drug (IND #61,880) for PET imaging at UCLA and Stanford University nuclear medicine clinics. UCLA’s medical internal review board (M-IRB) has approved [18F]FHBG PET imaging in normal volunteers, glioma patients and glioma patients who are enrolled in adoptive cellular gene therapy, when the infused cells express the PRG HSV1-tk. Stanford University’s M-IRB has approved [18F]FHBG PET imaging in glioma patients. COHNMC’s M-IRB has approved referral of the patient’s enrolled in the CTL therapy study for [18F]FHBG PET imaging at UCLA.
The patient gave informed consent and came to UCLA Nuclear Medicine clinic, where he was first administered a mini-mental status exam (MMSE) and a urine sample was collected for baseline urine-analysis. Two intravenous (iv) lines were inserted, one into each arm, from one of which was collected 2 ml blood for baseline CBC and 5 ml blood for baseline chemistry analysis. The patient’s baseline vital signs, including temperature, heart rate, blood pressure, blood oxygen %, respiratory rate and electrocardiogram were recorded. With the exception of EKG, which was recorded at approximately every 15 minutes (up to 2-hours) after [18F]FHBG injection, the other vital signs were recorded at approximately 5, 10, 15, 30, 60 and 120 minutes after [18F]FHBG injection. All of these vital signs were again measured and recorded the day after and one week after the imaging session. Furthermore, blood and urine was collected on those follow-up days for laboratory tests. These measurements are all included as supplementary data. Finally, another MMSE was taken a week after [18F]FHBG injection to rule out any effect on cognitive functions. The patient’s MMSE score was 25 at both baseline and follow-up.
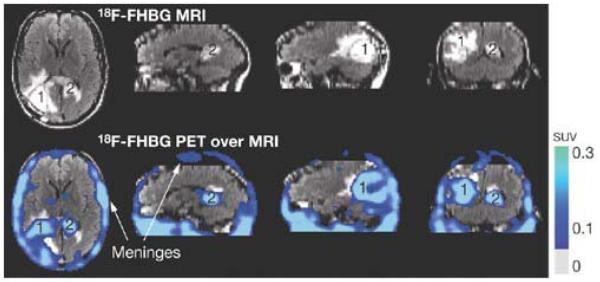
We injected 254.62 MBq (6.88 mCi) [18F]FHBG through the other iv line (FDA limit 7 mCi). At approximately two-hours and fifteen minutes after [18F]FHBG injection, the whole-body PET emission scan started from the patient’s head to bottom, using an ECAT EXACT HR+ PET scanner (CTI/Siemens, Inc., Knoxville, TN). The whole-body scan consisted of 7 bed positions (7-minutes each). Three minute transmission scans followed each 7 minute emission scans. A two bed position head scan immediately followed completion of the whole-body scan. Figure 1 shows enhanced [18F]FHBG accumulation within the tumor resection site, where CTLs had been infused. In addition, enhanced [18F]FHBG accumulation was observed near the patient’s corpus callosum. This indicates trafficking of infused CTLs to the remote corpus callosum tumor. The level of [18F]FHBG accumulation is greater than what we have observed in control GBM patients who had tumor resections or intact tumors. Table 1 includes quantitative data of tumor to background ratios, comparing [18F]FHBG accumulation levels of the CTL infused patient described in this report and control patients. Relative to similar controls [18F]FHBG accumulation is 2.6X higher in the patient’s tumor resection site and 2.8X higher in the remote corpus callosum tumor site. Cell culture uptake assays of the patient’s CTLs later confirmed their HSV1-tk expression, consistent with previous in vitro assays confirming CTL’s susceptibility to Ganciclovir induced cell death. Finally, a stereotactic biopsy of the left sided corpus callosum within a week of the [18F]FHBG PET scan confirmed the presence of malignant disease infiltrated with CD8+ T cells.
Figure 1.
MRI and PET over MRI superimposed brain images of the patient who had been infused autologous cytolytic T cells expressing IL13 zetakine and HSV1-tk genes. Images were acquired approximately two hours after [18F]FHBG injection. The patient had a surgically ressected tumor (1) in the left corner and a new non-ressected tumor in the center (2), near corpus callosum of his brain. The infused cells had localized at the site of tumor 1 and also trafficked to tumor 2. [18F]FHBG activity is higher than the brain background at both sites. Background [18F]FHBG activity is low within the Central Nervous System due to its inability to cross the blood brain barrier. Background activity is relatively higher in all other tissues. Activity can also be observed in the meninges. The tumor 1/meninges and tumor 2/meninges [18F]FHBG activity ratio in this patient was 1.75 and 1.57, respectively. Whereas the average resected tumor site/meninges and intact tumor site to meninges [18F]FHBG activity ratio in control patients was 0.86 and 0.44, respectively.
Table 1.
[18F]FHBG Activity Ratios of Tumors Over Other Tissues
| RATIOS | Patient with resected side tumor and intact center tumor, infused with CTLs (n=1) |
Patient with intact side and center tumor without CTL infusions (n=1)* |
Patients with resected side tumors, without CTL infusions (n=3)** |
|---|---|---|---|
| Side Tumor/ Brain BKGD |
4.82 | 1.54 | 1.82 |
| Side Tumor/ Meninges |
1.75 | 0.44 | 0.86 |
| Side Tumor/ Heart | 0.83 | 0.57 | 0.38 |
| Side Tumor/ Liver | 0.14 | 0.08 | 0.08 |
| Center Tumor/ Brain BKGD |
4.33 | 1.55 | |
| Center Tumor/ Meninges |
1.57 | 0.44 | |
| Center Tumor/ Heart |
0.74 | 0.57 | |
| Center Tumor/ Liver |
0.13 | 0.08 |
He was a control patient with two glioblastoma tumors that had not be surgically removed. This patient was scanned after receiving 7 mCi of [18F]FHBG.
Three control patients with surgically removed glioblastoma tumors, each were scanned with PET two hours after 4.2 mCi, 1.9 mCi and 5.6 mCi of [18F]FHBG injection.
DISCUSSION OF DIAGNOSIS
The patient was in his usual state of good health until December 2004 when he experienced his first grand mal seizure. The initial work up following neurologic stabilization with Dilantin, failed to reveal a causative etiology. Brain imaging revealed a 2 cm enhancing right occipital mass consistent in extra-axial location and imaging attributes with a meningioma. The patient was diagnosed with an idiopathic seizure disorder; due to bone pain he was switched to Lamictal and had further seizure activity. In March 2005 the patient experienced new headaches with physical exertion and had an MRI for evaluation. The scan revealed a new small (currently available records do not document size) but enhancing mass in the right parietal occipital cortex that surgical exploration revealed was GBM, which was gross totally resected. The patient recovered without new neurological deficits and was started on radiation therapy, with an initial larger field receiving 50 Gray and a smaller coned down field in the involved region receiving 60 Gray of radiation exposure. The patient was administered 75 mg/m2 daily doses of Temodar during radiation treatment followed by 6 months of adjuvant Temodar (200 mg/m2/day X 5 days every 4-weeks).
TREATMENT AND MANAGEMENT
This case describes the only cancer patient, being treated with genetically modified cytolytic T cells that express HSV1-tk/Hygromycin (HyTK), who has to our knowledge ever been imaged with [18F]FHBG. In fact, this is the first report of imaging therapeutic cells in a human, using a reporter gene/probe technology. Previously, dendritic cells were imaged in human patients by directly labeling them ex vivo with superparamagnetic iron oxide particles or 111In-Oxine.2, 3 Our patient was enrolled in an ACGT clinical trial, receiving autologous CTLs expressing IL-13 zetakine and HSV1-tk, following surgical removal of his GBM tumor recurrence. IL-13 zetakine specifically targets CTLs to kill residual glioblastoma cells.4 HSV1-tk serves two purposes. As a safety gene, HSV1-tk expressing CTLs, exposed to the drug Ganciclovir,undergo programmed cell death. The HSV1-TK enzyme can also mono-phosphorylate [18F]FHBG, which can be used to image cells expressing the HSV1-tk PRG in living animals and humans using PET.1
There are three currently conceived techniques for non-invasively imaging therapeutic cells in humans. The most conventional method is direct labeling of cells ex vivo with radionuclide or MRI probes, such as Indium-111 Oxine or Feridex (Berlex Laboratories, Wayne, NJ, USA). Easy implementation, reduced whole-body radiation exposure (radionuclide probes) and lowsignal to background ratio (if cells do not release the probe inside the patient) are these technique’s advantages.. Disadvantages are potential false positive images about cell location, lack of accurate information about cell survival, probe dilution after cell division and radionuclide probes’ activity decay; hence this is primarily a short term monitoring technique. Another approach is detecting therapeutic cells with a very specific probe for a receptor found only on their surface. This is not a general method, requiring development of specific imaging probes for potentially every type of therapeutic cells. Even then, sensitivity may be low. However, this is a long-term monitoring technique and can potentially allow imaging cell survival. We employed the reporter gene/probe based imaging technique. This technique requires stable incorporation of a radionuclide based or MRI reporter gene, regulated by a strong constitutive promoter into therapeutic cells, prior to administration into patients. The reporter probe is then injected anytime thereafter to image therapeutic cell location(s) and survival, providing a general solution for long-term cell monitoring. Genetic modification of therapeutic cells without significantly affecting the cell’s characteristics may be challenging. The sensitivity of this technique is determined by the reporter probe’s pharmacokinetics and the level of reporter gene expression per cell.
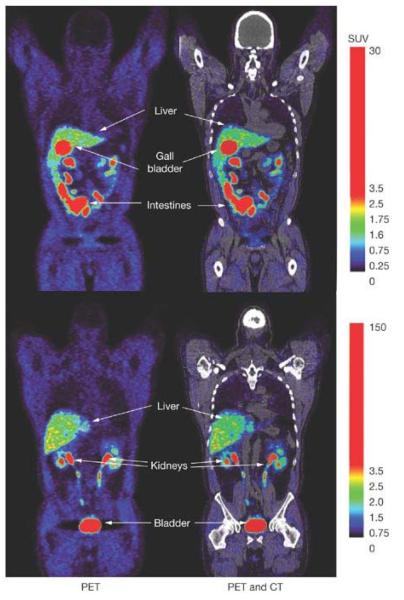
We have investigated [18F]FHBG in normal human volunteers5 and are now concurrently studying it in glioma patients without ACGT. We have also comprehensively assessed FHBG’s safety in rats and rabbits.6 Figure 2 illustrates two coronal slices of a control patient’s whole-body [18F]FHBG biodistribution PET image. The highest [18F]FHBG activity can be observed in organs involved in its clearance (bladder, kidney, ureters, liver, gall bladder and intestines). [18F]FHBG clears very rapidly from all other tissues. One always observes a sharp exponential decline of [18F]FHBG activity in both patient’s (representative graph in supplementary data) and normal human volunteer’s5 blood through time. [18F]FHBG does not cross the blood brain barrier (BBB), however we do observe a slight increase in [18F]FHBG background accumulation within intact glioma tumors or tumor resection sites of control patients (glioma patient’s who were not administered CTLs), perhaps due to a compromised BBB. However, quantitative analysis shows greater than two times higher [18F]FHBG accumulations in the resection site and intact tumor of the CTL infused patient.
Figure 2.
Whole-body PET and PET/CT images of [18F]FHBG biodistribution in a human, two hours after it’s intravenous injection. Two coronal slices are shown to illustrate activity within the liver, gall-bladder, intestines, kidney’s and bladder, which are organs involved with [18F]FHBG’s clearance from the body. Background activity in all other tissues is relatively low, due to the absence of HSV1-tk or HSV1-sr39tk expressing cells within the body of this human volunteer.
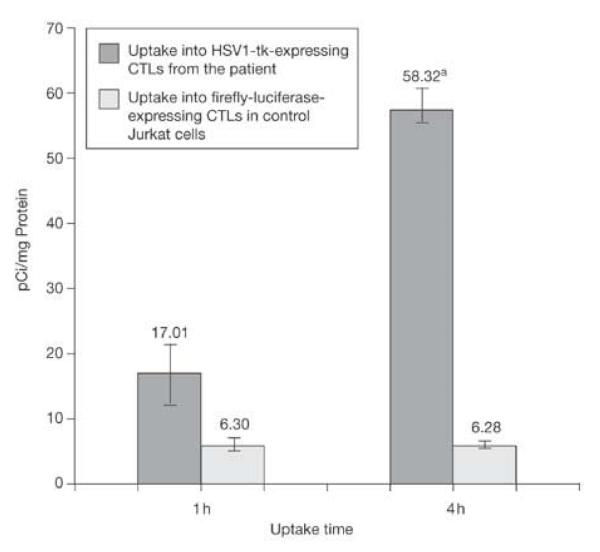
Our patient received a total of approximately 1 X 109 CTLs within a five-week period of direct infusions ( one week break between weeks 2 and 4). It is both possible that these cells proliferate or some die once injected into the patient. We were unable to quantify the number of cells present within the tumor resection site and the intact tumor near the corpus callosum. That would require a pharmacokinetic model7 for [18F]FHBG at brain tumor sites and knowing HSV1-tk expression levels per CTL at imaging time. We analyzed the level of specific HSV1-TK activity in the cultured CTLs that were injected into the patient by measuring the level of [3H]Penciclovir ([3H]PCV) uptake into the infused CTLs relative to control jurkat cells, which did not express HSV1-tk.8 Figure 3 shows that at one and four hours, approximately 3X and 9X [3H]PCV, respectively accumulated into the CTLs, relative to control cells. This low level of activity explains why [18F]FHBG accumulation in CTL infusion sites and intact tumor is only 2-3 X higher than background. In fact during uptake assay cultured cells were exposed to a relatively constant concentration of about 1 μCi/ml; whereas assuming homogeneous distribution of [18F]FHBG in 6 liters of blood, the maximum concentration CTLs would have been exposed to within the first 1-2 minutes after injection would have been about 1 μCi/ml, but then there is a rapid decline in blood concentration of [18F]FHBG within the first 20-40 minutes following it’s injection. Despite that, [18F]FHBG’s ability to detect the CTLs may be due to the low background within the surrounding brain tissue and the presence of a large number of CTLs.
Figure 3.
Uptake of [3H]Penciclovir into the genetically engineered CTL clone that was infused into the patient and control Jurkat cells. The autologous patient CTLs were genetically engineered to constitutively express HSV1-tk. The control cells expressed Firefly luciferase instead of HSV1-tk. Whereas control cells had a fixed uptake at both 1h and 4h, the CTL clone had a 2.7X higher uptake at 1h and 9.3X higher uptake at 4h. * indicates significant difference between uptake of [3H]PCV into patient CTLs vs control cells at 4 hours (P < 0.001).
Several changes to the clinical protocol are planned for future patients. In subsequent clinical trials we will explore concomitant CTL infusion and IL-2 injection to prolong persistence and therapeutic efficacy. We plan to improve imaging sensitivity by using a mutant HSV1-tk (HSV1-sr39tk), which should increase the rate of [18F]FHBG accumulation by CTLs, yielding much higher than 2.5X signal/background.9 Finally, comparing [18F]FHBG accumulation after CTL infusions to the level before CTL infusions in the same patient is preferable to comparing with average backgrounds in control patients. FDA now allows us to do two [18F]FHBG PET scans per patient in future studies.
CONCLUSION
Glioblastoma multiforme (GM) is the most common and malignant primary brain tumor10. The median GM patient’s survival is around 12 months and very few survive more than 3 years.10 Therefore, ACGT is a much needed treatment that should be investigated for extending GM patient survival. Long term imaging of therapeutic cells will be important for predicting long-term efficacy of this type of treatment. This case is the first ever reported reporter gene based imaging of therapeutic cells in a human patient. This case also illustrates that [18F]FHBG, which normally cannot cross the blood brain barrier, can accumulate within glioma tumors and should be able to detect HSV1-tk expressing therapeutic cells within these tumors.
Supplementary Material
ACKNOWLEDGEMENTS
Authors would like to acknowledge the funding supports from NIH NCI ICMIC P50 (PI: Gambhir) and RO1 CA103959 (PI:Jensen) and Doris Duke Charitable Foundation (PI: Gambhir). We would also like to thank Brenda Williams at COHNMC, Larry Pang and technologists at UCLA nuclear medicine clinics, the UCLA cyclotron crew, and Drs Martin Allen-Auerbach and Christian Schiepers for providing support with the patient study at UCLA. The supporting data on control patients used for comparison were collected and analyzed at Stanford University; hence we would also like to thank Yingbing Wang, Dr. Michael Goris, Dr. Fred Chin, Dr. Mohammad Namavari, Dr. Murugessan Subbarayan, Rhona Berganos, Dr. Erik Mitra, Dr. Andrei Iagaru, Dr. Larry Recht and the many involved Stanford University nuclear medicine clinic technologists.
References
- 1.Yaghoubi SS, Gambhir SS. PET imaging of herpes simplex virus type 1 thymidine kinase (HSV1-tk) or mutant HSV1-sr39tk reporter gene expression in mice and humans using [18F]FHBG. Nat Protoc. 2007;1:3069–3075. doi: 10.1038/nprot.2006.459. [DOI] [PubMed] [Google Scholar]
- 2.De Vries IJM, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat. Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 3.Morse MA, et al. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56–58. [PubMed] [Google Scholar]
- 4.Kahlon KS, et al. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–9166. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 5.Yaghoubi SS, et al. Human pharmacokinetic and dosimetry studies of [18F]FHBG: A reporter probe for imaging herpes simplex virus type-1 thymidine kinase reporter gene expression. J Nucl Med. 2001;42:1225–1234. [PubMed] [Google Scholar]
- 6.Yaghoubi SS, et al. Preclinical safety evaluation of 18F-FHBG: A PET reporter probe for imaging Herpes Simplex virus type 1 thymidine kinase (HSV1-tk) or mutant HSV1-sr39tk’s expression. J Nucl Med. 2006;47:706–715. [PubMed] [Google Scholar]
- 7.Green LA, et al. A tracer kinetic model for 18F-FHBG for quantitating Herpes Simplex virus type 1 thymidine kinase reporter gene expression in living animals using PET. J Nucl Med. 2004;45:1560–1570. [PubMed] [Google Scholar]
- 8.Yaghoubi SS, Gambhir SS. Measuring herpes simplex virus thymidine kinase reporter gene expression in vitro. Nat Protoc. 2006;1:2137–2142. doi: 10.1038/nprot.2006.334. [DOI] [PubMed] [Google Scholar]
- 9.Gambhir SS, et al. A Mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography. PNAS. 2000;97:2785–2790. doi: 10.1073/pnas.97.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krex D, et al. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.