Abstract
Dissemination of vector-transmitted pathogens depend on the survival and dispersal of the vector and the vector's ability to transmit the pathogen, while the host range of vector and pathogen determine the breath of transmission possibilities. In this study, we address how the interaction between dispersal and plant fidelities of a pathogen (stolbur phytoplasma tuf-a) and its vector (Hyalesthes obsoletus: Cixiidae) affect the emergence of the pathogen. Using genetic markers, we analysed the geographic origin and range expansion of both organisms in Western Europe and, specifically, whether the pathogen's dissemination in the northern range is caused by resident vectors widening their host-plant use from field bindweed to stinging nettle, and subsequent host specialisation. We found evidence for common origins of pathogen and vector south of the European Alps. Genetic patterns in vector populations show signals of secondary range expansion in Western Europe leading to dissemination of tuf-a pathogens, which might be newly acquired and of hybrid origin. Hence, the emergence of stolbur tuf-a in the northern range was explained by secondary immigration of vectors carrying stinging nettle-specialised tuf-a, not by widening the host-plant spectrum of resident vectors with pathogen transmission from field bindweed to stinging nettle nor by primary co-migration from the resident vector's historical area of origin. The introduction of tuf-a to stinging nettle in the northern range was therefore independent of vector's host-plant specialisation but the rapid pathogen dissemination depended on the vector's host shift, whereas the general dissemination elsewhere was linked to plant specialisation of the pathogen but not of the vector.
Introduction
The emergence of vector-transmitted plant symbionts/pathogens depends highly on vector dispersal and the host specificity of vector and pathogen [1]. For pathogens to emerge to epidemic levels, vector transmission to compatible host populations is required as are frequent encounters between vector and pathogen within a suitable environment [2]. While vector dispersal is essential for pathogens to encounter new host individuals and spread disease, vector feeding-behaviour (monophagous vs. polyphagous) can in distinct ways determine the pathogen's end host and the breadth of transmission potential [3]. Narrow host ranges may lead to specialised transmission cycles whereas polyphagous vectors have the potential to expose many host species to pathogens and different pathogens to specific hosts [4]–[6]. Broad feeding ranges may result in transmission to suboptimal or non-reservoir hosts with unsynchronised host-pathogen syndromes being the consequence [2], [5], [7]. These so-called spillover events, although having serious impacts on the infected non-reservoir hosts, are less important for pathogen dissemination, which is determined by the interaction between vector and pathogen and their common natural hosts. The incidence of these events has been related to relative host densities [8] and geographic range expansion of the vector [9], [10].
Phytoplasma are wall-less, non-helical prokaryotes that colonise plant phloem and depend of phloem-feeding insect vectors (leafhoppers, planthoppers, and psyllids) for transmission [11]. Phytoplasma are important plant pathogens that are known to induce disease in several hundred plant species worldwide, including major agricultural crops, ornamental plants and timber trees [reviewed in 3]. Phytoplasma of the stolbur (16Sr-XIIA) group [12] (proposed name: Candidatus Phytoplasma solani [13]) cause yellows diseases with high economic loss in grapevine (bois noir), maize (maize redness), lavender and various Solanaceous crops in Europe. Stolbur phytoplasmas have obligate vector-based transmission and stolbur diseases are highly or exclusively determined by pathogen and vector reservoirs in weedy host plants [14]–[19]. Stolbur phytoplasma has two main genetic clusters, tuf-a and tuf-b, characterised by a diagnostic SNP in the tuf-gene [16]. While tuf-a so far has been associated predominately with stinging nettle (Urtica dioica) [16], [20], tuf-b is found in a range of other weedy plants [17], field bindweed (Convolvolus arvensis) being the dominant reservoir plant throughout most of Europe [21].
The main, and in most regions only known, vector of stolbur phytoplasma is the planthopper Hyalesthes obsoletus Signoret, 1865 (Hemiptera: Cixiidae) [22]–[24]. Principal natural hosts of the vector correspond to the reservoir plants of stolbur, field bindweed again being the dominant host in most parts of Europe [21] but with stinging nettle being regarded as the natural host plant in northern Italy [25]. In the economically important grapevine disease bois noir, the epidemiology is coupled to the infection of these two herbaceous host plants and not to grapevine, since grapevine is not a nymphal substrate for H. obsoletus and, consequently, a dead-end host for stolbur. Vectors infected with stolbur nearly always (>95%) have stolbur type of the host plant from which they were collected [26].
The vector is predominantly Mediterranean. The northern distribution range limit is xerothermic habitats in Germany and northern France (Alsace) [27], mainly in vineyards on slopes of river valleys. Until about 25 years ago, the vector was rare in this area [28] and associated mainly with bindweed. Today, the vector's rapid population growth coincides with a host range expansion to stinging nettle, although an increase of vector populations on field bindweed, the traditional host, is also observed. The host shift to stinging nettle in the northern range has been accompanied by the emergence of tuf-a, which is now the dominant stolbur strain in most North-western European wine-growing regions [29]–[31].
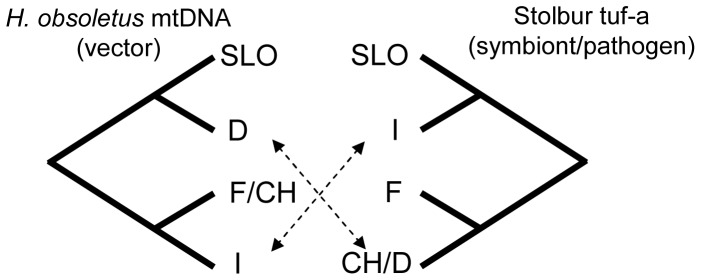
The northern populations of H. obsoletus from both host plants are fixed for the mtDNA haplotype “aa” [32] but are genetically divergent at microsatellite loci [33] (M. Imo, M. Maixner & J. Johannesen, unpublished data). This indicates that vector populations using stinging nettle in this area originated from local bindweed populations and that two genetic host-race populations have evolved perhaps since the host shift over the past 25 years (Fig. 1a,b). Haplotype “aa” has a historical origin east of the Italian-Slovenian karst divide and reached Germany and Alsace in a westward migration north of the European Alps. However, a northwards migration west of the Alps via France of vectors carrying the haplotype, “bb”, has reached the southernmost Germany and Alsace, establishing a secondary contact zone. Both haplotypes belong to a western European genealogical lineage that originated south of the European Alps and spread in a post-glacial circum Alpine expansion [32].
Figure 1. Schematic presentation of historical dispersal of H. obsoletus and expected co-dispersal scenarios of H. obsoletus and tuf-a stolbur.
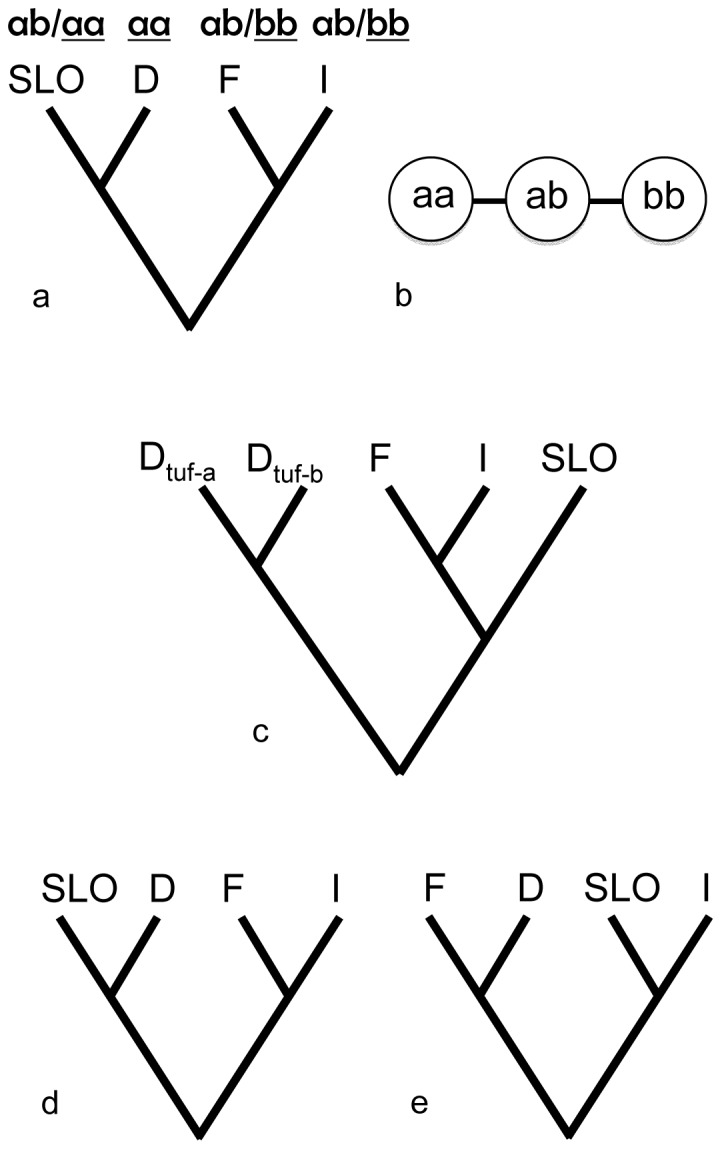
(a) Historical dispersal of H. obsoletus showing origin of German (D) H. obsoletus in Slovenia (SLO) inferred from (b) genealogical relationships of haplotypes aa, ab and bb. The haplotype aa evolved east of the Italian-Slovenian karst divide. Italian (I) and French (F) H. obsoletus exhibit the derived haplotype bb, which is found west of the divide only. The ancestral haplotype ab is found throughout southern Europe but not in Germany [32]. (c) Scenario 1: Stolbur tuf-a transferred from field bindweed to stinging nettle in the course of H. obsoletus' host shift. (d) Scenario 2: expected phylogeny of monophyletic tuf-a stolbur assuming historical co-dispersal of H. obsoletus and tuf-a stolbur from Slovenia into Germany. (e) Scenario 3: dispersal of monophyletic tuf-a stolbur from France into Germany that is independent of host race formation of H. obsoletus in Germany. An Italian ancestry is assumed in all scenarios.
The parallel associations among stolbur strains, vector populations and host plants in Germany and Alsace imply two independent vector transmission cycles of stolbur phytoplasma, one associated with stinging nettle as a new plant host and one associated predominately with field bindweed but also involving other host plants [16]. In contrast to the situation in Germany and Alsace, ancestral Mediterranean H. obsoletus populations associated with stinging nettle and field bindweed cannot be differentiated genetically at microsatellite loci (M. Imo, M. Maixner & J. Johannesen, unpublished data). The lack of plant specificity in H. obsoletus in southern countries thus contrasts the apparent global host specificity of stolbur strains.
The geographic range expansion of H. obsoletus coupled to an extant host-shift with specialised populations in a newly colonised area, and the acquisition and dissemination of a new obligate vectored pathogen-strain in this area offers the unique opportunity to study the interaction between geographic range expansion and host shift (plant fidelity) of a vector on the dissemination of an obligate vectored plant pathogen. In this study, we analyse the origin and dissemination of stinging nettle associated tuf-a stolbur in Western Europe where this strain has emerged as a major pathogen of grapevine. Specifically, we analyse whether the rapid dissemination of the pathogen in the novel geographic range, Germany and Alsace, is related to the vector's host-plant shift or influenced by geographic range expansion. We test three scenarios: 1) Stolbur was transferred from field bindweed to stinging nettle in the course of the vector's host shift between these two plants (Fig. 1c). In this case, monophyly of stolbur infecting field bindweed to stinging nettle in the geographic region of the host shift relative to regions outside is expected. Because tuf-a and tuf-b stolbur phytoplasma have been related to stinging nettle and field bindweed, respectively, on a European scale we do not expect this transfer to have happened. However, the transfer has so far not been subjected to a quantitative analysis in the area of the host shift. 2) Tuf-a stolbur co-migrated with resident, i.e. mtDNA haplotype “aa”, vectors into Germany (Fig. 1d). 3) The introduction of tuf-a stolbur is linked to the secondary contact between northwards emigrating “bb” vectors from France and resident “aa” vectors (Fig. 1e). In the second scenario, German tuf-a stolbur should be most related to Slovenian strains whereas the third scenario is supported if French and German tuf-a strains are the most related and these strains are associated with H. obsoletus of the “bb” haplotype. If scenario 2 is supported it suggests that stinging nettle intermediately may have been lost as a host, perhaps with stolbur tuf-a relying on an alternative vector. Scenario 3 implies that the dissemination on stinging nettle in Germany is caused by a new introduction of stolbur tuf-a. In the third scenario, the vector's host-shift is independent of stolbur infection but essential for the dissemination in a new environment.
Materials and Methods
The analyses were based on frequency distributions and genealogies of four stolbur genes and two H. obsoletus mtDNA genes. Stolbur gene sequences were obtained by direct sequencing of genomic DNA isolated from infected H. obsoletus collected on stinging nettle or field bindweed. Stolbur tuf-a was investigated from the circum Alpine geographic expansion range of H. obsoletus (Tables 1 and 2, Appendix S1, S2) and based on collections made between 2005 and 2010.
Table 1. Sample locations of tuf-a and -b strains sequenced at four genes, stamp, stol-11, secY and vmp1.
| Number of sequences | |||||||
| Stolbur | Country | Location | Region | stamp | stol-11 | secY | vmp1 |
| tuf-a | Germany | Lieser | Mosel | 3 | 4 | 3 | 4 |
| Bacharach | Rhine | 1 | 3 | 3 | 2 | ||
| Ungstein | Pfalz | 2 | 2 | 2 | 2 | ||
| Neuweier | Baden | 2 | 2 | 2 | 2 | ||
| Weinsberg | Württemberg | 2 | 2 | 2 | 2 | ||
| Randersacker | Franken | 2 | 2 | 1 | 1 | ||
| Switzerland, | Arlesheim | Basel | 0 | 1 | 1 | 1 | |
| north of Alps | Hallau | Schaffhausen | 1 | 4 | 3 | 4 | |
| Eglisau | Zürich | 0 | 1 | 1 | 1 | ||
| Bellerive | Murtensee | 1 | 2 | 2 | 1 | ||
| La Landeron | Bielersee | 1 | 4 | 5 | 3 | ||
| Espesse | Vaud | 1 | 3 | 3 | 4 | ||
| Russin | Geneve | 1 | 2 | 2 | 2 | ||
| Italy* | Rome | Lazio | 2 | 3 | 3 | 2 | |
| Pisa | Toscana | 1 | 2 | 2 | 1 | ||
| Verona | Venetio | 2 | 0 | 3 | 3 | ||
| Eisacktal | Trentino | 2 | 2 | 2 | 1 | ||
| Cembratal | Südtirol/Alto Adige | 4 | 6 | 6 | 2 | ||
| Gudo | Ticino | 0 | 1 | 1 | 1 | ||
| Serentina | Ticino | 0 | 2 | 2 | 0 | ||
| Biasca | Ticino | 2 | 2 | 2 | 2 | ||
| France | Bretenieres | Bourgogne | 1 | 1 | 1 | 1 | |
| Charentes | Charentes | 0 | 1 | 1 | 1 | ||
| Slovenia | Osevljek | Osijek-Baranja | 5 | 4 | 5 | 6 | |
| & Croatia | Nova Gorica | Goriška | 2 | 2 | 2 | 2 | |
| No information | - | 1 | 1 | 1 | 1 | ||
| Zeljezna Gora | Međimurje | 2 | 2 | 1 | 2 | ||
| tuf-b | Germany | Lieser | Mosel | 1 | 1 | 1 | 1 |
| Bacharach | Rhine | 1 | 1 | 1 | 1 | ||
| Boppard | Rhine | 1 | 1 | 1 | 1 | ||
| Neuweier | Baden | 1 | 1 | 1 | 1 | ||
| Norheim | Nahe | 1 | 1 | 1 | 1 | ||
| Italy | Eisacktal | Trentino | 1 | 1 | 1 | 1 | |
| Reggio | Emilia-Rom. | 1 | 1 | 1 | 1 | ||
| France | Beaune | Côte d'Or | 0 | 1 | 1 | 1 | |
| Slovenia | Nova Gorica | Goriška | 1 | 1 | 1 | 1 | |
The b-strains were used for outgroup comparisons. Map locations are shown in Appendix S1 and S2.
Includes the Swiss canton Ticino south of the Alps.
Table 2. Sample localities and mtDNA haplotypes for Hyalesthes obsoletus in the present study (A) and compared with summary data from [32] (B).
| Haplotype | |||||||||||||||||||
| Study | Locality | Country | Plant | n | aa | ab | ad | af | aj | bb | cd | db | ib | ig | kb | lb | nb | ob | tb |
| A | Neuweier | D | U | 3 | 3 | ||||||||||||||
| Neuweier | D | C | 3 | 3 | |||||||||||||||
| Arlesheim | CH | U | 3 | 1 | 1 | 1 | |||||||||||||
| Hallau | CH | U | 6 | 2 | 2 | 2 | |||||||||||||
| Bellerive | CH | U | 3 | 3 | |||||||||||||||
| Le Landeron | CH | U | 5 | 5 | |||||||||||||||
| Le Landeron | CH | C | 5 | 5 | |||||||||||||||
| Espesses | CH | C | 2 | 2 | |||||||||||||||
| Morges | CH | U | 5 | 5 | |||||||||||||||
| Russin | CH | U | 4 | 3 | 1 | ||||||||||||||
| Charentes | F | C | 5 | 5 | |||||||||||||||
| Beaune | F | C | 3 | 3 | |||||||||||||||
| Savigny les Beaunes | F | C | 3 | 3 | |||||||||||||||
| Pupilin | F | U | 2 | 2 | |||||||||||||||
| Mesnay | F | U | 1 | 1 | |||||||||||||||
| Gevingey | F | U | 1 | 1 | |||||||||||||||
| Mévouillon | F | L | 2 | 2 | |||||||||||||||
| Felthurns | I | C | 5 | 4 | 1 | ||||||||||||||
| Cembra | I | U | 3 | 2 | 1 | ||||||||||||||
| Modena | I | U | 5 | 1 | 1 | 1 | 2 | ||||||||||||
| Reggio | I | C | 4 | 2 | 2 | ||||||||||||||
| Rome | I | U | 3 | 2 | 1 | ||||||||||||||
| Osevljek | SLO | U | 5 | 4 | 1 | ||||||||||||||
| Total A | 81 | 9 | 11 | 1 | 1 | 50 | 1 | 1 | 1 | 3 | 2 | 1 | |||||||
| B | Germany | D | U/C | 34 | 31 | 3 | |||||||||||||
| France (Alsace) | F | U | 5 | 5 | |||||||||||||||
| France | F | U | 9 | 3 | 5 | 1 | |||||||||||||
| Italy | I | U | 22 | 12 | 4 | 2 | 1 | 2 | 1 | ||||||||||
| Slovenia/Austria | SLO/A | U/C | 24 | 13 | 11 | ||||||||||||||
| Grand total A+B | 175 | 58 | 37 | 1 | 4 | 1 | 60 | 1 | 1 | 3 | 1 | 1 | 1 | 3 | 2 | 1 | |||
Locations are shown in Fig. 1. The haplotype network of the sequences is presented in Appendix S9. Country: A, Austria, D, Germany; CH, Switzerland; F, France; I, Italy. Plant: C, Convolvulus arvensis; U, Urtica dioica; L, Lavandula angustifolia.
H. obsoletus was analysed for mtDNA sequences NADH dehydrogenase subunit I (400 bp), Cytochrome Oxidase subunit I (CO I) (198 bp), tRNA(Leu) (67 bp) and Cytochrome Oxidase subunit II (CO II) (523 bp), using the primers and protocol in [32]. New mtDNA sequences for the present study were obtained from the western area of tuf-a's putative origin, Italy and France, in the contact zone Switzerland, and at one south German location (Table 2). Samples from these areas west of the Alps were underrepresented in [32]. Individuals were collected between 2008 and 2010. We further sequenced the mtDNA from tuf-a positive H. obsoletus from Slovenia and Croatia. Thus, all stolbur tuf-a genotypes were related to a H. obsoletus mtDNA haplotype. The mtDNA distributions of the present study were combined with data from [32] to test demographic expansions of H. obsoletus. The haplotypes have the Genbank accession numbers: COII: EU155640–44, -48 and JX025159–63; ND1: EU155649–50, -52, 54–55 and JQ977743 [32]; this study].
The dispersal history of tuf-a stolbur was inferred from the genealogy and frequency distribution of partial gene sequences of two membrane proteins, vmp1 [34], [35] and stamp [36] and two house-keeping genes, stol-11 and secY [18], [37]. Stolbur infection in H. obsoletus was assessed with the stolbur specific primers stol-11: STOL-11f2: 5′-TAT-TTT-CCT-AAA-ATT-GAT-TGG-C-3′ and STOL-11r1: 5′-TGT-TTT-TGC-ACC-GTT-AAA-GC-3′ [37], vmp1: TYPH10F-AAC-GTT-CAT-CAA-CAA-TCA-GTC and TYPH10R 5′-CAC-TTC-TTT-CAG-GCA-ACT-TC-3 [18], secY: PosecF3 5′-GGA-TTG-ATA-GAT-GCT-GCC-CC-3′ and PosecR3 5′-GCC-CCT-ATA-ACG-GTG-ATT-TTG-A-3′ [18], stamp: stamp-F1 5′-TTC-TTT-AAA-CAC-ACC-AAG-AC-3′and stamp-R1 5′-AAG-CCA-GAA-TTT-AAT-CTA-CC-3′, using the published protocols [18], [36], [37].
DNA product amplification was performed in an end volume of 25 µl consisting of 1 µl forward and backward primer (10 pmol/µl), 1 µl DNA extract and 22 µl H2O sterile. Each sample was covered with 15 µl Chill Out 14 Liquid Wax (MJ Research). The PCR was performed with “Ready To Go™ PCR Beads” (0.5 ml tubes; Amersham Pharmacia Biotech) using a PTC-100 thermocycler (MJ Research).
Reference sequences of all stolbur genes were obtained by sequencing each gene in the sense and anti-sense direction. Hereafter, secY, stol-11 and stamp were sequenced in one direction. Vmp1, having an amplification product of about 1500 bp, was sequenced in both directions.
Data analysis
Sequence diversity of each of the four tuf-a genes was estimated within four pre-defined geographic regions, the host-shift population Germany (1), a putative transition population Switzerland (2), and the two putative regions of origin, Italy and southern France (3), and Slovenia and Croatia (4). Diversity was estimated as the number of sequences, nucleotide diversity and mean number of pair-wise differences between sequences using Arlequin 3.5 [38]. The number of synonymous and non-synonymous mutations was calculated with DnaSP v5.0 [39].
To test for monophyly of tuf-a genotypes, these were compared to nine strains of tuf-b from Germany (N = 5), Italy (N = 2), France (N = 1) and Slovenia (N = 1); all strains came from different sites (Table 1). Phylogenetic relationships among secY and stol-11 gene sequences were analysed with haplotype networks, TCS 1.2.1 [40]), due to low variability among these sequences. For the polymorphic vmp1 and stamp genes, we first determined the independence of of tuf-a and tuf-b (stinging nettle- and bindweed-associated) sequences with UPGMA phylogenetic analysis, as no appropriate out-group sequences were available.
After confirming monophyly of the stinging nettle associated sequences (see Results), the phylogenetic relationships of vmp1 and stamp genotypes were analysed with Neighborjoining (NJ), Maximum Likelihood (ML), and Maximum Parsimony (MP) with tuf-b genotypes as outgroups. For NJ and ML we used the HKY substitution model without gamma distribution, found by the online software “Findmodel” (Los Alamos National Laboratory, http://www.hiv.lanl.gov/). For MP, all characters were weighted equally. Significance of branch lengths was estimated in heuristic searches with random addition of sequences and TBR branch swapping in 2000 bootstrap replicates. 50%-majority rule consensus trees based on bootstrap search were computed for all tree algorithms. All phylogenetic analyses were performed with PAUP version 4.08b for the Macintosh [41].
We tested neutral molecular evolution of tuf-a genotypes for the genes vmp1, secY and stamp using Tajima's D [42] (the number of unique mutations among all sequences relative to the total number of mutations) and Fu's Fs (the probability of having a number of haplotypes greater or equal to the observed number of samples drawn from a constant-sized population) [43] with Arlequin 3.5. Diversifying selection has been shown for stamp across tuf-a and -b genotypes [36] but was not tested within tuf-a, the objective of the present study. We tested for clock-like evolution of branch lengths in vmp1 and stamp with PUZZLE 5.2 [44] using HKY substitution model without gamma distribution found by “Findmodel”. Rooting in PUZZLE was done by automatic search for best place.
The demographic history of the vector H. obsoletus was evaluated from population samples using D and Fs as demographic indices of the neutrally evolving mtDNA partial gene sequences, and by testing for deviation from a sudden demographic expansion (Arlequin 3.5). Because the outcomes of neutrality and/or demographic tests are influenced by how populations are defined [45] and sampling skew, we implemented the tests with and without a priori knowledge of the organisms, and relative to the sampling scheme. Hyalesthes obsoletus was considered both as a single population and as three populations based on the distribution of the mtDNA haplotypes “aa” and “bb”, which separate H. obsoletus at the Italian-Slovenian karst divide. The three populations were assigned to a western-lineage (France and Switzerland), an eastern-lineage (Germany, Slovenia, Croatia and Austria) population and the putative centre of origin, Italy. In the demographic analysis we assumed that vectors from the contact zone in Switzerland carrying either the “aa” or the “bb” haplotype each belonged to the eastern or western population group.
Results
Stolbur tuf-a genetic diversity
For tuf-a stolbur, we obtained one genotype in 61 stol-11 sequences (741 characters) (accession no. JQ977744), four genotypes in 62 secY sequences (829 characters) (JQ977707–10), thirteen genotypes in 54 vmp1 sequences (1308 characters) (JQ977721–33) and seven genotypes in 41 stamp sequences (459 characters) (JQ977713–19). The three genes secY, vmp1 and stamp were polymorphic in Italy and Slovenia whereas Swiss and German populations were polymorphic only for vmp1. The number of tuf-a secY, vmp1 and stamp genotypes in Italy was 3, 10 and 6, compared to 2, 2 and 3 in Slovenia and Croatia, and 1, 2 and 1 in both Switzerland and Germany. Genetic diversity in Italy compared to Slovenia and Croatia was c. 20 times higher in vmp1 (0.00979 vs. 0.00050) and c. 10 times higher in stamp (0.01028 vs. 0.00117) (Table 3). Genotypes of vmp1 were shared between Italy, Slovenia and Croatia (genotype N3) and between Germany and Switzerland (N1, N2) (Appendix S3). Stamp genotypes were shared between France, Switzerland, Germany and Italy (S1) and between Italy, Slovenia and Croatia (S2, S3). Switzerland and Germany never shared genotypes with Slovenia and Croatia. The frequency distributions in these two geographic regions were highly significantly different, vmp1: χ2 = 40.00, 3 df, P<0.0001; stamp: χ2 = 27.00, 3 df, P<0.0001.
Table 3. Genotypic diversity of stolbur genes secY, stamp and vmp1 in four predefined European regions, (1) the host-shift population Germany, (2) a putative transition population Switzerland, and the two putative regions of origin, (3) Italy and southern France, and (4) Slovenia and Croatia.
| Gene | Region | Number of isolates tested | Number of genotypes | Nucleotide diversity | Mean number of pair-wise differences |
| secY | Germany | 13 | 1 | 0 | 0 |
| Switzerland | 17 | 1 | 0 | 0 | |
| Slovenia/Croatia | 9 | 2 | 0.00027 | 0.22 | |
| Italy/France* | 23 | 3 | 0.00325 | 2.69 | |
| stamp | Germany | 12 | 1 | 0 | 0 |
| Switzerland | 5 | 1 | 0 | 0 | |
| Slovenia/Croatia | 10 | 3 | 0.00097 | 0.56 | |
| Italy/France* | 14 | 6 | 0.01028 | 5.88 | |
| vmp1 | Germany | 13 | 2 | 0.00012 | 0.15 |
| Switzerland | 16 | 2 | 0.00010 | 0.13 | |
| Slovenia/Croatia | 11 | 2 | 0.00050 | 0.66 | |
| Italy/France* | 14 | 10 | 0.00979 | 12.69 |
The region Switzerland includes Swiss samples north and west of the Alps. The region Italy/France includes samples from the Swiss canton Ticino, situated south of the Alps and part of the Italian Po Basin. The gene stol-11 was monomorphic in tuf-a stolbur and not included. Genotype frequencies are shown in Appendix S3.
includes the Swiss canton Ticino south of the Alps.
Stolbur tuf-a gene phylogenies
Tuf-a genotypes were monophyletic relative to tuf-b genotypes at all four genes (see below) and linked in two clusters corresponding to the two RFLP patterns found in the tuf gene, tuf-a and tuf-b. The tuf-a stol-11 genotype differed by 1 bp to the most related of the two observed tuf-b genotypes (JQ977745–46) (Fig. 2a). The tuf-a secY genotypes differed by minimum four substitutions to the two tuf-b genotypes (JQ977711–12) found in this survey (Fig. 2b).
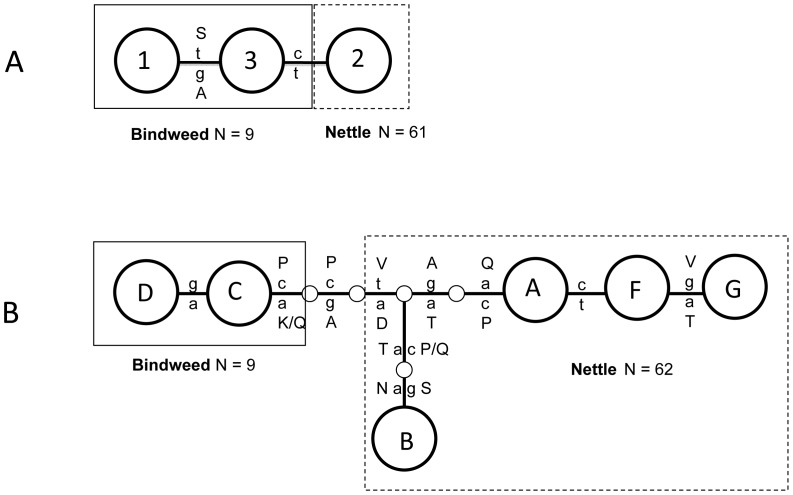
Figure 2. Haplotype networks for genotypes of stol-11 and secY.
The networks of stol-11 (A) and secY (B) show all genotypes observed in tuf-a (nettle) associated strains and in nine random, comparative tuf-b (bindweed) strains. Lower case letters: nucleotide substitutions; upper case letters: amino acid substitutions. The corresponding SEE-ERANET nomenclature of the secY genotypes is shown in Appendix S3.
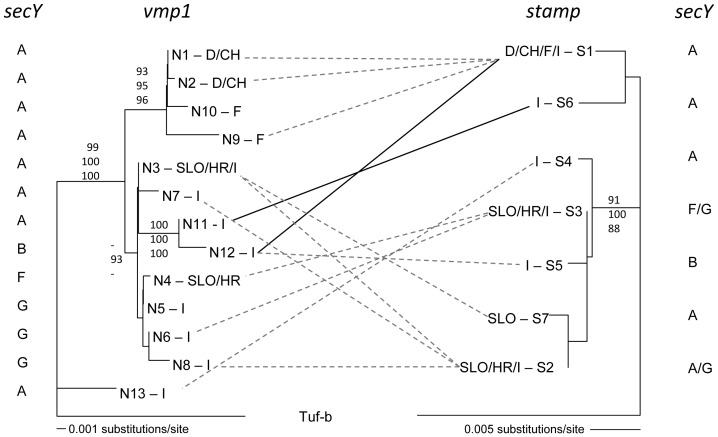
The thirteen tuf-a vmp1 genotypes were monophyletic relative to the nine paraphyletic tuf-b genotypes (JQ977734–42) observed in this survey (Appendix S4). All observed vmp1 genotypes exhibited three highly homologous repeated domains of which the first domain in the tuf-a cluster was characterised by a diagnostic penta-peptide (Asp-Val-Ala-Asn-Asn) (Appendix S5). Phylogenetic analyses with ML, NJ and MP produced identical topologies. Fig. 3 shows the phylogenetic relationship of tuf-a vmp1 with the tuf-b isolate Neuweiher57 as an out-group, ML (−ln = 2311.48036), NJ (ME score = 0.06674); MP analysis found three most parsimonious trees, each with identical main clusters. The MP analysis included 28 parsimony informative and 54 un-informative sites (tree length: 87; retention index: 0.90; consistency index: 0.94; homoplasy index: 0.06). The four tuf-a genotypes found in France, Germany and Switzerland clustered with high bootstrap scores, while genotypes found in Slovenia and Croatia were related (N3) with Italian ones.
Figure 3. Comparative phylogenetic relationships of stolbur tuf-a vmp1 and stamp genotypes based on isolates sequenced for both genes, and corresponding secY genotypes.
Bootstrap scores above 80 are given in following order, NJ, MP and ML. Lines show linked genotypes within isolates: congruent cluster = grey hatched line, incongruent cluster = bold full line. The Italian isolate Rome 7 exhibited the genotypes N11 (vmp1) and s6 (stamp), which belong to different clusters in the two phylogenetic trees (bold full line). Isolates with the vmp1 genotype N12 had stamp genotypes s1 (bold full line) or s5 (hatched line). Note that genotypes in France, Germany and Switzerland north of the Alps cluster together in both trees, and that secY genotypes cluster according to the vmp1 branching pattern. The secY genotype A is the ancestral tuf-a genotype. Geographic abbreviations: F = France, I = Italy, HR = Croatia, SLO = Slovenia, D = Germany, CH = Switzerland. The corresponding SEE-ERANET nomenclature of the tuf-a genotypes is given in Appendix S3.
The seven tuf-a stamp genotypes differed by at least 12 substitutions to the two observed tuf-b genotypes (JQ977720, FN813260.1 [36]). The phylogeny of tuf-a stamp corroborated the phylogeny of vmp1 genotypes in the geographic distribution of related genotypes where genotypes found in France/Germany/Switzerland (s1) and Slovenia/Croatia (s2, s3, s7) belonged to separate clades; ML (−ln = 810.33835), NJ (ME score = 0.04794); MP analysis found two most parsimonious trees, each with identical main clusters. The MP analysis included 5 parsimony informative and 17 un-informative sites (tree length: 45; retention index: 0.94; consistency index: 0.98; homoplasy index: 0.02). Although the bootstrap score separating the genotypes s1 and s6 from the remaining genotypes was slightly below 70, the two genotypes were characterised by a 6 bp deletion/insertion, as described in [36] (Appendix S6).
Multilocus genotypes including the three polymorphic genes were obtained from 33 tuf-a isolates (Appendix S7). Incongruent tuf-a phylogenetic relationships of vmp1 and stamp were observed in isolates with the vmp1 genotypes N11 and N12. The (single) Italian N11 isolate had the stamp genotype s6, which clustered with the western distributed genotype s1. For the genotype N12, the incongruence was more pronounced: N12 isolates from Cembra Valley (Italy) had the western distributed genotype s1 while N12 isolates from Biasca (Ticino, Switzerland) had the eastern distributed genotype s5. Both N12 isolates had the secY genotype B.
Molecular evolution and signals of hybridisation in tuf-a genotypes
Tuf-a stamp and secY genotypes did not deviate from neutral clock expectations (P>0.10), whereas the 13 tuf-a vmp1 genotypes did; vmp1 likelihood ratio test, P<0.001 (log L without clock: −2162.22, independent branch parameters: 22; log L with clock: −2189.02, independent branch parameters: 8; Likelihood ratio test statistic delta: 57.71, df = 14). Fu's Fs was significantly negative across the 13 tuf-a vmp1 genotypes (Fs = −4.90, P = 0.015) and marginally significant in seven stamp genotypes, Fs = −2.52 (Table 4). The ratio of non-synonymous to synonymous sites (DN/DS) for secY, stamp and vmp1 was 4, 3 and 3, respectively. The high non-synonymous to synonymous ratio in SecY was caused by non-synonymous substitutions between tuf-a and tuf-b and relative to genotype B, which was intermediate between the two major clades.
Table 4. Neutrality tests for vmp1, stamp and secY stolbur phytoplasma tuf-a genes, and demographic tests for the vector Hyalesthes obsoletus using the mtDNA genes ND1 and COII.
| Species | Gene/Level | N | Tajima's D | Fu's Fs | Demographic expansion, P value | Spatial expansion, P value |
| Stolbur | vmp1 | 13 | −1.27 | −4.90* | - | - |
| stamp | 7 | −0.49 | −2.52+ | - | - | |
| secY | 4 | −0.31 | −1.16 | - | - | |
| H. obsoletus | Total population | 175 | −1.32x | −7.44** | 0.006 | <0.001 |
| Italy | 42 | −1.25 | −4.89** | 0.12 | 0.09 | |
| Western Population (F-CH) | 59 | −1.37x | −3.12* | 0.44 | 0.11 | |
| Eastern population (SLO-A-D) | 74 | −0.15 | −0.02 | 0.23 | 0.03 |
N = number of sequences (molecular level) or individuals (population level) included in analysis. Note that the populations of H. obsoletus were grouped according to mtDNA distributions, F = France, CH = Switzerland, D = Germany, A = Austria, SLO = Slovenia. Significance:
P<0.05,
P<0.01,
P<0.001. Marginal significance:
0.05<P<0.10,
P = 0.047, Fu's Fs significant P<0.02. - not applicable.
A comparison of tuf-a and tuf-b vmp1 sequences suggests that the high number of amino acid substitutions is caused by a combination of insertions and deletions in the repeated domains (Appendix S5, see also [46]). Because vmp1 is characterised by highly similar repeated domains in both strains, events of hybridisation between strains rather than rearrangements within strains are difficult to verify. Based on the available sequences, there are two putative hybridisation events between tuf-a and tuf-b-strains involving the oligopeptide TPTQDTV. The oligopeptide sequence was found in the second repeat of four type-b genotypes (amino acid position 222–228) in combination with the sequence AGSLTV (position 235–240). Among all tuf-a sequences, this series was observed exclusively in the tuf-a N13 genotype. (It should be noted that the AGSLTV motif alone is uninformative of hybridisation as it is found in all genotypes in both strains.) The TPTQDTV peptide was further observed in the tuf-a genotypes N11 and N12 in the first repeated domain.
Putative signals of recombination in stamp were found in tuf-a genotypes s1 and s6, where the amino acid sequence at positions 35–60 was more tuf-b-like (Appendix S6).
Due to the signals of positive selection and/or hybridisation in tuf-a, a demographic analysis was omitted.
Genetic diversity and demography of H. obsoletus
Hyalesthes obsoletus mtDNA haplotype diversity and haplotype distributions of the combined COII/ND1 sequence (1130 bp) in the present study corroborated previous observations [32] (Table 2) showing that the ancestral haplotype “ab” was found throughout southern Western Europe, while “aa” and “bb” occurred east and west, respectively, of the Italian-Slovenia karst divide. Haplotype diversity was higher in Italy than in the western and eastern ranges, number of haplotypes: Italy = 10, western range = 5, eastern range = 3; nucleotide diversity×10−3: Italy = 1.004, western range = 0.274, eastern range = 0.314 (Appendix S8). The haplotypes “ab” and “bb” both are centres in star-like networks, whereas “aa” is not (Appendix S9). The frequency of the haplotype “bb” or “bb”-derived haplotypes increased towards its western range limit in France (0.83) and Switzerland (0.85). Haplotype “bb” was found in H. obsoletus caught on both stinging nettle and bindweed (Table 2), i.e. there was no evidence for plant associations in mtDNA, thus corroborating the lack of plant associations in the eastern haplotype “aa” [32]. The two northern most Swiss populations, Arlesheim and Hallau, had a mix of “bb” and “aa” haplotypes. Thus, the contact zone between “aa” and “bb” is northern Switzerland and southern Germany.
The analysis of demographic evolution of H. obsoletus populations found marginally significant negative D = −1.37 (P = 0.08) and significant negative Fs = −3.12 (P<0.02) for the western population, while deviations from demographic and spatial expansions were not significant (Table 4). The eastern population showed significant deviation from spatial expansion only (P = 0.03). The Italian population exhibited significant negative Fs = −4.89 (P<0.01) but no deviations from sudden expansion models (P>0.09).
The analysis of all individuals from the total sampling area produced a contradictory result: while D was marginally significant (D = −1.32, P = 0.08) and Fs significantly (Fs = −7.44, P<0.01) negative, indicating population expansions, the total population also deviated significantly from sudden demographic and spatial expansion models (P<0.006). The contrasting results are explained by two geographically separated and monomorphic haplotype distributions (“aa” and “bb”) which led to an excess of few steps and a deficit of large steps (mutation differences) in the expected mismatch distribution. The haplotype frequency distributions are shown in Appendix S8.
Co-dispersal of tuf-a stolbur phytoplasma and H. obsoletus
The historical co-dispersal of the vector H. obsoletus and tuf-a stolbur based on the phylogenies and genetic frequency distributions analysed above is summarised in Fig. 4. The figure combines the hypothesis settings of Fig. 1a (vector) and Fig. 1e (tuf-a stolbur) and shows non-concordant phylogenetic and geographic distributions between tuf-a (based on vmp1 and stamp genotypes) and vector mtDNA haplotypes for populations in North-western Europe (Germany). The vector's haplotype frequency distributions and genealogical relationships presented in Fig.1a and based on [32] were confirmed in the present study. Non-concordance was caused by German vectors of the eastern “aa” lineage, which originated in Slovenia, being infected with the western tuf-a genotype N1s1 found in France and Switzerland.
Figure 4. Summary co-dispersal analysis of H. obsoletus and tuf-a stolbur based on genealogies and frequency distributions of genetic markers for explaining dissemination of tuf-a in north-western Europe.
The figure combines Fig. 1a and e, showing incongruent historical dispersal events and indicates that dissemination of tuf-a in north-western Europe was caused by introduction of tuf-a by secondary immigration of vectors (scenario 3).
Discussion
In this study, we investigated the origin and population history of an emerging insect-vectored plant pathogen by considering how vector dispersal and plant (host) fidelity of both the pathogen and the vector interact on the dissemination process. Specifically, we analysed whether recent dramatic increase in infection pressure on grapevine in the northern, presumably suboptimal range of the vector could be explained by a local host-plant shift of the vector or by vector-mediated immigration of the pathogen. Our results imply that a combination of dispersal (range expansion) and new infections of the vector explain the general dissemination of stolbur tuf-a in Western Europe, and that an introduction of plant-specialised pathogens from plant-unspecialised to plant-specialised vectors during the range expansion explains high infection pressure in the north western range. These results highlight a complex epidemiology of stolbur tuf-a evolving after range expansion from a common source population.
Co-origins and population expansion
Parallel genetic diversity patterns in vector and tuf-a populations in Western Europe provide strong evidence for a common origin south of the European Alps. The vector was genetically most diverse in Italy where the ancestral haplotypes “ab” was frequent, diversity decreased towards the eastern and western range edges, which did not share the derived signature haplotypes “aa” and “bb”. These diversity patterns were repeated in tuf-a stolbur: genotypes were basal and diversity by far the highest in Italy. In Italy, vmp1 and/or stamp genotypes were shared with eastern (Slovenia/Croatia) and western (France/Germany/Switzerland) populations but not between these eastern and western populations, and diversity decreased towards both the western and the eastern range edges. Reduced genetic diversity in newly colonised regions supports founder effects of invasive populations, as shown in leafhopper-transmitted, invasive pathogen Xylella fastidiosa subsp. fastidiosa of Pierce's disease of grape in North America [47]. Positive selection in VMP1 via gene rearrangements [46] or the possibility of hybridisation between stolbur isolates might also question the phylogenetic rigor of the basal position of Italian vmp1 genotypes, e.g. N13. Despite this quandary, selection is not creating random phylogenetic signals at the geographic level because the genealogical and the geographic associations are correlated, although selection/adaptation may explain the dissemination of certain genotypes in different areas.
Signals of demographic expansion of vector populations detected in the western range and in Italy suggests that the dissemination of tuf-a in Western Europe is linked to a secondary range expansion of the vector with recently acquired tuf-a, which were not co-dispersed in prior migration events. Secondary expansion is implied by recent disease outbreaks related to tuf-a throughout Western Europe (see below), but is most readily seen in the vector's North-western range in Germany and Alsace where resident vector populations have the derived eastern “aa” (“Slovenian”) haplotype but lack tuf-a genotypes found in Slovenia. These distributions combined imply that “aa” evolved in Slovenia and spread to Germany and Alsace before tuf-a genotypes co-dispersed with the vector into Slovenia. The dispersal of tuf-a into Slovenia is supported by cohesion in microsatellite genetic variance among Italian and Slovenian vector populations (M. Imo, M. Maixner, J. Johannesen, unpublished data). A complicating issue in this secondary expansion scenario is the fact that the expanding haplotype “bb” is found neither in Slovenia nor in northern Germany. The paradox can be explained by male-biased gene flow or by mating success of local females. Both processes would preserve the original, maternally inherited mtDNA haplotypes. Male-biased gene flow is supported by field observations, which show that males move much more readily between plants than females (M. Maixner and J. Johannesen, unpublished data).
Pathogen dissemination has been linked with range expansions of the vector in other studies [9], [10], [48], [49] while yet others have failed to find associations. For example, the phylogeography of the Lyme disease pathogen, Borrelia burgdorferi is not correlated with its tick vectors, Ixodes scapularis [50] probably due to independent behaviour (dispersal) of the tick vectors by their avian hosts [51]. The two different outcomes of vector dispersal and pathogen dissemination, uncorrelated and correlated dispersal, are likely influenced by the trophic level of the interactions. However, as we show for H. obsoletus carrying tuf-a stolbur phytoplasma, dissemination in the same system may differ if host-specialisations of vector and pathogen are variable.
Plant fidelity and dissemination
The second part of our study addressed how plant fidelities of the vector and pathogen influence the emergence of tuf-a induced grapevine disease bois noir in North-western Europe (Fig. 4). In corroboration with the vector's range expansion in its western range, we found strong support for immigration of the tuf-a N1s1 genotype from France via Switzerland into Germany, and thus, in the North-western range, a new acquisition of tuf-a that is independent of the vector's host-shift but where the vector's host shift is essential for the dissemination of stolbur tuf-a (scenario 3, Fig. 1e). Monophyly and linkage of tuf-a genotypes at several genes precludes an associated host shift of vector and stolbur in North-western Europe (scenario 1) while at the same time providing support for an independent evolution of tuf-a and –b associated with the two plant complexes. Historical co-migration of unspecialised H. obsoletus and stolbur tuf-a from Slovenia (scenario 2) is also rejected because the stolbur tuf-a genotypes east of the Slovenian-Italian karst divide are unrelated to those found in North-western Europe.
The observed genetic pattern, scenario 3, suggests that immigrating plant-unspecialised vectors have transferred tuf-a (genotype N1s1) stolbur to plant-specialised vectors north of the contact zone. An expansion of genotype N1s1 vectored by resident German vectors into southern areas is unlikely because N1 is a tip-haplotype and is not related to Slovenian haplotypes. The complex nature of the vector-tuf-a interaction in the western geographic range is consistent with two general findings observed in host-pathogen systems, but at different interaction levels. First, we detected the emergence of an ecologically specialised pathogen, a phenomenon that might be common in specific host-pathogen associations [52], relative to its natural host plant. Second, we found no evidence that the evolution of plant fidelity of the vector facilitated divergence of tuf-a [53] on the natural host, although the time for genetic imprints thereof might be too short in the current system. Thus, divergence of the two stolbur strains tuf-a and -b primarily follow the natural host plants, while the transmission breadth to agricultural crops is influenced by the natural plant preference of the vector.
These complex interactions are related to the mosaic of co-evolution [54], which predicts different selection and/or historical idiosyncrasies in different geographic regions and thus the potential for divergent evolutionary trajectories in co-evolving systems. For H. obsoletus and tuf-a stolbur, we have shown that obligate vector-transmitted tuf-a stolbur are greatly influenced by geographic context in the sense of the vector's plant fidelity.
Ecology and history of tuf-a dissemination in North-western Europe
The epidemiology of stolbur disease in most agricultural crops is related to the pathogen's distribution in weedy host plants and should therefore be traced in the weeds rather than the crops. The first evidence for stolbur-induced (bois noir) symptoms in grapevine in North-western Europe come from the German Moselle Valley and probably dates back to the 1930's [55], which, perhaps coincidentally, coincides with the first report of the vector in Germany [56]. Bois noir, described at that time was confused with Flavescence dorée [57], remained restricted to a few locations until the late 1990's. Because the tuf-types were not distinguished until 2004 [16], the tuf-type of these first bois noir incidences is unknown but as H. obsoletus was observed with field bindweed prior to the present bois noir epidemics, bois noir was most likely caused by tuf-b before 1990.
The late awareness of the presence of two stolbur strains precludes a precise overview of the emergence of the new tuf-a strain before 2004. A survey of the abstract books of the European Bois Noir Workshops [58], [59] and the International Phytoplasmologist Working Group Meetings [60], [61] points towards high relative incidences in Northern Italy and North-western Europe and low relative incidences elsewhere. The dominance of tuf-a in the former region is associated with two observations. First, the emergence is correlated with rising mean temperatures [62], which may have mediated both the observed secondary range expansion into the area as well as survival and specialisation on stinging nettle in previously climatically adverse regions. The second aspect concerns why tuf-a dominates relative to tuf-b in the western compared with the eastern distribution range. Interestingly, the dominance is related to genotype N1s1, which might show traces of hybridisation, a phenomenon that has been linked to the rapid spread of emerging diseases in various organisms, e.g. Dutch Elms disease [63], swine flu [64] and possibly in the planthopper-transmitted Pierce's disease of grape [47]. The phylogenies of vmp1 and stamp genotypes in French, German and Swiss tuf-a isolates indicate traces of both within-tuf-strain (N11-s6 and N12-s1) and between-tuf-strain (stamp: s1 and tuf-b genotypes) hybridisation. The potential for hybridisation between the two tuf-strains is witnessed in the (rare) presence of both strains in stinging nettle as well as in the vector (M. Maixner, unpublished data), and may even occur between mollicute taxa [65]. Because N1s1 is found in stinging nettle populations of the vector that are genetically distinguishable (in Germany) as well as indistinguishable (in Switzerland) (M. Imo, M. Maixner & J. Johannesen, unpublished data) from field bindweed populations, immigration of the genotype into North-western Europe cannot explain host-race divergence of the vector per se although association with the genotype might be involved in the vector's ability to exploit the plant. Still, a genome scan of stolbur isolates is required to circumstantiate the hybridisation hypothesis.
The plant specificity of tuf-a raises the question of the vector's original association with stinging nettle. As far as we know, neither stinging nettle nor the vector shows symptoms of tuf-a infection, which suggests long co-evolutionary relationships. For stinging nettle, this is supported by diagnostic mutations in all analysed tuf-a genes [36] (this study). A long relationship between tuf-a and the vector may suggest that stinging nettle previously was a lower ranked host of the vector rather than tuf-a had another vector associated with stinging nettle before H. obsoletus acquired it as a new vector, as discussed for the origin of the grapevine yellows disease flavescence dorée (FD) [66]–[68]. For the situation today in North-western Europe where the vector utilises stinging nettle and field bindweed in distinct populations, the introduction of a new symbiont/pathogen has created independent epidemiological cycles resulting in grave disease outbreaks in an accidental foraging-host, grapevine.
Supporting Information
Sampling sites of the stolbur vector H. obsoletus in France, North Switzerland and Germany. Stolbur isolates were obtained from H. obsoletus locations in italics.* Sample sites from [32].
(PPT)
Sampling sites of the stolbur vector H. obsoletus in Italy, South Switzerland (Ticino), Slovenia, Croatia and Austria. Stolbur isolates were obtained from H. obsoletus locations in italics.* Sample sites from [32].
(PPT)
Genotype frequencies of stolbur tuf-a vmp1 , stamp and secY genotypes estimated in four pre-defined European regions, (1) the host-shift population Germany (D), (2) a putative transition population Switzerland (CH), and the two putative regions of origin, (3) Italy and southern France (I/F), and (4) Slovenia and Croatia (SLO/HR). The region Switzerland includes Swiss samples north and west of the Alps. The region Italy/France includes samples from the Swiss canton Ticino, situated south of the Alps and part of the Italian Po Basin. The gene stol-11 (accession no. JQ977744) was monomorphic in tuf-a stolbur and is not included. n = sample size. Genotype names of the present study are given with the corresponding SEE-ERANET nomenclature in brackets.
(DOC)
UPGMA consensus tree (2000 bootstrap replicates) showing monophyly of stolbur tuf-a vmp1 genotypes, N1–N13, nested within paraphyletic tuf-b genotypes. The vmp1 names in the present study are shown with the corresponding SEE-ERANET nomenclature in brackets.
(PPT)
Vmp1 peptide sequence evolution in 13 stolbur tuf-a genotypes associated with stinging nettle and 9 tuf-b genotypes associated with field bindweed observed in this study. Tuf-a is characterised by a diagnostic penta-peptide at position 165–169. The three repeated VMP1 domains begin at positions 115, 199, 281. Same coloured boxes show origins and putative rearrangement or hybrid motifs. The vmp1 names in the present study are shown with Genbank accession numbers and the corresponding SEE-ERANET nomenclature in brackets.
(XLS)
Stamp peptide sequence evolution in seven stolbur tuf-a genotypes associated with stinging nettle and two tuf-b genotypes associated with field bindweed observed in this study. Boxes indicate the putative signal of inter-strain hybridisatin between tuf-a (s1, s6) and tuf-b strains. The stamp names in the present study are shown with Genbank accession numbers and the corresponding SEE-ERANET nomenclature in brackets.
(XLS)
Multilocus genotypes of 33 stolbur tuf-a isolates for which all three polymorphic genes ( secY , vmp1 , stamp ) were scored. Genotype names are given for the present study with the corresponding SEE-ERANET nomenclature in brackets.
(DOC)
Hyalesthes obsoletus mtDNA haplotype frequencies in three predefined Western European geographic regions, the putative area of origin (Italy) and the two regions of expansion: West (France, Switzerland) and East (Slovenia, Croatia, Germany) of the European Alps. The table shows the frequency distributions used in the demographic expansion analysis. The regions were based on findings in [32] and confirmed in the present study, of an eastern and a western historical origin of the haplotypes aa and bb. The frequency distribution in each of the regions East and West are slightly biased towards an overrepresentation of aa or bb, respectively, because animals (haplotypes) in a secondary contact zone between the eastern and western lineages were grouped with the historical region of origin, not the current countries as listed in Table 2.
(DOC)
Mitochondrial DNA haplotypic network of the genes COII and ND1 (1311 bp) assayed in Western European Hyalesthes obsoletus . The haplotype “ab”, connected to the “Pannonian” haplotype “ec”, constitutes the root of the network. The haplotypes “ab” and “bb” are centres of star-like sub-networks, which show signals of demographic expansions in Italy and France/Switzwerland, respectively. The geographic distribution of the haplotypes is presented in Table 2 and Appendix S8.
(PPT)
Acknowledgments
We thank the organisers of the 2nd European Bois Noir Workshop (Castelbrando) where this paper was presented. We thank D. Klebsch, C. Gross, B. Maniyar, Y. Kappel, D. Kröhner, C. Neuerburg who provided valuable help in laboratory. This study could not have been done without the kind help of the people who shared H. obsoletus specimens with us for this study: M. Breuer, A. Schartl, M. Stark-Urnau, E. Angelini, A. Batlle, E. Boudon-Padieu, A. Bressan, P. Kuntzmann, G. Pasquini, H. Reisenzein, N. Zeisner, G. Seljak, D. Zsofia, E. Ibolya, J.-L. Danet, S. Malembic-Maher, S. Baric, N. Mori, Z. Budinscak, M. Elekes, Z. Der, J. Slobodan. This work benefitted from the SEE-ERANET network ”Epidemiology of phytoplasma disease of economical importance in South-Eastern Europe”. The authors greatly acknowledge I. Toševski and J. Jovic for discussions and an anonymous reviewer for improving the manuscript.
Funding Statement
This research was funded by “Stiftung Rheinland-Pfalz für Innovation,” grant number 0861 (http://www.stiftung-innovation.rlp.de/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hogenhout SA, Redinbaugh MG, Ammar E (2003) Plant and animal rhabdovirus host range: a bug's view. Trends Microbiol 11: 264–271. [DOI] [PubMed] [Google Scholar]
- 2. Weiver SC, Reisen WK (2010) Present and future arboviral threats. Antiviral Res 85: 328–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee IM, Davis RE, Gundersen-Rindal DE (2000) Phytoplasma: phytopathogenic mollicutes. Annu Rev Microbiol 2000 54: 221–55. [DOI] [PubMed] [Google Scholar]
- 4. Christensen NM, Axelsen KB, Nicolaisen M, Schulz A (2005) Phytoplasmas and their interactions with hosts. Trends Plant Sci 10: 526–535. [DOI] [PubMed] [Google Scholar]
- 5. Maixner M (2011) Recent advances in Bois noir research. Petria 21: 95–108. [Google Scholar]
- 6. Mannelli A, Bertolotti, Gern L, Gray J (2012) Ecology of Borrelia burgdorferi sensu lato in Europe: transmission dynamics in multi-host systems, influence of molecular processes and effects of climate change. FEMS Microbiol Rev 36: 837–861. [DOI] [PubMed] [Google Scholar]
- 7. Chen H, White DJ, Caraco TB, Stratton HH (2005) Epidemic and spatial dynamics of Lyme disease in New York State, 1990–2000. J Med Entomol 42: 899–908. [DOI] [PubMed] [Google Scholar]
- 8. Fabiszewski AM, Umbanhowar J, Mitchell CE (2010) Modelling landscape-scale pathogen spillover between domesticated and wild hosts: Asian soybean rust and kudzu. Ecol Appl 20: 582–592. [DOI] [PubMed] [Google Scholar]
- 9. Tatem AJ, Hay SI, Rogers DJ (2006) Global traffic and disease vector dispersal. Proc Natl Acad Sci 103: 6242–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aplin K, Suzuki H, Chinen AA, Chesser RT, ten Have J, et al. (2011) Multiple geographic origins of commensalism and complex dispersal history of black rats. Plos One 6: e26357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weintraub PG, Beanland L (2006) Insect vectors of phytoplasmas. Ann Rev Entomol 51: 91–111. [DOI] [PubMed] [Google Scholar]
- 12. Lee IM, Gundersen-Rindal DE, Davis RE, Bartoszyk IM (1998) Revised classification scheme of phytoplasmas based on ribosomal RFLP analyses of 16S rRNA and ribosomal protein gene sequences. Int J Syst Bacteriol 48: 1153–1169. [DOI] [PubMed] [Google Scholar]
- 13. IRPCM (2004) ‘Candidatus Phytoplasma’, a taxon for the wall-less, non-helical prokaryotes that colonize plant phloem and insects. Int J Syst Evol Microbiol 54: 1243–1255. [DOI] [PubMed] [Google Scholar]
- 14. Blattný C, Brčàk J, Pozde˘na J, Dlabola J, Limberk J, Bojňansky V (1954) Die Übertragung des Stolburvirus bei Tabak und Tomaten und seine virogeographischen Beziehungen. Phytopatol Zeitschrift 22: 381–416. [Google Scholar]
- 15. Bojnansky V, Karlikova V (1955) Rozsirenie stolburu a Hyalesthes obsoletus Sign. na Slovensku so zvlástnym zretefom na jeho prirodzené ohniska, skodlivost a moznosti boja proti nemu. Polnohospodarstvo 2: 326–345. [Google Scholar]
- 16. Langer M, Maixner M (2004) Molecular characterization of grapevine yellows associated phytoplasmas of the stolbur-group based on RFLP-analysis of non-ribosomal DNA. Vitis 43: 191–199. [Google Scholar]
- 17.Credi R, Terlizzi F, Milanesi L, Bondavalli R, Cavallini G, et al. (2006) Wild host plants of stolbur phytoplasma and its vector, Hyalesthes obsoletus, at sites of grapevine Bois noir occurrence in Emilia-Romagna, Italy. Extended abstracts 15th Meeting ICVG, Stellenbosch, South Africa, 3–7 April 2006. pp. 182–183.
- 18. Fialová R, Válová P, Balakishiyeva G, Danet J-L, Šafárová D, et al. (2009) Genetic variability of stolbur phytoplasma in annual crop and wild plant species in South Monrovia. J Plant Pathol 91: 411–416. [Google Scholar]
- 19. Kessler S, Schaerer S, Delabays N, Turlings TCJ, Trivellone V, et al. (2011) Host plant preferences of Hyalesthes obsoletus, the vector of the grapevine yellows disease ‘bois noir’ in Switzerland. Ent Exp Appl 139: 60–67. [Google Scholar]
- 20. Bressan A, Turata R, Maixner M, Spiazzi S, Boudon-Padieu E, et al. (2007) Vector activity of Hyalesthes obsoletus living on nettles and transmitting a stolbur phytoplasma to grapevines: a case study. Ann Appl Biol 150: 331–339. [Google Scholar]
- 21. Ember I, Acs Z, Munyaneza JE, Crosslin J, Kolber M (2011) Survey and molecular detection of phytoplasmas associated with potato in Romania and southern Russia. Eur J Plant Pathol 130: 367–377. [Google Scholar]
- 22. Fos A, Danet J-L, Zreik L, Garnier L, Bové LM (1992) Use of a monoclonal antibody to detect the stolbur mycoplasmalike organism in plants and insects and to identify a vector in France. Plant Disease 76: 1092–1096. [Google Scholar]
- 23. Maixner M, Ahrens U, Seemüller E (1995) Detection of the German grapevine yellows (Vergilbungskrankheit) MLO in grapevine, alternative hosts and a vector by specific PCR procedure. Eur J Plant Pathol 101: 241–250. [Google Scholar]
- 24. Sforza R, Clair D, Daire X, Larrue J, Boudon-Padieu E (1998) The role of Hyalesthes obsoletus (Hemiptera : Cixiidae) in the occurrence of bois noir of grapevines in France. J Phytopathol 146: 549–556. [Google Scholar]
- 25. Lessio F, Tedeschi R, Alma A (2007) Population dynamics, host plants and infection rate with stolbur phytoplasma of Hyalesthes obsoletus Signoret in north-western Italy. J Plant Pathol 89: 97–102. [Google Scholar]
- 26. Maixner M, Johannesen J, Michel K, Lux B, Seitz A (2007) Host plant specificity of Hyalesthes obsoletus and consequences for “bois noir” epidemiology”. Bull Insectology 60: 399–400. [Google Scholar]
- 27. Hoch H, Remane R (1985) Evolution und Speziation der Zikaden-Gattung Hyalesthes SIGNORET, 1865 (Homoptera Auchenorrhyncha Fulgoroidea Cixiidae). Marburger Entomol Pub 2: 1–427. [Google Scholar]
- 28. Sergel R (1986) Ein weiterer Nachweis der Cixiide Hyalesthes obsoletus Signoret in Franken (Homopetra: Auchenorrhyncha:Fulgoroidea). Abhand Naturwissenschaft Ver Würzburg 25: 81–82. [Google Scholar]
- 29. Kuntzmann K, Bogen E, Renel C (2008) Bois noir disease of the grapevine in Alsace: field transmission, observations made on symptomatology and reduction of transmission risk by the vector Hyalesthes obsoletus Signoret. IOBC/wprs Bull 36: 127–136. [Google Scholar]
- 30.Maixner M, Johannesen J, Seitz A (2009) Aspects of the interaction of stolbur phytoplasma, vectors and host plants in the two epidemic systems of bois noir. Progrès Agricole et Viticole, 2009, Hors Série – Extended abstracts 16th Meeting of ICVG, Dijon, France, 141–142.
- 31. Kehrli P, Kessler S, Schaerer S, Delabays N (2011) Distribution and host plant preferences of Hyalesthes obsoletus, the vector of bois noir disease, in Switzerland. Integrated protection and production in viticulture. IOBC/wprs Bull 67: 3–8. [Google Scholar]
- 32. Johannesen J, Lux B, Michel K, Seitz A, Maixner M (2008) Invasion biology and host specificity of the grapevine yellows disease vector Hyalesthes obsoletus in Europe. Entomol Exp Appl 126: 217–227. [Google Scholar]
- 33. Imo M, Lüneburg J, Hankeln T, Seitz A, Johannesen J (2011) Highly polymorphic di- and trinucleotide microsatellite markers for the grapevine yellows disease vector Hyalesthes obsoletus (Cixiidae). Eur J Entomol 108: 161–163. [Google Scholar]
- 34. Cimerman A, Arnaud G, Foissac X (2006) Stolbur phytoplasma genome survey achieved using a suppression substractive hybridization approach with high specificity. Appl Environ Microbiol 72: 3274–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cimerman A, Pacifico D, Salar P, Marzachì C, Foissac X (2009) Striking diversity of vmp1, a variable gene encoding a putative membrane protein of the Stolbur Phytoplasma. Appl Environ Microbiol 75: 2951–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fabre A, Danet J-L, Foissac X (2011) The stolbur phytoplasma antigenic membrane protein stamp is submitted to diversifying positive selection. Gene 472: 37–41. [DOI] [PubMed] [Google Scholar]
- 37. Daire X, Clair D, Reinert W, Boudon-Padieu E (1997) Detection and differentiation of grapevine yellows phytoplasmas belonging to the elm yellows group and to the stolbur subgroup by PCR amplification of non-ribosomal DNA. Eur J Plant Path 103: 507–514. [Google Scholar]
- 38. Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Res 10: 564–567. [DOI] [PubMed] [Google Scholar]
- 39. Librado P, Rozas J (2009) DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- 40. Clement M, Posada D, Crandall K (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9: 1657–1660. [DOI] [PubMed] [Google Scholar]
- 41.Swofford DL (1999) Phylogenetic analysis using parsimony (and other methods), version 4.0. Sunderland, MA: Sinauer Associates.
- 42. Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmidt HA, Strimmer K, Vingron M, von Haeseler A (2002) TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18: 502–504. [DOI] [PubMed] [Google Scholar]
- 45. Ray N, Currat M, Excoffier L (2003) Intra-deme molecular diversity in spatially expanding populations. Mol Biol Evol 20: 76–86. [DOI] [PubMed] [Google Scholar]
- 46.Malembic-Maher S, Desqué D, Fabre A, Carle P, Johannesen J, et al.. (2010) Insect vector-specific diversification of variable membrane proteins (VMP) of stolbur and flavescence dorée phytoplasmas. In: 18th International Congress of the International Organization for Mycoplasmology. Chianciano Terme, Italy. pp. 52–53.
- 47. Nunney L, Yuan X, Bromley R, Hartung J, Montero-Astúa M, et al. (2010) Population genomic analysis of a bacterial plant pathogen: novel insight into the origin of Pierce's disease of grapevine in the US. PLOS One 5: e15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marin M, Peisig O, Wingfield BD, Kirisits T, Wingfield MJ (2009) Single sequence repeat markers reflect diversity and geographic barriers in Eurasian populations of the conifer pathogen Ceratocystis polonica . Forest Pathology 39: 249–265. [Google Scholar]
- 49. Tsui CKM, Roe AD, El-Kassaby YA, Rice AV, Alamouti SM, et al. (2012) Population structure and migration pattern of a conifer pathogen, Grosmannia clavigera, as influenced by its symbiont, the mountain pine beetle. Mol Ecol 21: 71–86. [DOI] [PubMed] [Google Scholar]
- 50. Humphrey PT, Caporale DA, Brisson D (2010) Uncoordinated phylogeography of Borrelia burgdorferi and its tick vector, Ixodes scapularis . Evolution 64: 2653–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brinkerhoff RJ, Folsom-O'keefe CM, Tsao K, Diul-Wasser MA (2011) Do birds affect Lyme disease rick? Range expansion of the vector-pathogen Borrelia burgdorferi . Front Ecol Environ 9: 103–110. [Google Scholar]
- 52. Gladieux P, Guérin F, Giraud T, Caffier V, Lemarie C, et al. (2011) Emergence of novel fungal pathogens by ecological speciation: importance of reduced viability of immigrants. Mol Ecol 20: 4521–4532. [DOI] [PubMed] [Google Scholar]
- 53. Van Putten WF, Elzinga JA, Biere A (2007) Host fidelity of the pollinator guilds of Silene dioica and Silene latifolia: possible consequences fpr sympatric host race differentiation of a vectored plant disease. Int J Plant Sci 168: 421–434. [Google Scholar]
- 54.Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press, Chicago.
- 55. Herschler A (1937) Erfahrungen über Wachstumsstörungen an Reben durch Bodenverhältnisse. Deutscher Weinbau 16: 177–179. [Google Scholar]
- 56. Wagner W (1939) Die Zikaden des Mainzer Beckens (zugleich eine Revision der Kirschbaum'schen Arten in der Umgebung von Wiesbaden). Jahrb Nass Ver Naturkunde Wiesbaden 86: 77–212. [Google Scholar]
- 57. Mendgen K (1971) Untersuchungen über eine Vergilbungskrankheit der Reben an Rhein, Mosel und Saar. Weinberg und Keller 18: 345–431. [Google Scholar]
- 58.Kast WK, Stark-Urnau M, Bleyer K (2008) 1st European Bois noir Workshop 2008. Proceedings. Staatliche Lehr und Versuchungsanstatlt für Wein- und Obstbau, Weinsberg, Germany. 81 p.
- 59. Angelini E, Martini M, Mori N, Musetti R (2011) Proceedings of the workshop. 2nd Euroepan Bois noir Workshop 2011, Castelbrando, Cison do Valmarino (TV), Italy. Petria 21: 85–180. [Google Scholar]
- 60.Bertaccini A, Maini S (2007) First International Phytoplasmologist Working Group Meeting, Bologna (Italy). Bull Insectology, Vol. LX (2). 407 p.
- 61.Bertaccini A, Maini S (2011) Second International Phytoplasmologist Working Group Meeting, Bologna (Italy). Bulletin of Insectology, Vol. LXIV (Supplement). 304 p.
- 62.Boudon-Padieu E, Maixner M (2008) Potential effects of climate change on distribution and activity of insect vectors of grapevine pathogens. Global warming, which potential impacts on the vineyards? - Proceedings of the international and multi-disciplinary Colloquium, University of Dijon. pp. 1–8.
- 63. Brasier CM (2001) Rapid evolution of introduced plant pathogens via interspecific hybridization. Bioscience 51: 123–133. [Google Scholar]
- 64. Smith GJD, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, et al. (2009) Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459: 1122–1125. [DOI] [PubMed] [Google Scholar]
- 65. Bai X, Zhang J, Holford IR, Hogenhout SA (2004) Comparative genomics identifies genes shared by distantly related insect-transmitted plant pathogenic mollicutes. FEMS Microbiol Lett 235: 249–258. [DOI] [PubMed] [Google Scholar]
- 66. Maixner M, Reinert W, Darimont H (2000) Transmission of grapevine yellows by Oncopsis alni (Schrank) (Auchenorrhyncha : Macropsinae). Vitis 39: 83–84. [Google Scholar]
- 67. Arnaud G, Malembic-Maher S, Salar P, Bonnet P, Maixner M, et al. (2007) Multilocus sequence typing confirms the close genetic inter-relatedness between three distinct flavescence dorée phytoplasma strain clusters and group 16SrV phytoplasmas infecting grapevine and alder in Europe. Appl Environ Microbiol 73: 4001–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Filippin L, Jovic J, Cvrkovic T, Forte V, Clair D, et al. (2009) Molecular characteristics of phytoplasmas associated with Flavescence doree in clematis and grapevine and preliminary results on the role of Dictyophara europaea as a vector. Plant Pathol 58: 826–837. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sampling sites of the stolbur vector H. obsoletus in France, North Switzerland and Germany. Stolbur isolates were obtained from H. obsoletus locations in italics.* Sample sites from [32].
(PPT)
Sampling sites of the stolbur vector H. obsoletus in Italy, South Switzerland (Ticino), Slovenia, Croatia and Austria. Stolbur isolates were obtained from H. obsoletus locations in italics.* Sample sites from [32].
(PPT)
Genotype frequencies of stolbur tuf-a vmp1 , stamp and secY genotypes estimated in four pre-defined European regions, (1) the host-shift population Germany (D), (2) a putative transition population Switzerland (CH), and the two putative regions of origin, (3) Italy and southern France (I/F), and (4) Slovenia and Croatia (SLO/HR). The region Switzerland includes Swiss samples north and west of the Alps. The region Italy/France includes samples from the Swiss canton Ticino, situated south of the Alps and part of the Italian Po Basin. The gene stol-11 (accession no. JQ977744) was monomorphic in tuf-a stolbur and is not included. n = sample size. Genotype names of the present study are given with the corresponding SEE-ERANET nomenclature in brackets.
(DOC)
UPGMA consensus tree (2000 bootstrap replicates) showing monophyly of stolbur tuf-a vmp1 genotypes, N1–N13, nested within paraphyletic tuf-b genotypes. The vmp1 names in the present study are shown with the corresponding SEE-ERANET nomenclature in brackets.
(PPT)
Vmp1 peptide sequence evolution in 13 stolbur tuf-a genotypes associated with stinging nettle and 9 tuf-b genotypes associated with field bindweed observed in this study. Tuf-a is characterised by a diagnostic penta-peptide at position 165–169. The three repeated VMP1 domains begin at positions 115, 199, 281. Same coloured boxes show origins and putative rearrangement or hybrid motifs. The vmp1 names in the present study are shown with Genbank accession numbers and the corresponding SEE-ERANET nomenclature in brackets.
(XLS)
Stamp peptide sequence evolution in seven stolbur tuf-a genotypes associated with stinging nettle and two tuf-b genotypes associated with field bindweed observed in this study. Boxes indicate the putative signal of inter-strain hybridisatin between tuf-a (s1, s6) and tuf-b strains. The stamp names in the present study are shown with Genbank accession numbers and the corresponding SEE-ERANET nomenclature in brackets.
(XLS)
Multilocus genotypes of 33 stolbur tuf-a isolates for which all three polymorphic genes ( secY , vmp1 , stamp ) were scored. Genotype names are given for the present study with the corresponding SEE-ERANET nomenclature in brackets.
(DOC)
Hyalesthes obsoletus mtDNA haplotype frequencies in three predefined Western European geographic regions, the putative area of origin (Italy) and the two regions of expansion: West (France, Switzerland) and East (Slovenia, Croatia, Germany) of the European Alps. The table shows the frequency distributions used in the demographic expansion analysis. The regions were based on findings in [32] and confirmed in the present study, of an eastern and a western historical origin of the haplotypes aa and bb. The frequency distribution in each of the regions East and West are slightly biased towards an overrepresentation of aa or bb, respectively, because animals (haplotypes) in a secondary contact zone between the eastern and western lineages were grouped with the historical region of origin, not the current countries as listed in Table 2.
(DOC)
Mitochondrial DNA haplotypic network of the genes COII and ND1 (1311 bp) assayed in Western European Hyalesthes obsoletus . The haplotype “ab”, connected to the “Pannonian” haplotype “ec”, constitutes the root of the network. The haplotypes “ab” and “bb” are centres of star-like sub-networks, which show signals of demographic expansions in Italy and France/Switzwerland, respectively. The geographic distribution of the haplotypes is presented in Table 2 and Appendix S8.
(PPT)