Abstract
Previous studies in dementia epidemiology have reported higher Alzheimer's disease rates in African-Americans when compared with White Americans. To determine whether genetically determined African ancestry is associated with neuropathological changes commonly associated with dementia, we analyzed a population-based brain bank in the highly admixed city of São Paulo, Brazil. African ancestry was estimated through the use of previously described ancestry-informative markers. Risk of presence of neuritic plaques, neurofibrillary tangles, small vessel disease, brain infarcts and Lewy bodies in subjects with significant African ancestry versus those without was determined. Results were adjusted for multiple environmental risk factors, demographic variables and apolipoprotein E genotype. African ancestry was inversely correlated with neuritic plaques (P=0.03). Subjects with significant African ancestry (n=112, 55.4%) showed lower prevalence of neuritic plaques in the univariate analysis (odds ratio (OR) 0.72, 95% confidence interval (CI) 0.55–0.95, P=0.01) and when adjusted for age, sex, APOE genotype and environmental risk factors (OR 0.43, 95% CI 0.21–0.89, P=0.02). There were no significant differences for the presence of other neuropathological alterations. We show for the first time, using genetically determined ancestry, that African ancestry may be highly protective of Alzheimer's disease neuropathology, functioning through either genetic variants or unknown environmental factors. Epidemiological studies correlating African-American race/ethnicity with increased Alzheimer's disease rates should not be interpreted as surrogates of genetic ancestry or considered to represent African-derived populations from the developing nations such as Brazil.
Keywords: Alzheimer's disease, ancestry, dementia, ethnicity, neuropathology, race
Introduction
Self-declared race and skin color are often used as surrogates for genetic ancestry, despite being poor biological classifiers, especially in countries with admixed populations where significant overlaps between groups exist.1 Furthermore, racial categorization is modifiable by environmental factors such as sunlight exposure, socioeconomic level, physical appearance and cultural aspects.2, 3, 4 The marked epidemiological differences in health status between racial groups in countries such as Brazil and the United States are likely a combination of genetic and environmental factors, particularly socioeconomic levels.5, 6
Dementia is a complex phenotype caused by frequently overlapping neuropathological processes such as neuritic plaques and neurofibrillary tangles (Alzheimer's disease), small vessel disease and/or brain infarcts (vascular dementia) and synuclein deposits (Lewy body disease and Parkinson's disease), as well as other rarer alterations.7 The clinical diagnosis of dementia is further influenced by the educational level, language and cultural aspects. Several studies have shown that African Americans are more frequently diagnosed with dementia (in general) and Alzheimer's disease than Caucasians.8, 9, 10, 11, 12 These differences may be caused by genetic variants or the environment.13, 14, 15, 16 Few studies have focused on neuropathological post-mortem diagnosis and none on ancestry-informative marker (AIM) determined genetic ancestry.17, 18, 19
The quantitative assessment of ancestry using AIMs has been previously demonstrated to be useful in breast cancer epidemiology and lung-function prediction.20, 21 The population of Brazil is highly admixed, with major historic contributions from European immigrants, African slaves and native Amerindians.22, 23, 24 The genetic structure of the population of Brazil is approximately 80% European, 15% African and 5% Amerindian, with significant variation between regions.25, 26
Materials and methods
Study population
Brain samples from the Brazilian Aging Brain Study Group of the University of São Paulo Medical School, collected from 2004–2009, were studied.27 Exclusion criteria included age at death of less than 50 years, causes of death or tissue condition that impeded neuropathological analysis, informants without knowledge of the functional status of subjects (minimum 1 visit/week) and violent/criminal deaths. Tissue donations were obtained in the municipal São Paulo Autopsy Service, in Brazil. The population base includes all inhabitants of the city of São Paulo, ∼11 million people (5.6% of the population of Brazil), and study samples do not significantly deviate from census data for age, sex, race, years of schooling or socioeconomic levels28 (data not shown). All tissue donations were made by next-of-kin after providing informed consent, and the study was approved by the institutional review boards of all participating institutions. Knowledgeable informants were interviewed by nurses trained specifically for the questionnaires, including cognitive evaluation (Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) and Clinical Dementia Rating (CDR)) and demographics.29, 30
Neuropathological assessment
All neuropathological diagnoses were carried out by two trained specialists (LTG and MPA). Brains were inspected macroscopically, and lesions of Alzheimer's disease (neuritic plaques and neurofibrillary tangles), Lewy bodies and small vessel disease (microinfarcts, lacunes and small vessel disease) were scored according to the accepted criteria.31, 32, 33 Immunohistochemistry was done with antibodies against β-amyloid (4G8, 1/5000, Signet Laboratories, Dedhan, MA, USA), phospho-tau (PHF-1, 1/1000, gift from Peter Davies, New York City, NY, USA) and α-synuclein (EQV-1, 1/10 000, gift from Kenji Ueda, Tokyo, Japan). If TDP-43pathy was suspected, immunostaining for TDP-43 (1/500, ProteinTech Group, Chicago, IL, USA) was performed. All sections were submitted to antigenic retrieval. The reactions were detected using the Vectastain Elite ABC Kit method (Vector Laboratories, Burlingame, CA, USA). Neuritic plaques were classified as absent, mild, moderate or frequent. Neurofibrillary tangles were classified according to the Braak score of 0–VI (ref. 32).
Ancestry estimation and genotyping
Samples were genotyped for 90 single-nucleotide polymorphisms previously described from an AIM panel reported to efficiently separate Western European, African, Amerindian and East Asian populations.34 The AIMs were genotyped using Sequenom MassArray from the Genotyping Facility of the Broad Institute of MIT and Harvard. Samples with more than 90% call rate were included in the analysis. Single-nucleotide polymorphisms with >5% no-call rate were excluded from the analysis. APOE genotype (single-nucleotide polymorphisms rs429358 and rs7412) was determined by RealTime PCR, in duplicates, as previously described.35
We estimated ancestry by modeling four ancestral populations (k=4) with admixture in Structure version 2.3.3 (100 000 burn-ins, 200 000 iterations, LOCPRIOR=0), alongside samples from the Human Genome Diversity Panel and the HapMap (Phase I) project, both publicly available.36, 37, 38
Statistical analysis
Neuropathological variables (neuritic plaques, small vessel disease, infarcts, Lewy bodies) were dichotomized between presence and absence, and neurofibrillary tangles were divided between Braak score 0–III and IV–VI. Ancestry was analyzed as a quantitative variable and also dichotomized at ‘significant' African ancestry (>2%) to separate the two major ancestry groups present—those with European-only ancestry from admixed individuals (mainly European and African). This cutoff was defined based on the level of African ancestry present in 90% of Caucasian samples from the Human Genome Diversity and HapMap Projects. Subjects of Asian origin (n=6) were excluded from analysis. No subjects were identified as Amerindian by next-of-kin.
Kruskal–Wallis test was used in the initial analysis and confirmed using Spearman's rank correlation (two-sided). Logistic regression was used to model the effect of African ancestry on the presence of neuropathology. Each pathology was considered a separate outcome. Analysis was carried out with adjustment for age at death, sex, years of schooling, socioeconomic level, APOE genotype and family reported cardiovascular risk factors (hypertension, diabetes, hypercholesterolemia, heart failure, arrhythmia, smoking, alcohol consumption and sedentary lifestyle). A subgroup analysis using ancestry restricted to self-declared whites (75% of the samples) was also done (Supplementary Figure 2). Significant findings were considered when P<0.05.
Results
Ancestry and race
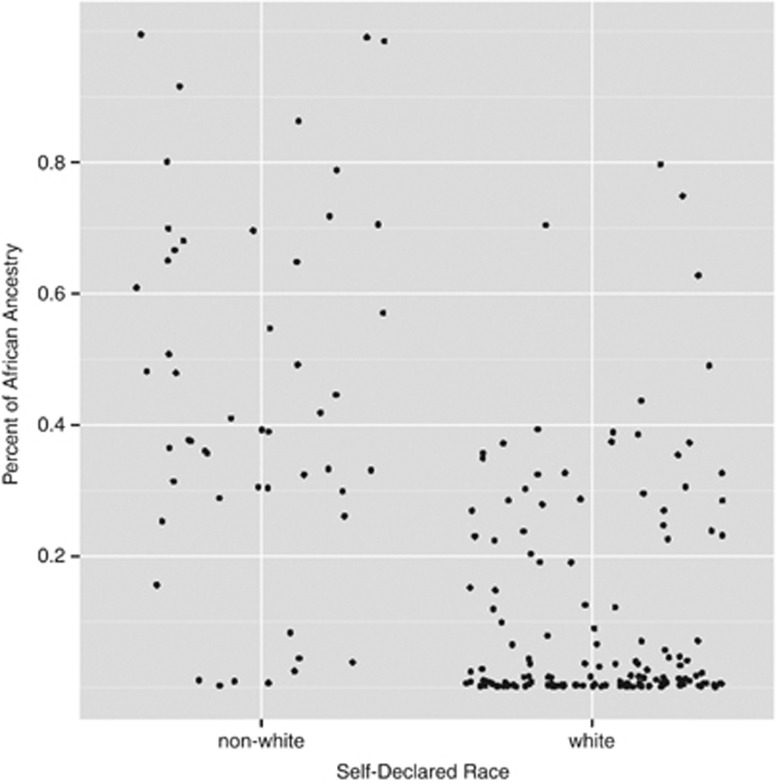
The study consisted of 202 brain samples with complete genotyping, neuropathology and close relatives' interviews. There were 112 individuals (55.4%) with significant African ancestry (Table 1, Supplementary Figure 2). Non-white race and genetically determined African ancestry were highly correlated (P<0.01). Some self-declared whites were over 70% African, while a few non-whites had more than 99% European ancestry (Figure 1). Table 1 shows demographic characteristics for subjects with significant (>2%) African ancestry and those without. African ancestry was associated with lower educational (P<0.05) and socioeconomic levels (P<0.01). There were no differences in cognitive status between groups, as measured by IQCODE or CDR scale.
Table 1. Study characteristics.
| African ancestry | Non-African ancestry | P-value | |
|---|---|---|---|
| Number | 112 | 90 | |
| Age at death (mean±s.d.) | 74.5±11.9 | 76.9±11.4 | 0.18 |
| Gender (% female) | 57.3% | 60.0% | 0.68 |
| Socioeconomic level (mean ABIPEME score ±s.d.) | 15.5±9.4 | 20.3±9.0 | <0.001 |
| Educational status (mean years of schooling ±s.d.) | 3.7±3.7 | 4.8±3.8 | 0.03 |
| Race (% self-declared White) | 58.0% | 95.5% | <0.001 |
| Cognitive status (% CDR=0) | 67.2% | 66.2% | 0.85 |
| Cognitive status (mean IQCODE ±s.d.) | 3.44±0.72 | 3.40±0.68 | 0.81 |
| APOE4 status (% positive) | 26.8% | 20.0% | 0.26 |
Figure 1.
Individual African ancestry estimates according to self-declared race in Brazil.
Ancestry and neuropathology
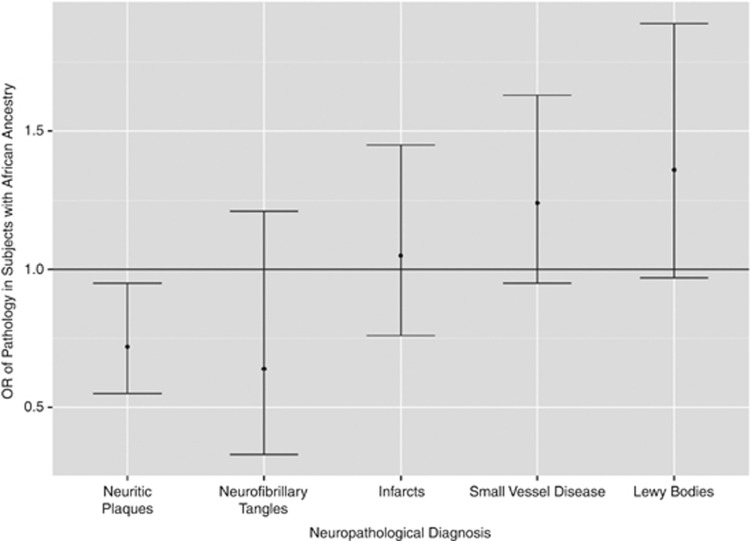
African ancestry was inversely correlated with neuritic plaques (P=0.03, two-sided Spearman's correlation), but not with other neuropathological changes (P>0.20 for neurofibrilary tangles, infarcts, small vessel disease and Lewy bodies). The odds ratio (OR) of each neuropathological change for African versus non-African ancestry is shown in Figure 2 and Table 2. The same analysis was done in self-declared Whites (75% of the sample), with similar results (Supplementary Figure 2).
Figure 2.
Odds ratio of neuropathological alterations.
Table 2. Odds ratio (OR) of pathology, African versus non-African.
| OR | Lower | Upper | P-value | |
|---|---|---|---|---|
| Neuritic plaques | 0.72 | 0.55 | 0.95 | 0.01 |
| Neurofibrillary tangles | 0.64 | 0.33 | 1.21 | 0.16 |
| Arteriolosclerosis | 1.24 | 0.95 | 1.63 | 0.18 |
| Infarcts | 1.05 | 0.76 | 1.45 | 0.78 |
| Lewy bodies | 1.36 | 0.97 | 1.89 | 0.14 |
The prevalence of Alzheimer's disease neuritic plaques was significantly lower in subjects with African ancestry (0.72, 95% confidence interval (CI) 0.55–0.95, P=0.01) (Figure 2). African ancestry also correlated with less pathology in the subgroup analysis restricted to self-declared whites (0.63, 95% CI 0.42–0.95, P=0.02) (Supplementary Figure 1). The prevalence of Alzheimer's related neurofibrilary tangles was higher in subjects with African compared with non-African ancestry, but this was not statistically significant. The prevalence of small vessel disease, brain infarcts and Lewy bodies was higher in subjects with African compared with non-African ancestry, but none were statistically significant. Self-declared race showed no statistically significant differences for any of the neuropathological end points (Supplementary Figure 1).
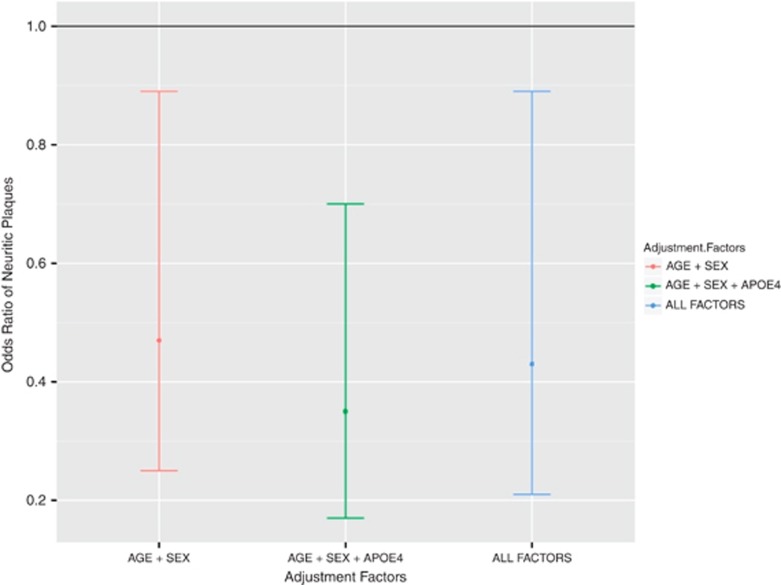
Adjustment for possible confounding factors did not alter the findings (Figure 3). Neuritic plaques were less prevalent in subjects with African ancestry when adjusted for age and sex (OR 0.47, 95% CI 0.25–0.89, P=0.02), when adjusting for age, sex and APOE4 status (OR 0.35, 95% CI 0.17–0.70, P<0.01), and when adjusting for all factors including socioeconomic level, educational level and cardiovascular risk factors (OR 0.43, 95% CI 0.21–0.89, P=0.02; Table 3, Figure 3)
Figure 3.
Odds ratio of presence of neuritic plaques, comparing African with non-African ancestry, adjusted for age and sex; age, sex and APOE4 status; and all factors.
Table 3. Odds ratio (OR) of subjects with African ancestry after adjustment for major factors.
|
Neuritic plaques |
||||
|---|---|---|---|---|
| OR | Lower | Upper | P-value | |
| Adjusted for age+sex | 0.47 | 0.25 | 0.89 | 0.02 |
| Adjusted for age+sex+APOE4 | 0.35 | 0.17 | 0.70 | 0.003 |
| Adjusted for Age+sex+APOE4+ socioeconomic, education levels and cardiovascular risk factors | 0.43 | 0.21 | 0.89 | 0.02 |
Discussion
The Brazilian population is highly admixed, with >90% of its ancestry derived from African slaves and European immigrants, which makes it ideal for ancestry-related studies. Moreover, Brazilian law mandates that autopsies be performed in all persons without a death certificate, which provides a large recruitment base for populational studies in neuropathology, without bias toward demented persons. The University of São Paulo alone performs more than 14 000 autopsies per year, encompassing the full range of demographic variation of the city. The centering of all samples in a single institution also greatly reduced inter-rater variation in both interviews and pathology.
Contrary to previous studies, our results show that African ancestry is highly protective of Alzheimer's disease neuropathology (neuritic plaques), with an adjusted OR of 0.43. This suggests that unknown genetic variants or environmental factors associated with African ancestry reduce the accumulation of β-amyloid or increase its clearance. The results are robust and are not altered when studying only those who self-defined themselves as whites, when adjusting for APOE4 status only or when adjusting for age and sex only.
Previous studies with the United States population reported a significantly higher prevalence of dementia in African Americans when compared with Caucasians.8, 9, 10, 11, 12 These differences could be due to cultural differences in the performance on cognitive screening tests, such as the Mini Mental Status Examination, genetic differences between races, environmental differences or likely a combination of factors.13, 14, 15, 16 In a study comparing dementia in Nigerians and African Americans, the former had significantly lower disease rates. Our data suggest that these results might be explained not only by environmental differences, but also by the European admixture present in African Americans.39 Further studies are needed to confirm this.
Cardiovascular disease risk and stroke also vary between races, but statistical significance often disappears when adjustments for socioeconomic levels are applied.14, 40, 41 Lower educational and socioeconomic levels in those with higher African ancestry may create important differences in disease susceptibility, which is independent of genetics, but confounds the analysis.
To our knowledge, few studies have compared autopsy-diagnosed cases in different races and none used AIMs.17, 18, 19 The growing clinical use of AIMs was recently shown by Kumar et al21 as a tool for improved lung-function prediction in African Americans, although this should only be considered an intermediary step toward the use of specific disease-related variants in personalized medicine.42, 43 Furthermore, dementia is a complex clinical phenotype that may be caused by widely diverse pathologies, and post-mortem diagnosis remains the gold-standard. The specific neuropathology of Alzheimer's disease, Lewy body dementia and vascular dementia may have different underlying biological and genetic causes, and should therefore be studied separately.
A few notes of caution regarding this study should be pointed out. First, although we adjusted for multiple environmental and social factors as well as APOE genotype, other untested environmental factors may be confounding our ancestry results. Second, African populations are highly variable (more so than Europeans), and therefore we cannot state from our data that this effect is applicable to all African populations.13 It is unknown if there are population subgroups within Africa with different risk profiles for neuropathological alterations. Actually, our results reinforce the need for more Alzheimer's disease studies in the developing world, for trends identified in the United States may not be universal. Third, we have adjusted our analysis using known cardiovascular risk factors, to focus on unknown genetic factors, but many authors have suggested that ethnic/racial differences in dementia prevalence may in fact be derived from differences in these factors (for example, hypertension, diabetes). A future expansion of this data set is required to answer these questions with confidence, especially regarding vascular dementia.
In conclusion, our study shows, for the first time, that Alzheimer's neuropathological findings depend on the ancestral genetic background. It clearly demonstrates that the presence of neuritic plaques are reduced in persons with African ancestry in a population-based sample, independently of known confounding factors. This should serve as a basis for future genetic studies of Alzheimer's disease, as well as alert against overinterpreting epidemiological studies using race and clinically diagnosed dementia.
Acknowledgments
This work was supported by grants from CEPID-FAPESP (Centro de Pesquisa, Inovação e Difusão-Fundação de Amparo a Pesquisa do Estado de São Paulo), INCT (Instituto Nacional de Ciência e Tecnologia), FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo), the Alzheimer's Association (NIRG-09-131502 to LTG), CNPq, CAPES, and by the generous donation from Renata and Robert Ruhman. We thank the respondents for all the help and especially for agreeing to participate in the donation program. We also thank Professor Carmen Saldiva André for assistance in statistical analysis.
Author contributions: Study concept and design: Schlesinger, Grinberg. Acquisition of data: Schlesinger, Grinberg, Farfel, Suemoto, Ferretti and Andrade. Analysis and interpretation of data: Schlesinger, Grinberg and Zatz. Drafting of the manuscript: Schlesinger, Grinberg and Zatz. Critical revision of the manuscript for important intellectual content: Schlesinger, Grinberg, Jacob-Filho, Nitrini and Zatz. Statistical analysis: Schlesinger. Obtained funding: Grinberg, Jacob-Filho and Zatz. Administrative, technical or material support: Alba, Naslavsky, Licinio, Leite, Santos, Brentani and Pasqualucci. Study supervision: Jacob-Filho, Nitrini and Zatz.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Parra FC, Amado RC, Lambertucci JR, Rocha J, Antunes CM, Pena SD. Color and genomic ancestry in Brazilians. Proc Natl Acad Sci USA. 2003;100:177–182. doi: 10.1073/pnas.0126614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FJ.Who is Black?: One Nation's Definition10th anniversary ed. Pennsylvania State University Press: University Park, PA; 2001 [Google Scholar]
- Mörner M. Race Mixture in the History of Latin America. Little: University Park, PA; 1967. [Google Scholar]
- Shriver MD, Parra EJ, Dios S, Bonilla C, Norton H, Jovel C, et al. Skin pigmentation, biogeographical ancestry and admixture mapping. Hum Genet. 2003;112:387–399. doi: 10.1007/s00439-002-0896-y. [DOI] [PubMed] [Google Scholar]
- Anderson NB, Bulatao RA, Cohen B, National Research Council (U.S.). Panel on Race Ethnicity and Health in Later Life . Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. National Academies Press: Washington, DC; 2004. [PubMed] [Google Scholar]
- Cooper RS, Kaufman JS, Ward R. Race and genomics. N Engl J Med. 2003;348:1166–1170. doi: 10.1056/NEJMsb022863. [DOI] [PubMed] [Google Scholar]
- Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. 2009;6:e1000180. doi: 10.1371/journal.pmed.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirovic J, Prineas R, Loewenstein D, Bean J, Duara R, Sevush S, et al. Prevalence of dementia in three ethnic groups: the South Florida program on aging and health. Ann Epidemiol. 2003;13:472–478. doi: 10.1016/s1047-2797(02)00437-4. [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Bogardus ST, Inouye SK. Dementia and race: are there differences between African Americans and Caucasians. J Am Geriatr Soc. 2001;49:477–484. doi: 10.1046/j.1532-5415.2001.49096.x. [DOI] [PubMed] [Google Scholar]
- Hou CE, Yaffe K, Pérez-Stable EJ, Miller BL. Frequency of dementia etiologies in four ethnic groups. Dement Geriatr Cogn Disord. 2006;22:42–47. doi: 10.1159/000093217. [DOI] [PubMed] [Google Scholar]
- Schoenberg BS, Anderson DW, Haerer AF. Severe dementia. Prevalence and clinical features in a biracial US population. Arch Neurol. 1985;42:740–743. doi: 10.1001/archneur.1985.04210090004002. [DOI] [PubMed] [Google Scholar]
- Heyman A, Fillenbaum G, Prosnitz B, Raiford K, Burchett B, Clark C. Estimated prevalence of dementia among elderly black and white community residents. Arch Neurol. 1991;48:594–598. doi: 10.1001/archneur.1991.00530180046016. [DOI] [PubMed] [Google Scholar]
- Tishkoff SA, Reed FA, Friedlaender FR, Ehret C, Ranciaro A, Froment A, et al. The genetic structure and history of Africans and African Americans. Science. 2009;324:1035–1044. doi: 10.1126/science.1172257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravata DM, Wells CK, Gulanski B, Kernan WN, Brass LM, Long J, et al. Racial disparities in stroke risk factors: the impact of socioeconomic status. Stroke. 2005;36:1507–1511. doi: 10.1161/01.STR.0000170991.63594.b6. [DOI] [PubMed] [Google Scholar]
- Cook BL, Manning WG. Measuring racial/ethnic disparities across the distribution of health care expenditures. Health Serv Res. 2009;44:1603–1621. doi: 10.1111/j.1475-6773.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology. 1997;8:621–628. [PubMed] [Google Scholar]
- Pytel P. Vascular and Alzheimer-type pathology in an autopsy study of African-Americans. Neurology. 2006;66:433–435. doi: 10.1212/01.wnl.0000196472.93744.57. [DOI] [PubMed] [Google Scholar]
- Riudavets MA, Rubio A, Cox C, Rudow G, Fowler D, Troncoso JC. The prevalence of Alzheimer neuropathologic lesions is similar in blacks and whites. J Neuropathol Exp Neurol. 2006;65:1143–1148. doi: 10.1097/01.jnen.0000248548.20799.a3. [DOI] [PubMed] [Google Scholar]
- Wilkins CH, Grant EA, Schmitt SE, McKeel DW, Morris JC. The neuropathology of Alzheimer disease in African American and white individuals. Arch Neurol. 2006;63:87–90. doi: 10.1001/archneur.63.1.87. [DOI] [PubMed] [Google Scholar]
- Fejerman L, John EM, Huntsman S, Beckman K, Choudhry S, Perez-Stable E, et al. Genetic ancestry and risk of breast cancer among U.S. Latinas. Cancer Res. 2008;68:9723–9728. doi: 10.1158/0008-5472.CAN-08-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Seibold MA, Aldrich MC, Williams LK, Reiner AP, Colangelo L, et al. Genetic ancestry in lung-function predictions. N Engl J Med. 2010;363:321–330. doi: 10.1056/NEJMoa0907897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha MCd, Salzano FM. História dos índios no Brasil. São Paulo, SP: Fundação de Amparo à Pesquisa do Estado de São Paulo: Companhia das Letras: Secretaria Municipal de Cultura, Prefeitura do Município de São Paulo. Companhia das Letras: São Paulo; 1992. [Google Scholar]
- Hall GM. Slavery and African Ethnicities in the Americas: Restoring the Links. University of North Carolina Press: Chapel Hill; 2005. [Google Scholar]
- Basto FLdB.Síntese da História da Imigração no Brasil2 edn. sn: Rio de Janeiro, Brasil; 1998 [Google Scholar]
- Lins TC, Vieira RG, Abreu BS, Grattapaglia D, Pereira RW. Genetic composition of Brazilian population samples based on a set of twenty-eight ancestry informative SNPs. Am J Hum Biol. 2009;22:187–192. doi: 10.1002/ajhb.20976. [DOI] [PubMed] [Google Scholar]
- Pena SD, Bastos-Rodrigues L, Pimenta JR, Bydlowski SP. DNA tests probe the genomic ancestry of Brazilians. Braz J Med Biol Res. 2009;42:870–876. doi: 10.1590/s0100-879x2009005000026. [DOI] [PubMed] [Google Scholar]
- Grinberg LT, Ferretti RE, Farfel JM, Leite R, Pasqualucci CA, Rosemberg S, et al. Brazilian Aging Brain Study Group Brain bank of the Brazilian aging brain study group—a milestone reached and more than 1,600 collected brains. Cell Tissue Bank. 2007;8:151–162. doi: 10.1007/s10561-006-9022-z. [DOI] [PubMed] [Google Scholar]
- Ferretti R, Damin A, Brucki S, Morillo L, Perroco T, Campora F, et al. Post-Mortem diagnosis of dementia by informant interview. Dement Neuropsychol. 2010;4:138–144. doi: 10.1590/S1980-57642010DN40200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol Aging. 1997;18:S91–SS4. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- Nassir R, Kosoy R, Tian C, White PA, Butler LM, Silva G, et al. An ancestry informative marker set for determining continental origin: validation and extension using human genome diversity panels. BMC Genet. 2009;10:39. doi: 10.1186/1471-2156-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero O, Hortiguela R, Bullido MJ, Calero M. Apolipoprotein E genotyping method by real time PCR, a fast and cost-effective alternative to the TaqMan and FRET assays. J Neurosci Methods. 2009;183:238–240. doi: 10.1016/j.jneumeth.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Li JZ, Absher DM, Tang H, Southwick AM, Casto AM, Ramachandran S, et al. Worldwide human relationships inferred from genome-wide patterns of variation. Science. 2008;319:1100–1104. doi: 10.1126/science.1153717. [DOI] [PubMed] [Google Scholar]
- HapMap A haplotype map of the human genome. Nature. 2005;437:1299–12320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunniyi A, Baiyewu O, Gureje O, Hall KS, Unverzagt F, Siu SH, et al. Epidemiology of dementia in Nigeria: results from the Indianapolis-Ibadan study. Eur J Neurol. 2000;7:485–490. doi: 10.1046/j.1468-1331.2000.00124.x. [DOI] [PubMed] [Google Scholar]
- Chiu M, Austin PC, Manuel DG, Tu JV. Comparison of cardiovascular risk profiles among ethnic groups using population health surveys between 1996 and 2007. Can Med Assoc J. 2010;182:E301–EE10. doi: 10.1503/cmaj.091676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwamm LH, Reeves MJ, Pan W, Smith EE, Frankel MR, Olson D, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121:1492–1501. doi: 10.1161/CIRCULATIONAHA.109.881490. [DOI] [PubMed] [Google Scholar]
- Race, Ethnicity, and Genetics Working Group The use of racial, ethnic, and ancestral categories in human genetics research. Am J Hum Genet. 2005;77:519–532. doi: 10.1086/491747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd J, Friedlaender F, Speed W, Pakstis A, De La Vega F, Kidd K. Analyses of a set of 128 ancestry informative single-nucleotide polymorphisms in a global set of 119 population samples. Invest Genet. 2011;2:1. doi: 10.1186/2041-2223-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.