Abstract
The bacteriocin produced by Mycobacterium smegmatis ATCC 14468 was isolated, and a study was made of its chemical, physical, and biological properties. No appreciable bacteriocin activity was found in the culture supernatant fluids, but it was released in appreciable quantities after disruption of the cells. The material was purified 49-fold by means of chromatography on diethylaminoethyl-cellulose, ammonium sulfate fractionation, gel filtration on Sephadex G-200, and chromatography on diethylaminoethyl-Sephadex A-50. Its molecular weight was determined to be approximately 75,000 from the elution profile on Sephadex G-200 chromatography. The bacteriocin was resistant to deoxyribonuclease, ribonuclease, lipase, ultraviolet irradiation, and freeze-thawing, whereas it was relatively less thermostable and was sensitive to proteolytic enzymes. The lethal effect of the bacteriocin was demonstrated by the decrease in viable counts of the bacteriocin-sensitive indicator strain, M. diernhoferi ATCC 19340. The bacteriocin preparation inhibited the growth of HeLa-S3 cells.
Full text
PDF

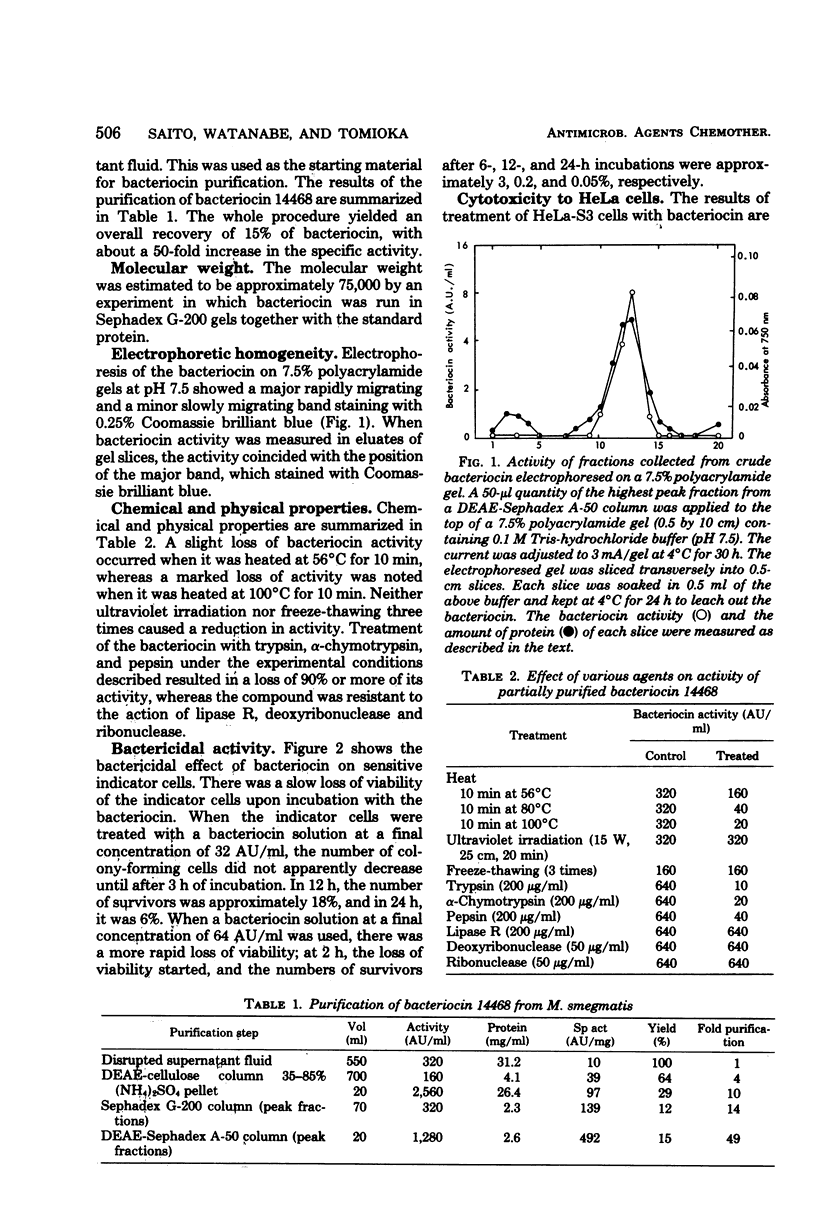
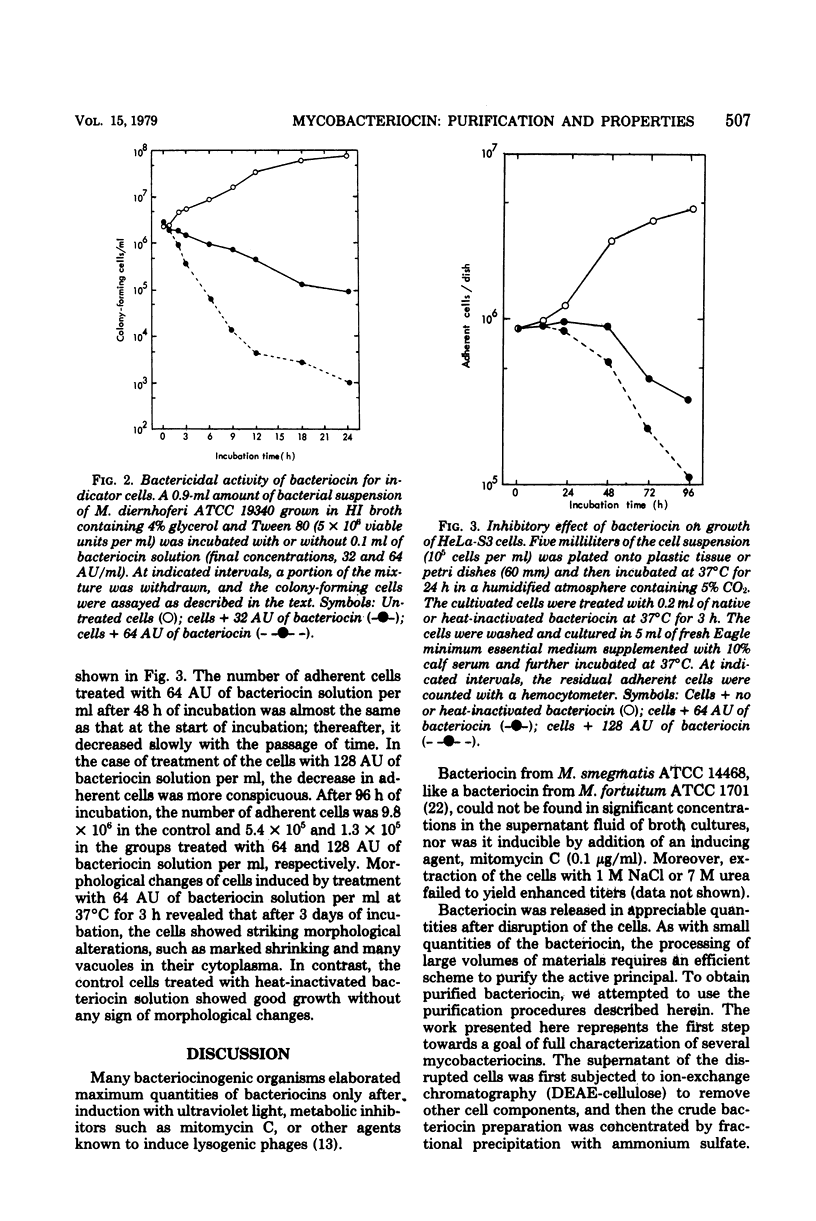


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adámek L., Trnka L., Mion P., Gutová M. Hemmstoffe vom Bakteriocin-Typus bei schnell wachsenden saphrophytischen Stämmen der Mycobakterien. Beitr Klin Erforsch Tuberk Lungenkr. 1968;138(1):51–55. [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Masi D. R., White J. C., Schnaitman C. A., Bradbeer C. Transport of vitamin B12 in Escherichia coli: common receptor sites for vitamin B12 and the E colicins on the outer membrane of the cell envelope. J Bacteriol. 1973 Aug;115(2):506–513. doi: 10.1128/jb.115.2.506-513.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano V. J., Hinsdill R. D. Characterization of a Staphylococcus aureus bacteriocin. J Bacteriol. 1970 Oct;104(1):117–125. doi: 10.1128/jb.104.1.117-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale E. M., Hinsdill R. D. Characterization of a bacteriocin from Staphylococcus aureus strain 462. Antimicrob Agents Chemother. 1973 Dec;4(6):634–640. doi: 10.1128/aac.4.6.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda T., Rieber M. Mitomycin C-induced phage-like particles in a mutant of Mycobacterium tuberculosis BCG. J Bacteriol. 1968 Aug;96(2):557–559. doi: 10.1128/jb.96.2.557-559.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovics G. BACTERIOCINS AND BACTERIOCIN-LIKE SUBSTANCES. Bacteriol Rev. 1962 Jun;26(2 Pt 1):108–118. [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Effects of colicin A and staphylococcin 1580 on amino acid uptake into membrane vesicles of Escherichia coli and staphylococcus aureus. Biochim Biophys Acta. 1973 Jul 18;311(4):483–495. doi: 10.1016/0005-2736(73)90124-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Mathieu L. G., Legault-Hetu D. Decreased sensitivity to polymyxin B in colicin K tolerant cells of Escherichia coli K-12 in the presence of colicin K. Can J Microbiol. 1973 Mar;19(3):345–351. doi: 10.1139/m73-057. [DOI] [PubMed] [Google Scholar]
- REEVES P. THE BACTERIOCINS. Bacteriol Rev. 1965 Mar;29:24–45. doi: 10.1128/br.29.1.24-45.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Maizel J. V., Jr T7-directed protein synthesis. Virology. 1969 Nov;39(3):575–586. doi: 10.1016/0042-6822(69)90105-6. [DOI] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya K., Tokiwa H. Bacteriocin-typing of Mycobacterium tuberculosis. Am Rev Respir Dis. 1974 Feb;109(2):304–305. doi: 10.1164/arrd.1974.109.2.304. [DOI] [PubMed] [Google Scholar]
- Tokiwa H. [Studies on mycobacteriocin. I. Characterization and classification of mycobacteriocin produced by rapidly growing mycobacteria (author's transl)]. Fukuoka Igaku Zasshi. 1974 Jul 25;65(7):473–480. [PubMed] [Google Scholar]


