Abstract
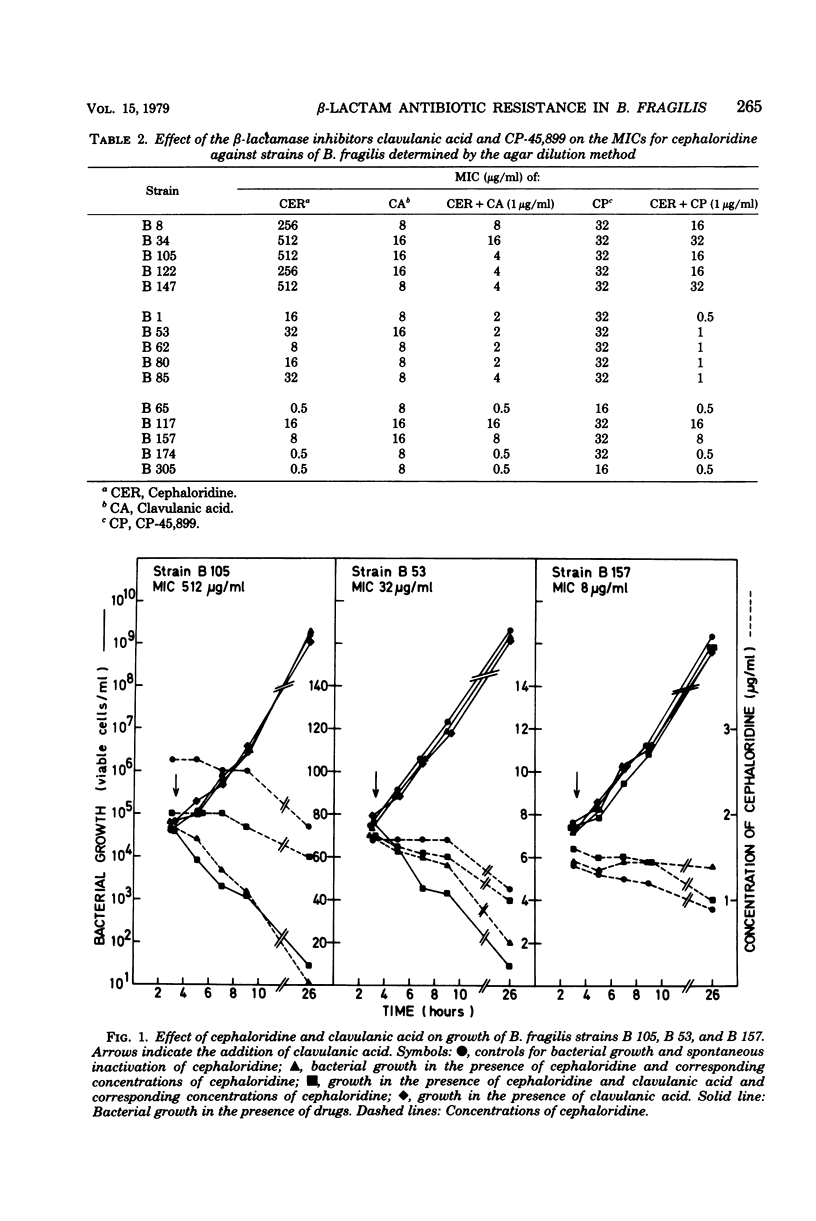
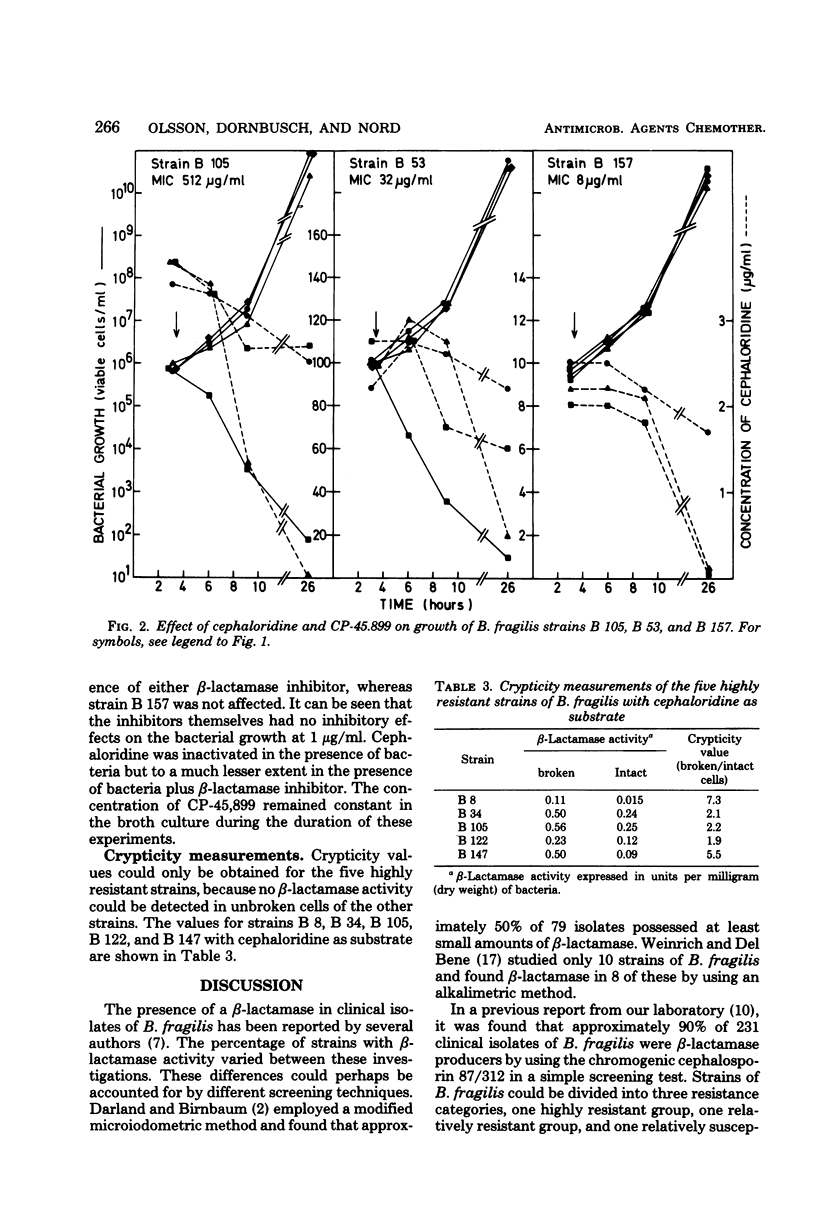
Fifteen strains of Bacteroides fragilis, five highly resistant, five moderately resistant, and five susceptible to benzylpenicillin and cephaloridine, were tested for β-lactamase production. In the highly resistant and the moderately resistant groups of strains, a correlation between formation of β-lactamase and minimal inhibitory concentrations was demonstrated. In the presence of the β-lactamase inhibitors clavulanic acid and CP-45,899 at concentrations of 1 μg/ml, the susceptibility to cephaloridine increased fourfold or more in the β-lactamase-producing strains. Crypticity measurements (β-lactamase activity broken/intact cells) with cephaloridine as substrate could indicate a diffusion barrier in the cell wall.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boman H. G., Nordström K., Normark S. Penicillin resistance in Escherichia coli K12: synergism between penicillinases and a barrier in the outer part of the envelope. Ann N Y Acad Sci. 1974 May 10;235(0):569–586. doi: 10.1111/j.1749-6632.1974.tb43291.x. [DOI] [PubMed] [Google Scholar]
- Darland G., Birnbaum J. Cefoxitin resistance to beta-lactamase: a major factor for susceptibility of bacteroides fragilis to the antibiotic. Antimicrob Agents Chemother. 1977 Apr;11(4):725–734. doi: 10.1128/aac.11.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Nord C. E., Olsson B. Antibiotic susceptibility testing of anaerobic bacteria by the standardized disc diffusion method with special reference to bacteroides fragilis. Scand J Infect Dis. 1975;7(1):59–66. doi: 10.3109/inf.1975.7.issue-1.11. [DOI] [PubMed] [Google Scholar]
- Nord C. E., Möllby R., Smyth C., Wadström T. Formation of phospholipase C and theta-haemolysin in pre-reduced media in batch anc continuous culture of Clostridium perfringens type A. J Gen Microbiol. 1974 Sep;84(1):117–127. doi: 10.1099/00221287-84-1-117. [DOI] [PubMed] [Google Scholar]
- Nord C. E., Olsson B., Dornbusch K. beta-lactamases in bacteroides. Scand J Infect Dis Suppl. 1978;(13):27–32. [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Dornbusch K., Nord C. E. Susceptibility to beta-lactam antibiotics and production of beta-lactamase in Bacteroides fragilis. Med Microbiol Immunol. 1977 Oct 7;163(3):183–194. doi: 10.1007/BF02126677. [DOI] [PubMed] [Google Scholar]
- Olsson B., Nord C. E., Wadström T. Formation of beta-lactamase in Bacteroides fragilis: cell-bound and extracellular activity. Antimicrob Agents Chemother. 1976 May;9(5):727–735. doi: 10.1128/aac.9.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading C., Cole M. Clavulanic acid: a beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother. 1977 May;11(5):852–857. doi: 10.1128/aac.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Clark D. C., Wotton S. Indirect method for assessing the penetration of beta-lactamase-nonsusceptible penicillins and cephalosporins in Escherichia coli strains. Antimicrob Agents Chemother. 1976 Aug;10(2):215–218. doi: 10.1128/aac.10.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Wotton S. Comparative study of seven cephalosporins: susceptibility to beta-lactamases and ability to penetrate the surface layers of Escherichia coli. Antimicrob Agents Chemother. 1976 Aug;10(2):219–222. doi: 10.1128/aac.10.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Weinrich A. E., Del bene V. E. Beta-lactamase activity in anaerobic bacteria. Antimicrob Agents Chemother. 1976 Jul;10(1):106–111. doi: 10.1128/aac.10.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. Clavulanic acid and susceptibility of Bacteroides fragilis to penicillin. Lancet. 1977 Jul 16;2(8029):145–145. doi: 10.1016/s0140-6736(77)90161-1. [DOI] [PubMed] [Google Scholar]
- Wüst J., Wilkins T. D. Effect of clavulanic Acid on anaerobic bacteria resistant to Beta-lactam antibiotics. Antimicrob Agents Chemother. 1978 Jan;13(1):130–133. doi: 10.1128/aac.13.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]


