Abstract
Reactive oxygen species (ROS) have been associated with various human diseases, and considerable attention has been paid to investigate their physiological effects. Various ROS are synthesized in the mitochondria and accumulate in the cytoplasm if the cellular antioxidant defense mechanism fails. The critical balance of this ROS synthesis and antioxidant defense systems is termed the redox system of the cell. Various cardiovascular diseases have also been affected by redox to different degrees. ROS have been indicated as both detrimental and protective, via different cellular pathways, for cardiac myocyte functions, electrophysiology, and pharmacology. Mostly, the ROS functions depend on the type and amount of ROS synthesized. While the literature clearly indicates ROS effects on cardiac contractility, their effects on cardiac excitability are relatively under appreciated. Cardiac excitability depends on the functions of various cardiac sarcolemal or mitochondrial ion channels carrying various depolarizing or repolarizing currents that also maintain cellular ionic homeostasis. ROS alter the functions of these ion channels to various degrees to determine excitability by affecting the cellular resting potential and the morphology of the cardiac action potential. Thus, redox balance regulates cardiac excitability, and under pathological regulation, may alter action potential propagation to cause arrhythmia. Understanding how redox affects cellular excitability may lead to potential prophylaxis or treatment for various arrhythmias. This review will focus on the studies of redox and cardiac excitation. Antioxid. Redox Signal. 18, 432–468.
I. Introduction
Partially reduced forms of oxygen (O2) lead to the formation of oxygen free radicals, generally called reactive oxygen species (ROS). Partially reduced forms of nitrogen (N2) are also described in living systems and are called reactive nitrogen species (RNS). The importance of ROS in physiological systems has been emphasized by several studies that determined that ROS and RNS could be deleterious or beneficial in living systems. Beneficial roles include cellular response against infectious agents, protection against reperfusion injury, and activation of a number of signaling pathways that regulate cellular process and responses (255). The deleterious effect of excessive ROS is called oxidative stress and can cause damage to the cellular lipids, proteins, and DNA, and thus inhibit normal functions (255). Several antioxidant systems exist to act as cellular mechanisms to protect against oxidative stress. A delicate balance of oxidants and antioxidants is necessary to maintain normal physiology in living cells.
Cardiac muscle tissue is characterized by automaticity and excitability. Cardiac excitability refers to the ease with which cardiac cells undergo a series of events characterized by sequential depolarization and repolarization, communication with adjacent cells, and propagation of electrical activity (22). The cardiac cell is coupled to this rhythmic excitability and contracts or relaxes in phase with the cardiac depolarization or repolarization. Excitability is the ability of a cardiac cell to generate an action potential at its membrane in response to depolarization and to transmit an impulse along the membrane. Cardiac contractility refers to the ability of a muscle tissue to contract when its thick (myosin) and thin (actin) filaments slide past each other in response to a stimulus. Since cardiac excitability and contractility are coupled, optimum and timely cardiac excitation is important for a proper contraction of the cardiac tissue, which may otherwise lead to various cardiac complications. Cardiac excitability arises from organized flow of ionic currents through ion-specific channels in the cell membrane, through the myoplasm and gap junctions that connect cells, and through the extracellular space (22). Each ion flow (current) possesses distinguishing kinetics, biochemical, or pharmacological properties, based upon the permeantion. These currents also determine the intracellular concentration of various ions and determine the potential across the cellular membrane (membrane potential).
The resting membrane potential of an adult cardiac myocyte is −90 mV, and with increased inward anionic currents, or decreased cationic currents, the membrane potential depolarizes. The upstroke of an action potential starts when the threshold potential of −55 to −60 mV has reached. The inward cationic and outward anionic currents maintain the plateau phase of the action potential, and gradually, the membrane potential decreases to negative voltages, and at the potential below −20 mV, voltage-dependent K+ channels open to fully repolarize the cell. During the action potential, a normal cell completely loses its excitability (capacity to respond with new stimulus). The action potential period during which the cell looses its excitability is also called the effective refractory period (138). This refractory period in cardiac myocytes is ∼300 ms depending upon the rate of the heartbeat (138). The duration between two subsequent action potentials is called the relative refractory period. During this time, also the cellular excitability is lost. Thus, longer refractory periods would make the cells nonexcitable for a longer time. However, exceptions do exist where cells get excited during these refractory periods to generate a type of triggered arrhythmia called early afterdepolarizations (EADs) and delayed afterdepolarizations (DADs), which eventualy can cause arrhythmia. For years, it has been observed that the kinetic, biochemical, and pharmacological properties of the ion channels are affected by ROS/RNS (39, 91, 114, 146). Therefore, an appropriate balance of ROS/RNS and antioxidants is necessary for a proper cardiac excitability that may otherwise lead to a cardiac arrhythmia.
II. Overview of ROS Effects on Cardiac Ion Currents, Action Potential, and Excitability
In cardiac myocytes, rapid changes in ROS/RNS can contribute to excitability by means of altering the ion-handling properties of the ion channels, without affecting the fundamental contractile machinery of the myocytes. This was demonstrated in early studies in which 20–30 min of superfusion of guinea pig ventricular strips with a ROS-generating system caused ∼10-mV reduction in the resting membrane potential, ∼10-mV reduction in the potential amplitude, and ∼50 V/s fall in the rate of depolarization (211). Spontaneous activity in 50% of the ventricular strip preparations was abolished by adding an antioxidant (211). This has also been shown in some of the other studies where ROS, such as hydrogen peroxide (H2O2) and hypochlorous acid (HOCl), induced the transient augmentation of twitch amplitude in myocytes followed by unexcitability (129, 170). Similarly, various hydroperoxides depolarized the cardiac resting membrane potential, increased the generation of an action potential, and ultimately rendered the cells unexcitable due to modulation of various ion channels (199). However, these ROS-induced alterations of excitability were not accompanied by any effects on the myofilaments (199, 220), suggesting that the basic contractile machinery was not compromised. In ventricular cells from guinea pig hearts, ROS induced membrane depolarization with a concomitant increase in action potential generation. This was suggested to have occurred due to the inhibition of the inward rectifier K+ channel activity and Ca2+ channel activity, by Nakaya et al. (199). Several studies confirmed that in general, exogenous ROS, such as H2O2, applied to isolated ventricular myocytes decreased the outward IK, and/or increased the inward ICa and/or INa (23, 91, 123, 167, 211, 263). In 1993, Jabr and Cole (126) explained a ROS-induced loss of excitability in four stages. In stage one, an early 5–10-mV membrane depolarization increases the action potential duration due to decreased resting inward rectifying K+ currents. In stage two, the activation of transient inward current mediated by a Na+/Ca+2 exchanger (NCX) causes DADs and triggered activity (TA). In stage three, cardiac cells fail to repolarize, and thus continuously depolarize between −35 and −20 mV, due to increased cationic currents; and lastly, in stage four, shortening of the action potential duration and decreased hyperpolarization cause loss of excitability. The study also confirmed that these alterations in electrical activity could be prevented by a ROS scavenger, N-(2-mercaptopropionyl)glycine (126). The possible effects of ROS on ionic currents and excitation were further emphasized by application of the chemical oxidants like tert-butyl hydroperoxide (t-BHP), dihydroxyfumarate (DHF), and xanthine/xanthine oxidase, which affected the K+, Na+, or Ca+2 currents and caused DADs and EADs in ventricular myocytes (3, 20, 200, 256, 262). Similarly, experimental inhibition of glutathione (GSH) synthesis with buthionine sulfoximine in canine hearts was associated with a decreased density of atrial ICa in myocytes (42), which would decrease the action potential duration. On the other hand, increasing the antioxidant capacity of the cells prevented the arrhythmogenic activity or proclivity of the cardiac tissue. For example, overexpression of catalase (CAT) prevented the oxidative stress-induced decrease in peak shortening, maximal velocity of shortening/relengthening, and increased time-to-90% relengthening in the mouse ventricular myocytes (64). A role for ROS toward cellular action potential generation, excitability, and associated cardiac diseases has also been observed clinically. Specifically, the levels of GSH are now said to be associated with the cardiovascular events in humans. For example, the risk of cardiovascular events is inversely associated with increasing quartiles of glutathione peroxidase (GPx) 1 activity in patients (32), and in myocytes from the atrial fibrillation patients, the GSH levels are reduced relative to controls (42). Moreover, atrial myocytes isolated from patients with atrial fibrillation when superfused with N-acetylcistein (NAC) (a clinically used antioxidant) showed increased L-type calcium currents (42). It can be inferred that the atrial fibrillation occurred due to decreased ICa that might shorten the action potential and increase the excitability.
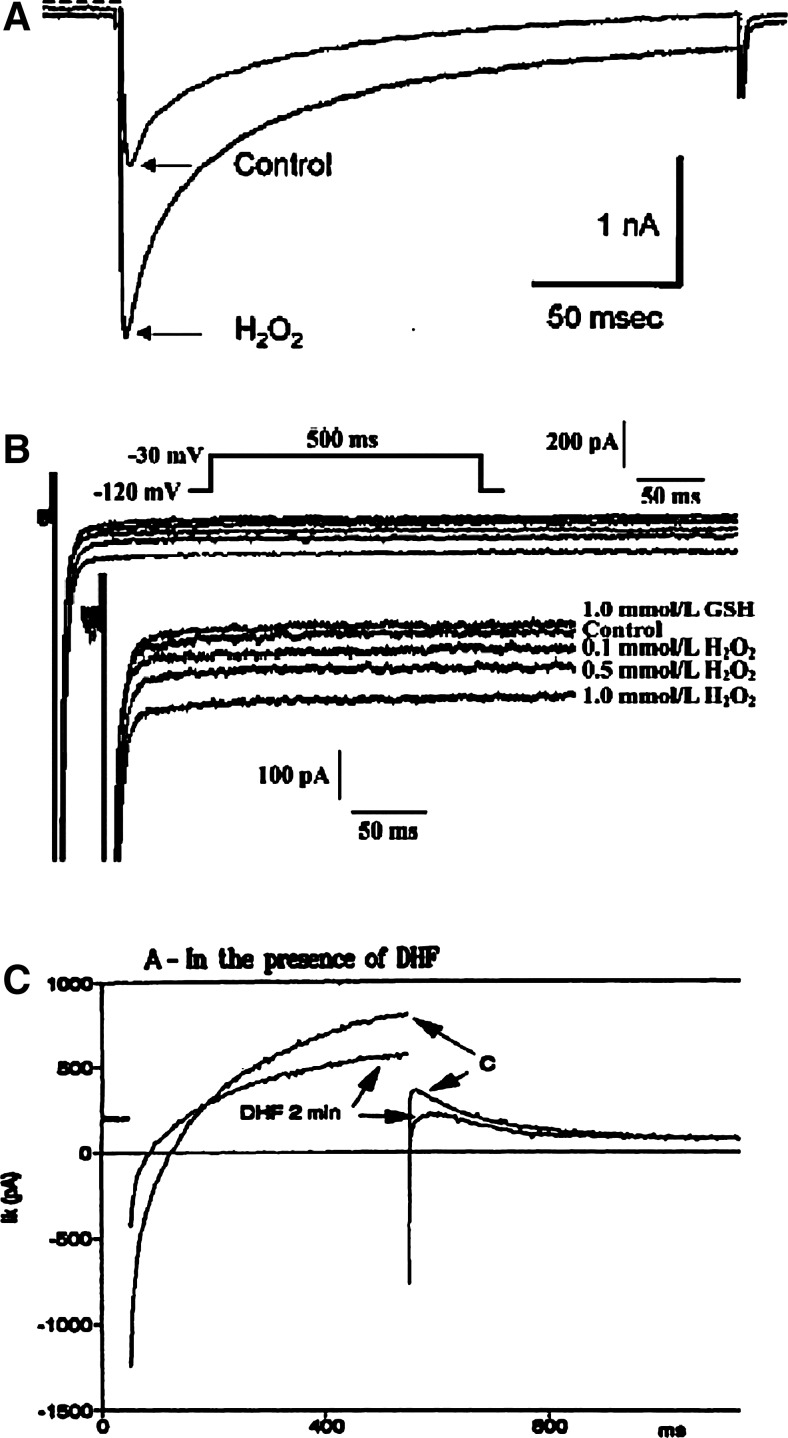
Overall, as ROS affect the ion-handling properties of the Ca+2, Na+, and K+ channels, it is expected that it will also affect the action potential of the cardiac cells by altering ICa, INa, or IK. Indeed, ROS-induced membrane depolarization occurred with increased Na+ window current (the window current results from the overlap of activation and inactivation steady-state curves due to depolarized membrane voltages that are sufficient to transiently open a few available channels) (27), increased activity of NCX (90), inhibition of the inward IK (199), activation of an inwardly directed nonselective cationic current (182), increased intracellular Ca+2 concentration associated with increased activity of the L-type Ca+2 channels (117) or ryanodine receptors (RyRs) (9), and decreased activity of the sarcoplasmic reticulum (SR) Ca2+ pump SERCA2a (149). An example from guinea pig myocytes (Fig. 1A–C) shows ROS-mediated increased ICa and INa and decreased IK (44, 167, 249). These alterations in currents cause prolongation of the action potential and EADs (44). In contrast to this example, shortening of the action potential duration has also been observed in an ischemic heart and is attributed to ROS-mediated increase in the delayed rectifying IK, activation of ATP-sensitive K+ (KATP) channels (mitochondrial or sarcolemal), or other mitochondrial K+ channels (30, 199, 275). In addition, ROS induced decreased cytosolic Ca+2 associated with decreased activity of L-type Ca2+ channels (100) and RyRs (287), or increased activity of NCX (219) has also been shown in other studies, and this may cause shortening of the action potential followed by loss of excitability as shown by Jabr and Cole (126). The contradictory results on ROS effects on ion channels and the action potential might be due to species differences (44, 276) in the concentration (89) and the type of ROS involved (41) and the number and type of channels affected (12, 149). In support of this idea, Tokube et al. (250) reported ROS-induced biphasic changes in the action potential duration, with initial lengthening of the action potential due to a rapid decrease in whole-cell IK and subsequent shortening due to a decrease of whole-cell ICa and increase in ATP-sensitive outward K+ current (IKATP). Apart from these time-dependent ROS-mediated increased or decreased Ca+2 concentrations, ROS-induced oscillation in intracellular Ca+2 concentrations has also been implicated in arrhythmogenic afterdepolarization (180) that would eventually lead to loss of excitability. These oscillations might be related to the ROS modulation of the RyR that are primarily responsible for the Ca+2-induced Ca+2 release in the cells. More recently, ROS-induced opening of the mitochondrial channels has been implicated in causing shortening of the action potential and thus arrhythmia (40). In this regard, Zhou et al. modeled a whole cell to demonstrate that increasing ROS production from the mitochondria triggers limit-cycle oscillations of the mitochondrial membrane potential (ΔΨm) and mitochondrial respiration, causing transient periods of mitochondrial uncoupling. These transient periods of mitochondrial uncoupling decrease the cytosolic ATP/ADP ratio and activate sarcolemal IKATP that shortens the cellular action potential duration and ultimately suppresses excitability (296).
FIG. 1.
Examples of the effects of reactive oxygen species (ROS) on various channel currents. (A, B) Hydrogen peroxide (H2O2) increased the Ca+2 currents from the L-type Ca2+ channels (ICaL) and the persistent Na+ currents from the cardiac Na+ channels (INaP). (C) Dihydroxyfumarate (DHF), an oxidant, decreased the inward rectifier K+ currents (IKr) from the Kv11.1 channels. All these currents were measured in the guinea pig myocytes, and are taken from (44, 167, 249), with copywrite permissions, respectively. The arrows indicate differences in the currents.
Given this overview, it can be seen that ROS effects on various ion channels and the interplay among these channels may affect cardiac action potential generation and thus excitability in complex ways. Therefore, pathophysiological processes that increase ROS synthesis might cause arrhythmogenic electrical dysfunctions and nonexcitability of the cardiac tissue. This review will first outline various ROS, their synthesis, their metabolism by antioxidants, and their roles in various pathways in the cardiomyocytes, followed by a more comprehensive review of the effects of ROS on various ion channels, individually, and finally, additional considerations for cardiac electrophysiology and arrhythmia.
III. ROS in Cardiac Physiology
A. Types of ROS and their synthesis in the heart
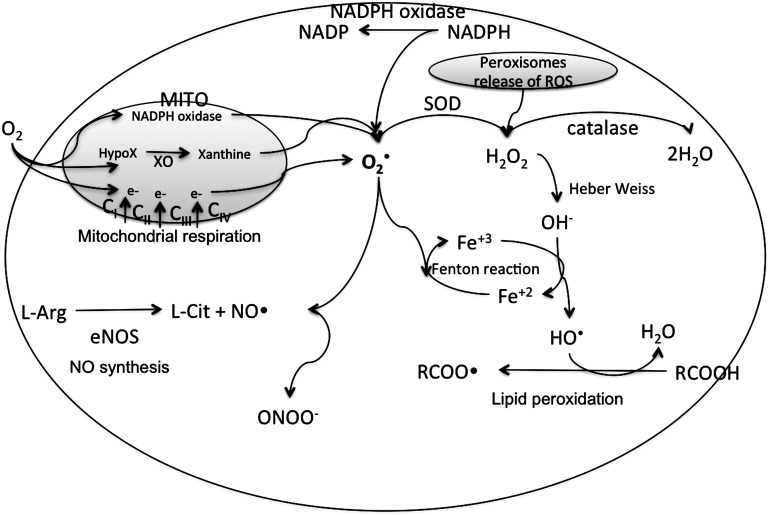
The major ROS in cardiac cell are superoxide radical ion (O2•−), singlet oxygen (O2•), H2O2, hydroxyl radical (OH•), and HOCl (176). The major sources of oxidative stress in cardiovascular system involve the enzymes (i) xanthine oxidoreductase (XOR), (ii) NADPH oxidase, (iii) nitric oxide synthase (NOS), and (iv) the mitochondrial cytochromes; and (v) hemoglobin (176). Various ROS in a cell and their synthesis are shown in Figure 2. The addition of one electron to dioxygen forms the superoxide anion radical (O2•−). Since mitochondria consume most of the O2 in the cells for their respiratory chain, the production of superoxide occurs mostly within the mitochondria of a cell.
FIG. 2.
Major ROS synthesis pathways in cardiac myocytes. In the mitochondria (MITO), a small percentage of electrons from the respiratory chain prematurely leak to O2 at complexes I, II, or III, resulting in the formation of the toxic superoxide radical ion (O2•−). O2•− is also generated by release of a free electron from the reactions where NADPH is oxidized to NADP by NADPH oxidase, and hypoxanthine is oxidized to xanthine (X) by xanthine oxidase (XO). The O2•− generated in the mitochondria can freely pass through the mitochondrial membrane into the cytosol. Cytosolic O2•− can interact with other intracellular molecules to generate (hydroxyl radical [OH•], a neutral form of the OH) from H2O2. This H2O2, but not O2•−, is mainly generated in the peroxisomes of the cells. When peroxisomes are damaged, their H2O2-consuming enzymes are downregulated, and H2O2 releases into the cytosol (62). In a Haber–Wiess reaction, O2•− reacts with H2O2 to form OH• and a hydroxyl anion (OH−). Moreover, under conditions of metabolic stress, iron-containing molecules in a cell, such as 4Fe-4S cluster-containing enzymes, release free iron. This iron participates in a Fenton reaction generating OH• radical. The Haber–Wiess and the Fenton reactions occur in conjunction, because the OH− released from the Haber–Wiess reaction feeds into the Fenton reaction to form more OH•. The reduction of Fe by O2•− yielding Fe2+ and O2 further propels the Fenton reaction. Other radicals derived from O2 that can be formed in living systems are lipid peroxyl radicals (RCOO•) that are synthesized due to peroxidation of the membrane lipids (6). Overproduction of nitrogen species is called nitrosative stress. Reactive nitrogen species (RNS) are formed from a single nitrogen radical (NO•) generated in cells by nitric oxide synthase (NOS), which metabolizes l-arginine (l-Arg) to l-citruline (l-Cit) with the formation of NO• (145). NO• and O2•− react together to produce a much more potent oxidative molecule peroxynitrite anion (ONOO−). The figure was created by adapting information from (176, 195, 255), respectively.
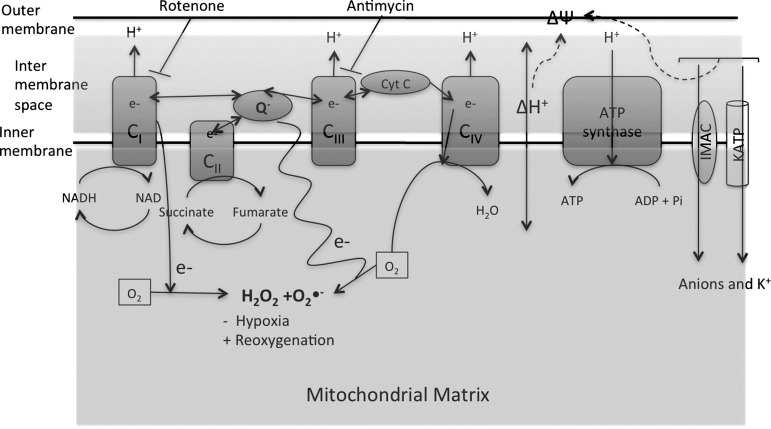
The transfer of electrons within the constituents of the mitochondrial respiratory chain is shown in Figure 3. In this respiratory chain, a small percentage of electrons prematurely leak to O2 at Complexes I, II, or III, resulting in the formation of the toxic O2•− (Fig. 3). For a direct evidence of ROS generation from the cardiac mitochondria, the synthesis of H2O2 from the isolated mitochondria from hearts, or mitochondria in intact cardiac cells, can be determined in a fluorescence-based assay (106). Within these fluorescence-based assays, inhibition of the mitochondrial respiratory chain at any stage causes generation of large amounts of ROS (Fig. 3). For example, in isolated heart mitochondria from beef or rodents, inhibition of Complex III by antimycin (218), or Complex I by rotenone (241), produces large amounts of ROS. Inhibition of the Complex III causes stabilization of the ubisemiquinone radical (218), which transfers a single electron to O2 to form O2•− in the intermembrane space in the mitochondria, whereas inhibition of Complex I produces O2•− from NADH (108). When uninhibited, the maximum rate of O2•− production by Complex III is generally smaller than that by Complex I. Moreover, it has also been confirmed that the primary site of O2•− generation in the mitochondrial electron transport chain is flavin mononucleotide of Complex I via a reverse electron flow, not forward electron flow via ubiquinone of Complex III (164, 241). Thus, it is generally accepted that in vivo Complex I is the major source of superoxide. In addition to respiratory complexes, many other enzymes associated with cardiac mitochondria have been shown to produce O2•− or H2O2 under various conditions. For example, mitochondrial ROS production is increased by high levels of the xanthine reductase (60) or NADPH oxidase (192).
FIG. 3.
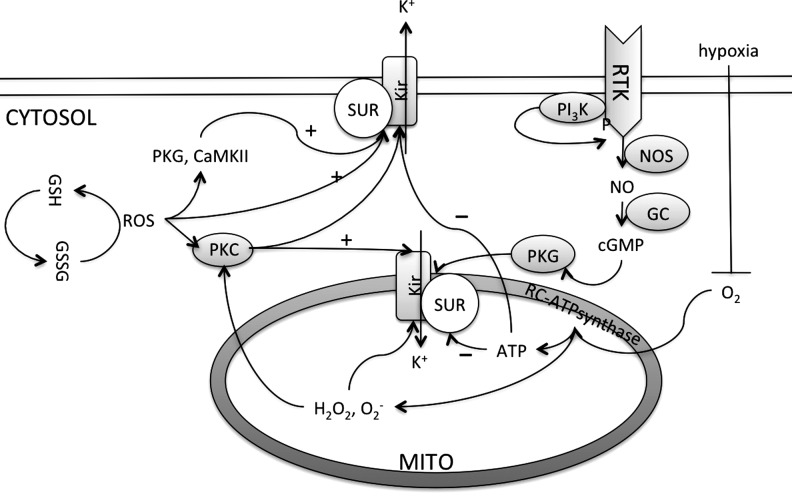
Synthesis of superoxide radical in the mitochondria. Electron transfer occurs between the Complex I through Complex IV of the respiratory chain. At the mitochondrial inner membrane, electrons from NADH (at Complex I) and succinate (at Complex II) pass to the coenzyme Q (ubiquinone [UQ]). UQ passes electrons to Complex III (cytochrome bc1 complex), which then passes the electrons to the cytochrome c (cyt c). Cyt c passes electrons to Complex IV (cyt c oxidase), which uses the electrons and hydrogen ions to reduce molecular oxygen to water. This enzymatic series produces a proton gradient across the mitochondrial membrane, producing a thermodynamic state that has the potential to do work. However, in this respiratory chain, a small percentage of electrons prematurely leak to O2 at complexes I, II, or III, resulting in the formation of the toxic O2•−. Reverse electron flow from Complex I also generated superoxide radicals. Inhibitors of Complex I and III that enhance the generation of ROS in cardiac mitochondria are Rotenone and Antimycin, respectively. Mitochondrial membrane potential (ΔΨm) is determined by the proton gradient across the membrane. Mitochondrial ion channels such as inner membrane anionic channel (IMAC) and ATP-sensitive K+ (KATP) channels cause ionic flux through the membrane and contribute toward mitochondrial membrane potential. The figure was created by adapting the information from (106, 195, 215), respectively.
Regardless of the source of O2•−, the mechanism and quantity of O2•− generated are dependent on the experimental substrate and energetic conditions (106, 183). For example, an elevated O2 concentration will also promote superoxide synthesis, as the rates of the nonenzymatic reactions of O2 with radical intermediates are proportional to the local O2 concentration (35). This is because larger proton gradient across the inner membrane causes increase in the rate of respiration, and thus increases utilization of the O2 (183). Hypoxia, as in ischemia, causes a decreased ROS production because of the decreased availability of O2. A decreased O2 availability halts the respiratory chain, decreases the proton gradient that further decreases the O2 utilization, and thus depolarizes the mitochondrial membrane potential (12, 183). The less utilization of O2 may form trace amounts of ROS. However, reoxygenation/reperfusion causes sudden burst of O2 into the mitochondria with a partially inactive respiratory activity, leads to an access to O2 unutilized by the respiratory chain, and thus causes a huge increase in the ROS production (144). This increased and decreased ROS synthesis with hypoxia/reoxygenation is associated with the modulation of the activities of several ion channels, alterations in action potentials, excitability, and thus arrhythmia (12, 183). In addition, such conditions favor O2•− production from Complex I by enhancing the reverse electron transport (106), and may also act by increasing the lifetime of the ubisemiquinone radical at Complex III (236). In this regard, mitochondrial ion channels, in particular ATP-sensitive K+ channels (KATP channels), may serve an important role in ROS generation in cardiac myocytes, and consequences of this increased ROS on myocyte excitability have been recently described (39). In summary, ROS are primarily synthesized in the mitochondria, depending upon the availability of the O2, and several components of the respiratory chain and ion channels are involved in this process. These ROS increase the overall cardiac oxidative stress leading to damage. This oxidative damage results whenever the ROS produced by mitochondria evade detoxification. This detoxification occurs due to the presence of the antioxidant systems as discussed below.
B. Antioxidants protect myocytes against oxidative stress
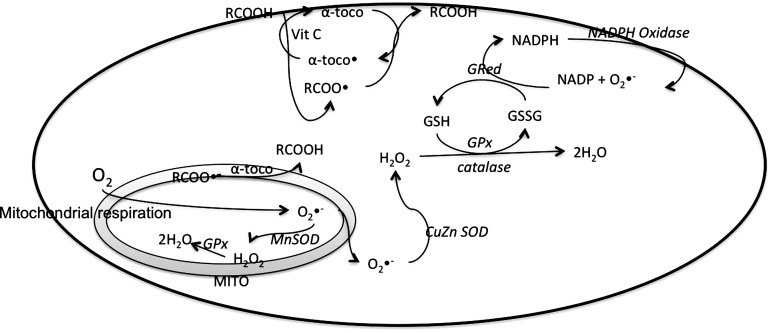
Defense mechanism against free radical-induced oxidative stress involves enzymatic and nonenzymatic antioxidant defense systems. Enzymatic antioxidant defenses include superoxide dismutase (SOD), GPx, and CAT. Nonenzymatic antioxidants are ascorbic acid (vitamin C), alpha-tocopherol (vitamin E), GSH, carotenoids, flavonoids, and other antioxidants (255). In addition, dietary antioxidants such as polyunsaturated fatty acids (PUFAs) and their effects on cardiac contractility and excitability have been extensively studied. Various antioxidants and how they regulate oxidative stress are shown in Figure 4. Under normal conditions, there is a balance between both the intracellular antioxidants and oxidants levels maintained by the activities of the antioxidant enzymes. For example, GPx reduces the cellular peroxides in a reaction in which GSH acts as a cofactor and gets oxidized oxidized GSH (GSSG) (Fig. 4) (179). GSSG is accumulated inside the cells, and the ratio of GSH/GSSG is a good measure of oxidative stress of an organism. GSH is highly abundant in the cytosol (3–15 mM) and mitochondria (5–11 mM), and is the major soluble antioxidant in the cell compartments (179). The levels of GSH in cultured rat heart cells were found to be about 148 nmoles/mg of protein, and these levels fall with stress (85). Levels of GSH are associated with the cardiovascular events in humans as well (32). Besides regulating other oxidants in the cell, GSH/GSSG also directly reduces the disulfide bond formed within cysteine residues of the proteins by serving as an electron donor, and has been shown to alter all the major ion channel activities in a cardiac cell (146). GSH is found almost exclusively in its reduced form, since the enzyme, GSH reductase, which reverts it from its oxidized form, is constitutively active as well as inducible upon oxidative stress. Because GSH is synthesized in the cytosol, its mitochondrial presence requires an active transport across the mitochondrial membrane. However, recently, it has been shown that externally added GSH is readily taken up by the mitochondria despite the 8 mM GSH present in the mitochondrial matrix (214). One of the possible mechanisms of this process is that the increased GSH levels can maintain the mitochondrial KATP channels in a closed state, thereby maintaining the mitochondrial respiration, and thus decreased synthesis of ROS (214).
FIG. 4.
Antioxidants in the cardiac cells. The major antioxidants are glutathione (GSH)/oxidized glutathione (GSSG) along with the enzymes glutathione reductase (GRed) and glutathione peroxidase (GPx), catalase, superoxide dismutase (SOD), NADPH oxidase, and α-tocopherol and vitamin C. MITO, mitochondria; RCOO• and RCOOH, lipid radical and lipid; α-toco• and α-toco, α-tocopheroxyl radical and α-tocopherol.
The second major antioxidant is SOD. In humans, there are three forms of SOD: (i) a homodimeric copper–zinc SOD (CuZnSOD) found primarily in the cytosol, (ii) a homotetrameric glycosylated CuZnSOD in the extracellular space, and (iii) a homotetrameric manganese SOD (MnSOD) in the mitochondrial matrix (184). Several lines of evidence suggest that the mitochondrial (MnSOD) plays a major role in limiting the synthesis of the ROS in the mitochondria of the cardiac cells (283) (see Fig. 4). For example, transgenic mouse lines expressing increased levels of human MnSOD in the cardiac mitochondria had decreased ROS levels and mitochondrial damage compared with the nontransgenic littermates, when treated with Adriamycin that increases mitochondrial ROS generation (283). Thus, increased activity or expression of SOD will prevent ROS-induced damage to the cardiac functions. However, clinical studies indicate that there is no relationship between the total SOD activity in erythrocytes and the risk of cardiovascular events (32). The impact of CAT, another major antioxidant, on cardiac dysfunction and oxidative damage was studied in transgenic mice that overexpressed CAT in a cardiac-specific manner (64). Increasing the blood glucose levels increased ROS generation in cardiac mycocytes of wild-type mice, but not the CAT-overexpressing mice (64). The increased ROS in control mice were associated with the intracellular Ca+2 changes and a decreased Ca+2 clearance, which was abrogated by the overexpression of CAT (64). Therefore, these results indicate that perhaps, ROS affected the activity or expression of various Ca+2 channels in the cardiac cells, in particular the sarcoendoplasmic reticulum Ca+2 ATPase (SERCA) channels and NCX that are responsible for removing Ca+2 from the cytosol (reviewed in the later sections), and that CAT overexpression abrogated this ROS effect. Increased intracellular Ca2+ concentrations do affect the action potential and excitability without affecting the contractile machinery (172) (also discussed later). In this regard, it is important to consider that CAT itself does not alter the activity or expression of the NCX, SERCA, or phospholamban (a protein that regulates the activity of the SERCA) (64). Such studies have shown that the GPx, SOD, and CAT are important in maintaining the oxidative stress of the cardiac myocytes, and thus regulate the cardiac membrane potential, action potential, and excitability indirectly by limiting the ROS-induced alteration of ionic balance. Other important antioxidant systems that are important in various tissues are thioreductase and methionine sufoxide reductase. Recently, two novel human thioredoxin reductase isoenzymes were cloned and sequenced, one mitochondrial and the other one predominantly expressed in testis. The major substrate for thioredoxin reductase is thioredoxin, which is as an electron carrier necessary for the catalytic cycles of biosynthetic enzymes and also protects cytosolic proteins from aggregation or inactivation via oxidative formation of intra- or intermolecular disulfides. Since many ion channels possess oxidizable cysteine residues, appropriate levels of reduced thioredoxin in cardiomyocyte are important, and to maintain this level, an active thioredoxin reductase is necessary. Paradoxically, thioredoxin reductase may possibly be (transiently or permanently) inactivated by oxidants such as H2O2. In cardiac myocytes, the thioredoxin/thioreductase system has an important implication on regulation of ion channel activity against ROS (discussed later under each ion channel section). For example, thioredoxin influence on various K+ channel activities has been studied (159, 160). Methionine sulfoxide reductase (MsrA) reverses oxidation of methionine in proteins. As shown by the use of an MsrA knockout (KO) mice, lack of MsrA in cardiac myocytes reduces the myocyte capability against ROS stress due to a significant increase in cellular oxidized protein levels resulting in a dysfunction (201). MsrA KO mice also revealed that under oxidative stress or physical stress, cellular contractility is significantly decreased (201). Conversely, overexpression of MsrA in primary neonatal rat cardiac myocytes protected myocytes against hypoxia-/reoxygenation-induced oxidative damage (216). Another study showed that the calmodulin (CaM) kinase II (CaMKII) can be activated by ROS directly without the Ca2+-CaM complex if MsrA is absent or inhibited (73). This activated CaMKII has many detrimental implications on various ion channels and ion fluxes in the myocytes (will be discussed in the later sections). Therefore, MsrA is related to myocardial apoptosis, impaired cardiac function, and increased mortality after myocardial infarction.
Among the nonenzymatic antioxidants, α-tocopherol has been studied. Because of its hydrophobic nature, almost all the cellular α-tocopherol is present in the membrane fraction of cells. In that location, it can readily donate an electron to a fatty acid hydroperoxyl radical, thus breaking the chain reaction associated with lipid peroxidation (132). Evidence suggests that the resulting α-tocopheroxyl radical can be recycled back to α-tocopherol by cytoplasmic ascorbic acid, and by ubiquinol in microsomes and mitochondrial membranes (132). It has been shown that increased lipid peroxides in the isolated myocytes caused an increase in the repolarizing IK, and thus in the arrhythmic phenotype, and that treatment with α-tocopherol prevents the IK modulation by reducing the lipid peroxide content of the cells (130). Dietary antioxidants, specially PUFAs, may act as antiarrhythmic agents principally, because they reduce Ca2+ entry by blocking ICa, act directly to decrease INa, and act indirectly to reduce the intracellular Ca2+ transients (267). For further details of the mechanism of action of PUFA and other dietary antioxidants, readers are encouraged to refer the literature.
IV. Redox Balance Affects Cardiac Physiology
The delicate balance of the reductants and the oxidants in a cell is called redox balance or simply redox. The ROS-induced oxidative stress in cardiac myocytes has been linked to various diseases such as ischemic heart disease, cardiomyopathies, cardiac hypertrophy, congestive heart failure, and arrhythmia (147). On the other hand, protective concentrations of ROS have been shown to be important in decreasing the postischemia/reperfusion damage to the heart (95). These paradoxical roles of ROS are explained by the findings that ROS can affect multiple pathways (protective or harmful), either by affecting the activities of various proteins/secondary messengers or by acting as a signaling molecule itself. For example, extracellular signal-regulated kinase (ERK), mitogen-activated protein kinase (MAPK), and specific isoforms of protein kinase C (PKC) are regulated by ROS in the cardiac cells (52, 61, 95, 194), and these may participate in favorable or detrimental pathways. These signaling molecules may be involved in the pathways that are responsible for either a short-term effect by modulating the activities of other proteins such as ion channels (289) or long-term effects by modulating the activities of transcription factors such as cJun that would ultimately alter the protein expression (52). Various signaling molecules and associated pathways affected by ROS in the cardiac cells are shown in Figure 5. Alterations of these signaling pathways are involved in pathogenic mechanisms for arrhythmia, hypertrophy, heart failure, and postischemic injury (61, 95, 194). In as far as direct effects are concerned, ROS alter the structural conformation of the proteins (including ion channels) by oxidizing thiols or nitrosylation of cysteines to modulate their functions. The effect of ROS on various ion channels is tabulated in Table 1.
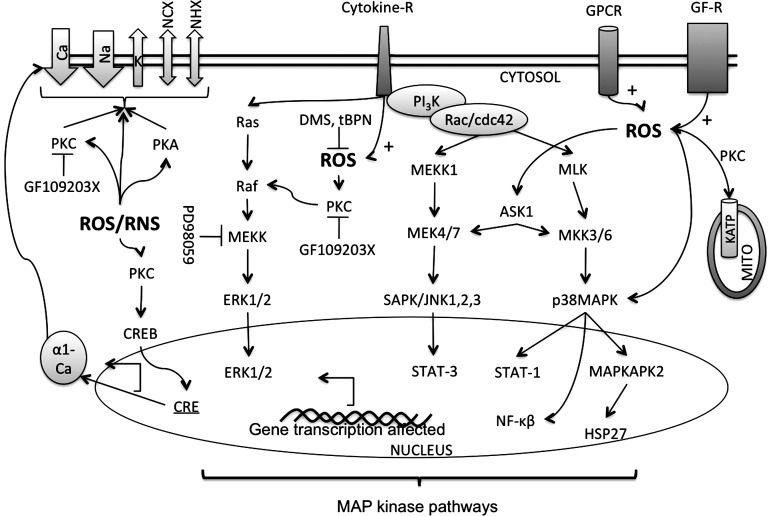
FIG. 5.
Pathways affected by ROS in cardiac myocytes. Apart from the hypoxia/reoxygenation-mediated ROS-synthesis, ROS are also produced after activation of cell surface receptors such as the cytokine receptors (Cytokine-R), the G-protein-coupled receptors (GPCR), and the growth factor receptor (GF-R) such as TGF-β1 (135, 290). Cellular ROS, such as H2O2, activate the three subfamilies of mitogen-activated protein kinases (MAPKs), namely the stress-activated protein kinases/c-Jun N-terminal kinases (SAPKs/JNKs), the extracellularly responsive kinases (ERK1/2), and p38-MAPK (52). ROS activate ERK in a protein kinase C (PKC)-dependent manner because the activation of ERKs can be inhibited by PD98059 [which inhibits the activation of ERK kinases (MEKKs)], and also by the PKC inhibitor GF109203X. ROS also activates p38-MAPK that then activates the downstream MAPK-activated protein kinase 2 (MAPKAPK2) that in turn phosphorylates HSP25/27 (52). Free-radical trapping agents such as dimethyl sulfoxide (DMS) and N-t-butyl-a-phenyl nitrone (tBPN) inhibit the activation of MAPKAPK2 (52). ROS were estimated as lipid peroxides, activate apoptosis-regulating signal kinase-1 (ASK-1), and Rac/cdc42 pathways. Activation of ASK-1 and Rac/cdc42 leads to activation of other kinases such as MEKK, SAPK/JNK, p38MAPKs, and Jun kinases, and this is reversed by addition of the reducing agents (290). Activation of such kinases activates several downstream nuclear transcription factors as NFkβ, STATs, and HSP. Therefore, dominant negative mutant ASK-1 can attenuate the agonist-mediated activation of NFkβ in cardiac cells in response to increased ROS (225). Activation of such transcription factors as Jun and NFkβ will lead to an altered gene expression. Indeed, DNA microarray studies show induction of nearly 100 genes in response to oxidant stress (203). Various ion channels such as Ca2+, Na+, and K+ channels as well as Na+/Ca+2 exchanger (NCX) and Na-H exchanger (NHX), and ATP sensitive K+ channels in the MITO are affected by these pathways. Inhibitors of MEKK, PKC, and ROS are shown.
Table 1.
Effect of Reactive Oxygen Species on Various Cardiac Ion-Channel Currents
| Currents | ROS system applied | Experimental systems | Effects on channel currents | References |
|---|---|---|---|---|
| ICaL | H2O2 application | Isolated guinea pig ventricular myocytes or recombinant Cav1.2 in HEK293 cells | Increased | (117, 257) |
| Hypoxia (decreased mitochondrial ROS) | Isolated guinea pig ventricular myocytes or recombinant Cav1.2 in HEK293 cells | Decreased | (76, 113, 117) | |
| HOCl application | Isolated hamster ventricular cardiomyocytes | Decrease | (100) | |
| Oxidizing agent, thimerosal, or buthionine sulfoxime | Isolated guinea pig or canine ventricular myocytes | Decrease | (42, 150) | |
| RyR | H2O2 application or application of oxidizing agents such as DTDP or DTNB | Reconstituted channels formed by the cardiac RyR purified from pig heart, or in intact rat cardiac myocytes, or in sheep myocardium, | Increased the open probability and increase in Ca2+ spark activity | (9, 63, 67, 278, 286) |
| GSNO and CysNO | Cardiomyocytes | Increased channel activity | (242) | |
| SERCA | H2O2 application | microsomes from HEK-293 cells overexpressing SERCA2b, or rat cardiomyocytes | Decreased activity | (96, 149) |
| Chemical NO application | SERCA reconstituted in phospholipid vesicles | Increased activity | (2) | |
| Unaffected IKto or IK1 by direct application of oxidants as t-BHP or H2O2 (27, 263) | ||||
| IK1 | ROS generated from the xanthine/xanthine oxidase system or H2O2 | Guinea pig cardiac ventricular myocytes | Decreased | (53, 199) |
| Diamide | Rat ventricular myocytes | Un affected | (159) | |
| Kv channels; Shaker K+ channels (Kv1.3, Kv1.4, and Kv1.5), one Shaw channel (Kv3.4) | Photoactivation of rose bengal | Expressed in Xenopus oocytes | Decreased | (66) |
| (KShIIIC, KShIIID and HukII | H2O2 application | Expressed in Xenopus oocytes | Inhibition of the time-dependent fast inactivation | (256) |
| IKs (Kv7.1) | H2O2 perfusion or diamide application | Expressed in CHO cells or in rat cardiac myocytes | Unaffected | (84, 159) |
| Expressed in Calu-3 cells | Increased | (58) | ||
| IKss (steady state outward K+ currents | DHF | Decreased | (44) | |
| Ipeak (Kv4.2, Kv4.3, or Kv1.4) and IKss (Kv1.5 and Kv2.1) | Diamide application | Ventricular myocytes of rats | Decreased | (160) |
| IKto | BCNU and BSO treatment of rats | Ventricular myocytes of rats | Decreased | (159) |
| IKr (Kv11.1) | FeSO4 and ascorbic acid generated ROS | Expressed in Xenopus oocytes | Increased outward currents Unaffected inward currents |
(244) |
| H2O2 application | Expressed in the CHO cells | Increased outward currents Decreased inward currents |
(23) | |
| Hypoxia induced ROS generation | Kv11.1 α-subunit expressed in HEK | Decreased conductance | (202) | |
| IKCa (BKCa) | H2S application | BKCa in rat glomus cells and the human recombinant BKCa channel expressed in HEK293 | Reduced conductance | (248) |
| IKATP | Hypoxia H2O2 or GSSG application |
Isolated myocytes | Increased | (123, 250, 277) |
| INa (Ipeak or INaT) | NADH application, hypoxia or application of GSSG | HEK cells stably expressing human cardiac Na+ channel subunits | Decreased | (163, 262) |
| Exogenous NO application | HEK cells stably expressing human cardiac Na+ channel subunits | Increased | (3, 253) | |
| INaP | H2O2, hypoxia or application of GSSG | Increase late | (167, 237, 238, 263) | |
| IP3R | H2O2, GSSG, t-BHP application | In endothelial cells or in permeabilized hepatocytes | Increased activity | (71, 72, 107, 294) |
| NCX | H2O2 application, or hypoxia reoxygenation | In rabbit ventricular myocytes | Increased activity | (70, 90, 149) |
| Na+/K+ ATPase | Xanthine plus xanthine oxidase, or hypoxia. [Cardiac glycosides have also been shown to increase cellular ROS (111, 271)] | In rat and bovine myocytes | Increased activity | (271, 272) |
ICaL, L-type Ca2+ currents; RyR, ryanodine receptors; INaP, persistent or late sodium currents; INaT, transient or peak sodium currents; IKs, slow component of delayed rectifier K-current; IK1, inward rectifier K-currents; IKto, transient outward K-currents; IKss, steady-state outward K-currents; NCX, Na+/Ca2+-exchanger; IP3R, IP3 receptor; SERCA, sarcoendoplasmic reticulum Ca+2-ATPase; IKATP, KATP channel current; IKCa, calcium-activated K-currents; BCNU, 1,3-bis-(2-chloroethyl)-1-nitrosourea; BSO, buthionine sulfoximine; CysNO, S-nitrocysteine; DHF, dihydroxyfumarate; DTDP, 2,2′-dihydropyridine; DTNB, 5,5′-dithio-bis(2-nitrobenzoic acid); GSNO, S-nitrosoglutathione; GSSG, oxidized glutathione; HOCl, hypochloric acid; H2O2, hydrogen peroxide; ROS, reactive oxygen species; t-BHP, tertbutyl hydroperoxide.
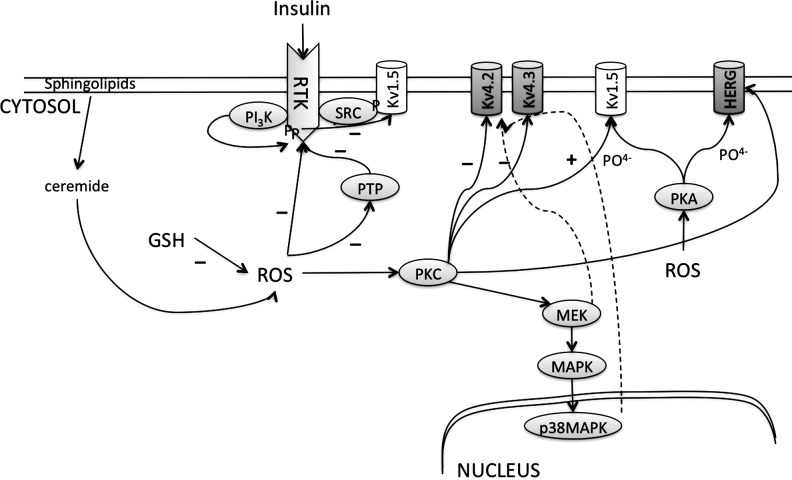
It is important to recognize that all ion channels are made up of macromolecular complexes involving many proteins. For example, the human cardiac Na+ channel, NaV1.5, which is a member of the family of voltage-gated sodium channels (NaV1 to 9), consists of a primary α-subunit and a secondary β-subunit (18). In a mammalian system, the α-subunit of NaV1.5 is sufficient to generate sodium currents; however, the β1-subunit increases the density of currents and regulates the gating of the channels (18). In addition, phosphorylation/dephosphorylation of α- or β-subunits, by the action of PKC, PKA, or Ca-CaM kinase, is important for activity of the Na+ channels (82). More importantly, ROS affect both the α- and the β-subunits either directly or indirectly to eventually alter INa and thus cellular excitability (163, 167, 263). Similarly, cardiac Ca2+ channels (L-type or the T-type) are formed by the combination of as many as 5 subunits, α1, α2, β, γ, and δ. The β subunit increases channel expression and accelerates the activation and inactivation kinetics. The α1-subunit is exclusively expressed in cardiac tissue, and its phosphorylation promotes a slow gating mode of the Ca2+ channels, increasing Ca2+entry, and resulting in cytotoxicity (74). In addition, a loss of function of the α1- or β2b-subunit of the L-type Ca2+ channels has been associated with many cardiac diseases. Needless to say, ROS-mediated direct or indirect modulation of the structure or functions of either of the subunits will alter the cardiac ICa, action potential, and thus excitability. The third major type of the ion channels, the K+ channels, is a complex group of channels divided into three major categories: the voltage-gated K+ channels, inward rectifier K+ channels, and the background K+ currents (155). Just like the Na+ and the Ca2+ channels, most of the K+ channels also consist of α-subunits and single/multiple β-subunits. The channel functional units also include the complementary proteins K+ channel-associated protein (KChAP) and the K+ channel-interacting protein (KChIP), which may increase channel activity and alter channel kinetics (94). K+ channel functions are regulated by various cellular proteins through processes such as phosphorylation and dephosphorylation (178). Once again, ROS affect the functions of various K+ channels by directly or indirectly modifying the subunit functions or structures, therefore reducing the repolarizing IK (84, 146, 160, 202, 244, 256). ROS may alter the structure and function of the channel-forming proteins by direct interaction (S-nitrosylation or formation of disulfide bonds) (50). Such direct modulations are normally irreversible and harmful to the cells.
The protective role of ROS in cardiac cells [as in ischemic hearts (95)] is exemplified by the ROS-induced activation of guanylyl cyclase (GC), which causes formation of the well-characterized second messenger cyclic guanosine monophosphate (cGMP) (187). The apparent paradox in the roles of the protective ROS as an essential biomolecules in the regulation of cellular functions and as toxic ROS causing damage, at least in part, is related to differences in the concentrations of ROS produced. This is analogous to the effects of NO, which has both regulatory functions and cytotoxic effects depending on the enzymatic source and relative amount of NO generated. NO functions as a signaling molecule mediating vasodilation when produced in low concentrations by the eNOS in vascular endothelial cells (212) and as a source of highly toxic oxidants utilized for microbial killing when produced in high concentrations by inducible NOS in macrophages (171). Thus, while discussing ROS effects, it is imperative to consider the mode of ROS activation (direct or indirect), and the source, concentration, and duration of ROS activity.
As mentioned earlier, ROS modulate a variety of signaling pathways in cardiac myocytes as shown in Figure 5. This interaction with the cell-signaling pathway occurs through a direct interaction with the thiol group of the regulatory proteins that may be essential secondary messengers and are necessary for a variety of normal cellular functions. Most important of these proteins are the kinases, phosphatases, and other transcription factors. Recent articles on the effects of ROS on various kinases/phosphatase signaling can be found in the literature (197). The most studied and important of these proteins is PKC. ROS-activated PKC in the cardiac cells regulates Ca+2 handling by activating L-type Ca+2 channels (mainly by affecting open probability) (151), phosphorylates, and thus activates Na+ channels (264), sarcolemmal Na+–hydrogen exchanger (16), NCX (232), and K+ channels (196). PKC-, and not PKA-, mediated activation of the L-type Ca+2 channels in adult ventricular myocytes depends on the activation of the ERK1/2 pathway (235). Similarly, the human inward rectifier K+ channel currents (IK1) in atrial cardiomyocytes and one of its underlying ion channels, the Kir2.1b channel, are inhibited by PKC-dependent signal transduction pathways, possibly contributing to arrhythmogenesis in patients with a structural heart disease in which PKC is activated (133). An isoform of PKC, PKCɛ, also activates the mitochondrial KATP channel (188). Another kinase, PKA, has been shown to activate the cardiac Na+ channels in the similar fashion; however, it phosphorylates a different site on the channels and then the PKC phosphorylation site (295). Furthermore, PKC activates the MAPK pathway that will ultimately lead to the increased transcription of the proteins. In fact, ROS increased the transcription of the human α1-subunit of cardiac Ca+2 channels in a PKC-dependent manner (251).
V. Redox Effect on Cardiac Calcium Channels and Excitability
The intracellular Ca+2 concentration is regulated by several types of channels, pores, and exchangers that work in a synchronous manner (114) (Fig. 6). It is well known that the concentration of intracellular calcium [Ca2+]I regulates cardiac contractility via its interaction with the contractile machinery that includes troponin, tropomyocin, myosin, and actin (114). However, the role of intracellular calcium toward cardiac excitability is relatively underappreciated. [Ca2+]I affects cellular excitability through direct or indirect modulation of ICa, IK, or INa via proteins as CaM or CaMKII that in turn are affected by ROS. While L-type Ca2+ channels are responsible for influx of Ca2+ into the cells, their activation and facilitation also depend on the initial [Ca2+]I (102). Initial increase in the local [Ca2+]I causes the formation of the Ca2+-CaM complex, which then activates autophosphorylation of the CaMKII (120). The activated CaMKII phosphorylates L-type Ca2+ channels and RyRs to increase [Ca2+]I (120, 233). Since ROS increase Ca2+ currents by directly modulating the α- and/or β- subunits of L-type Ca2+ channels to increase local [Ca2+]I, and since this initial increase in [Ca2+]I activates CaMKII that then activates more L-type Ca2+ channels, it could be expected that ROS facilitate ICaL via CaMKII activation. Indeed, H2O2 increased ICaL amplitude and slowed inactivation of ICaL, and this facilitation of ICaL was abolished by a CaMKII blocker KN-93 (239). Further investigations revealed that CaMKII-dependent phosphorylation of α1C subunits of L-type Ca2+ channels at S1512 and S1570 mediates this ICaL current facilitation (31). Thus, ROS-mediated activation of CaMKII would increase [Ca2+]I, which could depolarize the cells and affect the cellular excitation. In addition, ROS-mediated CaMKII activation due to initial Ca2+ increase also increases persistent Na+ currents (INaP) to prolong APD, and thus reduces the time that a cell can be excited again. For example, in rabbit myocytes, both acute and chronic CaMKIIδ (the predominant form in the heart) overexpression shifted voltage dependence of Na+ channel availability by −6 mV and slowed their recovery from inactivation (259) in a Ca2+-dependent manner. CaMKIIδ also increased INaP, and thus the total intracellular Na+ concentration [Na]I. This effect was completely prevented by CaMKII inhibition in the case of acute CaMKIIδ overexpression (259). Wagner et al. also reported that CaMKIIδ-mediated changes occurred due to a direct interaction of CaMKIIδ with Na+ channels and a subsequent phosphorylation of Na+ channels (259). Thus, [Ca2+]I levels regulate the INa via CaMKII. Indeed, in vivo, transgenic CaMKIIδ overexpression prolonged the QRS duration and QT intervals due to an increase in the INaP, which would prolong the effective refractory periods, and thus decrease the time that a cell can be excited again (259). In contrast to these results, in guinea pig ventricular myocytes, CaMKII (in the pipette solution) shifted the voltage dependence of Na+ channel availability by approximately +5 mV and enhanced the recovery from inactivation. CaMKII also increased persistent Na+ currents, thereby increasing the intracellular Na+ levels. Moreover, the Ca2+-CaM complex (in the pipette solution), in absence of CaMKII, also increased the voltage dependence of Na+ channel availability and the fraction of channels undergoing slow inactivation, but it did not alter the magnitude of the late current (5). Investigations have confirmed that CaM binds to the carboxy-terminal IQ domain1 of the human cardiac Na+ channel in a Ca2+-dependent manner (246).
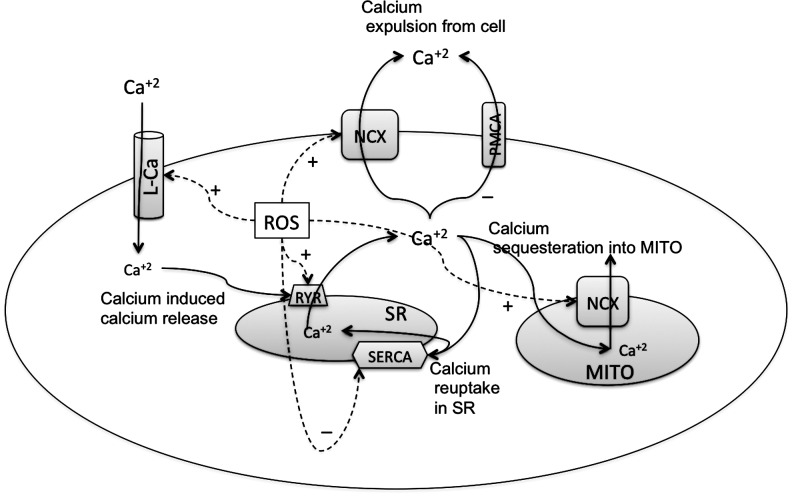
FIG. 6.
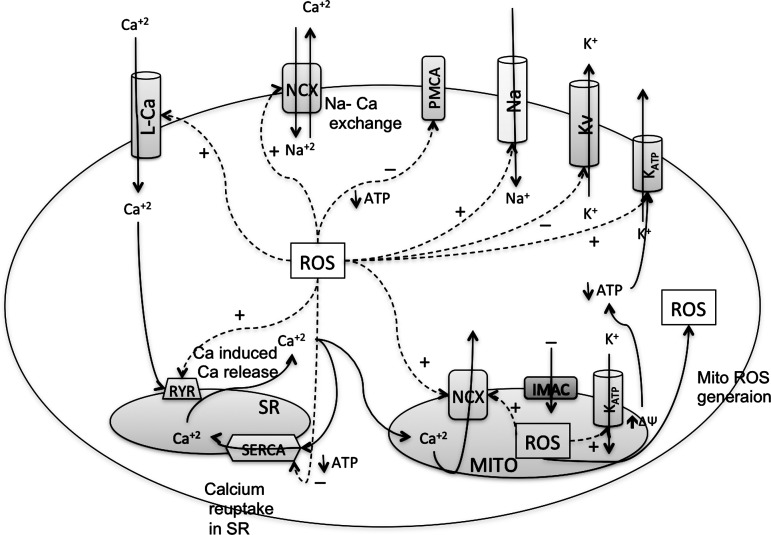
Effect of ROS on various Ca+2 handling channels in cardiac myocytes. Depolarization causes opening of the voltage-gated L-type Ca2+ channel (L-Ca) on sarcolemmal membranes, which produces the entry of a small amount of Ca+2 that triggers the opening of another Ca+2 channel on the sarcoplasmic reticulum (SR) called the ryanodine receptors (RyRs). This process, known as calcium-induced calcium release (CICR), increases the total Ca+2 content in the cytoplasm. Intracellular Ca+2 is decreased by reuptake into the SR by the sarcoendoplasmic reticulum Ca+2 ATPase (SERCA) pump and removed from the cell by NCX (114, 298). Each of these Ca+2 regulators is affected by the ROS in the cells to increase or decrease the cellular Ca+2. ROS induce the Ca+2 currents from the L-Ca, the Ca+2 release from RyR, and expulsion of Ca+2 out of the cytosol through the NCX on the sarcolemmal and mitochondrial membranes. ROS decrease the Ca+2 sequestering by SERCA or expulsion of Ca+2 by the plasma membrane Ca+2 ATPase (PMCA) pump.
Although the two studies mentioned above reported a number of contradictory results, they reported one consistent result. In both of the studies, CaMKII increased the persistent Na+ currents that would increase the [Na+]I. These experiments indicate that a local increase in [Ca2+]I prolongs the effective refractory period by increasing either ICaL or INa. The prolongation of the effective refractory period will reduce the availability of the cell to be excited again to generate a full action potential. Under abnormal circumstances, any depolarization during this prolonged effective refractory period will generate EAD that may be passed to the adjacent tissue that is not in its refractory period. This may cause arrhythmia. Possible explanations for controversial results in the two studies mentioned above might be the use of a different CaMKII isoform, species differences, or a possible existence of more than one Ca2+-dependent mechanism regulating the activity of Na+ channels. Nevertheless, these two studies did show that [Ca2+]I affects the Na+ channel activity, but did not emphasize the direct role of ROS. In this regard, Xie et al. reported that H2O2 treatment of rabbit ventricular myocytes increased [Ca+2]I (268), which enhanced the peak and late ICaL and increased SR Ca+2 release. This acute sarcoplasmic Ca2+ release may cause an increased CAMKII activity that will then lead to an increased INaP (259). Thus, ROS-induced increase in ICaL and INa will prolong the action potential duration (prolongs the effective refractory period) to decrease the excitability. Intracellular Ca+2 levels and its interaction with CaM also affect voltage-gated K+ channels. K+ currents through the KCNQ1 (Kv7.1) channels are inhibited by KCNE4 (a β-subunit), which interacts with CaM in a Ca2+-dependent manner (51). Disruption of the KCNE4-CaM interaction either by mutagenesis or by acute Ca2+ chelation impairs the ability of KCNE4 to inhibit KCNQ1, and thus increased the IK (51). Thus, in presence of ROS-induced increased [Ca2+]I, it can be assumed that the Ca2+-CaM complex will inhibit the Kv7.1 currents to prolong the relative refractory period and give rise to DADs. However, this needs to be tested directly. Nevertheless, it is clear that cellular Ca2+ levels regulate the refractory periods (by altering the activity of various channels) and alter cellular excitability. Thus, ROS effects on various Ca+2 channels are reviewed below.
A. Effect of ROS on the voltage-dependent L-type Ca+2 channel
Cardiac L-type Ca+2 channels are composed of polypeptide subunits (α1, β, and α2/δ) and form a heterotetrameric complex with a molecular mass of about 400 kDa (43). The hydrophobic α1 polypeptide is entrenched in the cell membrane, whereas the β-subunits are located in the cytoplasm. The δ-subunit is anchored in the cell membrane and has a single transmembrane segment with a short intracellular part and a long glycosylated extracellular part. The α2 peptide is an extracellular subunit of the Ca+2 channel (43). The L-type Ca+2 channels are involved in the ROS-induced modulation of Ca+2 currents by several pathways that may precipitate arrhythmia (Fig. 7). The Ca+2 channel activity was reduced when the pore-forming α1C-subunit of the human L-type Ca+2 channel, expressed in the HEK 293 cells, was exposed to acute hypoxia (76). Similarly, acute hypoxia decreased basal L-type Ca+2 channel currents (ICaL) in isolated guinea pig ventricular myocytes without shifting the current–voltage relationship (76, 113). Hypoxic effects may be mediated by a decreased generation of ROS in the mitochondria, because ROS generation is directly proportional to the local concentration of O2 available in the mitochondria (195). In guinea pig ventricular myocytes, direct H2O2 application or mitochondrial ROS (confirmed with inhibitors of mitochondrial respiration) increases the basal ICaL and sensitivity of ICaL to isoproterenol (257). This provided direct evidence of the role for the mitochondria and ROS generated by the mitochondria in regulation of the ICaL and suggests that an increase in ROS will increase the ICaL. This has been further confirmed by direct application of ROS (such as H2O2) to measure ICaL. H2O2 perfusion of the HEK 293 cells expressing the α1C-subunit of the human L-type Ca+2 channel (117) or the guinea pig ventricular myocytes (257) showed significantly enhanced depolarization-evoked Ca+2 currents in a voltage-dependent manner. Application of CAT to these cells/myocytes reduced the ICaL currents (117, 257). It was also revealed that the hypoxia effects on ICa were not due to the generation of mitochondrial H2O2 (117). Similarly, oxidized LDL has been shown to increase Ca+2 influx through the recombinant α1C-subunit of the L-type Ca2+ channel via enhanced production of ROS by the mitochondria (75). Contrary to this, application HOCl to isolated hamster ventricular cardiomyocytes caused a dose-dependent decrease in peak ICaL without affecting apparent reversal potential, activation, and inactivation kinetics (100). In addition, the NADPH oxidase inhibitors diphenylene iodonium (DIP) and phenylarsine oxide, which would decrease the ROS, did not alter the ICaL at basal or with hypoxia (117). These contrary results might be due to species differences or the type of ROS used. Nevertheless, it is now clear that ROS like H2O2 and HOCl do regulate the ICaL and may increase or decrease the currents.
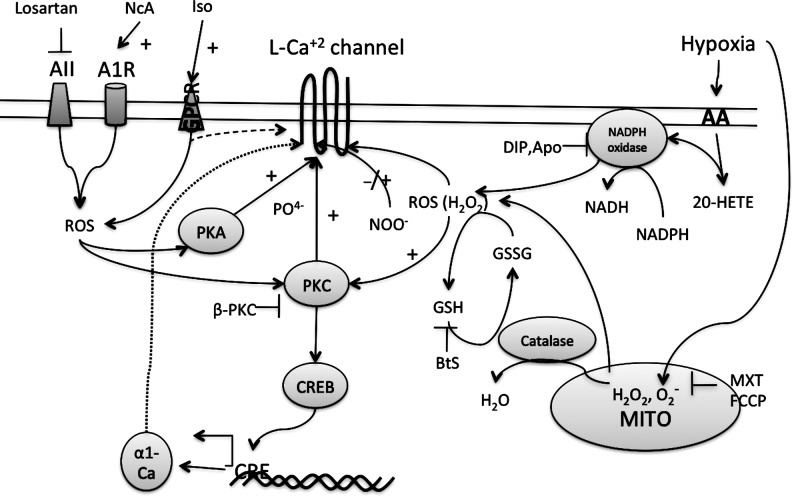
FIG. 7.
ROS induces the activity of L-type Ca+2 channels. Activation of various receptors such as angiotensin (AII) receptor, adenosine receptor (A1R), or GPCR increases ROS in the myocytes. Cytosolic ROS activate PKC or PKA that activates the channels by phosphorylation. PKC also activates CRE-binding protein (CREB) that binds to cAMP-response elements (CRE) on DNA to induce the expression of α-1 subunit of the L-type Ca+2 channels. BtS, buthionine sulfoxime; MXT, myxothiazol; FCCP, carbonylcyanide p-trifluoromethoxyphenylhydrazone, a protonophore and uncoupler of mitochondrial respiratory chain; DIP, diphenylene iodonium; Apo, apocynin; 20-HETE, 20-hydroxyeicosatetraenoic acid; AA, arachidonic acid; NcA, N-acetylcysteine; Iso, isoproterenol.
It is believed that ROS regulate ICaL by directly reacting with, and thus altering the conformation of the L-type Ca+2 channels. Indeed, the pore-forming subunit α1C contains more than 48 cysteine residues that can potentially undergo redox modification (169). Cysteine residues in the β-subunit may also be important for the trafficking of the L-type Ca+2 channels (48). Some of these cysteines make disulfide bonds while others are free and are available for redox modification so that direct ROS-induced modification of the subunits of the L-type Ca+2 channels may affect the conducting properties, and thus modulate the ICaL. As mentioned earlier, the results of ROS effects on ICaL have been controversial, and the results with the application of oxidizing sulfhydryl (SH) agents are also contradictory in several studies. Nevertheless, it evident that ICaL is modulated by various oxidizing agents. For example, thiol-oxidizing agents such as thimerosal decreased ICaL, through the human α1C-subunit expressed in HEK293 cells (76), through the rabbit cardiac Ca+2 channels expressed in HEK293 cells (116), and in isolated guinea pig ventricular myocytes (150). This inhibition of ICaL in guinea pig myocytes is reversible by the reducing agent such as dithiothreitol (DTT) (150). Treatment of the canine myocytes with an oxidizing agent, buthionine sulfoxime, also attenuated ICaL while this was reversed by treatment with GSH (42). On the contrary, several SH reagents induced ICaL in frog ventricular myocytes, and this effect was independent of cAMP production or G-protein stimulation, indicating that ROS increased the ICaL by directly modulating the Ca+2 channels (276). The effect of such oxidizing SH agents is also reversed by reducing agents such as GSH or DTT (276). Similarly, cGMP-independent direct application of NO causes S-nitrosylation of extracellular SH groups of the L-type Ca+2 channel, and therefore increases ICaL (41). Regarding the above-mentioned studies, it is important to mention that the reducing agents or the cellular antioxidant CAT is ineffective on ICaL by themselves under basal conditions.
These contradictory results might depend on the type, amount, and exposure time to ROS. For example, application of oxidizing agents such as 3-morpholinosydnonimine-HCl (SIN) to the ferret myocytes may cause an increase or a decrease in ICaL (41) depending on the dominant mechanism of action. The myocytes in which SIN inhibited ICaL the cellular redox conditions were such that the direct effects of NO were dominant, whereas in those myocytes in which SIN stimulated ICaL, the indirect effects of NO/O2•−, which S-nitrosylate and/or oxidize signaling molecules, were dominant (41). Thus, the type, mechanism, and amount of ROS determined the ICaL response. Similarly, in frog myocytes, methanethiosulfonate ethylammonium (MTSE) caused a biphasic increase and then decrease in ICaL (276). Since MTSE penetrates the cell membrane very slowly, it is hypothesized that the MTSE perfusion of the whole cell alters the intracellular redox balance and causes an initial increase in ICaL. After this initial increase in ICaL, MTSE reaches in the cell in time and causes a decrease in ICaL due to direct modulation of the channel proteins (276).
It is evident that direct application of ROS such as H2O2, HOCl, or several thiol-reducing or oxidizing compounds can alter channel function, but the response may vary. As previously mentioned, ROS may act by two different mechanisms, that is, (i) by direct modification of the channel proteins and (ii) by affecting the cellular signaling that in turn may affect the ICaL. In fact, ROS affect the L-type Ca+2 channels activity via PKC (45) (Fig. 7). The mechanism of the effect of ROS-induced PKC on the activity of cardiac L-type Ca+2 channels is not exactly known; however, it is established that the α1C- and β2-subunits of cardiac L-type Ca+2 channel can be phosphorylated by PKC in vitro (279). In fact, various isoforms of PKC (α, β1, β2, and ɛ) are detected in both neonatal and adult ventricular myocytes (36, 279). The effect of PKC on ICaL can be highly diverse. PKC can either decrease ICaL (228, 292), and may even cause an increase and decrease in ICaL during the same experiment in a biphasic response (151, 279). It is postulated that PKC phosphorylates the N′ terminus of the α1C-subunit of the L-type Ca+2 channel, and the effect on the channel can be either stimulating or suppressive (279). Recently, it was observed that the oxidation product of arachidonic acid, 20-hydroxyeicosatetraenoic acid (20-HETE), increases ICaL in a concentration-dependent manner (289). This effect of 20-HETE was mediated by the decreased activity of NADPH oxidase that increased the cellular ROS, and thus PKC activity (289) (Fig. 7). In addition, ROS-mediated increase in sensitivity of the ICaL to α-adrenergic receptor stimulation involves PKA. Since the activity of PKA can also be altered by ROS, PKA may be an important regulator of cell functions during changes in redox state (86, 119) (Fig. 7). In another study, angiotensin II increased ICaL through an increase in the α1C-subunit mRNA and protein levels in rat atrial myocytes. This increase in the α1C occurred due to serine-133 phosphorylation of CRE-binding protein (CREB) by PKC, NADPH oxidase, and the ROS pathway (251) (Fig. 7). This indicates that apart from the short-term effects of ROS, in a long term, ROS can also alter the protein expression to affect the ICaL. The ROS effects on ICaL become more complicated by the fact that a number of different splice variants of L-type Ca+2 are expressed in the heart that may have variable sensitivity to ROS (77).
Although often contradictory, the results mentioned above allow some insight into how ROS influence L-type Ca+2 channel function and the mechanisms for the calcium-mediated changes in the action potential, excitability, and arrhythmia in cardiac myocytes. For example, the gain-of-function mutations G406R and G402S cause reduced Cav1.2 channel inactivation, resulting in maintained depolarizing ICaL, prolongation of action potentials, and DADs (240). In another example, Mahajan et al. showed that ICaL can be targeted to increase dynamic wave stability by maintaining action potentials without depressing contractility (172). Mahajan et al. showed that in rabbit ventricular myocytes, when ICaL inactivation is inhibited/delayed, and when these myocytes were also treated with verapamil (an ICaL blocker), the intracellular Ca+2 transients were not affected; the action potential duration restitution slope was flattened; and action potential duration alternans (beat-to-beat alternation in action potential duration) were prevented (172). In fact, this approach has been proposed as the possible therapeutic way to increase the usability/safety of ICaL blockers as antiarrhythmic drugs. On the other hand, ROS-mediated decrease in ICaL and thus a decrease in Ca+2 influx during the plateau phase of the action potential may be necessary to prevent arrhythmias associated with prolongation of the QT interval during hypoxia. However, hypoxia and direct application of ROS also decrease the sensitivity of the L-type Ca+2 channel to the α-adrenergic receptor agonist isoproterenol, and this may be deleterious to the cell (115). Despite these studies, it is not clear if ROS cause increase/decrease in ICaL and how important this effect would be in arrhythmia biology. Nevertheless, for the most part, it has been observed that ROS cause a net increase in the cellular Ca+2 (91). This net increase in cytosolic Ca+2 is due to ROS affecting on other Ca-handling proteins as RYR, NCX, and SERCA, in addition to an increased/decreased ICaL.
B. Effects of ROS on the RyRs
Ca+2 release from the SR through RyR channels is essential for activation of cardiac and skeletal muscle contraction. Three isoforms of RyRs have been identified. The RyR1 isoform is dominant in skeletal muscles, whereas the RyR2 is predominantly expressed in the cardiac cells (79). At the amino acid level, the three mammalian RyR isoforms share 70% identity (79). The RyR has multiple sites for regulation, including cytosolic and luminal sites for regulation by Ca+2, and cytosolic sites for Mg2+, ATP, CaM, and FK-506-binding proteins (FKBP) (79).
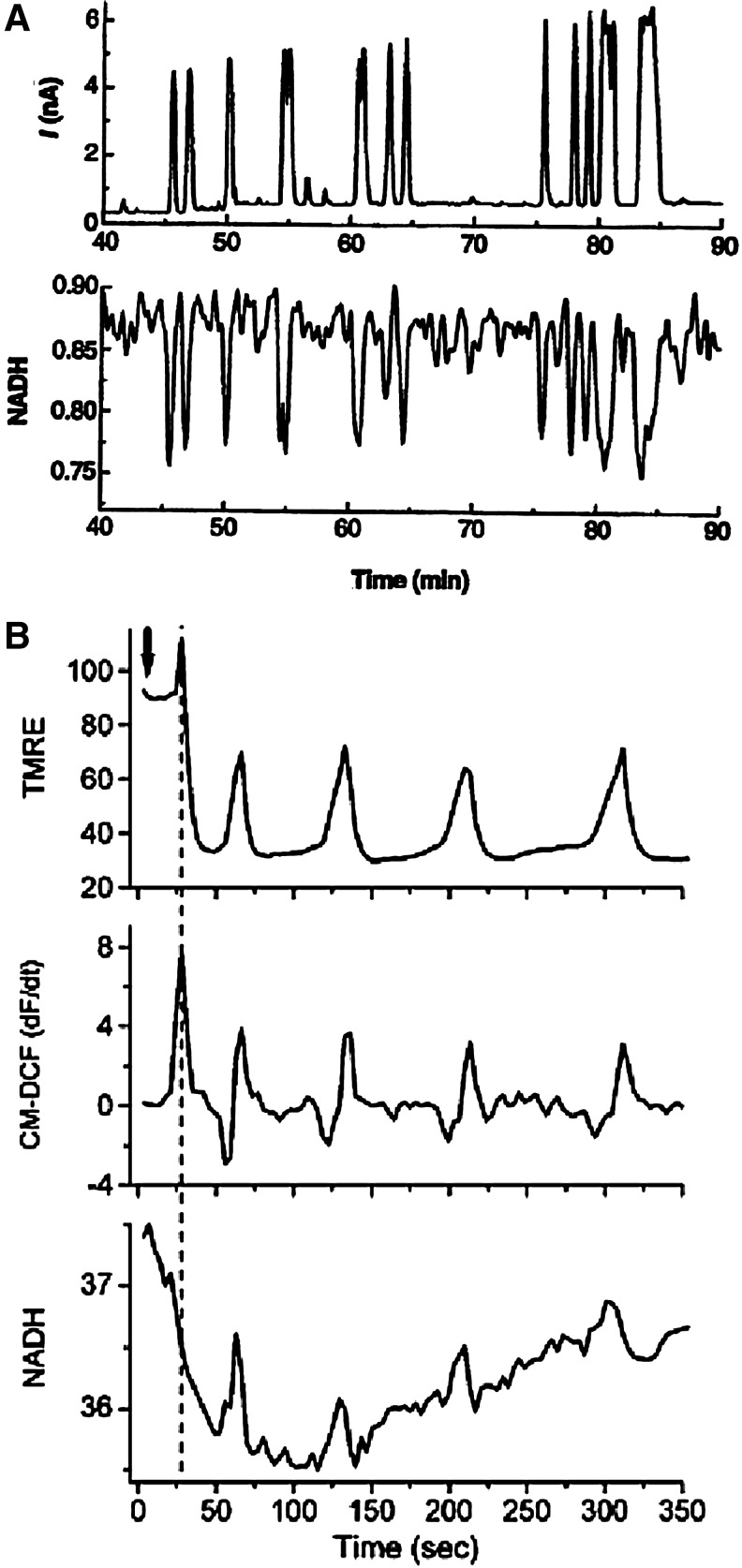
The radical O2•− increases Ca+2 efflux from the reconstituted RyR from the heavy fraction of cardiac SR vesicles due to decreased CaM content (134). In addition, OH• chemically generated by the reaction of H2O2 and Cu–ethylenediamine or the thiol-oxidizing agents also increased the open probability of the reconstituted channels formed by the cardiac RyR purified from the pig heart, and this effect was reversed by DTT (9). These results were supported by experiments in a native system of rat cardiomyocytes in which ROS production from mitochondria in intact rat cardiac myocytes was achieved by photostimulation and antimycin-A treatment (63, 278). Intracellular ROS and Ca+2 signals were simultaneously measured in fluorescence-based assays. Photoactivated or antimycin A-induced mitochondrial ROS production elicited a transient increase in Ca+2-spark activity from RyR (63, 278). Lowering basal mitochondrial ROS production, scavenging baseline ROS, and applying DTT diminished the spontaneous Ca+2 spark activities and abolished the Ca+2 spark responses to mitochondrial ROS (63, 278). Such findings suggest that OH• reacts with RyR and increases the Ca+2 release from the SR through the RyR.
The tetrameric RyR contains up to 89 cysteine residues per monomer (274). However, only a small number of hyperactive SH groups on the channel protein appears to have a redox-sensing function (274). Figure 8 shows the summary of the ROS effect on the functions of RyR directly or indirectly via other ancillary proteins. Oxidizing agents such as 2,2′-dihydropyridine (DTDP) or 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) activate the RyR channel (isolated from various species), and this effect was reversed by reducing agents such as DTT (67, 286). It has also been shown that H2O2 modulates the gating properties to increase the open probability of cardiac RyRs, and this effect was reversed by DTT (9, 208). In contrast, addition of NADH, which would increase O2•−, decreased the RyR activity that decreased the frequency and the amplitude of spontaneous Ca+2 sparks, and this inhibitory effect was reversed by NAD+(299). However, this might be due to the direct inhibitory effects of NADH on the RyR independent of the mitochondrial function (299). Regarding the effect of endogenously generated O2•−, it has been shown that exercise and tachycardia increase NADPH oxidase activity, which increases the cellular O2•− content, and thus enhanced the RyR activity (227) due to enhanced S-glutathionylation. The redox modulation of the RyR can also lead to alteration of the sensitivity of the channel to cytosolic Ca2+ and ATP. Redox modification of cysteine residues, for example by GSH/GSSG, alters the interaction of the RyR with triadin or CaM (19, 162). Triadin is a transmembrane ancillary protein that regulates the sensitivity of the RyR to luminal Ca+2 by stabilizing the binding of calsequestrin (an intra-SR Ca+2-binding protein) to the RyR (97), and oxidation/reduction of the thiols on RyR alters the interaction with triadin, and thus the sensitivity to the luminal Ca+2. Thus, oxidation of the SH groups in the triadin-binding site of RyR reduces triadin binding and may increase RyR activity (162). Similarly, oxidation of the RyR CaM-binding site by GSSG reduces the interaction with the CaM, and thus increases the RyR activity (19). Further information on regulation of RyR by CaM-CaMKII can be find in a review (173).
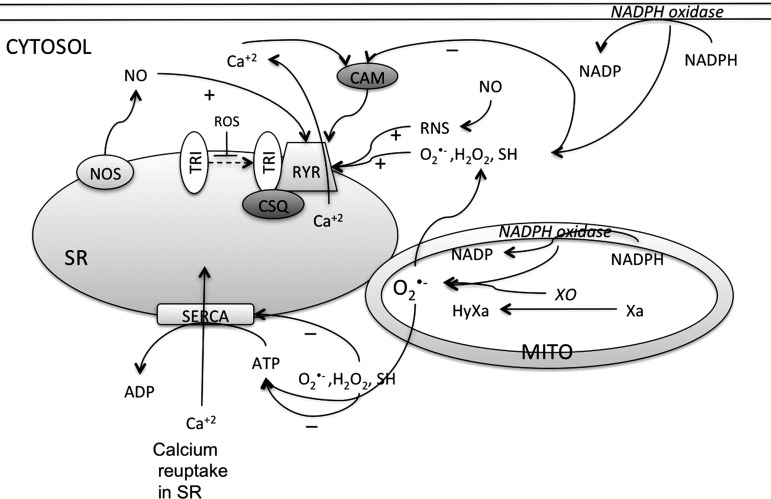
FIG. 8.
RyR activity is enhanced, and SERCA pump activity is decreased by ROS/RNS. ROS/RNS enhance the RyR activity directly or by inhibiting the interaction with triadin (TRI) that alters the Ca+2 sensitivity of receptor in the SR. Triadin stabilizes binding of calsequestrin (CSQ) to the RyR. ROS also decrease the ability of RyR to bind with calmodulin (CAM), thereby decreasing the activity. ROS decrease the activity of SERCA by direct interaction or by decreasing the cellular ATP content necessary for the SERCA activity. XO, xanthine oxidase; HyXa, hypoxanthine; Xa, xanthine.
In support of these results, altered ROS content in acquired diseases is also important in determining the intracellular Ca+2 via RyR activity. For example, in isolated intact and permeabilized ventricular myocytes from a canine model of heart failure, the ratio of GSH/GSSG as well as the level of free thiols on RyR decreased markedly, consistent with increased oxidative stress in heart failure. With this increased ROS, RyR-mediated SR Ca+2 leak was significantly enhanced in permeabilized myocytes from the heart failure compared to control cells, and this was reversed with reducing agents such as DTT (93). In dystrophin KO cardiomyocytes, slow elevations of the intracellular Ca+2 resulted in Ca+2 oscillations with an enhanced Ca+2 sensitivity. This is due to the elevated cellular ROS generation in dystrophy that causes the abnormal RyR sensitivity to increases in the intracellular Ca+2 concentration (131), which can be arrhythmogenic. Similarly, after ventricular fibrillation, the myocytes from a canine model had an increased rate of ROS production and increased RyR oxidation (21). Abnormal RyR function in ventricular fibrillation cells was indicated by increased fractional Ca+2 release for a given amplitude of Ca+2 current and elevated diastolic RyR-mediated SR Ca+2 leak. Treatment of ventricular fibrillation myocytes with reducing agents normalized the parameters of Ca+2 handling and shifted the threshold of Ca+2 alternans to higher frequencies (21).
Because Ca+2 release by RyR depends on the intraluminal SR Ca+2 content, a prolonged ROS activation of RyR would exhaust SR Ca+2 content, and thus decreases RyR activity. The evidence that RyR dysfunction depends on the concentration and the length of exposure to ROS and may be biphasic is now briefly reviewed. Experiments on single-ventricular myocytes indicate that H2O2 causes Ca+2 overload as a result of activation of Ca+2 release from intracellular stores (and not from activation of voltage-dependent Ca+2 channels or NCX) (89), and after an initial stimulation of release, prolonged exposure to O2•− caused a decrease in the Ca+2 release by RyRs probably due to the depletion of SR Ca+2. Similar biphasic responses were obtained when ROS synthesis in rat cardiomyocytes was induced by photoactivation or treatment with antimycin A (278). These treatments caused a transient increase in Ca+2 spark activity, followed by gradual spark suppression in phase with the mitochondrial ROS oscillations (278). Partial deletion of Ca+2 stores in the SR contributed in part to the gradual, but not the phasic, spark depression. H2O2 at 200 mM also elicited a bidirectional effect on sparks (278).
The mechanisms of NO action on the gating properties of RyR remain controversial. Nitrosylation of SH groups of cardiac RyR by low-molecular-weight S-nitrosothiols, such as or S-nitrosoglutathione (GSNO) and S-nitrocysteine (CysNO), stimulates channel activity (242). In NOS1-deficient mice, RyR hyponitrosylation leads to diastolic Ca+2 leak and a proarrhythmic phenotype (92). In addition, peroxynitrite (ONOO−) generated by SIN (3-morpholinosydnonimine) activates the channel several fold through thiol oxidation (274). On the other hand, NO produced endogenously by NOS localized to the cardiac SR decreases the open probability of the RyR (287). It seems that effects of NO or NO-related molecules also depend on the concentration and cytosolic environment. Low concentrations of NO donors have been shown to activate the RyR, whereas high concentrations cause inhibition of the channel (104). From these studies, it is evident that the nitrosylation of RyR enhances the activity. Together, these findings demonstrated that a nitrosoredox imbalance causes RyR oxidation, hyponitrosylation, and SR Ca+2 leak, a hallmark of cardiac dysfunction (93).
In summary, based on the studies, we speculate that increased cellular ROS/RNS content oxidizes the cysteine thiols of the RyR, which may decrease its interaction with the regulatory proteins CaM and triadin, or may alter the RyR conformation. This would increase the open probability of the RyR causing release of SR Ca+2, and thus increase the intracellular Ca+2 content. Thus, ROS-induced RyR activation might be associated with the depolarization of the membrane potential, prolongation of the action potential, and loss of excitability, to cause arrhythmia.
C. SERCA is affected by ROS
Because Ca+2 transport into the SR is tightly coupled to hydrolysis of ATP, inhibition of ATPase activity will ultimately decrease the Ca+2 pumping rate. Out of the three SERCA isoforms, SERCA1, SERCA2a and 2b, and SERCA3, SERCA2a is expressed in cardiac muscle. Redox agents are known to modulate SERCA (Fig. 8). The cardiac SERCA contains 25 cysteine residues (193). Reagents that oxidize thiols inhibit pump activity (opposite to the effect on RyR), whereas reducing agents (e.g., DTT and GSH) protect SERCA from this inhibition (229). H2O2 also inhibits the ATP-dependent Ca+2 uptake activity in the microsomes from HEK-293 cells overexpressing SERCA2b (96), perhaps by directly interfering with the ATP-binding site (273). In a rat cardiomyocyte system, short-term H2O2 application induced SR Ca+2 depletion via redox-mediated decreased activity of SERCA and increased activity of the NCX, and this was associated with thiol oxidation (149). NO does not appear to affect SERCA Ca+2 pump activity directly; however, nitroxyl (HNO; the 1-electron-reduced and protonated form of NO) increased the maximal rate of Ca+2 uptake mediated by SERCA due to a reversible oxidative modification of SERCA thiols (2). HNO increased the S-glutathiolation of SERCA, and adenoviral overexpression of glutaredoxin-1 prevented both the HNO-stimulated oxidative modification of SERCA and its activation, as did overexpression of a mutated SERCA in which cysteine 674 was replaced with serine. Thus, HNO increases the maximal activation of SERCA via S-glutathiolation at cysteine 674 (2, 153). More information on oxidative modulation came from G-alpha-q- (alpha-subunit of Gq protein) overexpressing mice (152). The isolated ventricular myocytes from G-alpha-q mice had marked abnormalities of myocyte calcium transients along with an increased oxidative stress. Increased ROS in these G-alpha-q myocardia caused irreversible sulfonylation at cysteine 674 and nitration at tyrosines 294/295 of SERCA protein without affecting the total expression (152). Due to these oxidative modulations of SERCA, the calcium-stimulated SERCA activity was decreased. Crossbreeding G-alpha-q mice with transgenic mice that have cardiac myocyte-specific overexpression of CAT decreased SERCA oxidative cysteine modifications, decreased SERCA cysteine-674 sulfonylation and tyrosine-294/295 nitration, restored SERCA activity, and improved myocyte calcium transients and contractile function (152). Thus, in contrast to the effects on the RyR, ROS/RNS decreased the activity of the SERCA and reduced the Ca+2 reuptake into the SR. This may increase the total Ca+2 and decreased Ca2+ release in the myocytes, causing prolongation of the action potential and thus decreased excitability. Further information on SERCA regulation by CaMKII can be found in a review (173).
VI. Redox and Potassium Channels and Excitability
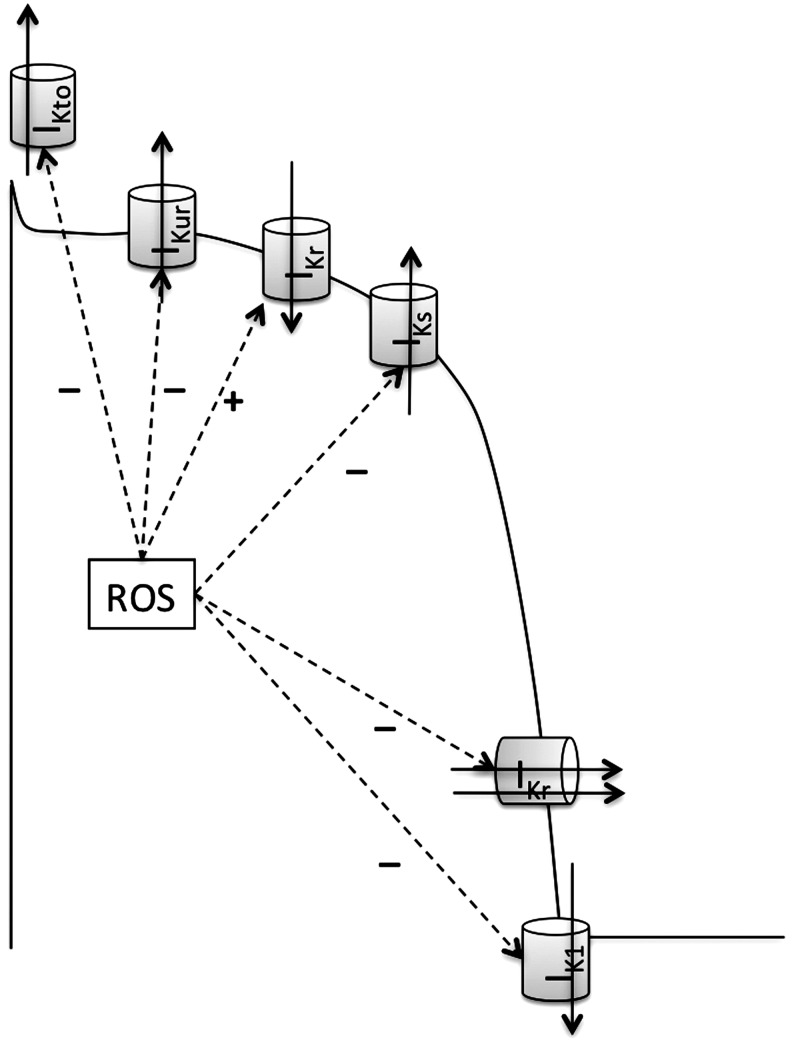
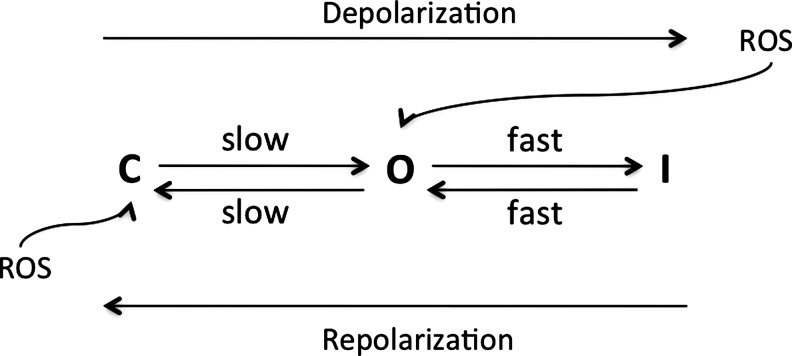
Cardiac K+ currents can be distinguished on the basis of differences in their functional and pharmacological properties. In mammalian cardiac cells, K+ channels can be categorized as voltage-gated (Kv) and ligand-gated K+ channels (155) (Table 2). In addition, in ventricular myocytes, it has been shown that the inhibition of leak K+ currents is sufficient to delay repolarization, causing prolongation of the action potential duration, and thus affects the excitability of the cells (24). Although the molecular identity of this large group of K-leak channels has been identified, their exact functions in cardiac cells, number, types, and effects of various intracellular signaling molecules, including ROS, are not very well known. Thus, these channels will not be reviewed further. The molecular constituents of various K+ channels are also shown in Table 2. K+ channels are responsible for the repolarization of the action potential, and thus any functional modulation of these K+ channels can disrupt excitation. Delay or inhomogeneity of the repolarization may initiate arrhythmias by causing EADs and TA or re-entrant arrhythmias, respectively. Different types of K+ channels are differently modified by ROS, an observation that might be of importance in disease states. ROS interaction and their effect on various K+ channel activities are reviewed below.
Table 2.
Various K+ Currents in the Cardiac Myocytes and Their Associated K+ Channel Subunits
| Class | Current | α-subunit | Gene | β-subunit | Gene |
|---|---|---|---|---|---|
| Kv channels | IKto | Kv4.3 | KCND3 | KChIP2 | KCNIP2 |
| Kv1.4 | KCNA4 | ||||
| Kv4.1 | KCND1 | KChIP1 | KCNIP1 | ||
| Kv4.2 | KCND2 | KChIP2 | KCNIP2 | ||
| IKUR | Kv1.5 | KCNA5 | Kvβ1 | KCNAB1 | |
| Kvβ2 | KCNAB2 | ||||
| IKr | Kv11.1 (HERG) | KCNH2 | minK | KCNE1 | |
| MiRP1 | KCNE2 | ||||
| IKs | Kv7.1 | KCNQ1 | minK | KCNE1 | |
| IK1 | Kir2.1, Kir2.2 | KCNJ2, KCNJ12 | |||
| IKCa | KCa1.1 (BKCa) | KCNMA1 | KCNMB | ||
| KCa2.1 (SKCa1) | KCNN1 | CaM | CALM | ||
| KCa2.2 (SKCa2) | KCNN2 | CaM | CALM | ||
| KCa2.3 (SKCa3) | KCNN3 | CaM | CALM | ||
| KCa3.1 (IKCa) | KCNN4 | CaM | CALM | ||
| Ligand Gated K+ channels | IKACH | Kir3.1, Kir3.4 | KCNJ3, KCNJ5 | ||
| IKATP | Kir6.2, Kir6.1 | KCNJ11, KCNJ8 | SUR2A, SUR2B | ABCC9 | |
| Leak K+ channels | K-Leak currents | K2P channels—TWIK, TREK, TASK, TALK, THIK, and TRESKSubfamilies | KCNK1,2,3,4,5,6,7,9,10,12,13,15,16,17,18 |
The K+ channels are made up of an α-subunit and a β-subunit. The proteins and genes associated that make these channels are tabulated. Voltage-gated K-channels open in response to a change in the membrane potential to permit transmembrane passage of K+ according to inherent voltage- and time-dependent properties. The Kv channels include the rapidly activating and inactivating transient outward current (IKto), the ultrarapid (IKur), rapid (IKr), and slow (IKs) components of the delayed rectifier and the inward rectifier (IK1), whereas the ligand-gated K+ channels include those that respond to a decrease in the intracellular concentration of ATP (KATP) or activated by ACH (KAch) (155). In addition, K+ channels that are activated by intracellular Ca2+ are called calcium-activated K+ channels (KCa channels). These channels are difficult to classify because of their structural dissimilarity with the other Kv channels, but because their activation follows opening of the voltage-dependent Ca2+ channels, functionally they have been classified as voltage-sensitive K+ channels. In addition, K-leak channels have also been defined in the excitable cardiac cells, which conduct voltage-independent ligand-independent leak K+ currents (247). CaM, calmodulin. The table was created by adapting the information from (24, 33, 245, 247, 285).
BKCa, large-conductance KCa; SKCa, small-conductance KCa; IKCa, intermediate-conductance KCa; KChIP, K+ channel-interacting protein; SUR, sulfonylurea receptors.
A. Effects of ROS on the Kv channels
At least five major K+ currents (IKto, IKur, IKr, IKs, and IK1) flowing through Kv channels possess different density and voltage- and time-dependent properties that contribute to repolarization in the ventricular myocardium (245) (see Fig. 9). While IKto causes early repolarization of the action potential plateau leading to a notch in the action potential shape, activation of the delayed rectifier currents, IKur, IKr, and IKs, together with a decrease in ICa, controls final repolarization. The late phase of the final repolarization is further supported by IK1 (245). ROS affect all the Kv channels as shown in Figure 9. ROS affect the activation and the inactivation kinetics of various IKv, and thus the net result of ROS depends on primarily whether the activation kinetics or the inactivation kinetics was affected. For example, a ROS-induced enhanced inactivation or slower activation will lead to more channels in the open state, and thus an increased IKv. Nevertheless, most of the studies have demonstrated that ROS decrease cardiac repolarizing K+ currents, which prolong the action potential, and thus prolong the effective refractory period, due to which the propensity of EAD increases (181, 211, 247). It is noteworthy that some of the studies have also demonstrated unaffected IKto or IK1 by direct application of oxidants as t-BHP or H2O2 (27, 263). The reasons for these contradictory results are unclear, but could presumably be direct effects of these oxidants on other channels/systems of the cell.
FIG. 9.
ROS modulate the activity of various voltage-dependent K+currents that contribute to the cardiac action potential. Various K+ currents such as the transient outward current (IKto), the ultrarapid (IKur), rapid (IKr), and slow (IKs) components of the delayed rectifier and the inward rectifier (IK1) are shown on the action potential when their activity is maximum. The outward currents IKto, IKur, IKs, and IKr and the inward IK1 are all inhibited, whereas the outward current by HERG, a depolarizing phase, is generally increased by ROS.
Several inward and outward K+ channels are affected by different ROS-generating systems. For example, in guinea pig cardiac ventricular myocytes, ROS generated from the xanthine/xanthine oxidase system or H2O2 decreased the inward K+ current (53, 199). In a comprehensive study, Duprat et al. (66) reported that when expressed in Xenopus oocytes, the activity of three Shaker K+ channels (Kv1.3, Kv1.4, and Kv1.5), one Shaw channel (Kv3.4), and one Kir channel (IRK3) was drastically inhibited by photoactivation of rose bengal, a classical generator of ROS. On the other hand, Shaker K+ channel Kv1.2; Shab channels Kv2.1 and Kv2.2; Shal channel Kv4.1; and inward rectifiers IRK1 and ROMK1, and hIsK were completely resistant to this treatment. In contrast, t-BHP, another generator of ROS, removed the fast inactivation processes of Kv1.4 and Kv3.4, but did not alter other channels. In Xenopus oocytes, external application of H2O2 was found to inhibit the time-dependent fast inactivation process of three cloned voltage-gated K+ channels, KShIIIC, KShIIID, and HukII (256), in a reversible manner. According to the authors, H2O2 caused a large increase in the current magnitude because of the inactivation block due to which some channels would still be opening while a significant fraction of channels would have been already inactivated. Other cloned voltage-gated K+ channels were not affected by H2O2 (256). Furthermore, transient nonlethal ionizing irradiation of the whole cell or H2O2 or heat stress induced whole-cell voltage-dependent outward K+ currents with no changes in the membrane potential of −70 mV, intracellular K+ concentration, or ATP levels (148). Similarly, Kv7.1-coded IKs currents were not modulated in CHO cells (84), but in the human submucosal gland serous cell line Calu-3, Kv7.1-coded IKs currents were increased by H2O2 perfusion (58). All of these studies were done in the heterologous system with exogenous expression of the subunits of the K+ channels and have provided complex results, suggesting that ROS may increase the activity of certain Kv channels while it may decrease activity of other Kv channels.
More comprehensive results came from the studies in native systems using isolated ventricular myocytes. For example, in isolated guinea pig ventricular myocytes, the ROS-generating system DHF reduced the outward K+ current (44) (Figs. 9 and 10). The effect of ROS on IKto [measured as a difference between peak current (Ipeak) and steady state current (Iss)] was demonstrated (160) in a native system. Exposure of rat ventricular myocytes to a thio-specific oxidant, diamide, decreased the Ipeak and Iss (measured at the end of the test pulse) by about 50% after depolarizing test pulses. The decrease in Ipeak, but not the decrease of Iss, was reversed by inhibiting the thioredoxin system (160). The ROS-induced decrease in Iss was reversed by inhibiting the glutaredoxin systems (Fig. 10). From this study, it might be inferred that the thioredoxin system controls the Kv4.2, Kv4.3, or Kv1.4 channels that constitute the Ipeak (see Table 1). Since Kv1.5 and Kv2.1 (IKur and IK1) channels largely contribute to Iss in rat myocytes, it may be postulated that one or both are regulated by the glutaredoxin system. In another study with the left ventricular myocytes of rats in which redox pathways were inhibited, GSH content was reduced; ROS were increased; and the maximum IKto density was reduced, whereas the IK1 and IKs (Kv7.1) were not significantly altered (159) (Fig. 10). Decreased IKto density may be due to ROS-induced decrease in mRNA and proteins levels of Kv4.2 and Kv1.4, which was reversed in vitro by exogenous GSH or N-acetylcysteine (a GSH precursor and antioxidant). This was also shown in the myocytes in which the increased oxidative stress decreases the mRNA and protein expression of Kv4.2 and Kv4.3 channel alpha-subunits as well as the accessory protein KChIP2 (159). Thus, results from the experiments in the native system suggest that ROS cause a decrease in the Kv currents (IKv; IKto, IKur, IKr, IKs, and IK1) in myocytes. One of the deadly dysfunctions of Kv current is Long-QT syndrome, in particular LQT1 (estimated 35% of LQT patients), which is caused by decreased IKs. The decrease in IKs decreases repolarizing currents, causing severe prolongation of the action potential (QT interval), and a reduced refractory period, both of which will render the myocytes unexcitable and will generate arrhythmia (28).
FIG. 10.
ROS effects on the voltage-gated K+channels. Phosphorylation of Kv1.5, Kv4.2, Kv4.3, or HERG decreases the currents from these channels. ROS activate PKC or PKA that are responsible for this phosphorylation. ROS also inactivate protein tyrosine phosphatase (PTP), causing increased phosphorylation of the receptor tyrosine kinases (RTK) that leads to activation of SRC-mediated phosphorylation of Kv1.5. ROS content is increased by cytosolic fatty acids such as ceramide and decreased by GSH.
The crucial role played by Kv channels in the control of cell excitability has gained considerable attention after discovering that the LQT2 was caused by mutations in the human ether-a-gogo-related gene (HERG) or KCNH2 encoding the channel Kv11.1 responsible for IKr (59). The main features of IKr are its modulation by the extracellular concentration of K+ ions, a peculiar inward rectification mechanism at depolarizing potentials, and its blockade by class III antiarrhythmics (230). However, at negative voltages, the channels recover from the inactivation faster than they deactivate, leading to more open channels and significant outward current (230) (see Fig. 11). When Xenopus oocytes, expressing Kv11.1, were exposed to an extracellular solution containing FeSO4 and ascorbic acid (Fe/Asc) (as a cellular ROS production system), outward IKr carried by Kv11.1 channels was increased, whereas inward currents were not modified (244). Currents carried by other K+ channels such as Kir, bEAG, rDRK1, and mIRK1 remained unaltered. This ROS-induced increase in outward IKr carried by Kv11.1 was due to a depolarizing shift of the voltage dependence of channel inactivation, with no change in channel activation (244). This effect would increase the availability of the channels to be opened in response to depolarization. At negative potentials, the channels are fully recovered from inactivation, and therefore the modulation by Fe/Asc of the inactivation process cannot be exerted, possibly explaining the lack of Fe/Asc-induced modulation of the inward current component of Kv11.1 channels (244). Fe/Asc-induced stimulation of Kv11.1 outward currents was completely prevented by scavenging ROS with a mixture of CAT, SOD, and mannitol (244). In the similar experiment, H2O2 enhanced the Kv11.1 outward currents by accelerating the activation at depolarizing voltages, whereas decreased the inward currents by accelerating the deactivation at repolarizing voltages in the CHO cells expressing human Kv11.1 channels (23). Effects of H2O2 superfusion were prevented by intracellular application of CAT, but SOD prevented only H2O2-induced acceleration of activating currents (23). Contrary to these results, hypoxia in a stimulus- and time-dependent manner increased ROS and decreased Kv11.1 protein with a marked reduction in Kv11.1 K+ conductance in HEK cells stably expressing the Kv11.1 α-subunit (202). Importantly, this downregulation of Kv11.1 by hypoxia was not due to increased proteasomal degradation or decreased transcription, but due to decreased synthesis of the protein (202). Antioxidants prevented hypoxia-evoked downregulation of Kv11.1 protein and exogenous oxidants (xanthine/xanthine oxidase system) that mimicked the effects of hypoxia, and yet the inhibitors of NADPH oxidase failed to prevent the effects of hypoxia (202). This study was somewhat confusing, because in this study, hypoxia increased ROS instead of decreasing, and thus the actual mechanism by which hypoxia modulated Kv11.1 currents is not clear.
FIG. 11.
Schematic of activation–inactivation kinetic model for HERG. ROS cause a depolarization shift in inactivation kinetics, and thus leads to more open channels to increase outward currents. At repolarizing potentials, most of the channels recover from inactivation to be active. However, ROS accelerate the deactivation at the repolarizing voltages, decreasing the inward currents. C, closed-deactivated; O, open; I, inactivation.
It is important to define whether ROS regulation of Kv channels occurs by direct thiol modification of Kv subunits or by indirect effects on redox-sensitive signaling molecules. In this regard, phorbol 12-myristate 13-acetate (PMA), a nonspecific activator of serine/threonine and tyrosine protein kinases, or phenylepherine, suppresses Kv4.2 and Kv4.3 currents in a heterologous system, and native IKto in rat ventricular myocytes, in a PKC-dependent manner (198) (Fig. 10). PKC decreases these currents by increasing the inactivation time constants and slows the time course of recovery from inactivation of IKto (198). Since cellular ROS activate PKC (47), increased ROS will reduce the IKto in a PKC-dependent manner. ROS cause transient oxidation of thiols in protein tyrosine phosphatases that leads to their inactivation, and this activates receptor tyrosine kinases (RTK) (47). Activation of RTK causes phosphorylation of the Kv channels (exemplified by Kv1.5 and Kv1.3) via SRC kinase that represses Kv currents (112). In this regard, diabetic rat ventricles showed decreased IKto due to a decreased GSH content. However, upon activation of RTK by insulin in these myocytes, GSH levels and IKto were restored (158). This was further confirmed in the myocytes from diabetic rats that were treated for several hours with GSH or NAC, to increase the cellular antioxidant capacity, and this treatment restored the IKto density (158). Therefore, ROS could also reduce the IKto and IKUR by indirectly activating the tyrosine kinase (receptor-mediated or nonreceptor-mediated, such as SRC). Overall, it can be conceived that increased ROS will decrease IKto and IKUR, whereas decreased cellular ROS would increase the IKto and IKUR. Furthermore, GSH upregulation due to activation of RTK by insulin was also blocked by inhibitors of PI3-kinase, MEK, and p38 MAP kinases (158), indicating that modulating these kinases could also decrease IKto. Human Kv1.5 (IKUR) also has one consensus site for phosphorylation by PKC located on the extracellular S4–S5 linker and 4 consensus sites for PKA located in the N- and C-terminal domains. Therefore, both PKC and PKA activation inhibited IKUR (156). Since PKC and PKA are stimulated by ROS, ROS might regulate IKUR in humans as well. PKA and PKC might also be involved in the Kv11.1 IKr regulation, because ceramide, a sphingolipid metabolite, depresses IKr mainly via ROS overproduction, and this suppression was reversed by PKA and PKC inhibitors, but not by tyrosine kinase inhibitors (17). These PKC and PKA effects occur presumably by direct phosphorylation of the Kv11.1. However, there are other modes by which ROS cause direct alteration of the Kv11.1 channels, and thus reduce Kv11.1 currents, which depend on the molecular makeup of the Kv11.1 itself. To localize the molecular regions involved in ROS-induced modulation of Kv11.1, segmental exchanges between the ROS-sensitive Kv11.1 and the ROS-insensitive bovine ether-a-gogo gene (bEAG) channels have been generated, and the sensitivity of these chimeric channels to ROS was studied (213). Results obtained suggest that histidines at positions 578 and 587 in the S5–S6 linker region of Kv11.1 channels are important in ROS-induced modulation of Kv11.1 channels (213). Moreover, mutagenesis revealed that cysteine 723, a conserved residue in a structural element linking the C-terminal domain to the channel's gate, is critical for ROS modification (141). For the most part, it is confirmed that the expression and activity of cardiac IKv are regulated by endogenous ROS, via receptor tyrosine kinase/PKC signaling, which functionally impacts K+ channel. However, effects of ROS on every K+ channel type and the functional impacts of ROS-induced alterations in cell signaling need further study. In summary, it seems like increased cellular ROS decreased the voltage-dependent K+ currents in the myocytes. This will reduce the total repolarizing currents that may lead to a depolarized membrane potential, an enhanced action potential duration, and decreased excitability.
B. Calcium-activated K+ channels
Calcium-activated K (KCa) channels are a key signaling target in smooth muscle cells, opening of which causes hyperpolarization. The three types of KCa channels, large conductance KCa (BKCa), the small conductance KCa (SKCa), and the intermediate conductance KCa (IKCa), might have a different expression pattern in various tissues (245). The BKCa channels are expressed in the adult and neonatal chick ventricular myocytes and conduct K+ currents (125). The function of the cardiac BKCa is debatable, because the α-subunit gene of the BKCa is not expressed in cardiomyocytes of certain species as rodent; however, BKCa channels have been reported in the guinea pig cardiomyocyte inner mitochondrial membrane, where it plays a cardioprotective role (118). SKCa channels are also expressed in human and mouse cardiac myocytes; however, SKCa channels were more abundant in the atria and pace-making tissues compared with the ventricles (252). All of the three isoforms of SKCa channel subunits (SK1, SK2, and SK3) are expressed. It has been shown that SKCa channels contribute functionally toward the shape and duration of cardiac action potentials (157). Therefore, genetic KO of the SK2 channels results in the delay in cardiac repolarization and atrial arrhythmias (157).
The effects of ROS on the activity of KCa have not been studied in detail; however, it has been shown that hypoxia causes repression of the BKCa β-subunit expression in cardiac mitochondria in a Hif-2α-mediated mechanism and prevents deleterious mitochondrial depolarization (38) (Fig. 12). Whether this hypoxia effect causes any decrease in BKCa currents and if the mechanisms involve ROS is unclear. In this regard, H2S has been shown to reduce the conductance from the native BKCa channel in rat glomus cells and from the human recombinant BKCa channel in HEK293 cells (248). In another study, NO stimulated the activation of BKCa channels in Xenopus oocytes, in which the α- and β-subunits of the human BKCa channels were expressed along with a receptor GC and rat atrial natriuretic peptide receptor (204). Site-directed mutation of consensus phosphorylation sites on the β-subunit (Ser855 and Ser869 to alanine) indicated that NO causes augmentation of the BKCa currents via GC-mediated stimulation of PKG, which phosphorylates the residues 855 and 869 (204). From these studies, it is clear that ROS/RNS do modulate the BKCa currents, but not by a direct redox modulation of the channel subunits. The effects of ROS on cardiac SK and IK channels need further investigation.
FIG. 12.
ROS enhance the activity of the large-conductance calcium-activated K-channels (BKCa). Activation by ROS occurs due to increase in cytosolic Ca+2, as well as directly by modulating channel subunits. Nitric oxide (NO) activates protein kinase G (PKG) that increases phosphorylation of the BKCa channels to increase the activity.
C. KATP channels
KATP channels are found at a high density in the heart (210). The cardiac KATP channels are formed by a Kir pore (6.1 or 6.2) and the sulfonylurea receptors (SURs) that act as the regulatory subunits (210). Expression of Kir6.1, Kir6.2, and the three isoforms of SUR viz SUR2A, SUR2B, and SUR1 has been demonstrated in the cardiac sarcolemma as well as the mitochondrial membranes (7, 154, 210). Moreover, it has been demonstrated indirectly by pharmacological techniques that active functional KATP channels do exist in both sarcolemma as well as mitochondria membranes (87). Despite ample evidence of KATP activity in sarcolemmal membranes (sarc-KATP) and mitochondrial membranes (mito-KATP), direct molecular evidence of the existence of the mito-KATP is weak (210). It is generally recognized that the predominant form of KATP channels in cardiac sarcolemma is Kir6.2-SUR2A (7). The identity of mitochondrial KATP channels has been a subject of investigation for years with variable results, suggesting that mito-KATP could be Kir6.1-SUR2A, Kir6.2-SUR2B, or other heteromeric combination of these subunits (7). This has been further complicated by the expression of various splice variants of the SURs recently discovered in the mitochondrial membranes (282).
Since the opening and K+ conductance of the KATP channel depends on the cellular ATP, the activity of the channels is directly correlated to the metabolic status of the cell and the availability of the O2 (Fig. 13). Therefore, KATP channels are also generally accepted as the metabolic sensors of the cell that play a major role in protecting the heart from ischemia–reperfusion-induced damage (87). The activation of sarc-KATP channels reduces the action potential duration (decreased excitability) and conserves energy during periods of ischemia (30). The activation of the mito-KATP channel increases the K+ influx in the mitochondria, depolarizes the mitochondrial membrane potential, increases the Ca+2 tolerance of the mitochondria, and conserves the structural integrity (87). However, this increase in potassium conductance due to the opening of sarc-KATP channels may predisposes heart to electrical dysfunction, and in some cases, the generation of fatal arrhythmia (30). Therefore, the opening of sarc-KATP channels, such as in an ischemic heart, can significantly shorten the action potential and promote arrhythmia, and if enough channels open, can render cells unexcitable (26, 109). These reductions in the action potential duration are exacerbated by KATP channel agonists (68, 122) and blocked by the KATP inhibitors such as glibenclamide, HMR 1833, or HMR 1098 (69, 81, 223). Various KATP inhibitors also reduce the incidence of postischemic ventricular arrhythmia in rat, rabbit, pig, dog, and in humans (15, 80, 254, 265); however, this blockade of the sarc-KATP causes increase in the infarct sizes of heart postischemia (95).
FIG. 13.
ROS activate KATP channels. KATP are formed by sulfonylurea receptors (SURs) and inward rectifier (Kir) on sarcolemma or mitochondrial membranes. ROS-mediated activation of Protein Kinase-C and G (PKC and PKG) activates KATP. ROS, such as H2O2, itself activate KATP directly. Hypoxia decreases mitochondrial ATP synthesis and thus cellular ATP content, which lead to increased opening of mitochondrial or sarcolemmal KATP, respectively.
Since hypoxia and the metabolic state of the cell regulate ROS generation, KATP channels are affected by ROS directly or indirectly via signaling. In fact, the first K+ channel that was found to be modulated by hypoxia and ROS in the heart was the KATP channel when hypoxia, presumably at a level able to cause metabolic inhibition, was shown to activate a K+ conductance that was called KATP (206). About 15 min of hypoxia increased the IKATP, whereas GSH could reverse the increased IKATP (123, 250, 277). In this regard, in the isolated myocytes, GSSG increased the IKATP, while GSH could reverse the increased IKATP during normoxia. The redox couple DTT/H2O2 produced results similar to the redox couple GSH/GSSG during normoxia (277). H2O2 increased the IKATP in a concentration-dependent manner, which was reversed by DTT or glibenclamide (123). H2O2 at concentrations of 5 mM, but not 1 mM, increased KATP channel activity in the presence of 0.3 mM ATP in ventricular cells isolated from guinea pig hearts. However, the presence of 10 μM ADP and Mg2+ together with 0.3 mM ATP increased the sensitivity to H2O2 and led to activation of the KATP channel by 1 mM H2O2 (123). The channel activation was due to an increase in the open-state probability and was not associated with a change in the single-channel conductance or the mean open and closed times. In addition, the ATP sensitivity of the channels was also decreased by 1 mM H2O2; therefore, H2O2 activates the KATP channel directly by decreasing the sensitivity to ATP, an effect potentiated by the presence of ADP (26, 123). OH• produced via the iron-catalyzed Haber–Weiss reaction in the guinea pig ventricular myocytes also activated KATP channels; however, this activation could not be prevented by even very high concentrations of glibenclamide (250). Several experiments have induced ventricular arrhythmias under normoxic conditions with delivery of ROS bursts, and scavenging the ROS with SOD mimetics, or mitochondrial-targeted antioxidant peptides were successful in decreasing the incidence of arrhythmia (49, 105, 143). The cellular mechanisms by which ROS cause KATP activation are not clear; however, it is believed that increased ROS deplete cellular ATP that causes KATP channels to open. Nevertheless, the IKATP augmented by hypoxia and GSSG was reversed by inhibition of the PKC by bisindolylmaleimide VI, inhibition of PKG by KT5823, and inhibition of CaMKII by KN-62 and KN-93 (277). Inhibition of PKA by H-89 did not reverse the hypoxia or GSSG effect (277) (Fig. 13). These results suggest that the effects of both GSSG and hypoxia on KATP channels involve the activation of the PKC, PKG, and CaMKII pathways, but not the PKA pathway. Whether there is also a direct modification of the KATP channels by the redox is unclear.
It has been observed that under conditions of metabolic stress, sarc-KATP currents and action potential durations oscillate in cardiac myocytes (12, 224). These fluctuations in sarc-KATP currents and, consequently, the action potential duration are intricately linked to the ΔΨm (12, 224). The ΔΨm collapses with an increased ROS generation in the intact myocyte or isolated mitochondria from various species. However, this collapse in ΔΨm is reversible, and thus oscillates, in isolated cells or the heart subjected to oxidative stress via modulating GSH/GSSG or NAD/NADH, ATP depletion, local generation of ROS by laser excitation, chemical oxidants, and respiratory inhibition, or to global ischemia/reperfusion (10, 12, 222, 224, 234). With this oscillatory ΔΨm, sarc-KATP current increases or decreases in phase with the ΔΨm, which may alter the action potential, and thus excitability, by altering K+ currents (Fig. 14). The exact mechanism of this ΔΨm-induced arrhythmia is not known yet, but has been postulated to be due to the local generation of mitochondrial ROS that induce further ROS generation by neighboring mitochondria in a spaciotemporal network (40). This term is coined as “ROS-induced ROS release.” The increase in ROS under conditions of oxidative stress can reach a critical level, after which cell-wide ΔΨm oscillations in the mitochondrial network are observed (12) (Fig. 15). Indeed, in isolated cardiac myocytes, oxidants caused oscillations in the ΔΨm, which were accompanied by ventricular tachycardia/fibrillation, but was reversed by GSH addition (10, 234). With all these lines of evidence, there are very few studies that have focused on the role of mitochondria and mito-KATP in regulating the ΔΨm and generation of arrhythmia. The ΔΨm and the mitochondrial ROS generation have been correlated to the increased K+ flux in the mitochondria. Although the identity of the mito-KATP is still under investigation, it is widely accepted that such a K+ flux across the mitochondrial membrane occurs due to a mito-KATP channel that opens upon metabolic inhibition and causes collapse of the ΔΨm with concomitant ROS generation (56, 87). In this regard, direct measurement of the mito-IKATP in fused mitoplasts or bilayer mitochondrial membranes has been demonstrated successfully (124, 127). Thus, it is evident that the ROS activate mito-KATP, which enhances K+ flux in to the mitochondria, depolarizes the mitochondria, and inhibits the respiratory chain. This causes even more increase in the ROS generation in presence of the trace amount of O2. Whether and how ROS actually open mito-KATP will remain a speculation until the identity of the mito-KATP has been confirmed. However, it has been observed that activation of PI3-kinase leads to phosphorylation of Akt and eNOS, which induced NO synthesis, which stimulates GC to produce cGMP, which activates PKG, ultimately leading to opening of mito-KATP in the mitochondrial inner membrane (55). Although the role of mito-KATP in protection against the I/R injury and postischemic arrhythmia has been widely studied by various groups (56, 87, 95), more studies are needed to understand the role of mito-KATP toward action potential, excitability, and arrhythmia.
FIG. 14.
Membrane excitability and mitochondrial energetics. (A) In-phase oscillations of the sarc-KATP currents correlate with the oscillations of the NADPH fluorescence in adult guinea pig myocytes. The NADPH fluorescence in shown in uncalibrated units, and the membrane currents were measured at −40 mV. This indicated that mitochondrial functions might be related to the cellular excitability. (B) Simultaneous, whole-cell recordings of the temporal evolution of the fluorescence intensity of TMRE (mito-membrane potential-sensitive), CM-DCF (ROS-sensitive), and the endogenous NADH signals (ROS-sensitive). Compounding these results, it has been suggested that the mitochondrial ROS production and mito-membrane potential influence the membrane sarc-KATP currents and thus membrane potential. Panels for this figure are taken from (54, 207), with copyright permission.
FIG. 15.
Characterization of cell-wide mitochondrial oscillations. (A) Images of a cardiomyocyte loaded with TMRE (for detection of mitochondrial membrane potential) and DCF (for detection of ROS generation at the frame interval of 7 s. The phasic increase in intensity of fluorescence is evident. (B) Time course of average whole-cell fluorescence of TMRE and NADH (as an indicator of ROS), and (C) DCF and the derivative of DCF signals (dF/dt, purple). The precise phase relationship between all signals can be clearly appreciated from the vertical reference line drawn in (B, C). The individual panels in the figure were originally published in Aon et al. (11) © The American Society for Biochemistry and Molecular Biology. Reprinted with permission.
VII. Redox Effects on Sodium Channels and Excitability
The cardiac Na+ channel is a member of the voltage-dependent family of Na+ channels. These channels consist of heteromeric assemblies of an α-subunit that forms the pore and ancillary β-subunits that modulate the function (25). The human cardiac Na+ channel α-subunit is a heavily glycosylated protein of ∼260 kDa consisting of 2016 amino acid residues encoded by the gene SCN5A, whereas the human β1-subunit is a 218-amino-acid protein encoded by SCN1B (88, 175). Several other isoforms of the α-subunit (Nav1.1–Nav1.3) encoded by SCN1A–SCN3A and β-subunit (β1, β2, β3 and β4.1, and β4.2) encoded by SCN1B, SCN2B, SCN3A, and SCN4A have also been described in the cardiomyocytes. The functions of various β-subunits in the heart are unclear and controversial in the literature. Investigations have revealed several functions of the β-subunits, including the increase in expression of α-subunits (Nav1.5) in the sarcolemma, augmentation of the amplitude of INa, modulation of the gating properties, and regulating the interaction of Nav1.5 proteins to extracellular matrix molecules, cytoplasmic cytoskeleton apparatus, and components of cardiac intercellular junctions (e.g., cadherins and connexins) (8). Regarding the existence of several subunit isoforms, studies have reported controversial results on the constitution of ventricular Na+ channels. Na+ channels composed of Nav1.5 and β2- and/or β4-subunits have been shown in intercalated disks of the myocytes (174), and channels composed of Nav1.1, Nav1.3, and Nav1.6 with β1- and/or β3-subunits have been shown in the transverse tubules (174). On the contrary, Nav1.1 or Nav1.5 with β1- and β2-subunits has been shown at the Z-line. Yet, the β2-extracellular domain did not modulate the functions of the Nav1.5 in a heterologous system. A β2 KO mice showed reduced neuronal Na+ currents, but effects on cardiac Na+ currents were not studied (46). β1 KO mice showed severely longer QT and RR intervals compared with the controls (165). Electrophysiological investigation in ventricular myocytes isolated from β1 KO mice revealed increased densities of both INa and INaP with no other changes in channel gating. A longer action potential duration and a slower rate of action potential repolarization were also found, consistent with the QT prolongation phenotype and the increase in INa. (165). The finding that the absence of β1 increased INa density contrasts with most in vitro reports, which show an increase of INa in the presence of β1. These discrepancies may be explained by the fact that the physiological heterologous expression systems do not fully recapitulate the situation in cardiac myocytes.
Confirming the key role of (-subunits in cardiac Na+ channel regulation, a novel mis-sense mutation (L179F) in SCN4B, the gene encoding the (β4-subunit, was recently identified in a 21-month-old girl affected by Long-QT syndrome (LQT10) (25). Patch-clamp studies in HEK293 cells stably expressing Nav1.5 and transfected with this mutant β4-subunit revealed increased INaL compared to Nav1.5 expressed alone or together with the wild-type β4-subunit (185). Although the functional biology of the (-subunit is not clear, these studies do indicate that functional defects in Na+ channel (-subunits are associated with arrhythmogenecity.
Despite the unclear and unestablished roles of β-subunits, it is clear that both the α- and β-subunits are highly expressed in the human heart (25), and that both the α- and β-subunits when expressed together in the heterologous system conduct larger currents, show accelerated activation and inactivation, and a more negative voltage dependence of steady-state inactivation compared with the α-subunit alone (25). The voltage-gated cardiac Na+ channel is responsible for the generation of the rapid upstroke of the action potential, and thereby plays a central role in excitability of myocardial cells. In addition, these channels also determine the velocity of the upstroke and the impulse conduction velocity in cardiac tissue, and therefore are also important for the impulse propagation. Mutations in the gene encoding this channel (SCN5A and SCN1B) cause mainly three forms of primary electrical disease—the LQTS, the Brugada syndrome, and cardiac conduction defects (18, 25, 231). Ample studies have demonstrated that the ROS-induced alterations in INa electrophysiology in cardiac myocytes are responsible for various arrhythmogenic conditions by affecting the action potential and excitability (237, 238, 263).
Na+ channels transit among various conformational states in the process of voltage-dependent gating. Depolarization from the resting membrane potential triggers activation (opening) of the Na+ channels. If the depolarization is maintained, the channels enter a nonconducting inactivated state. Subsequent to repolarization, the channels return to a closed state capable of being activated once again (18, 231). Therefore, INa increases rapidly on depolarization, reaching a transient peak lasting a few milliseconds (INaT), before beginning to inactivate. The inactivation of INa has a fast component (fast inactivation) that lasts a couple of milliseconds and is followed by a slowly inactivating component that can last hundreds of milliseconds (18, 25, 231). The current associated with the slow inactivating component has been referred to as late, sustained, or persistent INa (INaL, INasus, or INaP) to distinguish it from the peak transient INaT.
In a recent report, it has been demonstrated that elevated mitochondrial ROS generation, due to increased cytosolic NADH/NAD ratio, reduces INa in HEK cells stably expressing human cardiac Na+ channel subunits (163). The effect of ROS on Na+ channels is summarized in Figure 16. ROS, such as H2O2, increases INaP and thereby cause (i) prolongation of APD, and thus induction of EADs that decreases excitability; and (ii) a rise in intracellular Na+ that causes cellular Ca+2 overload due to Na+/Ca+2 exchange (237, 238, 263). As mentioned in previous sections, this Ca+2 overload in cardiomyocytes is associated with electrical instability and via affecting INa. Whole-cell and cell-attached patch-clamp techniques on isolated guinea pig ventricular myocytes showed that ROS regulate the INaP and INaT. Hypoxia for 15 min or application of GSSG increased INaP and decreased INaT at the same time, and this effect on INaP and INaT was reversed by GSH. Therefore, hypoxia for 15 min [here it was assumed that hypoxia increases ROS as in (300)] and GSSG decreased the action potential amplitude and shortened the action potential duration at 90% repolarization (APD90) of ventricular papillary cells simultaneously, while GSH could reverse the decreased amplitude and the shortened APD90 at the same time (262). It is not clear from this study how hypoxia causes increase in ROS, the effect of which could be reversed by antioxidants. It can be assumed that hypoxia caused an actual decrease in ROS (and not increase) and that decreases the propensity of the Na+ channels to inactivate or to remain inactivated causing an increased INaP. However, it is still not clear from this study how an increase in INaP could cause shortened action potential duration. Wang et al. (262) did mention that both the currents might be charged with the same channel with a different gating mode. Thus, when INaP increases, the INaT might decrease and vice versa. The net effect of this interplay between INaP and INaT after hypoxia causes action potential duration shortening. Here it should be noted that in wild-type Na+ channels, this noninactivating current component (INaP) is negligibly low (<0.3% of the transient current), but in the LQT3-mutant channels (where the action potential duration is prolonged), this fraction is increased (261). Regarding hypoxia and induction of INaP by hypoxia, drugs that are more-potent inhibitors of INaP have been in development. For example, ranolazine, flacainide, and mexiletin, which reduce INaP more potently than INaT, have been in study for the arrhythmias (due to increased INaP) caused by ischemia (i.e., hypoxia) (226). In another study, milrinone (a phosphodiesterase-III inhibitor) was shown to reduce the INaP induced by hypoxia and H2O2 (293). Milrinone also reversed the prolonged APD90 (action potential duration and 90% repolarization) due to hypoxia and H2O2 in guinea pig myocytes (293). Similarly, other studies have shown that H2O2 can cause increases of INaP (167, 237, 238, 263) and intracellular Na+ and Ca2+ (258), thereby increasing APD and thus the excitability. Therefore, ranolazine and tetrodotoxin (TTX) effectively reduced H2O2-induced INaP, action potential prolongation and EADs in isolated myocytes. Other oxidants such as t-BHP reduced INaT, by enhancing inactivation in HEK cells (which expressed cardiac Na+ channels) or myocytes (83) mimicking the effects of hypoxia and GSSG on INaT. Products of the cellular lipid peroxidation isoprostanes that are markers and the mediators of the oxidative stress mimicked the effects of t-BHP on Na+ channel function in HEK cells (83). In this regard, another reactive product of lipid peroxidation, 4-hydroxynonenal, was ineffective on the INaT, suggesting that physiologically generated ROS products do have a specific pathway by which they may or may not modulate INaT (83). In HEK cells stably expressing the cardiac Na+ channel or in cardiomyocytes, cytosolic NADH application (to increase mitochondrial ROS generation) caused the decrease of INa, and this effect was reversed by mitochondrial antioxidants or by inhibiting the mitochondrial respiratory chain that would inhibit the ROS generation (163).
FIG. 16.
ROS enhance persistent sodium currents (INaP), but decrease transient Na+ currents (INaT). Increased INaP increases cytosolic Na, which then increases the cytosolic Ca+2 via increase Na/Ca exchange by NCX. Decreased INaT decreases depolarization amplitude and may cause arrhythmia.
Although the mechanism is not clear, the results have been consistent showing that INaP and INaT in cardiac muscle can be modulated and regulated by ROS/RNS and other oxidants as well as with hypoxia. However, this is difficult to reconcile, as hypoxia is associated with a decreased synthesis of superoxide. It is possible that the increased production of NO reported during hypoxia/ischemia (137) contributes to the increase in INaP during hypoxia. Indeed, like hypoxia, exogenous NO also increases INaP in isolated hippocampal neurons (99) and in guinea pig myocytes (168), and these effects were abolished by GSH and DTT (3, 99, 168). These experiments suggest that NO acts by oxidizing the cardiac Na+ channel protein components that regulate INaP, or a closely associated regulatory protein, contained within the plasma membrane. On the contrary, another report showed that NO decreases the INa in guinea pig and mouse cardiomyocytes without affecting the activation inactivation or reactivation kinetics. Instead, NO decreases open probability and/or decreases the number of functional channels with no change in single-channel conductance (4). Additionally, application of cell-permeable analogs of cGMP or cAMP mimics the inhibitory effects of NO, and that these effects cannot be reversed by a sulfhydryl-reducing agent (4). This report suggests that the NO affects INa by the cGMP/cAMP pathway and not by direct modulation of the Na+ channels. In essence, NO and its RNS do alter INa through multiple pathways, and the results (although controversial) may alter the cellular excitability.
The fact that ROS/RNS-mediated INa can be reversed by the reducing agents suggests that the modulated Na+ channel activity occurs as a result of a ROS/RNS-induced covalent modification or disulfide bond formation at or near the channel protein, although the mechanism underlying is unclear. It is known that there are 42 cysteine residues in the α-subunit of the human cardiac Na+ channel (18). They distribute in the four S5–S6 linkers, intracellular linkers between domains I and II, II and III, and other regions of the channel (18). Thus H2O2, GSH/GSSG, DTT, and perhaps hypoxia could change the gating mode of the sodium channel by directly acting on one or more cysteine residues in the sodium channel (166). In this regard, NO and perhaps the RNS are shown to decrease the INaP and INaT possibly by S-nitrosylation of the cysteine residues on the α-subunit of the Na+ channel (3, 253). It is not clear if and how nitrosylation occurs, but inhibitors of NOS could prevent the increase in INaP and INaT. It is interesting to note that the Na+ channel co-immunoprecipitates with nNOS (253), and the increase in the Na+ channel activity is associated with an initial increase in the intracellular Ca+2, which in turn depends on the activity of plasma membrane Ca+2 ATPase (PMCA), syntropin, and caveolii (3, 253). The effects of ROS on β-subunits have not been studied as far as we know. However, in one study, experimental heart failure in dogs caused increased INaL due to slower decay, and this effect was concluded to be due to dysfunctioning of the β1-subunits (186). Regarding this study, it is to note that HF causes increased ROS generation in the myocytes and that might have caused the dysfunctioning of β-subunits. However, it was not clearly tested in this study or other studies possibly because the identity and function of the β-subunits in the myocytes are still unclear. There are several proteins that interact with the Na+ channels to alter/modulate Na+ channel functions. A latest review article (1) can be referred on this subject for a detailed update. In particular, signaling pathways that regulate cardiac Na+ channel function include activation of PKA, PKC, and CaMKII (Fig. 16), both of which as activated by ROS (166). The PKA phosphorylates two serine residues (S525 and S528 in the human cardiac isoform) within the DI–DII linker that augments the INa (82), whereas PKC phosphorylates S1505 in the DIII–DIV inactivation gate (217) that causes a reduction in the current amplitude at hyperpolarized (−94 mV) holding potential. Not only that, PKC activation also causes a negative shift in the voltage-dependence of steady-state inactivation (217). Thus, ROS may cause reduction in INaT indirectly by activating PKC. Therefore, ROS decrease INaT while increasing INaP in a reversible manner by directly interacting with the Na+ channel subunits or by activating kinases. This will cause in the prolongation of the action potential duration and increase in cellular Na+ and Ca2+ levels, which will ultimately lead to the impaired cardiac excitability. More information on ROS-mediated increased Na+ and Ca2+ levels in cardiomyocytes has been mentioned in section V. In short, increased intracellular Ca2+ content activates CaMKII that in turn activates Na+ channels to increase INaP. From several, it is indicative that presumably ROS alter Na+ channel activity via affecting various signaling molecules such as PKC. However, studies have not elucidated a clear mechanism.
It has been shown that ROS oxidize methionine 281/282 at the regulatory domain of CaMKII, and the oxidized CaMKII has properties equivalent to autophosphorylated CaMKII (73), which remains constitutively active to phosphorylate a number of proteins. It has also been shown that CaMKIIδ can phosphorylate cardiac Na+ channels, leading to increased INaP, and the consequent cellular Na+ and Ca2+ overload was significantly slowed in myocytes lacking CaMKIIδ (259). CaMKIIδ altered Na+ channel gating to reduce availability at a high heart rate, while enhancing INaP that prolongs the action potential duration. More recently, it has been shown that ROS require CaMKIIδ to increase INaP that ultimately increased intracellular Na+ and Ca2+ levels to cause arrhythmias (260). Conversely, an increase of INaP would be expected to cause elevation of intracellular Ca2+, and thus activation of CaMKII. Recently, in rat ventricular myocytes, it has been shown that an increased Nav1.5-dependent INaP activates CaMKII, and this effect was absent in the Nav1.5 KO mouse myocytes (280). These results suggest that the mechanism of increasing INaP by ROS is possibly a complex pathway that includes Ca2+ and several kinases (such as CaMKII and PKC) and a self-regulating ability. The increased activity of the INa that prolongs the influx of sodium and extends repolarization is also responsible for most of the LQT3 cases (185, 226).
VIII. Redox Effect on Other Ion Channels, Exchangers, and Pumps
A. IP3R receptors
Like the RyRs, there are three IP3R isoforms referred to as type-1, 2, and 3; however, only IP3R (type-2) is expressed in the heart (139). In ventricular, atrial, or Purkinje myocytes, immunofluorescence studies show that IP3Rs are found in the perinuclear region and in the nuclear membrane (121). The role of IP3R in cardiac excitation is controversial, because IP3R expression in the heart is very low compared to that of RyRs (139, 191). Therefore, majority of the intracellular Ca+2 release occurs through the RyR; however, Ca+2 release via IP3R in atrial and Purkinje myocytes has caught some attention (110, 161). In adult mammalian atrium, IP3-dependent Ca+2 release enhances atrial Ca+2 signaling and exerts a positive inotropic effect (297). In addition, by facilitating Ca+2 release, IP3R may also be an important component in the development of Ca+2-mediated changes in action potential, excitability (via inducing CaMKII-mediated INa and IK), and arrhythmias (297). Interestingly, IP3Rs, ankyrin-B, Na+/K+ ATPase, and NCX co-localized in cardiomyocyte T-tubules in discrete microdomains (190), and thus it is possible that the local Ca2+ release by IP3Rs causes activation of these channels to ultimately affect the action potential and excitability. Similarly, in Purkinje fiber cells, cell-wide Ca2+ waves have been suggested as a possible mechanism for increased excitability and spontaneous generation of action potential (37). Indeed, it has been demonstrated that activated IP3Rs promote the development of Ca2+ sparks and waves in Purkinje fiber cells that may be proarrhythmic (110). Evidence for redox modulation of the IP3R comes only from noncardiac tissue or in a heterologous system. For example, IP3Rs are downregulated when vascular smooth muscle cells are stimulated by H2O2 (177). This decrease in IP3R by H2O2 is accompanied by a reduction in calcium efflux induced by IP3 (177). Contrary to this, H2O2 has been shown to directly activate the IP3R in endothelial cells (294). In HeLa cells, the thiol reagent thimerosal increased the sensitivity of the IP3R to basal IP3 levels (129). In permeabilized hepatocytes, GSSG and t-BHP stimulate the IP3R by increasing the binding affinity of IP3 (71, 72, 107). These effects were also reversed by reducing agents such as DTT. In addition, endothelin-1 (ET-1) that activates IP3R also increases the ROS generation (78). Whether ET-1 induced IP3R and enhanced ROS generation are linked is not known (78). The effect of ROS on IP3R in cardiac myocytes needs further investigation, and if that has any influence on the cardiac excitability is to be determined.
B. Plasmalemmal Ca+2 ATPase
PMCA, like SERCA, is a member of P-type ATPases and is responsible for expulsion of Ca+2 from the cytosol of the cells. The PMCA pump isoforms, PMCA1 and PMCA2, are expressed in the heart, and are thought to specifically reduce Ca+2 in subsarcolemmal microdomains (243). However, PMCA activity is not significantly involved in cellular excitability, as the amount of Ca+2 extruded by this pump in adult myocytes is negligible (<1%) (243), and only PMCA4b has been suggested to regulate a membrane regional pool of Ca+2 involved in cardiomyocytes [as shown in the transgenic rats that overexpress PMCA4b in the heart (101, 266)]. However, it can be assumed that the lower expulsion of Ca2+ from the cell due to inactivity of PMCA could cause a localized subsarcolemmal increase in [Ca2+]I, which could affect various other channels to alter action potentials. ROS can substantially inhibit both Ca+2 transport and ATPase activity of PMCA presumably by decreasing the cellular ATP content in stressed conditions, or by direct modulation of the channel protein. For example, in brain synaptosomes, peroxyl radicals, H2O2, and ONOO− decreased the PMCA activity. The effect of ONOO− was the most potent, followed by peroxyl radicals and H2O2. These effects of ROS are reversible, and occur through oxidation of SH groups and peroxidation of membrane phospholipids (288). A similar effect may occur in cardiac tissue, but more studies are needed.
C. Sarcolemmal and mitochondrial Na+/Ca+2 exchanger
The Na+/Ca+2 exchanger (NCX) is an antiporter membrane protein that removes Ca+2 from cells in exchange of Na+ using the energy from ATP. The NCX removes a single Ca+2 ion in exchange for the import of three Na+ ions (136), and the current carried with it (INCX) contributes to the late phase of the action potential (281). Membrane depolarization or high intracellular Na+ can cause Ca+2 entry into the cell via reverse mode action of NCX, and thus NCX also serves important role in maintaining Na+/Ca+2 homeostasis (136) and cellular excitability. Increase in the [Na+]I or an increase in extracellular Ca2+ could cause an outward shift in INCX and thus a shortening of the action potential. On the other hand, increase in [Ca2+]I may cause an inward shift in INCX and thus the prolongation of action potential. Thus, the activity of NCX is important for the generation and morphology of action potential and thus cellular excitability. Indeed in the canine myocytes, NCX repolarizes the action potential at high [Na+]I (>10 mM), but contributes to the depolarization at low [Na+]I (<5mM) (14). Cardiac myocytes express sarcolemmal NCX protein at high levels, and when it is upregulated/overexpressed, it mediates Ca+2 influx (instead of efflux) during the cardiac action potential (140). Cardiac NCX (encoded by the gene NCX1) consists of nine transmembrane domains, and intramolecular disulfide bonds between cysteine residues of different domains have been implicated to be functionally relevant (136). The activity of the cardiac NCX is sensitive to modification by ROS. Indeed, NCX activity is profoundly inhibited by hypoxia in guinea pig ventricular myocytes, but is reactivated within 1–2 min of reoxygenation possibly due to rising ROS (70). Similarly, H2O2 perfusion or ROS generation by XOR system induced NCX activity in rabbit ventricular myocytes (90). Whether or not these changes occurred due to direct modulation of NCX is unknown. However, a recent study showed that H2O2 increased the activity of NCX in adult rat ventricular myocytes (149) due to thiol modification. Furthermore, CAT overexpression increased NCX expression in cardiac myocytes (221), suggesting that NCX expression might have been decreased by ROS. Again, whether this is due to direct effects of ROS is unknown. Similarly, NCX activity in bovine cardiac sarcolemmal vesicles was stimulated up to 10-fold by preincubating the vesicles with FeSO4 and DTT in an NaCl medium, which was then reversed by GSH (219). The combination of FeSO4 and DTT removed Na+-dependent inactivation of NCX and activated the exchanger, and this effect was reversed by CAT. Therefore, changes in the NCX activity depend on a rearrangement of disulfide bonds between cysteine residues that is modulated by the ROS or ROS scavengers. Because significant bursts of ROS generation occur during reperfusion of the ischemic heart, changes of NCX activity are likely to play an important role in ischemia-related Ca+2 overload.
Mitochondrial Ca+2 fluxes play a very important role in cell physiology. During cell activation, the increase in cytosolic Ca+2 triggers influx of Ca+2 in the mitochondrial matrix through a mitochondrial calcium uniporter, which is then released out of mitochondria by a mitochondrial NCX (34). The role of mitochondrial Ca+2 fluxes and mitochondrial NCX in the generation of arrhythmia is much less clear. It is known that H2O2 or the XOR system increased the mitochondrial Ca+2 concentration ([Ca+2]m) at least in the endothelial cells, and that this raised [Ca+2]m was secondary to the increases in the cytosolic Ca+2 increases (284), and due to inhibition of the NCX of the mitochondria (128). Similarly in intact hearts, perfusion with oxygen radicals resulted in a reduction in [Ca+2]m, which was partly reversed by SOD and CAT (284). Further studies are need to elucidate if ROS affect NCX by a direct or an indirect mechanism and how that effects the cardiac excitability.
D. Sodium–potassium ATPase pump
The sodium–potassium ATPase (Na/K-ATPase) is an ATP-dependent ion pump and serves as a receptor for the cardiotonic steroids (CTS) like digoxin. The CTS inhibit the Na/K-ATPase, elevating [Na+]i that in turn raises [Ca2+]i via NCX. However, lower concentration of CTS (concentration that does not inhibit the Na/K-APTase) can stimulate the Na/K-ATPase to exert activating or deactivating effects on several signaling pathways in the myocytes. For details on these effects, the readers are encouraged to refer the review by Zhang et al. (291). Various pathways affected by the Na/K-ATPase include Src binding that makes a complex with epidermal growth factor receptor (EGFR) [Na/K-ATPase-Src-EGFR complex], the Ras/Raf/MEK/ERK1/2 pathway, mitochondrial ROS production, the activation of the IP3 pathway that eventually leads to transcriptional activation of several proteins, and the activation of the PKC pathway (98, 142, 189, 269, 271). Thus, the functions of Na/K-ATPase are much more complex than just increasing the intracellular Ca2+ to promote contracture. In addition, it has been shown that factors such as PKC, Ca2+, and ROS can modulate the activity of the Na/K-ATPase directly or through various signaling pathways. ROS directly induce the endocytosis of the Na/K-ATPase, thereby reducing its plasma membrane density, and hence its activity at the cell surface (270, 272). It is assumed that this occurs due to a direct interaction of ROS with the Na/K-ATPase pump to alter the conformation. Additionally, ROS affect the Na/K-ATPase pump indirectly via PKC, CaMKII, and intracellular Ca2+. Ca2+/CaM-dependent CaMKII activity phosphorylates the Na/K-ATPase and inhibits its activity (205). The effects of PKC on the Na/K-ATPase activity have been controversial. PKC has been shown to phosphorylate the Na/K-ATPase α1-subunits to promote the exocytosis of Na/K-ATPase molecules from the late endosomes into the sarcolemmal membrane, causing an increased Na/K-ATPase activity (103). On the other hand, PKC has also been shown to induce endocytosis of the Na/K-ATPase, thereby reducing its density in the plasma membranes, and hence Na/K-ATPase activity at the cell surface (209). The reasons to these controversial results are not clear, but it is indeed clear, and PKC regulates the activity of the Na/K-ATPase, which in turn is regulated by ROS. Since the Na/K-ATPase pump is the primary regulator of the resting membrane potential (by exchange of 3Na+ to 2K+), it does regulate the excitability. ROS-induced decrease in Na/K-ATPase activity may prolong the refractory period, and thus may reduce cellular excitability. This may rise to EADs or DADs that will cause arrhythmia. An inactivated Na/K-ATPase (for example, by CTS) increases the cellular Na+ that inhibits NCX activity, and thus retains cellular Ca2+ to increase cardiac contracture. However, it is noteworthy that ROS increase the activity of the NCX (as reviewed earlier). Therefore, it can be assumed that ROS could increase the efficacy of CTS (toxicity of CTS) by increasing cellular Na+ and greater retention of Ca2+ due to a subsequent action of NCX. It is also important to consider that ROS increase the activity of RYR that will further increase [Ca2+]i. By the action of ROS on the Na/K-ATPase, ROS will eventually accumulate cations in the cells that could cause the prolongation of the effective refractory periods and thus reduction in the excitability. However, increase in the total depolarizing currents may give rise to EADs that will propagate as arrhythmias. Thus, in presence of ROS, the same amount of CTS will cause toxicity and arrhythmogenesis. The effect of CTS and ROS on the Na/K-ATPase activity and RYR to cause arrhythmogenesis has been recently studied by Ho et al. (111) (here CTS were shown to increase ROS).
E. Mitochondrial inner membrane anion channel
Several distinct energy-dissipating ion channels in the inner membrane have been proposed to be involved in the ΔΨm collapse, contributing to the generation of arrhythmia. One of these channels is the inner membrane anion channel (IMAC) (40). Although the identity of the IMAC is unknown, electrophysiologically, it is known that the IMAC is responsible for anionic currents across the mitochondrial membrane (12). Due to these currents, the IMAC regulates the ΔΨm, its reversible collapse, and thus membrane potential oscillations in cardiac myocytes (12). This indicates that targeting the IMAC may be effective in preventing arrhythmias by stopping ROS-induced ROS release. A confirmatory role for IMAC involvement in arrhythmia was provided in a series of studies where inhibiting the IMAC prevented arrhythmias in intact mammalian hearts (39). Whether or not ROS affect the IMAC function is not known partly, because the identity of the IMAC is unknown, and this needs further studies.
IX. Overall Effect of ROS on Action Potential, Excitability, and Arrhythmia
In summary, under pathological conditions, such as heart failure and ischemia–reperfusion, ROS levels can become elevated and predispose the heart to arrhythmias by altering the cardiac action potential. ROS induce functional impairment of various ion channels, including Na+ channels (INaT and INaP), L-type Ca2+ current (ICaL), K+ currents (IKto, IKr, and IKATP), RyR, SERCA2a, and the NCX (Fig. 17 and Table 1). This impairment might be due to a direct modulation of the cysteine SH–SH on the channel subunits or indirectly by affecting the signaling molecules in the myocytes that may ultimately affect the functions of these ion channels. In ventricular myocytes, INa, ICaL, RYR, and NCX cause inward currents, with a net increase in intracellular Ca+2 and Na+, influencing depolarization, whereas the rapid and slow delayed rectifier K+ currents (IKur, IKr, and IKs), the transient outward current (IKto), and the inward rectifier potassium current (IK1) cause outward currents. Any irregularity in these currents will lead to an impaired ionic balance in the cell, alteration in action potential, and may cause EADs, DADs, and TA, that is, arrhythmia. Eventually, this may affect the cellular excitability.
FIG. 17.
Effects of ROS on cardiac ion channels, cytosolic ionic balance, and excitation-contraction coupling. ROS increase (although controversial) the activation of L-type Ca2+ channels, RyR, and mitochondrial NCX, but decrease the activity of SERCA and PMCA. This increases cytosolic Ca+2 content. Increased cytosolic Ca+2 is expelled out of the cytosol by ROS-mediated increased activity of NCX that increases cytosolic Na+ content. ROS also increase Na+ content by enhancing persistent Na+ currents (INaP) from Na+ channels. The increase in positive charge is balanced by expulsion of cytosolic K+ content. However, ROS decrease K+ efflux from the cytosol by inactivating voltage-dependent K+ channels. ROS-mediated activation of the sarc-KATP and mito-KATP channels, which increases K-efflux from the cytosol or sequesteration into the mitochondria, respectively. This may compensate for some of the increased positive charge in the cytosol. Overall, increased positive charge in the cytosol may prolong the depolarization phase of the action potential and reduce the excitability.
From various studies, it can be summarized that ROS increase or decrease ICaL with a net result of an increased intracellular Ca+2 via its action on other Ca+2-handling proteins such as RyR, SERCA, and NCX. Since ROS reduce the ability of SERCA and PMCA to sequester the cytosolic Ca+2 back into the SR or expel out of the cytosol, respectively, the cytosolic Ca+2 would remain high. In this regard, it is to be considered that although ROS affect the functions of PMCA and SERCA by direct modulation, the activities of these channels depend upon the cellular ATP (149) as well. Enhanced ROS in the cytosol is accompanied by a decreased ATP synthesis due to less utilization of the cellular O2 by the respiratory change (13, 65). Therefore, in presence of ROS, decreased ATP could cause the reduced activity of the ATPase pumps such as SERCA and PMCA. Regarding intracellular Ca+2, NCX plays a major role in expulsion of Ca+2 out of the cytosol in exchange of Na+. ROS induce the activity of NCX directly, and thus may cause expulsion of some of the Ca+2. However, under the conditions of increased cellular ROS, INaP is enhanced (83). Enhanced INaP leads to an increased intracellular Na+ that triggers the reverse mode of the NCX. Thus, instead of clearing Ca+2 out of the cell, in presence of ROS, NCX may actually increase the cytosolic Ca+2 in exchange of Na+ (140). In addition, elevated cytosolic Na+ also leads to a decreased Ca+2 uptake by the mitochondria, perhaps due to the impairment of the mitochondrial NCX. The interplay between cytosolic and mitochondrial NCX and increased intracellular Na+ is complex and not well understood primarily, because their activities are modulated by the intracellular ROS differently. Overall, ROS enhance the depolarizing inward currents and thus cause increase in the intracellular Ca+2 and Na+ in the cell. This may result in a decrease in the repolarizing reserve (defined as the ability of the cells to repolarize as ion channels or current density varies) and thus decrease opportunity for the cells to return to the resting potential that decreases excitability, and may lead to action potential prolongation promoting arrhythmias.
The majority of the repolarizing reserve of the cardiac cells is provided by the K+ channels that cause an outward K+ current. ROS reduce all the voltage-dependent K+ currents IKto, IKur, and IKs, thereby further reducing the repolarizing reserve. However, ROS increase the outward IKr at depolarizing voltages that may contribute to normalizing repolarization reserve. The Kv11.1 channels and thus IKr are not activated until a substantial repolarizing voltage has been reached by the activity of IKs. Therefore, in a myocyte in which inward currents (INa and ICa) have increased and majority of the outward currents (in particular IKto and IKs) have decreased, the depolarizing phase of the action potential has already prolonged before even the IKr gets activated. Nevertheless, it has been shown that inhibition of all the inward K+ currents together has a more potent effect on reducing the repolarization reserve in the myocytes than reduction of only one type of K+ current (29). Furthermore, a decrease in membrane conductance during the plateau phase due to the inhibition of IKv would make the cell more sensitive to even small depolarizing currents such as the INCX. The net effect would be prolonged action potential duration. In this regard, previous studies analyzing the mechanisms of ROS-induced prolongation of the action potential have implicated not only the role of increased INaP and a decreased ICaL that ultimately causes increased intracellular Ca+2 but also the role of decreased IKv (57). Indeed, the time course of IKv reduction by ROS closely paralleled the effects on the action potential duration as shown by Cerbai et al. (44). This is consistent with the notion that a decrease in the K+ current responsible for membrane repolarization (at potential values around the plateau phase) may favor early after depolarization development.
Another important aspect of the cellular ROS is the induction of KATP channels. KATP channels when open, due to an increase in cellular pH or a decrease in ATP content, contribute to the cellular repolarizing currents by expulsion of K+ from the cells (30). Cellular ROS directly open the sarc-KATP channels causing a net outward K+ current, thereby depolarizing the membranes and prolonging the action potential. Furthermore, ROS also cause opening of the mito-KATP channels, which increases the K+-flux into the mitochondria, causing the respiratory chain inhibition due to shifting of ionic balance and thus shuts the ATP synthesis resulting in a collapse of the ΔΨm (87). Because the respiratory chain is inhibited, and ATP synthesis has stopped, the synthesis of ROS increases even further. This has been shown to occur in a network of mitochondria that are closely attached to each other in the cells, wherein the ROS from one mitochondria influence the ROS generation from the neighboring mitochondria, and this leads to the oscillations of ΔΨm (13). The net effect is collapse of the ΔΨm, decreased cellular ATP that opens sarc-KATP, and mitochondrial ROS-induced ROS release that causes oscillations of the ΔΨm (12). Decreased cellular ATP will contribute to a decreased SERCA and PMCA activity as well as opening of the sarc-KATP, both of which cause prolongation of the depolarizing phase of the action potential. Moreover, collapse of the ΔΨm and oscillation of the ΔΨm will further lead to arrhythmogenesis as explained by O'Rourke et al. (13). In summary, ROS generation in the cardiac cells alters the activity of various ion channels in a way such that the net inward currents are increased, and the net outward currents are decreased. This results in the enhanced cellular Na+ and Ca2+ ions, prolongation of the action potential duration, reduction of the refractory phase, and thus reduced excitability that promotes arrhythmogenesis. Therapies targeting decrease in ROS may lead to a safer and more general cure for various arrhythmogenic diseases, as has already been indicated in several clinical studies.
Abbreviations Used
- ASK-1
apoptosis-regulating signal kinase-1
- BCNU
1,3-bis-(2-chloroethyl)-1-nitrosourea
- bEAG
bovine ether-a-gogo gene
- BKCa,
large conductance KCa
- BSO
buthionine sulfoximine
- Ca-CaM
calcium–calmodulin
- CaMKII
CaM kinase II
- CAT
catalase
- cGMP
cyclic guanosine monophosphate
- CREB
CRE-binding protein
- CTS
cardiotonic steroids
- DADs
delayed afterdepolarizations
- DHF
dihydroxyfumarate
- DIP
diphenylene iodonium
- DTDP
2,2′-dihydropyridine
- DTNB
5,5′-dithio-bis(2-nitrobenzoic acid)
- DTT
dithiothreitol
- EADs
early afterdepolarizations
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- ET-1
endothelin-1
- FKBP
FK-506-binding proteins
- GPx
glutathione peroxidase
- GSH
glutathione
- GSSG
oxidized glutathione
- HERG
human ether-a-gogo-related gene
- 20-HETE
20-hydroxyeicosatetraenoic acid
- HOCl
hypochlorous acid
- IKCa
intermediate conductance KCa
- IMAC
inner membrane anion channel
- KCa
calcium-activated K
- KChAP
K-channel-associated protein
- KChIP
K-channel-interacting protein
- KO
knockout
- MAPK
mitogen-activated protein kinase
- MsrA
methionine sulfoxide reductase
- MTSE
methanethiosulfonate ethylammonium
- NAC
N-acetylcysteine
- NCX
Na+/Ca+2 exchanger
- NOS
nitric oxide synthase
- O2•−
superoxide radical ion
- OH•
hydroxyl radical
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- PUFAs
polyunsaturated fatty acids
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinases
- RyR
ryanodine receptor
- SERCA
sarcoendoplasmic reticulum Ca+2-ATPase
- SH
sulfhydryl
- SIN
3-morpholinosydnonimine-HCl
- SKCa
small conductance KCa
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum
- SUR
sulfonylurea receptor
- t-BHP
tertbutyl hydroperoxide
- TA
triggered activity
- TTX
tetrodotoxin
- XOR
xanthine oxidoreductase
Acknowledgments
The authors acknowledge the numerous scientists for the work that is reviewed here. N.T.A. and J.C.M. also acknowledge the NIH grant support (R01HL-57414) to J.C.M.
References
- 1.Abriel H. Cardiac sodium channel Na(v)1.5 and interacting proteins: Physiology and pathophysiology. J Mol Cell Cardiol. 2010;48:2–11. doi: 10.1016/j.yjmcc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Adachi T. Weisbrod RM. Pimentel DR. Ying J. Sharov VS. Schoneich C. Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 3.Ahern GP. Hsu SF. Klyachko VA. Jackson MB. Induction of persistent sodium current by exogenous and endogenous nitric oxide. J Biol Chem. 2000;275:28810–28815. doi: 10.1074/jbc.M003090200. [DOI] [PubMed] [Google Scholar]
- 4.Ahmmed GU. Xu Y. Hong Dong P. Zhang Z. Eiserich J. Chiamvimonvat N. Nitric oxide modulates cardiac Na(+) channel via protein kinase A and protein kinase G. Circ Res. 2001;89:1005–1013. doi: 10.1161/hh2301.100801. [DOI] [PubMed] [Google Scholar]
- 5.Aiba T. Hesketh GG. Liu T. Carlisle R. Villa-Abrille MC. O'Rourke B. Akar FG. Tomaselli GF. Na+ channel regulation by Ca2+/calmodulin and Ca2+/calmodulin-dependent protein kinase II in guinea-pig ventricular myocytes. Cardiovasc Res. 2010;85:454–463. doi: 10.1093/cvr/cvp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aikens J. Dix TA. Perhydroxyl radical (HOO.) initiated lipid peroxidation. The role of fatty acid hydroperoxides. J Biol Chem. 1991;266:15091–15098. [PubMed] [Google Scholar]
- 7.Akrouh A. Halcomb SE. Nichols CG. Sala-Rabanal M. Molecular biology of K(ATP) channels and implications for health and disease. IUBMB Life. 2009;61:971–978. doi: 10.1002/iub.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin AS. Asghari-Roodsari A. Tan HL. Cardiac sodium channelopathies. Pflugers Arch. 2010;460:223–237. doi: 10.1007/s00424-009-0761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anzai K. Ogawa K. Kuniyasu A. Ozawa T. Yamamoto H. Nakayama H. Effects of hydroxyl radical and sulfhydryl reagents on the open probability of the purified cardiac ryanodine receptor channel incorporated into planar lipid bilayers. Biochem Biophys Res Commun. 1998;249:938–942. doi: 10.1006/bbrc.1998.9244. [DOI] [PubMed] [Google Scholar]
- 10.Aon MA. Cortassa S. Maack C. O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007;282:21889–21900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aon MA. Cortassa S. Marban E. O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 12.Aon MA. Cortassa S. O'Rourke B. Mitochondrial oscillations in physiology and pathophysiology. Adv Exp Med Biol. 2008;641:98–117. doi: 10.1007/978-0-387-09794-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aon MA. Cortassa S. O'Rourke B. Redox-optimized ROS balance: a unifying hypothesis. Biochim Biophys Acta. 2010;1797:865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armoundas AA. Hobai IA. Tomaselli GF. Winslow RL. O'Rourke B. Role of sodium-calcium exchanger in modulating the action potential of ventricular myocytes from normal and failing hearts. Circ Res. 2003;93:46–53. doi: 10.1161/01.RES.0000080932.98903.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aronson D. Mittleman MA. Burger AJ. Effects of sulfonylurea hypoglycemic agents and adenosine triphosphate dependent potassium channel antagonists on ventricular arrhythmias in patients with decompensated heart failure. Pacing Clin Electrophysiol. 2003;26:1254–1261. doi: 10.1046/j.1460-9592.2003.t01-1-00177.x. [DOI] [PubMed] [Google Scholar]
- 16.Avkiran M. Haworth RS. Regulatory effects of G protein-coupled receptors on cardiac sarcolemmal Na+/H+ exchanger activity: signalling and significance. Cardiovasc Res. 2003;57:942–952. doi: 10.1016/s0008-6363(02)00782-4. [DOI] [PubMed] [Google Scholar]
- 17.Bai Y. Wang J. Shan H. Lu Y. Zhang Y. Luo X. Yang B. Wang Z. Sphingolipid metabolite ceramide causes metabolic perturbation contributing to HERG K+ channel dysfunction. Cell Physiol Biochem. 2007;20:429–440. doi: 10.1159/000107527. [DOI] [PubMed] [Google Scholar]
- 18.Balser JR. The cardiac sodium channel: gating function and molecular pharmacology. J Mol Cell Cardiol. 2001;33:599–613. doi: 10.1006/jmcc.2000.1346. [DOI] [PubMed] [Google Scholar]
- 19.Balshaw DM. Xu L. Yamaguchi N. Pasek DA. Meissner G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor) J Biol Chem. 2001;276:20144–20153. doi: 10.1074/jbc.M010771200. [DOI] [PubMed] [Google Scholar]
- 20.Barrington PL. Meier CF., Jr. Weglicki WB. Abnormal electrical activity induced by free radical generating systems in isolated cardiocytes. J Mol Cell Cardiol. 1988;20:1163–1178. doi: 10.1016/0022-2828(88)90596-2. [DOI] [PubMed] [Google Scholar]
- 21.Belevych AE. Terentyev D. Viatchenko-Karpinski S. Terentyeva R. Sridhar A. Nishijima Y. Wilson LD. Cardounel AJ. Laurita KR. Carnes CA. Billman GE. Gyorke S. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc Res. 2009;84:387–395. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 23.Berube J. Caouette D. Daleau P. Hydrogen peroxide modifies the kinetics of HERG channel expressed in a mammalian cell line. J Pharmacol Exp Ther. 2001;297:96–102. [PubMed] [Google Scholar]
- 24.Besana A. Robinson RB. Feinmark SJ. Lipids and two-pore domain K+ channels in excitable cells. Prostaglandins Other Lipid Mediat. 2005;77:103–110. doi: 10.1016/j.prostaglandins.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Bezzina CR. Rook MB. Wilde AA. Cardiac sodium channel and inherited arrhythmia syndromes. Cardiovasc Res. 2001;49:257–271. doi: 10.1016/s0008-6363(00)00272-8. [DOI] [PubMed] [Google Scholar]
- 26.Bhatnagar A. Contribution of ATP to oxidative stress-induced changes in action potential of isolated cardiac myocytes. Am J Physiol. 1997;272:H1598–H1608. doi: 10.1152/ajpheart.1997.272.4.H1598. [DOI] [PubMed] [Google Scholar]
- 27.Bhatnagar A. Srivastava SK. Szabo G. Oxidative stress alters specific membrane currents in isolated cardiac myocytes. Circ Res. 1990;67:535–549. doi: 10.1161/01.res.67.3.535. [DOI] [PubMed] [Google Scholar]
- 28.Bianchi L. Priori SG. Napolitano C. Surewicz KA. Dennis AT. Memmi M. Schwartz PJ. Brown AM. Mechanisms of I(Ks) suppression in LQT1 mutants. Am J Physiol Heart Circ Physiol. 2000;279:H3003–H3011. doi: 10.1152/ajpheart.2000.279.6.H3003. [DOI] [PubMed] [Google Scholar]
- 29.Biliczki P. Virag L. Iost N. Papp JG. Varro A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br J Pharmacol. 2002;137:361–368. doi: 10.1038/sj.bjp.0704881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billman GE. The cardiac sarcolemmal ATP-sensitive potassium channel as a novel target for anti-arrhythmic therapy. Pharmacol Ther. 2008;120:54–70. doi: 10.1016/j.pharmthera.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Blaich A. Welling A. Fischer S. Wegener JW. Kostner K. Hofmann F. Moosmang S. Facilitation of murine cardiac L-type Ca(v)1.2 channel is modulated by calmodulin kinase II-dependent phosphorylation of S1512 and S1570. Proc Natl Acad Sci U S A. 2010;107:10285–10289. doi: 10.1073/pnas.0914287107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blankenberg S. Rupprecht HJ. Bickel C. Torzewski M. Hafner G. Tiret L. Smieja M. Cambien F. Meyer J. Lackner KJ. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 33.Bond CT. Maylie J. Adelman JP. Small-conductance calcium-activated potassium channels. Ann N Y Acad Sci. 1999;868:370–378. doi: 10.1111/j.1749-6632.1999.tb11298.x. [DOI] [PubMed] [Google Scholar]
- 34.Bondarenko OI. Sahach VF. [Mitochondrial Na(+)-Ca(2+)-exchanger inhibitor CGP37157 produces endothelial cell depolarization with membrane potential oscillations] Fiziol Zh. 2011;57:9–16. [PubMed] [Google Scholar]
- 35.Boveris A. Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowling N. Walsh RA. Song G. Estridge T. Sandusky GE. Fouts RL. Mintze K. Pickard T. Roden R. Bristow MR. Sabbah HN. Mizrahi JL. Gromo G. King GL. Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 37.Boyden PA. Barbhaiya C. Lee T. ter Keurs HE. Nonuniform Ca2+ transients in arrhythmogenic Purkinje cells that survive in the infarcted canine heart. Cardiovasc Res. 2003;57:681–693. doi: 10.1016/s0008-6363(02)00725-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brick JE. Brick JF. Elnicki DM. Musculoskeletal disorders. When are they caused by hormone imbalance? Postgrad Med. 1991;90:129–132. doi: 10.1080/00325481.1991.11701106. 135–126. [DOI] [PubMed] [Google Scholar]
- 39.Brown DA. Aon MA. Frasier CR. Sloan RC. Maloney AH. Anderson EJ. O'Rourke B. Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing mitochondrial depolarization. J Mol Cell Cardiol. 2010;48:673–679. doi: 10.1016/j.yjmcc.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown DA. O'Rourke B. Cardiac mitochondria and arrhythmias. Cardiovasc Res. 2010;88:241–249. doi: 10.1093/cvr/cvq231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell DL. Stamler JS. Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J Gen Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carnes CA. Janssen PM. Ruehr ML. Nakayama H. Nakayama T. Haase H. Bauer JA. Chung MK. Fearon IM. Gillinov AM. Hamlin RL. Van Wagoner DR. Atrial glutathione content, calcium current, and contractility. J Biol Chem. 2007;282:28063–28073. doi: 10.1074/jbc.M704893200. [DOI] [PubMed] [Google Scholar]
- 43.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 44.Cerbai E. Ambrosio G. Porciatti F. Chiariello M. Giotti A. Mugelli A. Cellular electrophysiological basis for oxygen radical-induced arrhythmias. A patch-clamp study in guinea pig ventricular myocytes. Circulation. 1991;84:1773–1782. doi: 10.1161/01.cir.84.4.1773. [DOI] [PubMed] [Google Scholar]
- 45.Chai Y. Zhang DM. Lin YF. Activation of cGMP-dependent protein kinase stimulates cardiac ATP-sensitive potassium channels via a ROS/calmodulin/CaMKII signaling cascade. PLoS One. 2011;6:e18191. doi: 10.1371/journal.pone.0018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen C. Bharucha V. Chen Y. Westenbroek RE. Brown A. Malhotra JD. Jones D. Avery C. Gillespie PJ., 3rd Kazen-Gillespie KA. Kazarinova-Noyes K. Shrager P. Saunders TL. Macdonald RL. Ransom BR. Scheuer T. Catterall WA. Isom LL. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel beta 2-subunits. Proc Natl Acad Sci U S A. 2002;99:17072–17077. doi: 10.1073/pnas.212638099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiarugi P. PTPs versus PTKs: the redox side of the coin. Free Radic Res. 2005;39:353–364. doi: 10.1080/10715760400027987. [DOI] [PubMed] [Google Scholar]
- 48.Chien AJ. Carr KM. Shirokov RE. Rios E. Hosey MM. Identification of palmitoylation sites within the L-type calcium channel beta2a subunit and effects on channel function. J Biol Chem. 1996;271:26465–26468. doi: 10.1074/jbc.271.43.26465. [DOI] [PubMed] [Google Scholar]
- 49.Cho J. Won K. Wu D. Soong Y. Liu S. Szeto HH. Hong MK. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron Artery Dis. 2007;18:215–220. doi: 10.1097/01.mca.0000236285.71683.b6. [DOI] [PubMed] [Google Scholar]
- 50.Choudhary G. Dudley SC., Jr Heart failure, oxidative stress, and ion channel modulation. Congest Heart Fail. 2002;8:148–155. doi: 10.1111/j.1527-5299.2002.00716.x. [DOI] [PubMed] [Google Scholar]
- 51.Ciampa EJ. Welch RC. Vanoye CG. George AL., Jr KCNE4 juxtamembrane region is required for interaction with calmodulin and for functional suppression of KCNQ1. J Biol Chem. 2011;286:4141–4149. doi: 10.1074/jbc.M110.158865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clerk A. Michael A. Sugden PH. Stimulation of multiple mitogen-activated protein kinase sub-families by oxidative stress and phosphorylation of the small heat shock protein, HSP25/27, in neonatal ventricular myocytes. Biochem J. 1998;333(Pt 3):581–589. doi: 10.1042/bj3330581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coetzee WA. Opie LH. Effects of oxygen free radicals on isolated cardiac myocytes from guinea-pig ventricle: electrophysiological studies. J Mol Cell Cardiol. 1992;24:651–663. doi: 10.1016/0022-2828(92)91049-b. [DOI] [PubMed] [Google Scholar]
- 54.Cortassa S. Aon MA. Winslow RL. O'Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J. 2004;87:2060–2073. doi: 10.1529/biophysj.104.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costa AD. Garlid KD. West IC. Lincoln TM. Downey JM. Cohen MV. Critz SD. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 56.Costa AD. Jakob R. Costa CL. Andrukhiv K. West IC. Garlid KD. The mechanism by which the mitochondrial ATP-sensitive K+ channel opening and H2O2 inhibit the mitochondrial permeability transition. J Biol Chem. 2006;281:20801–20808. doi: 10.1074/jbc.M600959200. [DOI] [PubMed] [Google Scholar]
- 57.Couchonnal LF. Anderson ME. The role of calmodulin kinase II in myocardial physiology and disease. Physiology (Bethesda) 2008;23:151–159. doi: 10.1152/physiol.00043.2007. [DOI] [PubMed] [Google Scholar]
- 58.Cowley EA. Linsdell P. Oxidant stress stimulates anion secretion from the human airway epithelial cell line Calu-3: implications for cystic fibrosis lung disease. J Physiol. 2002;543:201–209. doi: 10.1113/jphysiol.2002.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Curran ME. Splawski I. Timothy KW. Vincent GM. Green ED. Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 60.de Jong JW. van der Meer P. Nieukoop AS. Huizer T. Stroeve RJ. Bos E. Xanthine oxidoreductase activity in perfused hearts of various species, including humans. Circ Res. 1990;67:770–773. doi: 10.1161/01.res.67.3.770. [DOI] [PubMed] [Google Scholar]
- 61.De Windt LJ. Lim HW. Haq S. Force T. Molkentin JD. Calcineurin promotes protein kinase C and c-Jun NH2-terminal kinase activation in the heart. Cross-talk between cardiac hypertrophic signaling pathways. J Biol Chem. 2000;275:13571–13579. doi: 10.1074/jbc.275.18.13571. [DOI] [PubMed] [Google Scholar]
- 62.del Rio LA. Sandalio LM. Palma JM. Bueno P. Corpas FJ. Metabolism of oxygen radicals in peroxisomes and cellular implications. Free Radic Biol Med. 1992;13:557–580. doi: 10.1016/0891-5849(92)90150-f. [DOI] [PubMed] [Google Scholar]
- 63.Deng J. Wang G. Huang Q. Yan Y. Li K. Tan W. Jin C. Wang Y. Liu J. Oxidative stress-induced leaky sarcoplasmic reticulum underlying acute heart failure in severe burn trauma. Free Radic Biol Med. 2008;44:375–385. doi: 10.1016/j.freeradbiomed.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 64.Dong F. Fang CX. Yang X. Zhang X. Lopez FL. Ren J. Cardiac overexpression of catalase rescues cardiac contractile dysfunction induced by insulin resistance: Role of oxidative stress, protein carbonyl formation and insulin sensitivity. Diabetologia. 2006;49:1421–1433. doi: 10.1007/s00125-006-0230-7. [DOI] [PubMed] [Google Scholar]
- 65.Drahota Z. Krivakova P. Cervinkova Z. Kmonickova E. Lotkova H. Kucera O. Houstek J. Tert-butyl hydroperoxide selectively inhibits mitochondrial respiratory-chain enzymes in isolated rat hepatocytes. Physiol Res. 2005;54:67–72. doi: 10.33549/physiolres.930578. [DOI] [PubMed] [Google Scholar]
- 66.Duprat F. Guillemare E. Romey G. Fink M. Lesage F. Lazdunski M. Honore E. Susceptibility of cloned K+ channels to reactive oxygen species. Proc Natl Acad Sci U S A. 1995;92:11796–11800. doi: 10.1073/pnas.92.25.11796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eager KR. Dulhunty AF. Activation of the cardiac ryanodine receptor by sulfhydryl oxidation is modified by Mg2+ and ATP. J Membr Biol. 1998;163:9–18. doi: 10.1007/s002329900365. [DOI] [PubMed] [Google Scholar]
- 68.Edwards G. Ibbotson T. Weston AH. Levcromakalim may induce a voltage-independent K-current in rat portal veins by modifying the gating properties of the delayed rectifier. Br J Pharmacol. 1993;110:1037–1048. doi: 10.1111/j.1476-5381.1993.tb13918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edwards G. Weston AH. Induction of a glibenclamide-sensitive K-current by modification of a delayed rectifier channel in rat portal vein in insulinoma cells. Br J Pharmacol. 1993;110:1280–1281. doi: 10.1111/j.1476-5381.1993.tb13955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eigel BN. Gursahani H. Hadley RW. ROS are required for rapid reactivation of Na+/Ca2+ exchanger in hypoxic reoxygenated guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol. 2004;286:H955–H963. doi: 10.1152/ajpheart.00721.2003. [DOI] [PubMed] [Google Scholar]
- 71.Elliott SJ. Doan TN. Henschke PN. Reductant substrate for glutathione peroxidase modulates oxidant inhibition of Ca2+ signaling in endothelial cells. Am J Physiol. 1995;268:H278–H287. doi: 10.1152/ajpheart.1995.268.1.H278. [DOI] [PubMed] [Google Scholar]
- 72.Elmoselhi AB. Samson SE. Grover AK. SR Ca2+ pump heterogeneity in coronary artery: free radicals and IP3-sensitive and -insensitive pools. Am J Physiol. 1996;271:C1652–C1659. doi: 10.1152/ajpcell.1996.271.5.C1652. [DOI] [PubMed] [Google Scholar]
- 73.Erickson JR. Joiner ML. Guan X. Kutschke W. Yang J. Oddis CV. Bartlett RK. Lowe JS. O'Donnell SE. Aykin-Burns N. Zimmerman MC. Zimmerman K. Ham AJ. Weiss RM. Spitz DR. Shea MA. Colbran RJ. Mohler PJ. Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Erxleben C. Liao Y. Gentile S. Chin D. Gomez-Alegria C. Mori Y. Birnbaumer L. Armstrong DL. Cyclosporin and Timothy syndrome increase mode 2 gating of CaV1.2 calcium channels through aberrant phosphorylation of S6 helices. Proc Natl Acad Sci U S A. 2006;103:3932–3937. doi: 10.1073/pnas.0511322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fearon IM. OxLDL enhances L-type Ca2+ currents via lysophosphatidylcholine-induced mitochondrial reactive oxygen species (ROS) production. Cardiovasc Res. 2006;69:855–864. doi: 10.1016/j.cardiores.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 76.Fearon IM. Palmer AC. Balmforth AJ. Ball SG. Varadi G. Peers C. Hypoxic and redox inhibition of the human cardiac L-type Ca2+ channel. Adv Exp Med Biol. 2000;475:209–218. doi: 10.1007/0-306-46825-5_20. [DOI] [PubMed] [Google Scholar]
- 77.Fearon IM. Varadi G. Koch S. Isaacsohn I. Ball SG. Peers C. Splice variants reveal the region involved in oxygen sensing by recombinant human L-type Ca(2+) channels. Circ Res. 2000;87:537–539. doi: 10.1161/01.res.87.7.537. [DOI] [PubMed] [Google Scholar]
- 78.Fellner SK. Parker L. Endothelin-1, superoxide and adeninediphosphate ribose cyclase in shark vascular smooth muscle. J Exp Biol. 2005;208:1045–1052. doi: 10.1242/jeb.01506. [DOI] [PubMed] [Google Scholar]
- 79.Fill M. Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 80.Fischbach PS. White A. Barrett TD. Lucchesi BR. Risk of ventricular proarrhythmia with selective opening of the myocardial sarcolemmal versus mitochondrial ATP-gated potassium channel. J Pharmacol Exp Ther. 2004;309:554–559. doi: 10.1124/jpet.103.060780. [DOI] [PubMed] [Google Scholar]
- 81.Fosset M. Schmid-Antomarchi H. de Weille JR. Lazdunski M. Somatostatin activates glibenclamide-sensitive and ATP-regulated K+ channels in insulinoma cells via a G-protein. FEBS Lett. 1988;242:94–96. doi: 10.1016/0014-5793(88)80992-x. [DOI] [PubMed] [Google Scholar]
- 82.Frohnwieser B. Chen LQ. Schreibmayer W. Kallen RG. Modulation of the human cardiac sodium channel alpha-subunit by cAMP-dependent protein kinase and the responsible sequence domain. J Physiol. 1997;498(Pt 2):309–318. doi: 10.1113/jphysiol.1997.sp021859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fukuda K. Davies SS. Nakajima T. Ong BH. Kupershmidt S. Fessel J. Amarnath V. Anderson ME. Boyden PA. Viswanathan PC. Roberts LJ., 2nd Balser JR. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ Res. 2005;97:1262–1269. doi: 10.1161/01.RES.0000195844.31466.e9. [DOI] [PubMed] [Google Scholar]
- 84.Gamper N. Zaika O. Li Y. Martin P. Hernandez CC. Perez MR. Wang AY. Jaffe DB. Shapiro MS. Oxidative modification of M-type K(+) channels as a mechanism of cytoprotective neuronal silencing. EMBO J. 2006;25:4996–5004. doi: 10.1038/sj.emboj.7601374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao G. Ollinger K. Brunk UT. Influence of intracellular glutathione concentration of lipofuscin accumulation in cultured neonatal rat cardiac myocytes. Free Radic Biol Med. 1994;16:187–194. doi: 10.1016/0891-5849(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 86.Gao T. Yatani A. Dell'Acqua ML. Sako H. Green SA. Dascal N. Scott JD. Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 87.Garlid KD. Costa AD. Quinlan CL. Pierre SV. Dos Santos P. Cardioprotective signaling to mitochondria. J Mol Cell Cardiol. 2009;46:858–866. doi: 10.1016/j.yjmcc.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gellens ME. George AL., Jr. Chen LQ. Chahine M. Horn R. Barchi RL. Kallen RG. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc Natl Acad Sci U S A. 1992;89:554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gen W. Tani M. Takeshita J. Ebihara Y. Tamaki K. Mechanisms of Ca2+ overload induced by extracellular H2O2 in quiescent isolated rat cardiomyocytes. Basic Res Cardiol. 2001;96:623–629. doi: 10.1007/s003950170014. [DOI] [PubMed] [Google Scholar]
- 90.Goldhaber JI. Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol. 1996;271:H823–833. doi: 10.1152/ajpheart.1996.271.3.H823. [DOI] [PubMed] [Google Scholar]
- 91.Goldhaber JI. Ji S. Lamp ST. Weiss JN. Effects of exogenous free radicals on electromechanical function and metabolism in isolated rabbit and guinea pig ventricle. Implications for ischemia and reperfusion injury. J Clin Invest. 1989;83:1800–1809. doi: 10.1172/JCI114085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gonzalez DR. Beigi F. Treuer AV. Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A. 2007;104:20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonzalez DR. Treuer AV. Castellanos J. Dulce RA. Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem. 2010;285:28938–28945. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009;2:185–194. doi: 10.1161/CIRCEP.108.789081. [DOI] [PubMed] [Google Scholar]
- 95.Gross GJ. Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol. 2003;285:H921–930. doi: 10.1152/ajpheart.00421.2003. [DOI] [PubMed] [Google Scholar]
- 96.Grover AK. Samson SE. Misquitta CM. Sarco(endo)plasmic reticulum Ca2+ pump isoform SERCA3 is more resistant than SERCA2b to peroxide. Am J Physiol. 1997;273:C420–C425. doi: 10.1152/ajpcell.1997.273.2.C420. [DOI] [PubMed] [Google Scholar]
- 97.Gyorke I. Hester N. Jones LR. Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haas M. Askari A. Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem. 2000;275:27832–27837. doi: 10.1074/jbc.M002951200. [DOI] [PubMed] [Google Scholar]
- 99.Hammarstrom AK. Gage PW. Nitric oxide increases persistent sodium current in rat hippocampal neurons. J Physiol. 1999;520(Pt 2):451–461. doi: 10.1111/j.1469-7793.1999.t01-1-00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hammerschmidt S. Wahn H. The effect of the oxidant hypochlorous acid on the L-type calcium current in isolated ventricular cardiomyocytes. J Mol Cell Cardiol. 1998;30:1855–1867. doi: 10.1006/jmcc.1998.0749. [DOI] [PubMed] [Google Scholar]
- 101.Hammes A. Oberdorf-Maass S. Rother T. Nething K. Gollnick F. Linz KW. Meyer R. Hu K. Han H. Gaudron P. Ertl G. Hoffmann S. Ganten U. Vetter R. Schuh K. Benkwitz C. Zimmer HG. Neyses L. Overexpression of the sarcolemmal calcium pump in the myocardium of transgenic rats. Circ Res. 1998;83:877–888. doi: 10.1161/01.res.83.9.877. [DOI] [PubMed] [Google Scholar]
- 102.Han DY. Minobe E. Wang WY. Guo F. Xu JJ. Hao LY. Kameyama M. Calmodulin- and Ca2+-dependent facilitation and inactivation of the Cav1.2 Ca2+ channels in guinea-pig ventricular myocytes. J Pharmacol Sci. 2010;112:310–319. doi: 10.1254/jphs.09282fp. [DOI] [PubMed] [Google Scholar]
- 103.Han F. Bossuyt J. Despa S. Tucker AL. Bers DM. Phospholemman phosphorylation mediates the protein kinase C-dependent effects on Na+/K+ pump function in cardiac myocytes. Circ Res. 2006;99:1376–1383. doi: 10.1161/01.RES.0000251667.73461.fb. [DOI] [PubMed] [Google Scholar]
- 104.Hart JD. Dulhunty AF. Nitric oxide activates or inhibits skeletal muscle ryanodine receptors depending on its concentration, membrane potential and ligand binding. J Membr Biol. 2000;173:227–236. doi: 10.1007/s002320001022. [DOI] [PubMed] [Google Scholar]
- 105.Hearse DJ. Kusama Y. Bernier M. Rapid electrophysiological changes leading to arrhythmias in the aerobic rat heart. Photosensitization studies with rose bengal-derived reactive oxygen intermediates. Circ Res. 1989;65:146–153. doi: 10.1161/01.res.65.1.146. [DOI] [PubMed] [Google Scholar]
- 106.Heinen A. Aldakkak M. Stowe DF. Rhodes SS. Riess ML. Varadarajan SG. Camara AK. Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2007;293:H1400–H1407. doi: 10.1152/ajpheart.00198.2007. [DOI] [PubMed] [Google Scholar]
- 107.Henschke PN. Elliott SJ. Oxidized glutathione decreases luminal Ca2+ content of the endothelial cell ins(1,4,5)P3-sensitive Ca2+ store. Biochem J. 1995;312(Pt 2):485–489. doi: 10.1042/bj3120485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herrero A. Barja G. Localization of the site of oxygen radical generation inside the complex I of heart and nonsynaptic brain mammalian mitochondria. J Bioenerg Biomembr. 2000;32:609–615. doi: 10.1023/a:1005626712319. [DOI] [PubMed] [Google Scholar]
- 109.Hicks MN. Cobbe SM. Effect of glibenclamide on extracellular potassium accumulation and the electrophysiological changes during myocardial ischaemia in the arterially perfused interventricular septum of rabbit. Cardiovasc Res. 1991;25:407–413. doi: 10.1093/cvr/25.5.407. [DOI] [PubMed] [Google Scholar]
- 110.Hirose M. Stuyvers BD. Dun W. ter Keurs HE. Boyden PA. Function of Ca(2+) release channels in Purkinje cells that survive in the infarcted canine heart: a mechanism for triggered Purkinje ectopy. Circ Arrhythm Electrophysiol. 2008;1:387–395. doi: 10.1161/CIRCEP.107.758110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ho HT. Stevens SC. Terentyeva R. Carnes CA. Terentyev D. Gyorke S. Arrhythmogenic adverse effects of cardiac glycosides are mediated by redox modification of ryanodine receptors. J Physiol. 2011;589:4697–4708. doi: 10.1113/jphysiol.2011.210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holmes TC. Fadool DA. Ren R. Levitan IB. Association of Src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science. 1996;274:2089–2091. doi: 10.1126/science.274.5295.2089. [DOI] [PubMed] [Google Scholar]
- 113.Hool LC. Hypoxia increases the sensitivity of the L-type Ca(2+) current to beta-adrenergic receptor stimulation via a C2 region-containing protein kinase C isoform. Circ Res. 2000;87:1164–1171. doi: 10.1161/01.res.87.12.1164. [DOI] [PubMed] [Google Scholar]
- 114.Hool LC. What cardiologists should know about calcium ion channels and their regulation by reactive oxygen species. Heart Lung Circ. 2007;16:361–372. doi: 10.1016/j.hlc.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 115.Hool LC. Arthur PG. Decreasing cellular hydrogen peroxide with catalase mimics the effects of hypoxia on the sensitivity of the L-type Ca2+ channel to beta-adrenergic receptor stimulation in cardiac myocytes. Circ Res. 2002;91:601–609. doi: 10.1161/01.res.0000035528.00678.d5. [DOI] [PubMed] [Google Scholar]
- 116.Hu H. Chiamvimonvat N. Yamagishi T. Marban E. Direct inhibition of expressed cardiac L-type Ca2+ channels by S-nitrosothiol nitric oxide donors. Circ Res. 1997;81:742–752. doi: 10.1161/01.res.81.5.742. [DOI] [PubMed] [Google Scholar]
- 117.Hudasek K. Brown ST. Fearon IM. H2O2 regulates recombinant Ca2+ channel alpha1C subunits but does not mediate their sensitivity to acute hypoxia. Biochem Biophys Res Commun. 2004;318:135–141. doi: 10.1016/j.bbrc.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 118.Huhn R. Heinen A. Weber NC. Schlack W. Preckel B. Hollmann MW. Ischaemic and morphine-induced post-conditioning: impact of mK(Ca) channels. Br J Anaesth. 2010;105:589–595. doi: 10.1093/bja/aeq213. [DOI] [PubMed] [Google Scholar]
- 119.Humphries KM. Deal MS. Taylor SS. Enhanced dephosphorylation of cAMP-dependent protein kinase by oxidation and thiol modification. J Biol Chem. 2005;280:2750–2758. doi: 10.1074/jbc.M410242200. [DOI] [PubMed] [Google Scholar]
- 120.Hund TJ. Rudy Y. A role for calcium/calmodulin-dependent protein kinase II in cardiac disease and arrhythmia. Handb Exp Pharmacol. 2006:201–220. doi: 10.1007/3-540-29715-4_7. [DOI] [PubMed] [Google Scholar]
- 121.Hund TJ. Ziman AP. Lederer WJ. Mohler PJ. The cardiac IP3 receptor: uncovering the role of “the other” calcium-release channel. J Mol Cell Cardiol. 2008;45:159–161. doi: 10.1016/j.yjmcc.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ibbotson T. Edwards G. Noack T. Weston AH. Effects of P1060 and aprikalim on whole-cell currents in rat portal vein; inhibition by glibenclamide and phentolamine. Br J Pharmacol. 1993;108:991–998. doi: 10.1111/j.1476-5381.1993.tb13496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ichinari K. Kakei M. Matsuoka T. Nakashima H. Tanaka H. Direct activation of the ATP-sensitive potassium channel by oxygen free radicals in guinea-pig ventricular cells: its potentiation by MgADP. J Mol Cell Cardiol. 1996;28:1867–1877. doi: 10.1006/jmcc.1996.0179. [DOI] [PubMed] [Google Scholar]
- 124.Inoue I. Nagase H. Kishi K. Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- 125.Iribe G. Jin H. Kaihara K. Naruse K. Effects of axial stretch on sarcolemmal BKCa channels in post-hatch chick ventricular myocytes. Exp Physiol. 2010;95:699–711. doi: 10.1113/expphysiol.2009.051896. [DOI] [PubMed] [Google Scholar]
- 126.Jabr RI. Cole WC. Alterations in electrical activity and membrane currents induced by intracellular oxygen-derived free radical stress in guinea pig ventricular myocytes. Circ Res. 1993;72:1229–1244. doi: 10.1161/01.res.72.6.1229. [DOI] [PubMed] [Google Scholar]
- 127.Jiang MT. Nakae Y. Ljubkovic M. Kwok WM. Stowe DF. Bosnjak ZJ. Isoflurane activates human cardiac mitochondrial adenosine triphosphate-sensitive K+ channels reconstituted in lipid bilayers. Anesth Analg. 2007;105:926–932. doi: 10.1213/01.ane.0000278640.81206.92. table of contents. [DOI] [PubMed] [Google Scholar]
- 128.Jornot L. Maechler P. Wollheim CB. Junod AF. Reactive oxygen metabolites increase mitochondrial calcium in endothelial cells: implication of the Ca2+/Na+ exchanger. J Cell Sci. 1999;112(Pt 7):1013–1022. doi: 10.1242/jcs.112.7.1013. [DOI] [PubMed] [Google Scholar]
- 129.Josephson RA. Silverman HS. Lakatta EG. Stern MD. Zweier JL. Study of the mechanisms of hydrogen peroxide and hydroxyl free radical-induced cellular injury and calcium overload in cardiac myocytes. J Biol Chem. 1991;266:2354–2361. [PubMed] [Google Scholar]
- 130.Jude S. Bedut S. Roger S. Pinault M. Champeroux P. White E. Le Guennec JY. Peroxidation of docosahexaenoic acid is responsible for its effects on I TO and I SS in rat ventricular myocytes. Br J Pharmacol. 2003;139:816–822. doi: 10.1038/sj.bjp.0705308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jung C. Martins AS. Niggli E. Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signalling and reactive oxygen species-generating pathways. Cardiovasc Res. 2008;77:766–773. doi: 10.1093/cvr/cvm089. [DOI] [PubMed] [Google Scholar]
- 132.Kagan V. Serbinova E. Packer L. Antioxidant effects of ubiquinones in microsomes and mitochondria are mediated by tocopherol recycling. Biochem Biophys Res Commun. 1990;169:851–857. doi: 10.1016/0006-291x(90)91971-t. [DOI] [PubMed] [Google Scholar]
- 133.Karle CA. Zitron E. Zhang W. Wendt-Nordahl G. Kathofer S. Thomas D. Gut B. Scholz E. Vahl CF. Katus HA. Kiehn J. Human cardiac inwardly-rectifying K+ channel Kir(2.1b) is inhibited by direct protein kinase C-dependent regulation in human isolated cardiomyocytes and in an expression system. Circulation. 2002;106:1493–1499. doi: 10.1161/01.cir.0000029747.53262.5c. [DOI] [PubMed] [Google Scholar]
- 134.Kawakami M. Okabe E. Superoxide anion radical-triggered Ca2+ release from cardiac sarcoplasmic reticulum through ryanodine receptor Ca2+ channel. Mol Pharmacol. 1998;53:497–503. doi: 10.1124/mol.53.3.497. [DOI] [PubMed] [Google Scholar]
- 135.Kevin LG. Novalija E. Stowe DF. Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesth Analg. 2005;101:1275–1287. doi: 10.1213/01.ANE.0000180999.81013.D0. [DOI] [PubMed] [Google Scholar]
- 136.Kimura J. Ono T. Sakamoto K. Ito E. Watanabe S. Maeda S. Shikama Y. Yatabe MS. Matsuoka I. Na+-Ca2+ exchanger expression and its modulation. Biol Pharm Bull. 2009;32:325–331. doi: 10.1248/bpb.32.325. [DOI] [PubMed] [Google Scholar]
- 137.Kitakaze M. Node K. Komamura K. Minamino T. Inoue M. Hori M. Kamada T. Evidence for nitric oxide generation in the cardiomyocytes: its augmentation by hypoxia. J Mol Cell Cardiol. 1995;27:2149–2154. doi: 10.1016/s0022-2828(95)91335-1. [DOI] [PubMed] [Google Scholar]
- 138.Klabunde RE. Cardiovascular Physiology Concepts. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2005. pp. 10–40. [Google Scholar]
- 139.Kockskamper J. Zima AV. Roderick HL. Pieske B. Blatter LA. Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol. 2008;45:128–147. doi: 10.1016/j.yjmcc.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kohlhaas M. Maack C. Adverse bioenergetic consequences of na+-Ca2+ exchanger-mediated Ca2+ influx in cardiac myocytes. Circulation. 2010;122:2273–2280. doi: 10.1161/CIRCULATIONAHA.110.968057. [DOI] [PubMed] [Google Scholar]
- 141.Kolbe K. Schonherr R. Gessner G. Sahoo N. Hoshi T. Heinemann SH. Cysteine 723 in the C-linker segment confers oxidative inhibition of hERG1 potassium channels. J Physiol. 2010;588:2999–3009. doi: 10.1113/jphysiol.2010.192468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kometiani P. Li J. Gnudi L. Kahn BB. Askari A. Xie Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem. 1998;273:15249–15256. doi: 10.1074/jbc.273.24.15249. [DOI] [PubMed] [Google Scholar]
- 143.Konya L. Kekesi V. Juhasz-Nagy S. Feher J. The effect of superoxide dismutase in the myocardium during reperfusion in the dog. Free Radic Biol Med. 1992;13:527–532. doi: 10.1016/0891-5849(92)90147-9. [DOI] [PubMed] [Google Scholar]
- 144.Korge P. Ping P. Weiss JN. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ Res. 2008;103:873–880. doi: 10.1161/CIRCRESAHA.108.180869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Koshland DE., Jr The molecule of the year. Science. 1992;258:1861. doi: 10.1126/science.1470903. [DOI] [PubMed] [Google Scholar]
- 146.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol. 1998;275:C1–C24. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- 147.Kukreja RC. Hess ML. The oxygen free radical system: from equations through membrane-protein interactions to cardiovascular injury and protection. Cardiovasc Res. 1992;26:641–655. doi: 10.1093/cvr/26.7.641. [DOI] [PubMed] [Google Scholar]
- 148.Kuo SS. Saad AH. Koong AC. Hahn GM. Giaccia AJ. Potassium-channel activation in response to low doses of gamma-irradiation involves reactive oxygen intermediates in nonexcitatory cells. Proc Natl Acad Sci U S A. 1993;90:908–912. doi: 10.1073/pnas.90.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kuster GM. Lancel S. Zhang J. Communal C. Trucillo MP. Lim CC. Pfister O. Weinberg EO. Cohen RA. Liao R. Siwik DA. Colucci WS. Redox-mediated reciprocal regulation of SERCA and Na+-Ca2+ exchanger contributes to sarcoplasmic reticulum Ca2+ depletion in cardiac myocytes. Free Radic Biol Med. 2010;48:1182–1187. doi: 10.1016/j.freeradbiomed.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lacampagne A. Duittoz A. Bolanos P. Peineau N. Argibay JA. Effect of sulfhydryl oxidation on ionic and gating currents associated with L-type calcium channels in isolated guinea-pig ventricular myocytes. Cardiovasc Res. 1995;30:799–806. [PubMed] [Google Scholar]
- 151.Lacerda AE. Rampe D. Brown AM. Effects of protein kinase C activators on cardiac Ca2+ channels. Nature. 1988;335:249–251. doi: 10.1038/335249a0. [DOI] [PubMed] [Google Scholar]
- 152.Lancel S. Qin F. Lennon SL. Zhang J. Tong X. Mazzini MJ. Kang YJ. Siwik DA. Cohen RA. Colucci WS. Oxidative posttranslational modifications mediate decreased SERCA activity and myocyte dysfunction in Galphaq-overexpressing mice. Circ Res. 2010;107:228–232. doi: 10.1161/CIRCRESAHA.110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lancel S. Zhang J. Evangelista A. Trucillo MP. Tong X. Siwik DA. Cohen RA. Colucci WS. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lefer DJ. Nichols CG. Coetzee WA. Sulfonylurea receptor 1 subunits of ATP-sensitive potassium channels and myocardial ischemia/reperfusion injury. Trends Cardiovasc Med. 2009;19:61–67. doi: 10.1016/j.tcm.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li GR. Dong MQ. Pharmacology of cardiac potassium channels. Adv Pharmacol. 2010;59:93–134. doi: 10.1016/S1054-3589(10)59004-5. [DOI] [PubMed] [Google Scholar]
- 156.Li GR. Feng J. Wang Z. Fermini B. Nattel S. Adrenergic modulation of ultrarapid delayed rectifier K+ current in human atrial myocytes. Circ Res. 1996;78:903–915. doi: 10.1161/01.res.78.5.903. [DOI] [PubMed] [Google Scholar]
- 157.Li N. Timofeyev V. Tuteja D. Xu D. Lu L. Zhang Q. Zhang Z. Singapuri A. Albert TR. Rajagopal AV. Bond CT. Periasamy M. Adelman J. Chiamvimonvat N. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009;587:1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li S. Li X. Li YL. Shao CH. Bidasee KR. Rozanski GJ. Insulin regulation of glutathione and contractile phenotype in diabetic rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2007;292:H1619–H1629. doi: 10.1152/ajpheart.00140.2006. [DOI] [PubMed] [Google Scholar]
- 159.Li X. Tang K. Xie B. Li S. Rozanski GJ. Regulation of Kv4 channel expression in failing rat heart by the thioredoxin system. Am J Physiol Heart Circ Physiol. 2008;295:H416–H424. doi: 10.1152/ajpheart.91446.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liang H. Li X. Li S. Zheng MQ. Rozanski GJ. Oxidoreductase regulation of Kv currents in rat ventricle. J Mol Cell Cardiol. 2008;44:1062–1071. doi: 10.1016/j.yjmcc.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lipp P. Laine M. Tovey SC. Burrell KM. Berridge MJ. Li W. Bootman MD. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol. 2000;10:939–942. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- 162.Liu G. Abramson JJ. Zable AC. Pessah IN. Direct evidence for the existence and functional role of hyperreactive sulfhydryls on the ryanodine receptor-triadin complex selectively labeled by the coumarin maleimide 7-diethylamino-3-(4′-maleimidylphenyl)-4-methylcoumarin. Mol Pharmacol. 1994;45:189–200. [PubMed] [Google Scholar]
- 163.Liu M. Liu H. Dudley SC., Jr Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circ Res. 2010;107:967–974. doi: 10.1161/CIRCRESAHA.110.220673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu Y. Fiskum G. Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 165.Lopez-Santiago LF. Meadows LS. Ernst SJ. Chen C. Malhotra JD. McEwen DP. Speelman A. Noebels JL. Maier SK. Lopatin AN. Isom LL. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol. 2007;43:636–647. doi: 10.1016/j.yjmcc.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Luo A. Ma J. Zhang P. Zhou H. Wang W. Sodium channel gating modes during redox reaction. Cell Physiol Biochem. 2007;19:9–20. doi: 10.1159/000099188. [DOI] [PubMed] [Google Scholar]
- 167.Ma JH. Luo AT. Zhang PH. Effect of hydrogen peroxide on persistent sodium current in guinea pig ventricular myocytes. Acta Pharmacol Sin. 2005;26:828–834. doi: 10.1111/j.1745-7254.2005.00154.x. [DOI] [PubMed] [Google Scholar]
- 168.Ma JH. Wang XP. Zhang PH. [Nitric oxide increases persistent sodium current of ventricular myocytes in guinea pig during normoxia and hypoxia] Sheng Li Xue Bao. 2004;56:603–608. [PubMed] [Google Scholar]
- 169.Macdonald WA. Hool LC. The effect of acute hypoxia on excitability in the heart and the L-type calcium channel as a therapeutic target. Curr Drug Discov Technol. 2008;5:302–311. doi: 10.2174/157016308786733546. [DOI] [PubMed] [Google Scholar]
- 170.MacFarlane NG. Miller DJ. Effects of the reactive oxygen species hypochlorous acid and hydrogen peroxide on force production and calcium sensitivity of rat cardiac myofilaments. Pflugers Arch. 1994;428:561–568. doi: 10.1007/BF00374578. [DOI] [PubMed] [Google Scholar]
- 171.MacMicking JD. Nathan C. Hom G. Chartrain N. Fletcher DS. Trumbauer M. Stevens K. Xie QW. Sokol K. Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 172.Mahajan A. Sato D. Shiferaw Y. Baher A. Xie LH. Peralta R. Olcese R. Garfinkel A. Qu Z. Weiss JN. Modifying L-type calcium current kinetics: consequences for cardiac excitation and arrhythmia dynamics. Biophys J. 2008;94:411–423. doi: 10.1529/biophysj.106.98590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Maier LS. Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 174.Maier SK. Westenbroek RE. McCormick KA. Curtis R. Scheuer T. Catterall WA. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation. 2004;109:1421–1427. doi: 10.1161/01.CIR.0000121421.61896.24. [DOI] [PubMed] [Google Scholar]
- 175.Makita N. Sloan-Brown K. Weghuis DO. Ropers HH. George AL., Jr Genomic organization and chromosomal assignment of the human voltage-gated Na+ channel beta 1 subunit gene (SCN1B) Genomics. 1994;23:628–634. doi: 10.1006/geno.1994.1551. [DOI] [PubMed] [Google Scholar]
- 176.Mandelker L. Introduction to oxidative stress and mitochondrial dysfunction. Vet Clin North Am Small Anim Pract. 2008;38:1–30. doi: 10.1016/j.cvsm.2007.10.005. v. [DOI] [PubMed] [Google Scholar]
- 177.Martin-Garrido A. Boyano-Adanez MC. Alique M. Calleros L. Serrano I. Griera M. Rodriguez-Puyol D. Griendling KK. Rodriguez-Puyol M. Hydrogen peroxide down-regulates inositol 1,4,5-trisphosphate receptor content through proteasome activation. Free Radic Biol Med. 2009;47:1362–1370. doi: 10.1016/j.freeradbiomed.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 178.Marx SO. Kurokawa J. Reiken S. Motoike H. D'Armiento J. Marks AR. Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 179.Masella R. Di Benedetto R. Vari R. Filesi C. Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16:577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 180.Matsuda H. Noma A. Kurachi Y. Irisawa H. Transient depolarization and spontaneous voltage fluctuations in isolated single cells from guinea pig ventricles. Calcium-mediated membrane potential fluctuations. Circ Res. 1982;51:142–151. doi: 10.1161/01.res.51.2.142. [DOI] [PubMed] [Google Scholar]
- 181.Matsuura H. Shattock MJ. Effects of oxidant stress on steady-state background currents in isolated ventricular myocytes. Am J Physiol. 1991;261:H1358–H1365. doi: 10.1152/ajpheart.1991.261.5.H1358. [DOI] [PubMed] [Google Scholar]
- 182.Matsuura H. Shattock MJ. Membrane potential fluctuations and transient inward currents induced by reactive oxygen intermediates in isolated rabbit ventricular cells. Circ Res. 1991;68:319–329. doi: 10.1161/01.res.68.2.319. [DOI] [PubMed] [Google Scholar]
- 183.Matsuzaki S. Szweda PA. Szweda LI. Humphries KM. Regulated production of free radicals by the mitochondrial electron transport chain: Cardiac ischemic preconditioning. Adv Drug Deliv Rev. 2009;61:1324–1331. doi: 10.1016/j.addr.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.McCord JM. Fridovich I. Superoxide dismutase: the first twenty years (1968–1988) Free Radic Biol Med. 1988;5:363–369. doi: 10.1016/0891-5849(88)90109-8. [DOI] [PubMed] [Google Scholar]
- 185.Medeiros-Domingo A. Kaku T. Tester DJ. Iturralde-Torres P. Itty A. Ye B. Valdivia C. Ueda K. Canizales-Quinteros S. Tusie-Luna MT. Makielski JC. Ackerman MJ. SCN4B-encoded sodium channel beta4 subunit in congenital long-QT syndrome. Circulation. 2007;116:134–142. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mishra S. Undrovinas NA. Maltsev VA. Reznikov V. Sabbah HN. Undrovinas A. Post-transcriptional silencing of SCN1B and SCN2B genes modulates late sodium current in cardiac myocytes from normal dogs and dogs with chronic heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H1596–H1605. doi: 10.1152/ajpheart.00948.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mittal CK. Murad F. Activation of guanylate cyclase by superoxide dismutase and hydroxyl radical: a physiological regulator of guanosine 3′,5′-monophosphate formation. Proc Natl Acad Sci U S A. 1977;74:4360–4364. doi: 10.1073/pnas.74.10.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Miura T. Liu Y. Kita H. Ogawa T. Shimamoto K. Roles of mitochondrial ATP-sensitive K channels and PKC in anti-infarct tolerance afforded by adenosine A1 receptor activation. J Am Coll Cardiol. 2000;35:238–245. doi: 10.1016/s0735-1097(99)00493-3. [DOI] [PubMed] [Google Scholar]
- 189.Mohammadi K. Kometiani P. Xie Z. Askari A. Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem. 2001;276:42050–42056. doi: 10.1074/jbc.M107892200. [DOI] [PubMed] [Google Scholar]
- 190.Mohler PJ. Davis JQ. Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Moschella MC. Marks AR. Inositol 1,4,5-trisphosphate receptor expression in cardiac myocytes. J Cell Biol. 1993;120:1137–1146. doi: 10.1083/jcb.120.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Murdoch CE. Zhang M. Cave AC. Shah AM. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res. 2006;71:208–215. doi: 10.1016/j.cardiores.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 193.Murphy AJ. Sulfhydryl group modification of sarcoplasmic reticulum membranes. Biochemistry. 1976;15:4492–4496. doi: 10.1021/bi00665a025. [DOI] [PubMed] [Google Scholar]
- 194.Murphy E. Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Murray KT. Fahrig SA. Deal KK. Po SS. Hu NN. Snyders DJ. Tamkun MM. Bennett PB. Modulation of an inactivating human cardiac K+ channel by protein kinase C. Circ Res. 1994;75:999–1005. doi: 10.1161/01.res.75.6.999. [DOI] [PubMed] [Google Scholar]
- 197.Nabeebaccus A. Zhang M. Shah AM. NADPH oxidases and cardiac remodelling. Heart Fail Rev. 2011;16:5–12. doi: 10.1007/s10741-010-9186-2. [DOI] [PubMed] [Google Scholar]
- 198.Nakamura TY. Coetzee WA. Vega-Saenz De Miera E. Artman M. Rudy B. Modulation of Kv4 channels, key components of rat ventricular transient outward K+ current, by PKC. Am J Physiol. 1997;273:H1775–1786. doi: 10.1152/ajpheart.1997.273.4.H1775. [DOI] [PubMed] [Google Scholar]
- 199.Nakaya H. Takeda Y. Tohse N. Kanno M. Mechanism of the membrane depolarization induced by oxidative stress in guinea-pig ventricular cells. J Mol Cell Cardiol. 1992;24:523–534. doi: 10.1016/0022-2828(92)91841-r. [DOI] [PubMed] [Google Scholar]
- 200.Nakaya H. Tohse N. Kanno M. Electrophysiological derangements induced by lipid peroxidation in cardiac tissue. Am J Physiol. 1987;253:H1089–H1097. doi: 10.1152/ajpheart.1987.253.5.H1089. [DOI] [PubMed] [Google Scholar]
- 201.Nan C. Li Y. Jean-Charles PY. Chen G. Kreymerman A. Prentice H. Weissbach H. Huang X. Deficiency of methionine sulfoxide reductase A causes cellular dysfunction and mitochondrial damage in cardiac myocytes under physical and oxidative stresses. Biochem Biophys Res Commun. 2010;402:608–613. doi: 10.1016/j.bbrc.2010.10.064. [DOI] [PubMed] [Google Scholar]
- 202.Nanduri J. Wang N. Bergson P. Yuan G. Ficker E. Prabhakar NR. Mitochondrial reactive oxygen species mediate hypoxic down-regulation of hERG channel protein. Biochem Biophys Res Commun. 2008;373:309–314. doi: 10.1016/j.bbrc.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 203.Napoli C. de Nigris F. Palinski W. Multiple role of reactive oxygen species in the arterial wall. J Cell Biochem. 2001;82:674–682. doi: 10.1002/jcb.1198. [DOI] [PubMed] [Google Scholar]
- 204.Nara M. Dhulipala PD. Ji GJ. Kamasani UR. Wang YX. Matalon S. Kotlikoff MI. Guanylyl cyclase stimulatory coupling to K(Ca) channels. Am J Physiol Cell Physiol. 2000;279:C1938–C1945. doi: 10.1152/ajpcell.2000.279.6.C1938. [DOI] [PubMed] [Google Scholar]
- 205.Netticadan T. Kato K. Tappia P. Elimban V. Dhalla NS. Phosphorylation of cardiac Na+-K+ ATPase by Ca2+/calmodulin dependent protein kinase. Biochem Biophys Res Commun. 1997;238:544–548. doi: 10.1006/bbrc.1997.7305. [DOI] [PubMed] [Google Scholar]
- 206.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 207.O'Rourke B. Ramza BM. Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994;265:962–966. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- 208.Oba T. Koshita M. Yamaguchi M. H2O2 modulates twitch tension and increases Po of Ca2+ release channel in frog skeletal muscle. Am J Physiol. 1996;271:C810–818. doi: 10.1152/ajpcell.1996.271.3.C810. [DOI] [PubMed] [Google Scholar]
- 209.Ogimoto G. Yudowski GA. Barker CJ. Kohler M. Katz AI. Feraille E. Pedemonte CH. Berggren PO. Bertorello AM. G protein-coupled receptors regulate Na+, K+-ATPase activity and endocytosis by modulating the recruitment of adaptor protein 2 and clathrin. Proc Natl Acad Sci U S A. 2000;97:3242–3247. doi: 10.1073/pnas.060025597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Olson TM. Terzic A. Human K(ATP) channelopathies: diseases of metabolic homeostasis. Pflugers Arch. 2010;460:295–306. doi: 10.1007/s00424-009-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pallandi RT. Perry MA. Campbell TJ. Proarrhythmic effects of an oxygen-derived free radical generating system on action potentials recorded from guinea pig ventricular myocardium: a possible cause of reperfusion-induced arrhythmias. Circ Res. 1987;61:50–54. doi: 10.1161/01.res.61.1.50. [DOI] [PubMed] [Google Scholar]
- 212.Palmer RM. Ferrige AG. Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 213.Pannaccione A. Castaldo P. Ficker E. Annunziato L. Taglialatela M. Histidines 578 and 587 in the S5–S6 linker of the human Ether-a-gogo Related Gene-1 K+ channels confer sensitivity to reactive oxygen species. J Biol Chem. 2002;277:8912–8919. doi: 10.1074/jbc.M111353200. [DOI] [PubMed] [Google Scholar]
- 214.Pastore A. Federici G. Bertini E. Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta. 2003;333:19–39. doi: 10.1016/s0009-8981(03)00200-6. [DOI] [PubMed] [Google Scholar]
- 215.Perier C. Tieu K. Guegan C. Caspersen C. Jackson-Lewis V. Carelli V. Martinuzzi A. Hirano M. Przedborski S. Vila M. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci U S A. 2005;102:19126–19131. doi: 10.1073/pnas.0508215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Prentice HM. Moench IA. Rickaway ZT. Dougherty CJ. Webster KA. Weissbach H. MsrA protects cardiac myocytes against hypoxia/reoxygenation induced cell death. Biochem Biophys Res Commun. 2008;366:775–778. doi: 10.1016/j.bbrc.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 217.Qu Y. Rogers JC. Tanada TN. Catterall WA. Scheuer T. Phosphorylation of S1505 in the cardiac Na+ channel inactivation gate is required for modulation by protein kinase C. J Gen Physiol. 1996;108:375–379. doi: 10.1085/jgp.108.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Raha S. McEachern GE. Myint AT. Robinson BH. Superoxides from mitochondrial complex III: the role of manganese superoxide dismutase. Free Radic Biol Med. 2000;29:170–180. doi: 10.1016/s0891-5849(00)00338-5. [DOI] [PubMed] [Google Scholar]
- 219.Reeves JP. Bailey CA. Hale CC. Redox modification of sodium-calcium exchange activity in cardiac sarcolemmal vesicles. J Biol Chem. 1986;261:4948–4955. [PubMed] [Google Scholar]
- 220.Reid MB. Khawli FA. Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol. 1993;75:1081–1087. doi: 10.1152/jappl.1993.75.3.1081. [DOI] [PubMed] [Google Scholar]
- 221.Ren J. Li Q. Wu S. Li SY. Babcock SA. Cardiac overexpression of antioxidant catalase attenuates aging-induced cardiomyocyte relaxation dysfunction. Mech Ageing Dev. 2007;128:276–285. doi: 10.1016/j.mad.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Romashko DN. Marban E. O'Rourke B. Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc Natl Acad Sci U S A. 1998;95:1618–1623. doi: 10.1073/pnas.95.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ruiz Petrich E. Leblanc N. deLorenzi F. Allard Y. Schanne OF. Effects of K+ channel blockers on the action potential of hypoxic rabbit myocardium. Br J Pharmacol. 1992;106:924–930. doi: 10.1111/j.1476-5381.1992.tb14436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ryu SY. Lee SH. Ho WK. Generation of metabolic oscillations by mitoKATP and ATP synthase during simulated ischemia in ventricular myocytes. J Mol Cell Cardiol. 2005;39:874–881. doi: 10.1016/j.yjmcc.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 225.Sabri A. Hughie HH. Lucchesi PA. Regulation of hypertrophic and apoptotic signaling pathways by reactive oxygen species in cardiac myocytes. Antioxid Redox Signal. 2003;5:731–740. doi: 10.1089/152308603770380034. [DOI] [PubMed] [Google Scholar]
- 226.Saint DA. The cardiac persistent sodium current: an appealing therapeutic target? Br J Pharmacol. 2008;153:1133–1142. doi: 10.1038/sj.bjp.0707492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sanchez G. Escobar M. Pedrozo Z. Macho P. Domenech R. Hartel S. Hidalgo C. Donoso P. Exercise and tachycardia increase NADPH oxidase and ryanodine receptor-2 activity: possible role in cardioprotection. Cardiovasc Res. 2008;77:380–386. doi: 10.1093/cvr/cvm011. [DOI] [PubMed] [Google Scholar]
- 228.Satoh H. Depression of the spontaneous activity by phorbol esters in young embryonic chick cardiomyocytes. Jpn J Pharmacol. 1995;67:297–304. doi: 10.1254/jjp.67.297. [DOI] [PubMed] [Google Scholar]
- 229.Scherer NM. Deamer DW. Oxidative stress impairs the function of sarcoplasmic reticulum by oxidation of sulfhydryl groups in the Ca2+-ATPase. Arch Biochem Biophys. 1986;246:589–601. doi: 10.1016/0003-9861(86)90314-0. [DOI] [PubMed] [Google Scholar]
- 230.Schonherr R. Heinemann SH. Molecular determinants for activation and inactivation of HERG, a human inward rectifier potassium channel. J Physiol. 1996;493(Pt 3):635–642. doi: 10.1113/jphysiol.1996.sp021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schott JJ. Alshinawi C. Kyndt F. Probst V. Hoorntje TM. Hulsbeek M. Wilde AA. Escande D. Mannens MM. Le Marec H. Cardiac conduction defects associate with mutations in SCN5A. Nat Genet. 1999;23:20–21. doi: 10.1038/12618. [DOI] [PubMed] [Google Scholar]
- 232.Shigekawa M. Katanosaka Y. Wakabayashi S. Regulation of the cardiac Na+/Ca2+ exchanger by calcineurin and protein kinase C. Ann N Y Acad Sci. 2007;1099:53–63. doi: 10.1196/annals.1387.059. [DOI] [PubMed] [Google Scholar]
- 233.Sigalas C. Mayo-Martin MB. Jane DE. Sitsapesan R. Ca2+-calmodulin increases RyR2 open probability yet reduces ryanoid association with RyR2. Biophys J. 2009;97:1907–1916. doi: 10.1016/j.bpj.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Slodzinski MK. Aon MA. O'Rourke B. Glutathione oxidation as a trigger of mitochondrial depolarization and oscillation in intact hearts. J Mol Cell Cardiol. 2008;45:650–660. doi: 10.1016/j.yjmcc.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Smani T. Calderon-Sanchez E. Gomez-Hurtado N. Fernandez-Velasco M. Cachofeiro V. Lahera V. Ordonez A. Delgado C. Mechanisms underlying the activation of L-type calcium channels by urocortin in rat ventricular myocytes. Cardiovasc Res. 2010;87:459–466. doi: 10.1093/cvr/cvq063. [DOI] [PubMed] [Google Scholar]
- 236.Smith RA. Kelso GF. Blaikie FH. Porteous CM. Ledgerwood EC. Hughes G. James AM. Ross MF. Asin-Cayuela J. Cocheme HM. Filipovska A. Murphy MP. Using mitochondria-targeted molecules to study mitochondrial radical production and its consequences. Biochem Soc Trans. 2003;31:1295–1299. doi: 10.1042/bst0311295. [DOI] [PubMed] [Google Scholar]
- 237.Song Y. Shryock JC. Belardinelli L. A slowly inactivating sodium current contributes to spontaneous diastolic depolarization of atrial myocytes. Am J Physiol Heart Circ Physiol. 2009;297:H1254–H1262. doi: 10.1152/ajpheart.00444.2009. [DOI] [PubMed] [Google Scholar]
- 238.Song Y. Shryock JC. Wagner S. Maier LS. Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 239.Song YH. Cho H. Ryu SY. Yoon JY. Park SH. Noh CI. Lee SH. Ho WK. L-type Ca(2+) channel facilitation mediated by H(2)O(2)-induced activation of CaMKII in rat ventricular myocytes. J Mol Cell Cardiol. 2010;48:773–780. doi: 10.1016/j.yjmcc.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 240.Splawski I. Timothy KW. Decher N. Kumar P. Sachse FB. Beggs AH. Sanguinetti MC. Keating MT. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci U S A. 2005;102:8089–8096. doi: 10.1073/pnas.0502506102. discussion 8086–8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.St-Pierre J. Buckingham JA. Roebuck SJ. Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 242.Stoyanovsky D. Murphy T. Anno PR. Kim YM. Salama G. Nitric oxide activates skeletal and cardiac ryanodine receptors. Cell Calcium. 1997;21:19–29. doi: 10.1016/s0143-4160(97)90093-2. [DOI] [PubMed] [Google Scholar]
- 243.Strehler EE. Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 244.Taglialatela M. Castaldo P. Iossa S. Pannaccione A. Fresi A. Ficker E. Annunziato L. Regulation of the human ether-a-gogo related gene (HERG) K+ channels by reactive oxygen species. Proc Natl Acad Sci U S A. 1997;94:11698–11703. doi: 10.1073/pnas.94.21.11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tamargo J. Caballero R. Gomez R. Valenzuela C. Delpon E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 246.Tan HL. Kupershmidt S. Zhang R. Stepanovic S. Roden DM. Wilde AA. Anderson ME. Balser JR. A calcium sensor in the sodium channel modulates cardiac excitability. Nature. 2002;415:442–447. doi: 10.1038/415442a. [DOI] [PubMed] [Google Scholar]
- 247.Tarr M. Arriaga E. Goertz KK. Valenzeno DP. Properties of cardiac I(leak) induced by photosensitizer-generated reactive oxygen. Free Radic Biol Med. 1994;16:477–484. doi: 10.1016/0891-5849(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 248.Telezhkin V. Brazier SP. Cayzac SH. Wilkinson WJ. Riccardi D. Kemp PJ. Mechanism of inhibition by hydrogen sulfide of native and recombinant BKCa channels. Respir Physiol Neurobiol. 2010;172:169–178. doi: 10.1016/j.resp.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 249.Thomas GP. Sims SM. Cook MA. Karmazyn M. Hydrogen peroxide-induced stimulation of L-type calcium current in guinea pig ventricular myocytes and its inhibition by adenosine A1 receptor activation. J Pharmacol Exp Ther. 1998;286:1208–1214. [PubMed] [Google Scholar]
- 250.Tokube K. Kiyosue T. Arita M. Effects of hydroxyl radicals on KATP channels in guinea-pig ventricular myocytes. Pflugers Arch. 1998;437:155–157. doi: 10.1007/s004240050760. [DOI] [PubMed] [Google Scholar]
- 251.Tsai CT. Wang DL. Chen WP. Hwang JJ. Hsieh CS. Hsu KL. Tseng CD. Lai LP. Tseng YZ. Chiang FT. Lin JL. Angiotensin II increases expression of alpha1C subunit of L-type calcium channel through a reactive oxygen species and cAMP response element-binding protein-dependent pathway in HL-1 myocytes. Circ Res. 2007;100:1476–1485. doi: 10.1161/01.RES.0000268497.93085.e1. [DOI] [PubMed] [Google Scholar]
- 252.Tuteja D. Rafizadeh S. Timofeyev V. Wang S. Zhang Z. Li N. Mateo RK. Singapuri A. Young JN. Knowlton AA. Chiamvimonvat N. Cardiac small conductance Ca2+-activated K+ channel subunits form heteromultimers via the coiled-coil domains in the C termini of the channels. Circ Res. 2010;107:851–859. doi: 10.1161/CIRCRESAHA.109.215269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ueda K. Valdivia C. Medeiros-Domingo A. Tester DJ. Vatta M. Farrugia G. Ackerman MJ. Makielski JC. Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proc Natl Acad Sci U S A. 2008;105:9355–9360. doi: 10.1073/pnas.0801294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Vajda S. Baczko I. Lepran I. Selective cardiac plasma-membrane K(ATP) channel inhibition is defibrillatory and improves survival during acute myocardial ischemia and reperfusion. Eur J Pharmacol. 2007;577:115–123. doi: 10.1016/j.ejphar.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 255.Valko M. Leibfritz D. Moncol J. Cronin MT. Mazur M. Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 256.Vega-Saenz de Miera E. Rudy B. Modulation of K+ channels by hydrogen peroxide. Biochem Biophys Res Commun. 1992;186:1681–1687. doi: 10.1016/s0006-291x(05)81602-x. [DOI] [PubMed] [Google Scholar]
- 257.Viola HM. Arthur PG. Hool LC. Transient exposure to hydrogen peroxide causes an increase in mitochondria-derived superoxide as a result of sustained alteration in L-type Ca2+ channel function in the absence of apoptosis in ventricular myocytes. Circ Res. 2007;100:1036–1044. doi: 10.1161/01.RES.0000263010.19273.48. [DOI] [PubMed] [Google Scholar]
- 258.Wagner HH. Ulbricht W. The rates of saxitoxin action and of saxitoxin-tetrodotoxin interaction at the node of Ranvier. Pflugers Arch. 1975;359:297–315. doi: 10.1007/BF00581441. [DOI] [PubMed] [Google Scholar]
- 259.Wagner S. Dybkova N. Rasenack EC. Jacobshagen C. Fabritz L. Kirchhof P. Maier SK. Zhang T. Hasenfuss G. Brown JH. Bers DM. Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wagner S. Ruff HM. Weber SL. Bellmann S. Sowa T. Schulte T. Anderson ME. Grandi E. Bers DM. Backs J. Belardinelli L. Maier LS. Reactive oxygen species-activated Ca/calmodulin kinase IIdelta is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555–565. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wang Q. Shen J. Splawski I. Atkinson D. Li Z. Robinson JL. Moss AJ. Towbin JA. Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 262.Wang W. Ma J. Zhang P. Luo A. Redox reaction modulates transient and persistent sodium current during hypoxia in guinea pig ventricular myocytes. Pflugers Arch. 2007;454:461–475. doi: 10.1007/s00424-007-0219-1. [DOI] [PubMed] [Google Scholar]
- 263.Ward CA. Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol. 1997;500(Pt 3):631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.West JW. Numann R. Murphy BJ. Scheuer T. Catterall WA. A phosphorylation site in the Na+ channel required for modulation by protein kinase C. Science. 1991;254:866–868. doi: 10.1126/science.1658937. [DOI] [PubMed] [Google Scholar]
- 265.Wirth KJ. Rosenstein B. Uhde J. Englert HC. Busch AE. Scholkens BA. ATP-sensitive potassium channel blocker HMR 1883 reduces mortality and ischemia-associated electrocardiographic changes in pigs with coronary occlusion. J Pharmacol Exp Ther. 1999;291:474–481. [PubMed] [Google Scholar]
- 266.Wu X. Chang B. Blair NS. Sargent M. York AJ. Robbins J. Shull GE. Molkentin JD. Plasma membrane Ca2+-ATPase isoform 4 antagonizes cardiac hypertrophy in association with calcineurin inhibition in rodents. J Clin Invest. 2009;119:976–985. doi: 10.1172/JCI36693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Xiao YF. Gomez AM. Morgan JP. Lederer WJ. Leaf A. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc Natl Acad Sci U S A. 1997;94:4182–4187. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Xie LH. Chen F. Karagueuzian HS. Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Xie Z. Askari A. Na(+)/K(+)-ATPase as a signal transducer. Eur J Biochem. 2002;269:2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- 270.Xie Z. Jack-Hays M. Wang Y. Periyasamy SM. Blanco G. Huang WH. Askari A. Different oxidant sensitivities of the alpha 1 and alpha 2 isoforms of Na+/K(+)-ATPase expressed in baculovirus-infected insect cells. Biochem Biophys Res Commun. 1995;207:155–159. doi: 10.1006/bbrc.1995.1166. [DOI] [PubMed] [Google Scholar]
- 271.Xie Z. Kometiani P. Liu J. Li J. Shapiro JI. Askari A. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J Biol Chem. 1999;274:19323–19328. doi: 10.1074/jbc.274.27.19323. [DOI] [PubMed] [Google Scholar]
- 272.Xie ZJ. Wang YH. Askari A. Huang WH. Klaunig JE. Studies on the specificity of the effects of oxygen metabolites on cardiac sodium pump. J Mol Cell Cardiol. 1990;22:911–920. doi: 10.1016/0022-2828(90)90122-i. [DOI] [PubMed] [Google Scholar]
- 273.Xu KY. Zweier JL. Becker LC. Oxygen-free radicals directly attack the ATP binding site of the cardiac Na+,K(+)-ATPase. Ann N Y Acad Sci. 1997;834:680–683. doi: 10.1111/j.1749-6632.1997.tb52349.x. [DOI] [PubMed] [Google Scholar]
- 274.Xu L. Eu JP. Meissner G. Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 275.Xu W. Liu Y. Wang S. McDonald T. Van Eyk JE. Sidor A. O'Rourke B. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 276.Yamaoka K. Yakehiro M. Yuki T. Fujii H. Seyama I. Effect of sulfhydryl reagents on the regulatory system of the L-type Ca channel in frog ventricular myocytes. Pflugers Arch. 2000;440:207–215. doi: 10.1007/s004249900242. [DOI] [PubMed] [Google Scholar]
- 277.Yan XS. Ma JH. Zhang PH. Modulation of K(ATP) currents in rat ventricular myocytes by hypoxia and a redox reaction. Acta Pharmacol Sin. 2009;30:1399–1414. doi: 10.1038/aps.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yan Y. Liu J. Wei C. Li K. Xie W. Wang Y. Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc Res. 2008;77:432–441. doi: 10.1093/cvr/cvm047. [DOI] [PubMed] [Google Scholar]
- 279.Yang L. Doshi D. Morrow J. Katchman A. Chen X. Marx SO. Protein kinase C isoforms differentially phosphorylate Ca(v)1.2 alpha(1c) Biochemistry. 2009;48:6674–6683. doi: 10.1021/bi900322a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yao L. Fan P. Jiang Z. Viatchenko-Karpinski S. Wu Y. Kornyeyev D. Hirakawa R. Budas GR. Rajamani S. Shryock JC. Belardinelli L. Nav1.5-dependent persistent Na+ influx activates CaMKII in rat ventricular myocytes and N1325S mice. Am J Physiol Cell Physiol. 2011;301:C577–C586. doi: 10.1152/ajpcell.00125.2011. [DOI] [PubMed] [Google Scholar]
- 281.Yaras N. Turan B. Interpretation of relevance of sodium-calcium exchange in action potential of diabetic rat heart by mathematical model. Mol Cell Biochem. 2005;269:121–129. doi: 10.1007/s11010-005-3439-8. [DOI] [PubMed] [Google Scholar]
- 282.Ye B. Kroboth SL. Pu JL. Sims JJ. Aggarwal NT. McNally EM. Makielski JC. Shi NQ. Molecular identification and functional characterization of a mitochondrial sulfonylurea receptor 2 splice variant generated by intraexonic splicing. Circ Res. 2009;105:1083–1093. doi: 10.1161/CIRCRESAHA.109.195040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yen HC. Oberley TD. Vichitbandha S. Ho YS. St Clair DK. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest. 1996;98:1253–1260. doi: 10.1172/JCI118909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ytrehus K. Rotevatn S. Lovaas E. Saetersdal T. Mjos OD. Mitochondrial calcium in hearts subjected to lipid peroxidation with contracture development. Basic Res Cardiol. 1989;84:646–652. doi: 10.1007/BF01906949. [DOI] [PubMed] [Google Scholar]
- 285.Yuan P. Leonetti MD. Pico AR. Hsiung Y. MacKinnon R. Structure of the human BK channel Ca2+-activation apparatus at 3.0 A resolution. Science. 2010;329:182–186. doi: 10.1126/science.1190414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zable AC. Favero TG. Abramson JJ. Glutathione modulates ryanodine receptor from skeletal muscle sarcoplasmic reticulum. Evidence for redox regulation of the Ca2+ release mechanism. J Biol Chem. 1997;272:7069–7077. doi: 10.1074/jbc.272.11.7069. [DOI] [PubMed] [Google Scholar]
- 287.Zahradnikova A. Minarovic I. Venema RC. Meszaros LG. Inactivation of the cardiac ryanodine receptor calcium release channel by nitric oxide. Cell Calcium. 1997;22:447–454. doi: 10.1016/s0143-4160(97)90072-5. [DOI] [PubMed] [Google Scholar]
- 288.Zaidi A. Fernandes D. Bean JL. Michaelis ML. Effects of paraquat-induced oxidative stress on the neuronal plasma membrane Ca(2+)-ATPase. Free Radic Biol Med. 2009;47:1507–1514. doi: 10.1016/j.freeradbiomed.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zeng Q. Han Y. Bao Y. Li W. Li X. Shen X. Wang X. Yao F. O'Rourke ST. Sun C. 20-HETE increases NADPH oxidase-derived ROS production and stimulates the L-type Ca2+ channel via a PKC-dependent mechanism in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2010;299:H1109–H1117. doi: 10.1152/ajpheart.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhang GX. Kimura S. Nishiyama A. Shokoji T. Rahman M. Yao L. Nagai Y. Fujisawa Y. Miyatake A. Abe Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res. 2005;65:230–238. doi: 10.1016/j.cardiores.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 291.Zhang L. Zhang Z. Guo H. Wang Y. Na+/K+-ATPase-mediated signal transduction and Na+/K+-ATPase regulation. Fundam Clin Pharmacol. 2008;22:615–621. doi: 10.1111/j.1472-8206.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- 292.Zhang ZH. Johnson JA. Chen L. El-Sherif N. Mochly-Rosen D. Boutjdir M. C2 region-derived peptides of beta-protein kinase C regulate cardiac Ca2+ channels. Circ Res. 1997;80:720–729. doi: 10.1161/01.res.80.5.720. [DOI] [PubMed] [Google Scholar]
- 293.Zheng J. Ma J. Zhang P. Hu L. Fan X. Tang Q. Milrinone inhibits hypoxia or hydrogen dioxide-induced persistent sodium current in ventricular myocytes. Eur J Pharmacol. 2009;616:206–212. doi: 10.1016/j.ejphar.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 294.Zheng Y. Shen X. H2O2 directly activates inositol 1,4,5-trisphosphate receptors in endothelial cells. Redox Rep. 2005;10:29–36. doi: 10.1179/135100005X21660. [DOI] [PubMed] [Google Scholar]
- 295.Zhou J. Yi J. Hu N. George AL., Jr. Murray KT. Activation of protein kinase A modulates trafficking of the human cardiac sodium channel in Xenopus oocytes. Circ Res. 2000;87:33–38. doi: 10.1161/01.res.87.1.33. [DOI] [PubMed] [Google Scholar]
- 296.Zhou L. Cortassa S. Wei AC. Aon MA. Winslow RL. O'Rourke B. Modeling cardiac action potential shortening driven by oxidative stress-induced mitochondrial oscillations in guinea pig cardiomyocytes. Biophys J. 2009;97:1843–1852. doi: 10.1016/j.bpj.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zima AV. Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca(2+) signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol. 2004;555:607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zima AV. Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 299.Zima AV. Copello JA. Blatter LA. Effects of cytosolic NADH/NAD(+) levels on sarcoplasmic reticulum Ca(2+) release in permeabilized rat ventricular myocytes. J Physiol. 2004;555:727–741. doi: 10.1113/jphysiol.2003.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zuo L. Clanton TL. Reactive oxygen species formation in the transition to hypoxia in skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C207–C216. doi: 10.1152/ajpcell.00449.2004. [DOI] [PubMed] [Google Scholar]