Abstract
The radiosynthesis and in vivo evaluation of 5-(5-(6-[11C]methyl-3,6-diazabicyclo[3.2.0]heptan-3-yl)pyridin-2-yl)-1H-indole [11C]rac-(1), a potential PET tracer for α7 nicotinic acetylcholine receptors (α7-nAChR), are described. Syntheses of the nonradioactive standard rac-1 and corresponding desmethyl precursor 7 were achieved in several reaction steps. Radiomethylation of 7 with [11C]CH3I afforded [11C]rac-1 in an average radiochemical yield of 30 ± 5% (n = 5) with high radiochemical purity and an average specific radioactivity of 444 ± 74 GBq/µmol (n = 5). The total synthesis time was 30 min from end-of-bombardment. Biodistribution studies in mice showed that [11C]rac-1 penetrates the blood-brain barrier and specifically labels neuronal α7-nAChRs.
Keywords: positron emission tomography, radioligand, nicotinic acetylcholine receptors, α7-nAChR
1. Introduction
A large body of experimental evidence associates cerebral nicotinic acetylcholine receptors (nAChRs) with the pathophysiology of a variety of disorders of central nervous system (CNS) disorders.1–4 Because of the importance of α7-nAChR as potential drug target for treatment of various central disorders including schizophrenia,5, 6 synthesis and pre-clinical examination of α7-nAChR subtype selective compounds receives substantial attention in industry and academia.7–10
Substantial effort of PET researchers in the last decade has been devoted to imaging nAChRs in human brain. This imaging work in turn is linked to the development of PET radioligands for nAChR. Several PET radioligands for the major cerebral nAChR subtype, α4β2-nAChR, are currently available for human and animal studies (see for review11, 12). In contrast, exploration of PET radioligands for imaging the second major cerebral nicotinic receptor subtype α7-nAChR so far has been less successful (see for review13, 14). Recent studies revealed new interesting compounds,15–17 but more quality radioligands for PET imaging of α7-nAChR remains to be discovered.
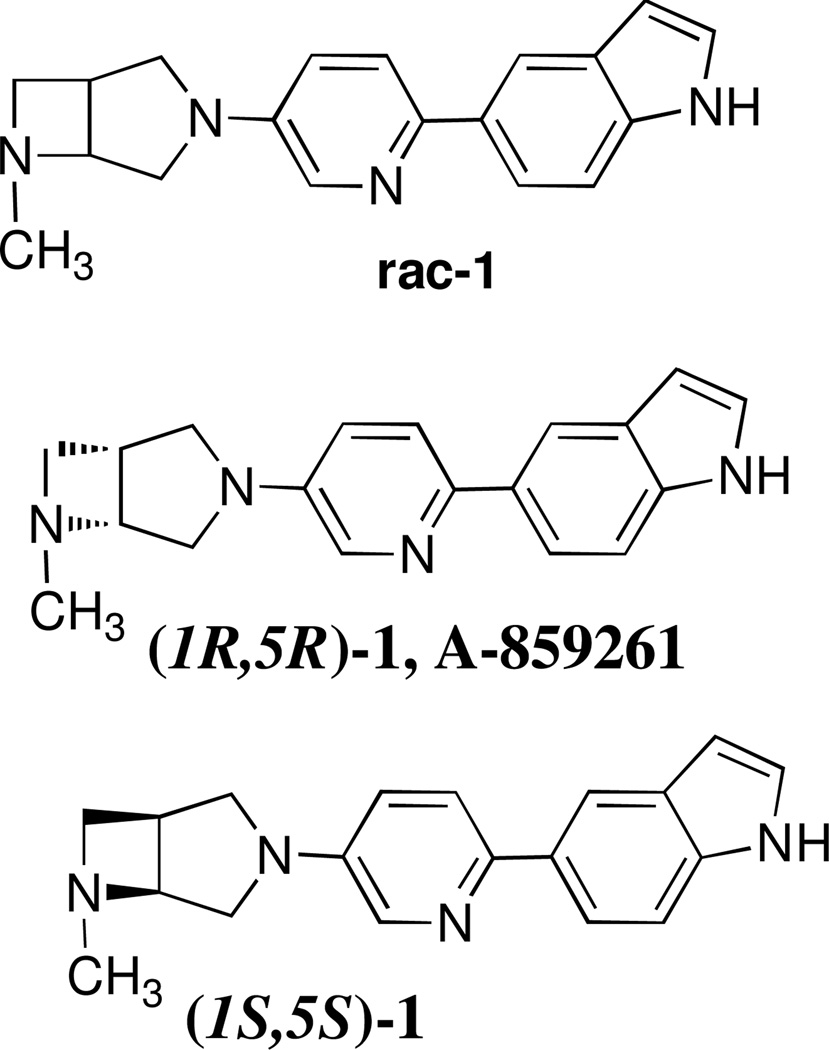
Recently, a series of biarylsubstituted 3,6-diazabicyclo[3.2.0]heptanes derivatives was discovered by Abbott Laboratories as potent and selective α7-nAChR agonists with low binding affinities at other cerebral receptors and nicotinic receptor subtypes.18, 19 Two members of the series, 5-(5-((1R,5R)-6-methyl-3,6-diazabicyclo[3.2.0]heptan-3-yl)pyridin-2-yl)-1H-indole ((1R,5R)-1, A-859261) and 5-(5-((1S,5S)-6-methyl-3,6-diazabicyclo[3.2.0]heptan-3-yl)pyridin-2-yl)-1H-indole ((1S,5S)-1) exhibit highest α7-nAChR binding affinities (Ki = 0.5 and 0.6 nM,19 respectively, α7 Ki displacement of [3H]-A-585539 ([3H]-(S,S)-2,2-dimethyl-5-(6-phenylpyridazin-3-yl)-5-aza-2-azoniabicyclo[2.2.1]heptane iodide) from membrane enriched fractions of rat brain minus cerebellum or cortex; n ≥ 3; SEM < 10%.) (Figure 1). A-859261 and analogs showed no interaction at 5HT3 receptors. In the CEREP profiling, A-859261 exhibited none or weak interaction up to 10 µM. Also, A-859261 exhibited low activity at the hERG channel, its [3H]dofetilide binding Ki is 1.9 µM. An in vivo study demonstrated that A-859261 readily enters the mouse brain after intraperitoneal administration and exhibit cognition-enhancing properties that are attributable to α7-nAChR agonists.19 We hypothesized that racemic 1 (rac-1) (Figure 1) should also manifest good α7-nAChR binding affinity and could penetrate the blood–brain barrier. Thus radiolabeled [11C]rac-1 might be suitable for PET imaging the cerebral α7-nAChR.
Figure 1.
Structures of potent and selective α7-nAChR ligands.
In this study we report the synthesis of rac-1 (Scheme 1), radiosynthesis of ([11C]rac-1) (Scheme 2) and its in vivo evaluation as a potential PET radioligand for imaging α7-nAChR.
Scheme 1.
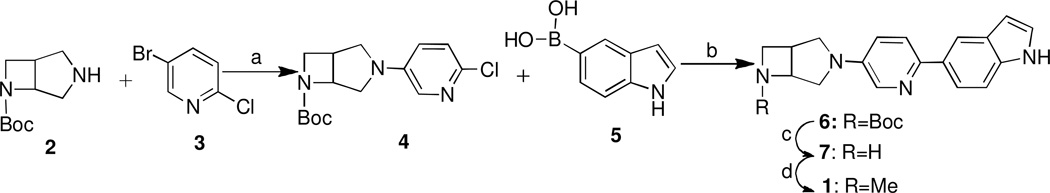
Synthesis of 5-(5-(6-methyl-3,6-diazabicyclo[3.2.0]heptan-3-yl)pyridin-2-yl)-1H-indole and its desmethyl precursor. Reagents and conditions: (a) Pd2(dba)3, BINAP, t-BuONa in toluene, at 85 °C 20 h, 83%; (b) Pd(PPh3)4, dioxane/Na2CO3 aq. 2M)=4:1, at 115 °C 1.5 h, 77%; (c) CF3CO2H-CH2Cl2, 27%; (d) HCHO, NaBH(OAc)3, CH3CN, 31%.
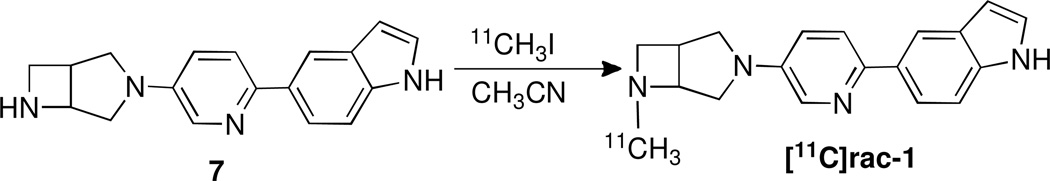
Scheme 2.
Radiosynthesis of [11C]rac-1.
2. Results and discussion
2.1. Chemistry
As shown in Scheme 1, racemic tert-butyl 3,6-diazabicyclo[3.2.0]heptane-6-carboxylate (2) was condensed with 5-bromo-2-chloropyridine (3) via the Buchwald-Hartwig amination procedure18, 20, 21 to yield the corresponding intermediate, tert-butyl 3-(6-chloropyridin-3-yl)-3,6-diazabicyclo[3.2.0]heptane-6-carboxylate (4) in high yield. A transformation of 4 under the Suzuki coupling reaction conditions with indol-5-ylboronic acid (5) gave 6. The N-Boc deprotection of 6 with trifluoroacetic acid (TFA) yielded the corresponding 7. Reductive methylation of 7 proceeded well to give the corresponding N-Me analogue rac-1.
The radiosynthesis of [11C]rac-1 was accomplished via N-methylation of the nor-methyl precursor 7 with the [11C]CH3I in acetonitrile as shown in Scheme 2 followed by semi-preparative HPLC purification and C18 solid phase extraction and formulation. The final product [11C]rac-1 was prepared with an average radiochemical yield of 30 ± 5% (n = 5), radiochemical purity greater than 98% and an average specific radioactivity of 444 ± 74 GBq/µmol mCi/µmol (n = 5). The total synthesis time was 30 min from the end-of-bombardment (EOB). The identity of the radiotracer [11C]rac-1 was confirmed by co-injection with non-radioactive reference compound rac-1 onto the analytical HPLC system. The final product [11C]rac-1 was formulated as a sterile and apyrogenic solution in saline with 7% ethyl alcohol.
2.2. In vivo experiments in CD1 mice
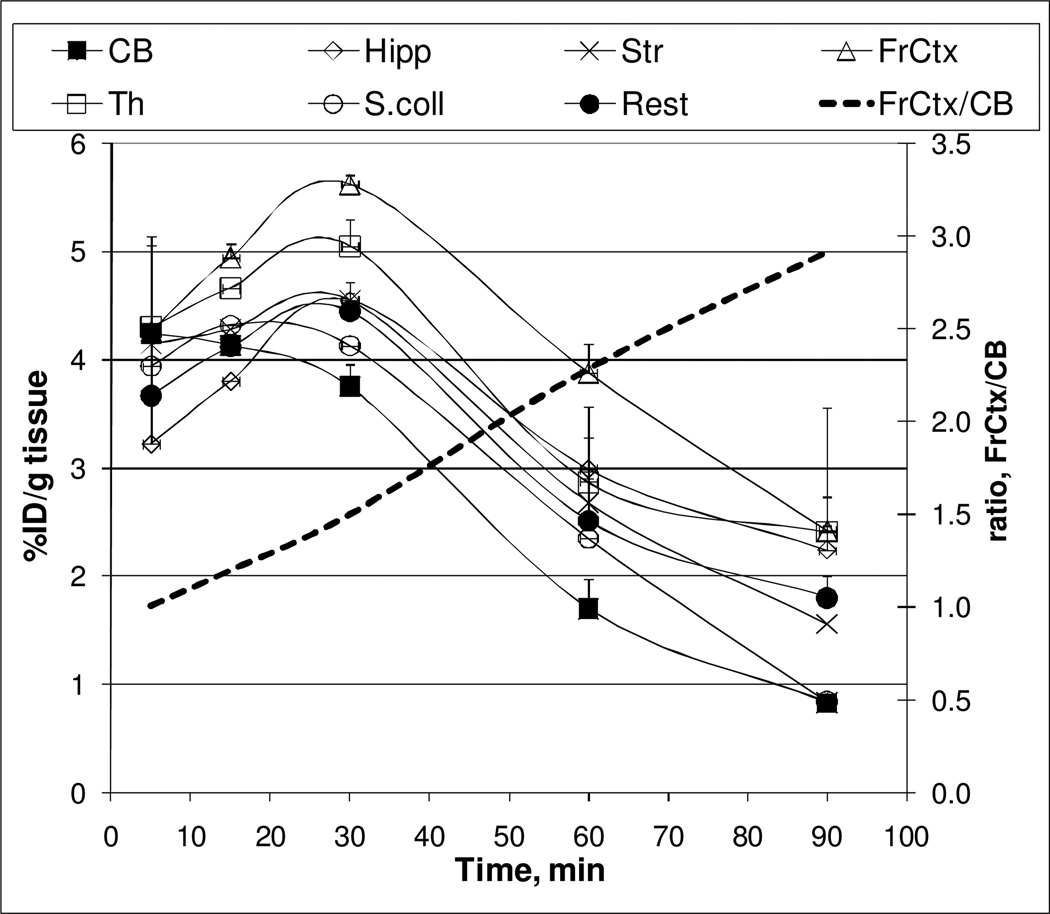
The radioactivity accumulation for [11C]rac-1 was determined as %ID/g tissue in six regions of the CD1 mouse brain after intravenous injection of the radioligand (Figure 2). The highest accumulation of [11C]rac-1 radioactivity was seen in four brain regions: the frontal cortex, thalamus, striatum, and hippocampus with peak at 30 min post-injection. A gradual decline of radioactivity in these regions was observed throughout the rest of the experiment. The rate of uptake and washout of radioactivity in the mouse cerebellum, a region with the lowest concentration of radioactivity, was more rapid. The regional radioactivity concentrations of [11C]rac-1 in the mouse brain agreed with in vitro and in vivo data on the distribution of α7-nAChRs14. The ratios of tissue to cerebellum increased steadily over the observation period, reaching values of 3 in the frontal cortex at 90 min after injection (Figure 2).
Figure 2.
Regional brain distribution of [11C]rac-1 in CD1 mice. CB = cerebellum; Hipp = hippocampus; Str = striatum; FrCtx = frontal cortex; Th = thalamus, S.coll = superior colliculus; Rest = the rest of the brain) in the baseline study. The frontal cortex to cerebellum ratio (----) reached a value of 3 at 90 minutes post i.v. injection.
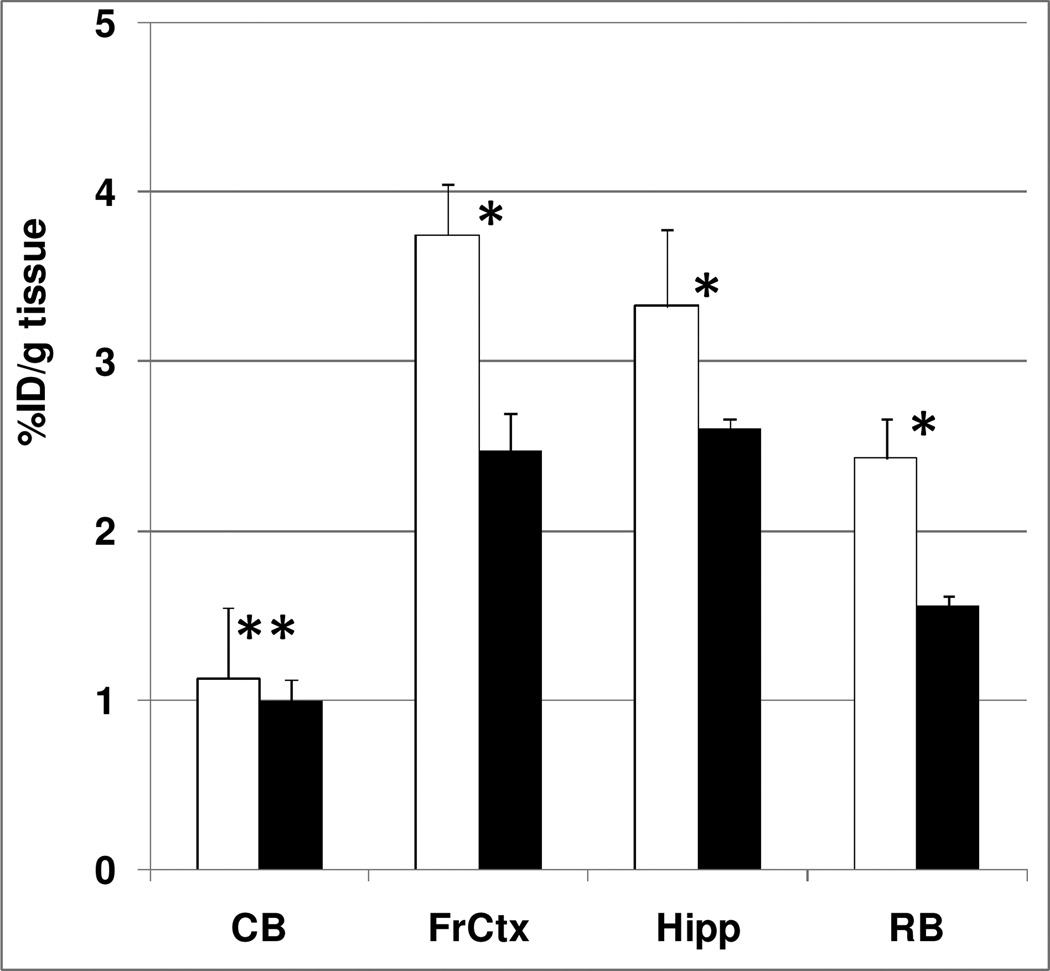
To investigate the specific binding for α7-nAChRs, a blocking study was performed using the selective α7-nAChRs agonist PHA543613.22 A blocking dose of PHA543613 significantly inhibited [11C]rac-1 binding in the hippocampus and frontal cortex suggesting that the binding in these regions is specific and it is mediated by α7-nAChRs. As expected, the blockade in the cerebellum was minimal since the known density of α7-nAChRs is low in this region14 (Figure 3). When the specific binding was estimated by using the radioactivity concentration in the blocked cerebellum as a measure of nonspecific binding, the decrease in the frontal cortex was 48% and the decrease in the hippocampus was 33% (Figure 3).
Figure 3.
At 90 min after injection in controls (open bars) and blocking experiments with the selective α7-nAChRs agonist PHA543613 (0.5 mg/kg, subcutaneous) (black bars), the regional brain uptake of [11C]rac-1 in mice (cerebellum (CB), frontal cortex (FrCtx), hippocampus (Hipp), and the rest of the brain (RB) were compared. There was significant blocking in all regions except cerebellum. Data are mean ± SD. *P < 0.05, significantly different from controls; **P > 0.05, insignificantly different from controls (ANOVA single factor analysis)
The regional brain distribution and blockade results suggest that [11C]rac-1 is a potentially useful radioligand for measuring α7-nAChRs in the mouse brain.
3. Materials and methods
3.1. Chemistry
Most chemicals were purchased from Sigma–Aldrich (Milwaukee, WI) and used without further purification. Compound 2 was obtained from Astatech (Bristol, PA) and PHA543613 hydrochloride was purchased from Tocris. Column flash chromatography was carried out using E. Merck silica gel 60F (230–400 mesh). Analytical thin layer chromatography (TLC) was performed on plastic sheets coated with Silica Gel 60 F254 (0.25 mm thickness, Macherey-Nagel). 1H NMR and 13C NMR (nuclear magnetic resonance) spectra were recorded with a Varian-400 MHz in CDCl3. The spectra were acquired at 293 K. The chemical shifts were recorded on the ppm scale and were referenced to the internal standard trimethylsilane (TMS) at δH 0 ppm. Coupling constant (J) values were given in Hertz. Multiplicity was defined by s (singlet), d (doublet), t (triplet), and m (multiplet).
3.1.1. 3-(6-Chloro-3-pyridinyl)-3,6-diazabicyclo[3.2.0]-heptane-6-carboxylate tert-butyl ester (4)
A mixture of tert-butyl 3,6-diazabicyclo[3.2.0]heptane-6-carboxylate (0.648 g, 3.27 mmol), 5-bromo-2-chloropyridine (0.738 g, 3.83 mmol), tris(dibenzylideneacetone)dipalladium(0) [Pd2(dba)3] (66 mg, 0.072 mmol), racemic 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl (rac-BINAP) (135 mg, 0.22 mmol), sodium tert-butoxide (t-BuONa) (0.367 g, 3.82 mmol) in toluene (3 mL) was heated at 85 °C for 20 h. The mixture was cooled and concentrated under reduced pressure. The crude material was purified by flash chromatography on silica gel (Hexanes/Ethyl acetate 3:1) to give product 4 as pale yellow oil (840 mg, 83%). 1H NMR (CDCl3, 400 MHz) δ 7.86 (d, J=3.2 Hz, 1H), 7.17 (d, J=9.2 Hz, 1H), 7.02 (dd, J=3.2 Hz, 8.8 Hz, 1H), 4.83 (s, 1H), 4.11 (t, J=8 Hz, 1H), 3.98-3.89 (m, 1 H), 3.69-3.62 (m, 2H), 3.24-3.17 (m, 1H), 3.03 (dd, J=6.6 Hz, 10.2 Hz, 1H), 2.90 (dd, J=4.4 Hz, 10.8 Hz, 1H), 1.45 (s, 9H); 13C NMR (CDCl3, 100 MHz) δ 148.6, 144.1, 139.7, 135.2, 124.0, 123.9, 79.9, 65.4, 64.4, 54.5, 53.3, 32.8, 28.5; HRMS calculated for C15H20ClN3O2 [M+H] m/z=310.1322; found, 310.1344.
3.1.2. 3-[6-(1H-indol-5-yl)-3-pyridinyl]-6-Boc-3,6-diazabicyclo[3.2.0]heptane (6)
A mixture of 4 (392 mg, 1.27 mmol), 5 (316 mg, 1.96 mmol) and tetrakis(triphenylphosphine)palladium(0) (150 mg, 0.13 mmol) were dissolved in 1,4-dioxane (12 mL). After 10 min stirring, a solution of sodium carbonate (519 mg, 4.9 mmol) in water (3 mL) was added. The mixture was heated to reflux and allowed to react at 115 °C for 1.5 h. TLC showed the starting material disappeared. After cooling to room temperature, the mixture was poured into a 1:1 mixture of water/ethyl acetate (50:50). The organic layer was separated, and the aqueous layer was further extracted with 30 mL of ethyl acetate twice. The combined organic layers were dried over Na2SO4, filtered, and concentrated to dryness. The residue was purified by silica gel chromatography (Ethyl acetate/Hexanes 1:1 to 1:2) to give product 6 as pale yellow solid (381 mg, 77%). M.p. 121–123 °C; 1H NMR (CDCl3, 400 MHz) δ 8.55 (s, 1H), 8.27 (s, 1H), 8.21 (s, 1H), 7.81 (br s, 1H), 7.72-7.67 (m, 1H), 7.46 (d, J=8 Hz, 1H), 7.24-7.22 (m. 1H), 7.09 (br s, 1H), 6.62–6.61 (m, 1H), 4.85 (s, 1H), 4.13 (t, J=8 Hz, 1H), 4.05 (s, 1H), 3.75 (d, J=10.4 Hz, 1H), 3.69 (dd, J=4.4 Hz, 8.4 Hz, 1H), 3.24-3.18 (m, 1H), 3.07-3.03 (m, 1H), 2.91 (s, 1H), 1.49 (s, 9H). 13C NMR (CDCl3, 100 MHz) δ 148.8, 143.3, 135.8, 131.7, 128.3, 124.9, 121.9, 120.8, 120.5, 118.3, 111.2, 103.1, 79.8, 65.5, 64.5, 54.5, 53.2, 32.8, 28.5; HRMS calculated for C23H26N4O2 [M+H] m/z=391.2134; found, 391.2146.
3.1.3. 3-[6-(1H-indol-5-yl)-3-pyridinyl]-3,6-diazabicyclo[3.2.0]heptane (7)
TFA (5 mL) was added to a solution of 6 (380 mg, 0.97 mmol) in CH2Cl2 (10 mL) at 0 °C. The mixture was stirred at 0 °C for 15 min, then at room temperature for 2 h until TLC showed the starting material disappeared. The solvent was evaporated off. The residue was purified by silica gel chromatography using CH2Cl2/MeOH/NH4OH 9:1:0.1 to give product 7 as pale yellow oil (76 mg, 27%). 1H NMR (CDCl3, 400 MHz) δ 8.27-8.25 (m, 2H), 8.19-8.17 (m, 1H), 7.83 (dd, J=1.6 Hz, 8.4 Hz, 1H), 7.67 (d, J=8.4 Hz, 1H), 7.45 (d, J=8.8 Hz, 1H), 7.22 (t, J=2.8 Hz, 1H), 7.12 (dd, J=3.0 Hz, 9.0 Hz, 1H), 6.61-6.59 (m, 1H), 4.58-4.56 (m, 1H), 3.88 (t, J=8 Hz, 1H), 3.78 (d, J=10 Hz, 1H), 3.72 (d, J=10.8 Hz, 1H), 3.43-3.35 (m, 2H), 3.13-3.03 (m, 2H), 1.77 (br s, 1H); 13C NMR (CDCl3, 100 MHz) δ 143.7, 135.9, 131.9, 128.3, 124.7, 121.7, 120.9, 120.4, 118.3, 111.1, 103.3, 62.2, 57.5, 54.5, 51.0, 37.6; HRMS calculated for C18H18N4, [M+H] m/z=291.1610; found, 291.1628.
3.1.4. 5-(5-(6-methyl-3,6-diazabicyclo[3.2.0]heptan-3-yl)pyridin-2-yl)-1H-indole (rac-1)
To a solution of 7 (75 mg, 0.25 mmol) in 37% HCHO (0.6 mL) and CH3CN (0.6 mL) was added NaBH(OAc)3 (0.11 g, 0.52 mmol). This mixture was stirred at ambient temperature for 4 h and then quenched with NaHCO3 (0.25 g). CHCl3 (15 mL) was added, the layers were separated and the aqueous layer was extracted with CHCl3 (3 × 15 mL). The combined organic layers were dried over Na2SO4, concentrated under reduced pressure and purified via flash column chromatography on silica gel (CH2Cl2/MeOH/NH4OH 15:1:0.1) to give the product rac-1 as yellow oil (22 mg, 31%). 1H NMR (CDCl3, 400 MHz) δ 8.20 (d, J=2.8 Hz, 1H), 8.18-8.16 (m, 1H), 7.83 (dd, J=1.6 Hz, 8.4 Hz, 1H), 7.65 (d, J=8.4 Hz, 1H), 7.44 (d, J=8.8 Hz, 1H), 7.22 (t, J=2.8 Hz, 1H), 7.06 (dd, J=2.8 Hz, 8.4 Hz, 1H), 6.61-6.59 (m, 1H), 3.95-3.92 (m, 1H), 3.80 (dd, J=2 Hz, 10 Hz, 1H), 3.73 (d, J=10.8 Hz, 1H), 3.24-3.37 (m, 3H), 3.17-3.15 (m, 1H), 2.99 (dd, J=4 Hz, 10.8 Hz, 1H), 2.42 (s, 3H), 1.69 (br s, 1H); 13C NMR (CDCl3, 100 MHz) δ 143.2, 135.4, 132.0, 128.3, 124.6, 121.1, 120.9, 120.3, 118.2, 111.1, 103.2, 74.8, 70.2, 59.5, 53.7, 43.1, 33.1; HRMS calculated for C19H20N4, [M+H] m/z=305.1766; found, 305.1785.
3.2. Radiochemistry
The semi-preparative high performance liquid chromatography (HPLC) system consisted of a Waters model 610 pump, a Valco injector, a Varian Prostar 325 LC detector set to 254 nm, a Bioscan Flow-Count PMT radioactivity detector. Analytical HPLC was performed using a Varian Prostar 210 pump with a Prostar 410 Autosampler, a Varian Prostar 325 LC detector set to 254 nm, and a Bioscan Flow-Count PMT radioactivity detector. All HPLC were recorded and analyzed with Varian Galaxie Chromatography Data System software (version 1.9.302.952). A dose calibrator (Capintec 15R) was used for all radioactivity measurements. [11C]Methyl iodide was prepared from 11CO2 using a Tracerlab FX MeI module (General Electric) and a PETtrace biomedical cyclotron (General Electric).
3.2.1. Synthesis of 5-(5-(6-[11C]methyl-3,6-diazabicyclo[3.2.0]heptan-3-yl)pyridin-2-yl)-1H-indole ([11C]rac-1)
Precursor 7 (0.5 mg) was dissolved in 200 µL of anhydrous acetonitrile, capped in a small V-vial and cooled to −40 °C. [11C]Methyl iodide was swept by argon flow into the solution. After the radioactivity reached a plateau, the vial was assayed in the dose calibrator and then heated at 80 °C for 5 min. Water (200 µL) was added and the solution was injected onto the semi-preparative HPLC column (Phenomenex Luna C-18 10 µm column, semi-preparative 10 × 250 mm, 32/68/0.2 v/v/v CH3CN/0.1 M aqueous ammonium formate/Et3N, at a flow-rate of 10 mL/min). The retention time of 7 was 3.92 min. The product [11C]rac-1 peak, having a retention time of 8.85 min, was collected into a flask containing 50 mL water. The mixture was transferred through a Waters C-18 Sep-Pak Plus. The product was eluted with 1 mL ethanol into a vial and diluted with 14 mL of 0.9% saline. The final product [11C]rac-1 was then analyzed by analytical HPLC (Phenomenex Luna C-18 10 µm columns, analytical 4.6 × 250 mm, 32/68/0.2 v/v/v CH3CN/0.1 M aqueous ammonium formate/Et3N, 4 mL/min, tR = 3.6 min) to determine the radiochemical purity (>98%) and the specific radioactivity at the end of synthesis. The total synthesis time was 30 min from EOB with an average radiochemical yield of 30% and an average specific radioactivity of 444 GBq/µmol (non-decay corrected from the end of 11CH3I synthesis).
3.3. In vivo experiments
3.3.1. Baseline dissection studies in mice
CD1 mice (all males, 23–28 g) from the Charles River Laboratories (Wilmington, MA) were used in the animal experiments. The animals were sacrificed by cervical dislocation at various times following injection of [11C]rac-1 (0.1 mCi in 0.2 mL saline, specific radioactivity ~5000 mCi/µmol) into a lateral tail vein. The brains were rapidly removed and dissected on ice. The brain regions of interest were weighed and their radioactivity content was determined in a γ-counter with a counting error below 3%. Aliquots of the injectate were used as standards and their radioactivity content was counted along with the tissue samples. The percent of injected dose per gram of tissue (%ID/g tissue) was calculated. All animal protocols were approved by the Animal Care and Use Committee of the Johns Hopkins University.
3.3.2. Blocking dissection studies in mice
In vivo blocking studies were performed by subcutaneous administration of PHA543613 (0.5 mg/kg) followed 15 min later by an intravenous injection of the radiotracer (3.7 MBq in 0.2 mL saline; specific radioactivity ~185 GBq/µmol). The blocker was dissolved in a vehicle solution (saline/alcohol 9:1) and administered in a volume of 0.1 mL. Control animals were injected with 0.1 mL of the vehicle solution. Ninety minutes after administration of the radiotracer, brain tissues were harvested, and the radioactivity content was determined. There were three animals per time-point in the baseline and blockade cohorts.
4. Conclusion
In summary, 5-(5-(6-[11C]methyl-3,6-diazabicyclo[3.2.0]heptan-3-yl)pyridin-2-yl)-1H-indole ([11C]rac-1), a radioligand with α7-nAChRs binding affinity in the subnanomolar range, has been synthesized. In the mouse brain, [11C]rac-1 specifically accumulated in the frontal cortex and hippocampus, regions with an elevated density of α7-nAChRs, whereas the uptake in cerebellum a region with low densities of α7-nAChRs, was non-specific. The results of cerebral distribution of [11C]rac-1 in CD1 mice suggest that the PET radioligand should be further studied with PET imaging in non-human primates.
Acknowledgments
The authors would like to thank Dr. William H. Bunnelle (Abbott Laboratories) for helpful discussions and Mrs. Judy Buchanan for editorial assistance. This research was supported in part by the Division of Nuclear Medicine of Johns Hopkins University School of Medicine and by NIH Grants DA020777 and MH079017 (A.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Philip NS, Carpenter LL, Tyrka AR, Price LH. Psychopharmacology (Berl) 2010;212:1. doi: 10.1007/s00213-010-1932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrero MB, Bencherif M, Lippiello PM, Lucas R. Pharm. Res. 2011;28:413. doi: 10.1007/s11095-010-0283-7. [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa M, Hashimoto K. Curr. Pharm. Des. 2011;17:121. doi: 10.2174/138161211795049561. [DOI] [PubMed] [Google Scholar]
- 4.Parri HR, Hernandez CM, Dineley KT. Biochem. Pharmacol. 2011;82:931. doi: 10.1016/j.bcp.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 5.Chiamulera C, Fumagalli G. Cent Nerv Syst Agents Med Chem. 2007;7:269. [Google Scholar]
- 6.Hajos M, Rogers BN. Curr. Pharm. Des. 2010;16:538. doi: 10.2174/138161210790361434. [DOI] [PubMed] [Google Scholar]
- 7.Dani JA. Biol. Psychiatry. 2001;49:166. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- 8.Van Kampen M, Selbach K, Schneider R, Schiegel E, Boess F, Schreiber R. Psychopharmacology (Berl) 2004;172:375. doi: 10.1007/s00213-003-1668-7. [DOI] [PubMed] [Google Scholar]
- 9.Li T, Bunnelle WH, Ryther KB, Anderson DJ, Malysz J, Helfrich R, Gronlien JH, Hakerud M, Peters D, Schrimpf MR, Gopalakrishnan M, Ji J. Bioorg. Med. Chem. Lett. 2010;20:3636. doi: 10.1016/j.bmcl.2010.04.105. [DOI] [PubMed] [Google Scholar]
- 10.Bunnelle WH, Tietje KR, Frost JM, Peters D, Ji J, Li T, Scanio MJ, Shi L, Anderson DJ, Dyhring T, Gronlien JH, Ween H, Thorin-Hagene K, Meyer MD. J. Med. Chem. 2009;52:4126. doi: 10.1021/jm900249k. [DOI] [PubMed] [Google Scholar]
- 11.Horti AG, Gao Y, Kuwabara H, Dannals RF. Life Sci. 2010;86:575. doi: 10.1016/j.lfs.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horti AG, Wong DF. PET Clin. 2009;4:89. doi: 10.1016/j.cpet.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horti AG, Villemagne VL. Curr. Pharm. Des. 2006;12:3877. doi: 10.2174/138161206778559605. [DOI] [PubMed] [Google Scholar]
- 14.Toyohara J, Wu J, Hashimoto K. Curr Top Med Chem. 2010;10:1544. doi: 10.2174/156802610793176828. [DOI] [PubMed] [Google Scholar]
- 15.Ettrup A, Mikkelsen JD, Lehel S, Madsen J, Nielsen EØ, Palner M, Timmermann DB, Peters D, Knudsen GM. J. Nucl. Med. 2011;52:1449. doi: 10.2967/jnumed.111.088815. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa M, Nishiyama S, Tsukada H, Hatano K, Fuchigami T, Yamaguchi H, Matsushima Y, Ito K, Magata Y. Nucl. Med. Biol. 2010;37:347. doi: 10.1016/j.nucmedbio.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 17.(a) Deuther-Conrad W, Fischer S, Hiller A, Becker G, Cumming P, Xiong G, Funke U, Sabri O, Peters D, Brust P. Eur J Nucl Med Mol Imaging. 2011;38:1541. doi: 10.1007/s00259-011-1808-y. [DOI] [PubMed] [Google Scholar]; (b) Deuther-Conrad W, Fischer S, Hiller A, Ostergaard Nielsen E, Brunicardi Timmermann D, Steinbach J, Sabri O, Peters D, Brust P. Eur J Nucl Med Mol Imaging. 2009;36:791. doi: 10.1007/s00259-008-1031-7. [DOI] [PubMed] [Google Scholar]
- 18.Basha A, Bunnelle WH, Dart MJ, Gallagher ME, Ji J, Li T, Pace JM, Ryther KB, Tietje KR, Mortell KH, Nersesian DL, Schrimpf MR. 2005/0101602 Al. US Patent. 2005
- 19.Ji J, Bunnelle WH, Bitner RS, Gopalakrishnan M, Li T. 236th ACS National Meeting; Philadelphia, PA, US. 2008. Poster, MEDI-045. [Google Scholar]
- 20.Hartwig JF. Acc. Chem. Res. 1998;31:852. [Google Scholar]
- 21.Muci AR, Buchwald SL. Top. Curr. Chem. 2002;219:131. [Google Scholar]
- 22.Wishka DG, Walker DP, Yates KM, Reitz SC, Jia S, Myers JK, Olson KL, Jacobsen EJ, Wolfe ML, Groppi VE, Hanchar AJ, Thornburgh BA, Cortes-Burgos LA, Wong EHF, Staton BA, Raub TJ, Higdon NR, Wall TM, Hurst RS, Walters RR, Hoffmann WE, Hajós M, Franklin S, Carey G, Gold LH, Cook KK, Sands SB, Zhao SX, Soglia JR, Kalgutkar AS, Arneric SP, Rogers B. J. Med. Chem. 2006;49:4425. doi: 10.1021/jm0602413. [DOI] [PubMed] [Google Scholar]