Abstract
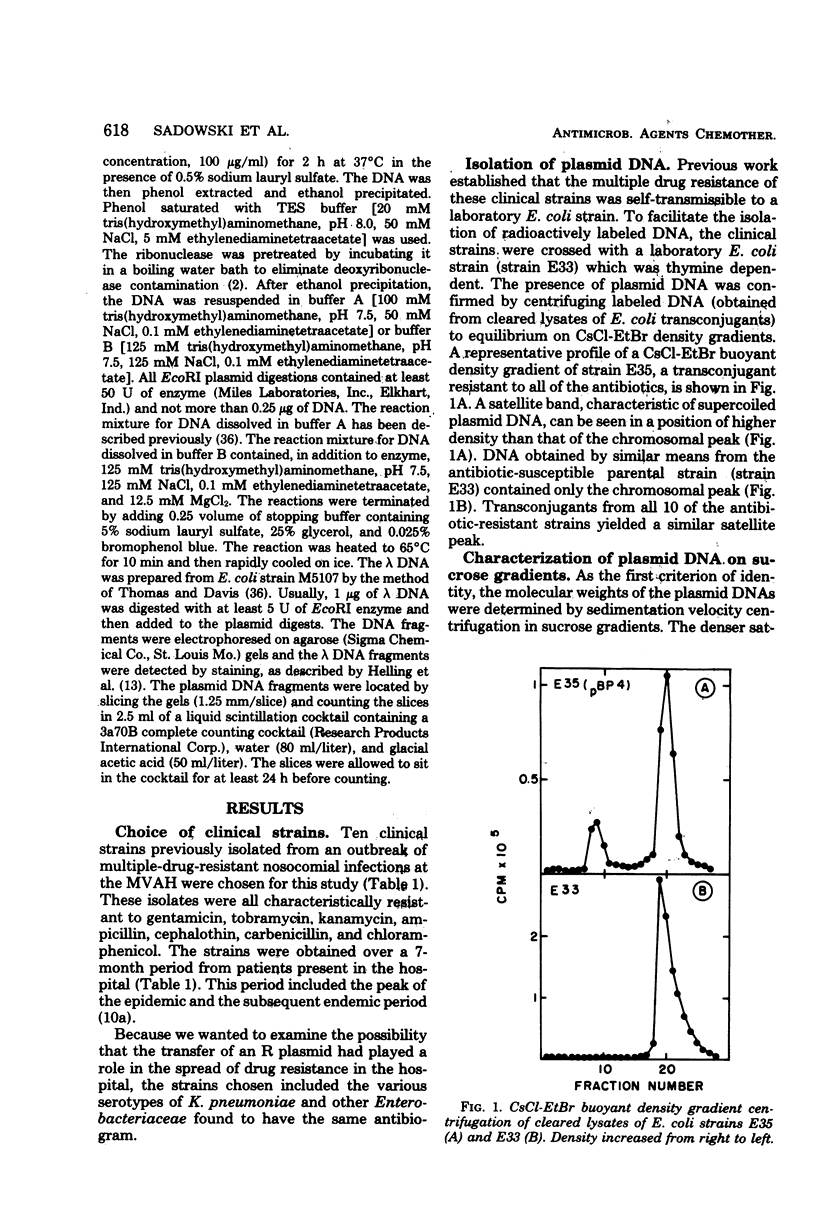
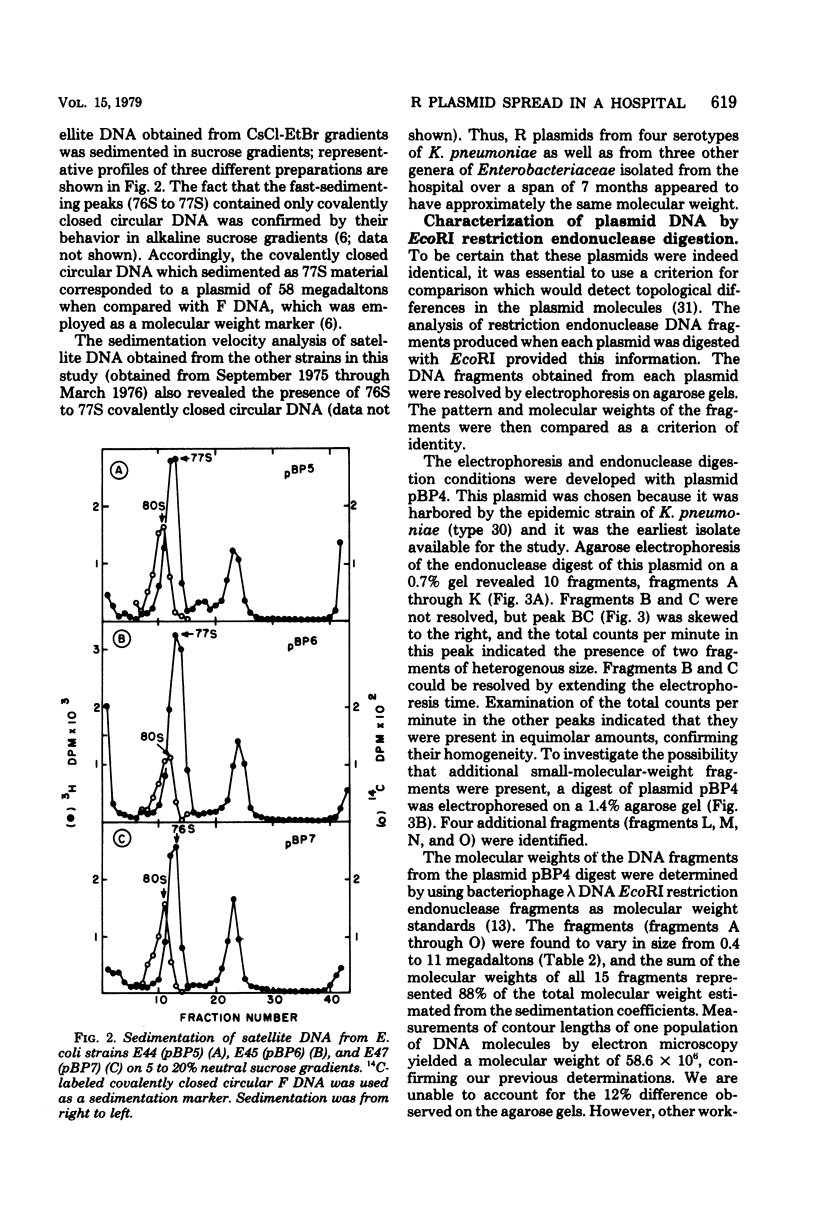
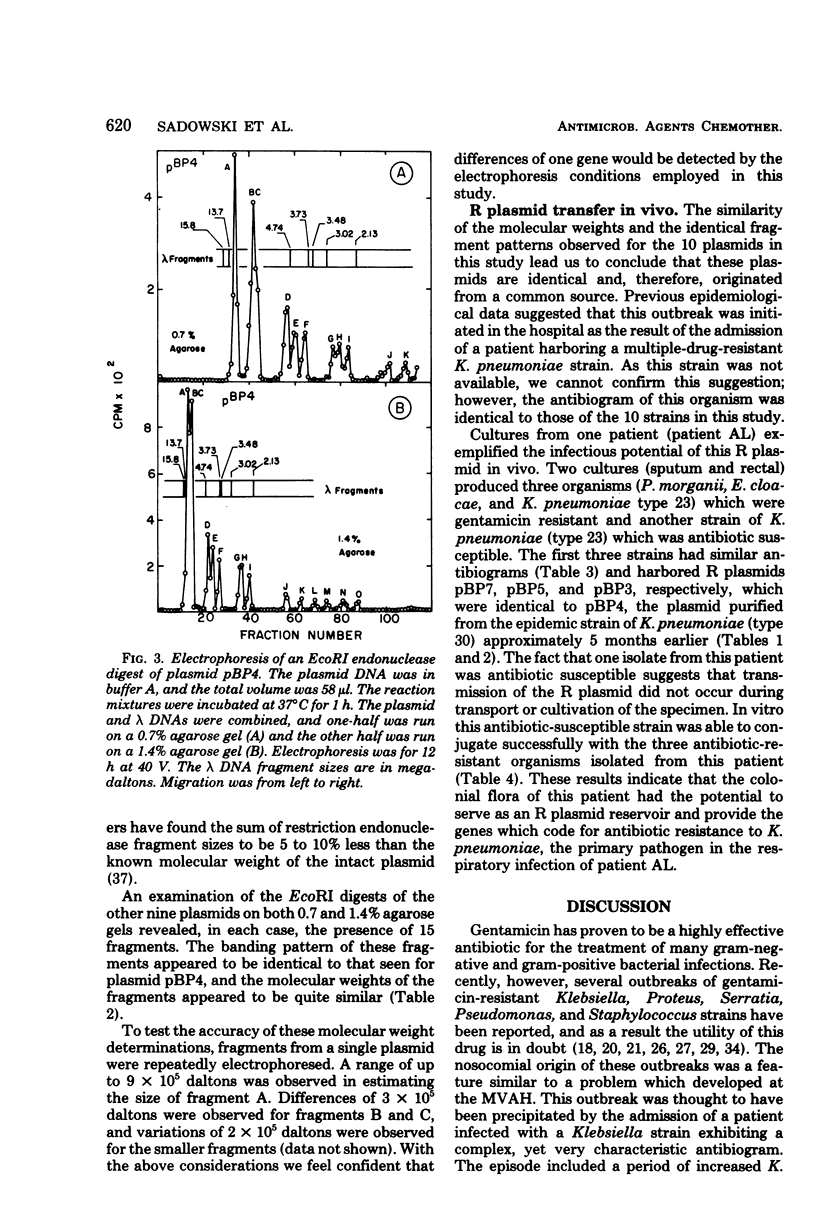
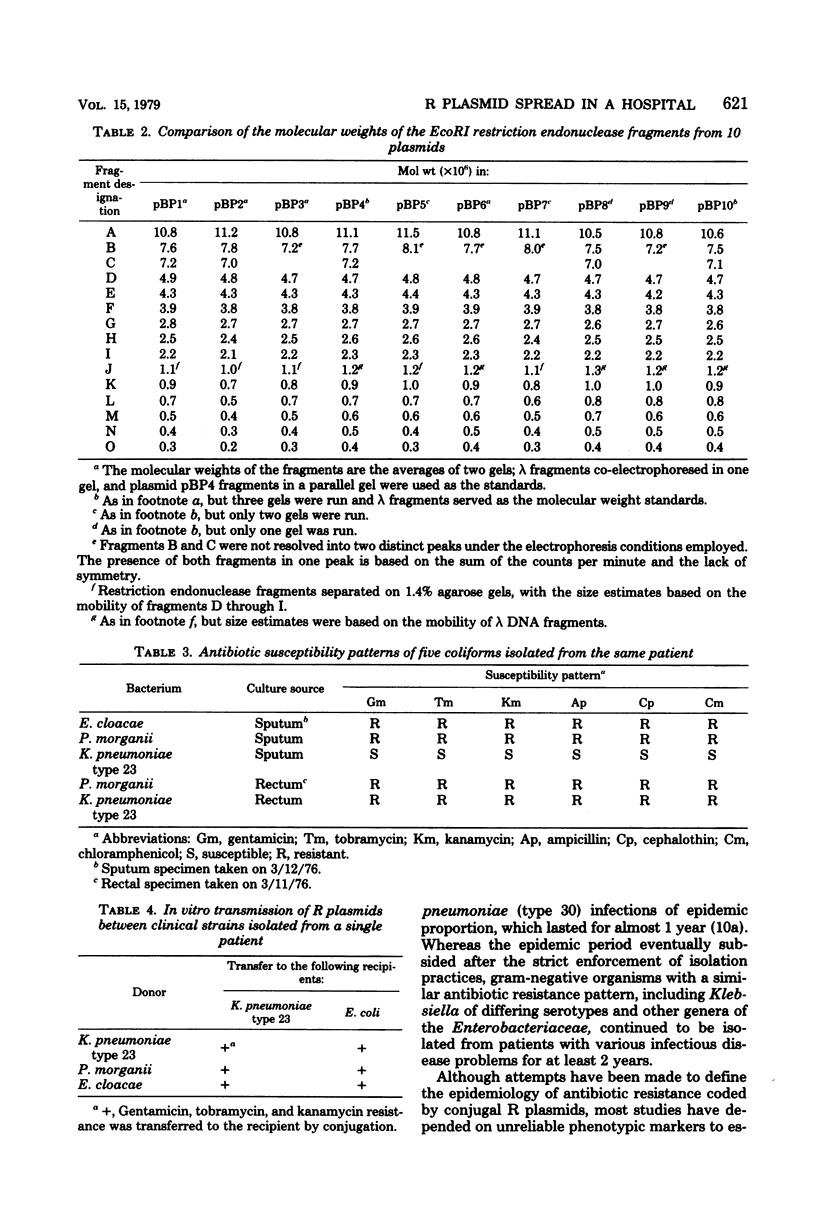
Gentamicin resistance in Klebsiella pneumoniae involved in an outbreak at the Minneapolis Veterans Administration Hospital was due to a transmissible R plasmid. In addition to gentamicin, this plasmid conferred resistance to tobramycin, kanamycin, ampicillin, carbenicillin, cephalothin, chloramphenicol, and sulfathiazole. R plasmids which transferred this complex antibiogram were identified in several clinical isolates, including four different serotypes of K. pneumoniae, Escherichia coli, Enterobacter cloacae, and Proteus morganii. The covalently closed circular form of all R plasmids isolated had a sedimentation coefficient of 76S to 77S, corresponding to a molecular weight of 58 × 106. The possibility that a single R plasmid was responsible for the dissemination of multiple drug resistance among all of these different clinical strains was examined by characterizing the plasmids by using EcoRI restriction endonuclease. The same 15 fragments were obtained from each of the 10 plasmids analyzed. Their molecular weights ranged from 4 × 105 to 11 × 106. Thus, we conclude that each of the 10 plasmids present in the various clinical strains isolated from the hospital over a 7-month period originated from a common source and that R plasmid transfer was important in their spread.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., Sherratt D. J., Clewell D. B., Helinski D. R. Isolation of supercoiled colicinogenic factor E 1 DNA sensitive to ribonuclease and alkali. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2518–2522. doi: 10.1073/pnas.69.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Paper strip method for assaying gradient fractions containing radioactive macromolecules. Anal Biochem. 1971 Oct;43(2):427–432. doi: 10.1016/0003-2697(71)90272-7. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. A minute circular DNA from Escherichia coli 15. Proc Natl Acad Sci U S A. 1968 Jul;60(3):992–999. doi: 10.1073/pnas.60.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N. Drug resistance and R factors in the bowel bacteria of London patients before and after admission to hospital. Br Med J. 1969 May 17;2(5654):407–411. doi: 10.1136/bmj.2.5654.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N. Infection caused by Proteus mirabilis strains with transferrable gentamicin-resistance factors. Lancet. 1975 Jun 21;1(7921):1355–1357. doi: 10.1016/s0140-6736(75)92262-x. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., Inamine J. M., Minshew B. H. Common plasmid specifying tobramycin resistance found in two enteric bacteria isolated from burn patients. Antimicrob Agents Chemother. 1978 Feb;13(2):312–317. doi: 10.1128/aac.13.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P., Smith D. H. Studies on the epidemiology of resistance (R) factors. I. Analysis of Klebsiella isolates in a general hospital. II. A prospective study of R factor transfer in the host. Ann Intern Med. 1969 Jul;71(1):1–9. doi: 10.7326/0003-4819-71-1-1. [DOI] [PubMed] [Google Scholar]
- Gerding D. N., Buxton A. E., Hughes R. A., Cleary P. P., Arbaczawski J., Stamm W. E. Nosocomial multiply resistant Klebsiella pneumoniae: epidemiology of an outbreak of apparent index case origin. Antimicrob Agents Chemother. 1979 Apr;15(4):608–615. doi: 10.1128/aac.15.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinter N. J., Barth P. T. Characterization of SmSu plasmids by restriction endonuclease cleavage and compatibility testing. J Bacteriol. 1976 Oct;128(1):394–400. doi: 10.1128/jb.128.1.394-400.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Transposition of a plasmid deoxyribonucleic acid sequence that mediates ampicillin resistance: identity of laboratory-constructed plasmids and clinical isolates. J Bacteriol. 1977 Jan;129(1):530–533. doi: 10.1128/jb.129.1.530-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. C., Richmond M. H., Sykes R. B. Molecular characterization of the R factors implicated in the carbenicillin resistance of a sequence of Pseudomonas aeruginosa strains isolated from burns. Antimicrob Agents Chemother. 1973 Feb;3(2):279–288. doi: 10.1128/aac.3.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg H. D., Berkman J. I. Prevalence of extrachromosomal drug resistance. The role of drug-resistant and drug-selected bacteria in nosocomial disease. Ann N Y Acad Sci. 1971 Jun 11;182:52–58. doi: 10.1111/j.1749-6632.1971.tb30642.x. [DOI] [PubMed] [Google Scholar]
- Kline B. C., Helinski D. R. F 1 sex factor of Escherichia coli. Size and purification in the form of a strand-specific relaxation complex of supercoiled deoxyribonucleic acid and protein. Biochemistry. 1971 Dec 21;10(26):4975–4980. doi: 10.1021/bi00802a022. [DOI] [PubMed] [Google Scholar]
- Kline B. C. Mechanism and biosynthetic requirements for F plasmid replication in Escherichia coli. Biochemistry. 1974 Jan 1;13(1):139–146. doi: 10.1021/bi00698a022. [DOI] [PubMed] [Google Scholar]
- Korfhagen T. R., Loper J. C., Ferrel J. A. Pseudomonas aeruginosa R factors determining gentamicin plus carbenicillin resistance from patients with urinary tract colonizations. Antimicrob Agents Chemother. 1975 Jan;7(1):64–68. doi: 10.1128/aac.7.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton K. B., Richmond M. H., Bevan R., Gillespie W. A. Antibiotic resistance and R factors in coliform bacilli isolated from hospital and domestic sewage. J Med Microbiol. 1974 Feb;7(1):91–103. doi: 10.1099/00222615-7-1-91. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., HARADA K., HASHIMOTO H. Multiple resistance of enteric bacteria and transmission of drug-resistance to other strain by mixed cultivation. Jpn J Exp Med. 1960 Jun;30:179–184. [PubMed] [Google Scholar]
- Meyer R. D., Lewis R. P., Halter J., White M. Gentamicin-resistant Pseudomonas aeruginosa and Serratia marcescens in a general hospital. Lancet. 1976 Mar 13;1(7959):580–583. doi: 10.1016/s0140-6736(76)90370-6. [DOI] [PubMed] [Google Scholar]
- Moller J. K., Bak A. L., Bülow P., Christiansen C., Christiansen G., Stenderup A. Transferable and non-transferable drug resistance in enteric bacteria from hospital and from general practice. Scand J Infect Dis. 1976;8(2):112–116. doi: 10.3109/inf.1976.8.issue-2.09. [DOI] [PubMed] [Google Scholar]
- Moorhouse E. C. Transferable drug resistance in enterobacteria isolated from urban infants. Br Med J. 1969 May 17;2(5654):405–407. doi: 10.1136/bmj.2.5654.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D., Smith H. O. Restriction endonucleases in the analysis and restructuring of dna molecules. Annu Rev Biochem. 1975;44:273–293. doi: 10.1146/annurev.bi.44.070175.001421. [DOI] [PubMed] [Google Scholar]
- Porthouse A., Brown D. F., Smith R. G., Rogers T. Gentamicin resistance in Staphylococcus aureus. Lancet. 1976 Jan 3;1(7949):20–21. doi: 10.1016/s0140-6736(76)92912-3. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie R. P., Duncan I. B. Emergence of gentamicin-resistant Klebsiella in a general hospital. Antimicrob Agents Chemother. 1977 Feb;11(2):179–184. doi: 10.1128/aac.11.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H. Some environmental consequences of the use of antibiotics: or 'what goes up must come down'. J Appl Bacteriol. 1972 Jun;35(2):155–176. doi: 10.1111/j.1365-2672.1972.tb03687.x. [DOI] [PubMed] [Google Scholar]
- Roe E., Jones R. J., Lowbury E. J. Transfer of anibioic resistanceetween Pseudomonas aeruginosa, Escherichia coli, and other gram-negative bacilli in rns. Lancet. 1971 Jan 23;1(7691):149–152. doi: 10.1016/s0140-6736(71)91930-1. [DOI] [PubMed] [Google Scholar]
- Shaw E. J., Datta N., Jones G., Marr F. M., Froud W. J. Effect of stay in hospital and oral chemotherapy on the antibiotic sensitivity of bowel coliforms. J Hyg (Lond) 1973 Sep;71(3):529–534. doi: 10.1017/s0022172400046519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Thompson R., Hughes S. G., Broda P. Plasmid identification using specific endonucleases. Mol Gen Genet. 1974;133(2):141–149. doi: 10.1007/BF00264835. [DOI] [PubMed] [Google Scholar]


