Abstract
Bombyx mori nucleopolyhedrovirus (BmNPV) that infects the silkworm, B. mori, accounts for >50% of silk cocoon crop losses globally. We speculated that simultaneous targeting of several BmNPV essential genes in transgenic silkworm would elicit a stable defense against the virus. We introduced into the silkworm germline the vectors carrying short sequences of four essential BmNPV genes in tandem, either in sense or antisense or in inverted-repeat arrangement. The transgenic silkworms carrying the inverted repeat-containing transgene showed stable protection against high doses of baculovirus infection. Further, the antiviral trait was incorporated to a commercially productive silkworm strain highly susceptible to BmNPV. This led to combining the high-yielding cocoon and silk traits of the parental commercial strain and a very high level of refractoriness (>75% survival rate as compared to <15% in nontransgenic lines) to baculovirus infection conferred by the transgene. We also observed impaired infectivity of the occlusion bodies derived from the transgenic lines as compared to the wild-type ones. Currently, large-scale exploitation of these transgenic lines is underway to bring about economic transformation of sericulture.
Keywords: BmNPV, piggyBac, RNAi, silkworm, transfer of transgene
RNA interference (RNAi) is a mechanism by which cells silence the expression of foreign genes. This process often provides an adaptive innate immunity against viruses where double-stranded RNAs encoded by the viruses during infection act as pathogen trigger after they are taken up by the cellular RNAi machinery (Wang et al. 2006). Alternatively, this natural defense mechanism is exploited as an antiviral therapy via the artificial inhibition of the expression of essential viral genes (Leonard and Schaffer 2006). Multiple protocols of delivery of dsRNA or of constructs encoding dsRNAs in the organism are currently under assay to combat infections of various viruses. The efficiency of the assays is still challenged by the delicate setting of the proper dosage of the RNAi, the relative longevity of the effect, the occurrence of RNAi driven toxicity, and the virus-intrinsic susceptibility. A few attempts have been made in animal and plant models where the antiviral trait was installed by transgenesis to confer stable protection to the transformed individuals and to their progeny. Successes have been reached in plants (Bucher et al. 2006; Bonfim et al. 2007; Zhang 2010) but, to our knowledge, no case has yet been reported in animals showing a stable and robust protection against a virus after a RNAi-aided antiviral trait was introduced through germline transformation.
The baculovirus, Bombyx mori nucleopolyhedrovirus (BmNPV), is a major pathogen that affects silkworm rearings and hampers silk cocoon production in Asia. In India alone >50% of silk cocoon crop losses are attributed to baculovirus infection (Khurad et al. 2006). Effective treatment against the virus has been elusive due to its sturdy nature and the lack of control strategies. Interestingly, the biology of the virus is reasonably well studied, and its entire genome sequence and gene annotation are unraveled. Baculovirus infectious cycle is characterized by the occurrence of two forms of the virus: the so-called budded viruses (BV) that spread systemic infection within the host and the occlusion-derived viruses (ODV) that are encapsulated within polyhedral inclusion bodies (PIBs/OBs) and spread the baculovirus from host to host through per os infection.
During proliferation in culturo, baculoviruses express genes in a cascade form with early classes of proteins accumulating immediately upon infection, while late and very late classes of proteins appear subsequently (Huh and Weaver 1990a,b; Hoopes and Rohrmann 1991). Transient plasmid-based analyses have led to recognition of several essential genes. Among these, one codes for IE-1, a transregulator that activates a number of late expression factors (encoded by lef genes), another encodes LEF-1, a DNA primase of the replication complex (Mikhailov and Rohrmann 2002), and a third codes for LEF-3, which carries single-stranded DNA-binding capabilities (Evans et al. 1997). All these three genes are critical for the accomplishment of viral DNA replication. Another gene codes for the per os infectivity factor, P74, a conserved ODV structural protein, among baculoviridae, which, though not required for the making of budded viruses, is necessary for oral infectivity and the contamination from individual to individual (Kuzio et al. 1989; Peng et al. 2012).
The availability of a well-established method of transgenesis in Bombyx mori (Royer et al. 2005; Tomita 2011) has already led to the construction of transgenic silkworm lines harboring a dsRNA-encoding transgene targeting the essential viral gene lef-1 (Isobe et al. 2004) or ie-1 (Kanginakudru et al. 2007). Both attempts resulted in moderate but short-lasting effects, which were also reproduced in cultured BmN cells. It is thought that incomplete gene silencing may be the cause of the rapid recovery of viral proliferation.
Targeting simultaneously several viral genes from a single transgene construct is possible since only a few hundred-basepair long double-strand RNA sequence is sufficient to elicit RNA interference in the silkworm (Gandhe et al. 2007; Mrinal and Nagaraju 2010; Shukla and Nagaraju 2010; Terenius et al. 2011). With the aim of installing a strong antiviral trait, we assayed the properties of a transgene carrying four tandem sequences arising from four essential genes of the baculovirus (ie1, lef1, lef3, and p74). The transgene was equipped with silkworm cytoplasmic actin A3 gene promoter, to deliver dsRNA in all tissues and cells. The dsRNA-encoding transgene was shown to confer a high level of baculovirus resistance in transgenic silkworm. Further, the incorporation of the antiviral trait to a commercial productive silkworm strain highly susceptible to baculovirus led to combining the sericultural traits of interest and the transgene-based baculovirus resistance. The following presentation shows that this experimental strategy proved very efficacious to the point that it can elicit stable refractoriness over generations of silkworms to the infection of the virus. Further, our study also demonstrates that the baculovirus-infected transgenic silkworms produce OBs that are far less infective compared to wild-type OBs and, hence, are likely to be less potent in horizontal transmission.
Materials and Methods
Silkworm strains and virus stock
The B. mori strain, Nistari, a nondiapausing, polyvoltine, low-silk-yielding and moderately resistant to baculoviral infection (LD50 ∼4000 OBs/larva), was used in the study. A diapausing, high-yielding silkworm strain, CSR2, highly susceptible to baculoviral infection (LD50 ∼250 OBs/larva) was used as a recurrent parent for the introduction of the transgene from the transgenic Nistari strain. TAFib6, a transgenic Nistari line that expresses nonviral dsRNA, and nontransgenic Nistari and CSR2 strains were maintained as controls. The transgenic lines targeting the BmNPV ie1 gene were used and are referred to as IE 126A, IE 126B, and IE 58E, as already described (Kanginakudru et al. 2007).
The baculovirus used for infection is the wild-type BmNPV that is usually prevalent in farmers’ silkworm rearing house. The OBs were harvested from the infected hemolymph by brief centrifugation at 1000 rpm for 1 min and the pellet was washed in water and suspended in phosphate buffer saline (PBS). The silkworm larvae that were out of third molt were starved for 12 hr before infection. The OBs in 50 µl suspension were spread on to a 1-cm-diameter piece of mulberry leaf at 6000 OBs/leaf piece and fed to each larva. A total of 50 larvae were fed and only those larvae that consumed the entire leaf piece were maintained. The virus-fed larvae were subsequently reared on fresh mulberry leaves and observed for symptoms of viral infection. The dead larvae were removed immediately to prevent spreading of the secondary viral infection. Our initial per os infection experiments showed that 6000 OBs/larva ingested at third instar resulted in ∼100% mortality of CSR2 strain and >50% mortality of Nistari strain (data not shown). We thus used 6000 OBs/larva to infect third instar larva.
Construction of sense, antisense, and inverted repeat vectors
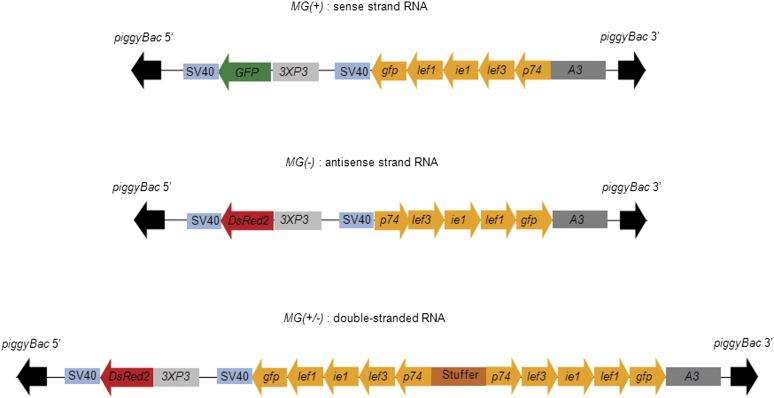
The sense, antisense and inverted-repeat vectors were constructed using a piggyBac vector backbone. The constructs were made in such a way that dsRNA is generated for the four essential viral genes ie1, lef1, lef3, and p74 in the host cells in sense, antisense orientation, or inverted-repeat arrangement under the control of the silkworm cytoplasmic actin A3 gene promoter. The coding sequence of green fluorescent protein (gfp) was also introduced along with the stretches of viral gene sequences, in the same respective orientation. The constructs pPiggyMG(+)3XP3–GFP (sense), pPiggyMG(−)3XP3–DsRed2 (antisense), and pPiggyMG(+/−)3XP3–DsRed2 (inverted repeat) are represented in Figure 1. The targeted viral gene nucleotide sequence information is provided in Supporting Information, File S2. The gene encoding GFP driven by a 3XP3 promoter in the MG(+) vector was used as an eye-color selection marker. Similarly, a DsRed coding sequence downstream of the 3XP3 promoter in the antisense and inverted repeat vectors enabled identification of transgenic individuals by their DsRed eye (Uchino 2006). The various steps involved in the construction of the vectors are schematically represented in Figure S1 and detailed in File S1.
Figure 1 .
Representation of piggybac-inserted sequences in the various silkworm transgenic lines. The transgenes were carried in the vector constructs pPiggyMG(+)3XP3–GFP (9677 bp), pPiggyMG(−)3XP3–DsRed2 (10097 bp), and pPiggyMG(+/−)3XP3–DsRed2 (12422 bp). PiggyBac 5′ and piggyBac 3′ indicate the left and right terminal inverted repeats of the piggyBac element that flank the inserts. Each transgene comprised a segment of four BmNPV genes, namely ie1 (310 bp), lef1 (326 bp), lef3 (316 bp), and p74 (310 bp) and the entire coding sequence of green fluorescent protein (800 bp) in the same relative orientation. SV40 represents a 200-bp fragment carrying the SV40 polyadenylation site. The MultiGene(+) transgene, abbreviated MG(+), consisted of segments of the four targeted viral genes and gfp gene, transcribed as a sense-strand RNA under the control of the silkworm cytoplasmic actin A3 promoter. The marker gene, gfp, under the control of the 3XP3 promoter is expressed in ommatidia, and embryonic nervous tissues facilitates identification of the transgenic individuals. The MG(−) transgene consists of segments of four viral target genes and gfp gene transcribed as an antisense strand RNA with the same A3 gene promoter. The marker gene encoded red fluorescent protein (DsRed). The gfp gene in the MG(+) and the MG(−) transgenes helped to verify the RNAi-aided extinction of GFP accumulation in transgenic hybrid lines that carried both constructs. MG(+/−) transgene harbored a portion of each of the essential baculoviral genes in sense and antisense orientation separated by a 323-bp-long stuffer sequence and encoded a double-strand RNA of the baculovirus partial gene sequences.
Germline transformation of B. mori
Germline transgenesis was carried out essentially as described previously (Tamura et al. 2000; Royer et al. 2005) using Nistari strain. Freshly laid silkworm eggs were micro-injected with 1 µg/µl of any of the three piggyBac-derived constructs and the helper plasmid pHA3PIG in equimolar ratio. Eggs were incubated at 25° in humidified chambers for 10 days until hatching. The transgenic lines obtained by using pPiggyMG(+)3XP3GFP, pPiggyMG(−)3XP3–DsRed2, and pPiggyMG(+/−)3XP3–DsRed2 are hereafter referred to as MG(+) (sense), MG(−) (antisense), and MG(+/−) (inverted repeat), respectively.
Generation of G0 and G1 broods
The G0 larvae from the injected eggs were reared on fresh mulberry leaves under standard rearing conditions. G0 adults originating from injected eggs were mated among themselves and the G1 progeny was screened under a fluorescence stereomicroscope (Leica MZFLIII) for the expression in ommatidia of either GFP or DsRed as described previously (Thomas et al. 2002). Data on the number of eggs injected and the final transgenics obtained with the three constructs were collected. G1 adults were sib-mated to obtain G2 offspring.
Generation of homozygous lines and F1 hybrids
Homozygous silkworm lines carrying a single insertion were generated for each transgenic group by sib-mating for three generations. Based on the intensities of the selection markers, lines from each group were selected for making F1 hybrids or for testing their antiviral property. Crosses were also made between MG(+) and MG(−) transgenic lines, which also express GFP in sense and antisense orientation, to bring together sense- and antisense-producing loci in the hybrid offspring.
Viral inoculation and mortality analyses in transgenic silkworms and their hybrids
For per os viral infection, OBs were spread onto fresh leaf pieces as described above. One hundred day 1 third-instar larvae of each of the Nistari transgenic lines, MG(+), MG(−), MG(+/−) and MG(+) × MG(−), along with control lines, were fed with OBs and only the larvae that consumed all the leaf pieces were retained and reared on fresh mulberry leaves until the end of larval life. BmNPV infection was triggered per os using 6000 OBs/larva. Mortality of the silkworm larvae that ingested OBs was estimated by counting the number of dead ones until the time of eclosion of moths from the infected batches. Larvae of single viral gene target transgenic lines (IE 126A, 126B, 58E), nonviral target transgenic line, TAFib6, and nontransgenic strains, Nistari and CSR2, as well as their hybrids (Nistari × CSR2 and TAFib6 × CSR2), were used as controls.
Viral titer determination and viral DNA quantification in the infected transgenic and the nontransgenic lines
Transgenic lines as well as the control lines were infected with BmNPV as mentioned above. For each line, 100 larva of day 1 of third instar were infected individually with 6000 OBs/larva. The larvae that completely consumed the OBs were further reared until eclosion of the moths. Hemolymph was collected from each larva and the number of OBs were counted using Neubauer hemocytometer. The baculovirus accumulation was measured at day 4 of the fifth-instar larvae as the concentration of the OBs in the hemolymph of the infected larvae.
The virus accumulation level was also measured by viral DNA quantification. Briefly, the viral DNA was isolated from the hemolymph collected from five larvae each of the BmNPV-infected transgenic lines as well as their nontransgenic lines using DNeasy kit (Qiagen). The 25 µl qPCR reaction mixture included 20 ng DNA, 12.5 µl of 2× SYBER Green Master Mix (Applied Biosystems), 5 µM of viral lef3 gene primers, and nuclease-free water to make up the total volume. The PCR was performed on a RT-7500 system (Applied Biosystems) with the initial denaturation for 2 min at 50° and 10 min at 95° followed by 40 cycles at 95° for 15 sec and 60° for 1 min. Each of the reactions was performed three times independently, in duplicate, and the results were normalized against 18S rRNA gene of B. mori. The lef3 primers used for amplification of viral DNA do not overlap with the hairpin of lef3 in the transgenic vector. The viral primer sequences used are provided in Table S1. The qPCR data were analyzed by using RT-7500 system software and plotted by using standard delta delta-Ct (ddCt) method.
Western blot analysis by using antibodies against GP64
Total pupal protein was isolated from IE, MG(+/−), MG(+), and MG(−) transgenic and control (Nistari, CSR2, TAFib6) lines infected with 6000 OBs/larva and western blotting was carried out as described previously (Kanginakudru et al. 2007). We incubated the membrane with primary antibody GP64 and secondary antibody conjugated to horseradish peroxidase. The primary antibody against GP64 coat protein of baculovirus was obtained from Novagen (Millipore, India). This antibody was found to cross-react with the GP64 of BmNPV. After addition of HRP substrate, the chemiluminescent signal was detected with X-ray film. The same membrane was stripped and reused for detection of α-tubulin as a loading control. The intensity of bands was quantified using QuantJ software. Each band density was first normalized by dividing it by the density of the α-tubulin band in the same lane and expressed as fold change.
Chromosomal localization of transgene in the transgenic lines
The site of insertion of the transgenes in the MG(+/−) lines was determined using transposable element display (TED) method following the modified protocol of van der Linden and Plasterk (2004). This method was used to target the left arm of the piggyBac transposon. The expected size of the amplicon after the second-round PCR was >120 bp, which comprises the transgene portion and the genomic DNA of unknown length. The PCR product from the second amplification step was used for sequencing. Formation of a single PCR amplification product after the second PCR reaction showed the presence of single-copy insertion of the transgene in the MG(+/−) lines (Figure S2).
We examined the genomic sequence at the insertion sites to verify whether the insertion of the transgenes was piggyBac transposon mediated. For all insertions, the piggyBac inverted terminal repeats were recovered and found to be bordered by the characteristic piggyBac target TTAA sequence (File S3). The location of insertion was further confirmed by the PCR product sequences, which were blasted against the complete genome sequence of B. mori, and the relative position of the transgene on the chromosome was determined for each of the transgenic lines (Figure S3 and Table S3).
Introduction of pPiggyMG(+/−)3XP3-DsRed2 to the high-yielding commercial strain, CSR2 through recurrent backcross strategy
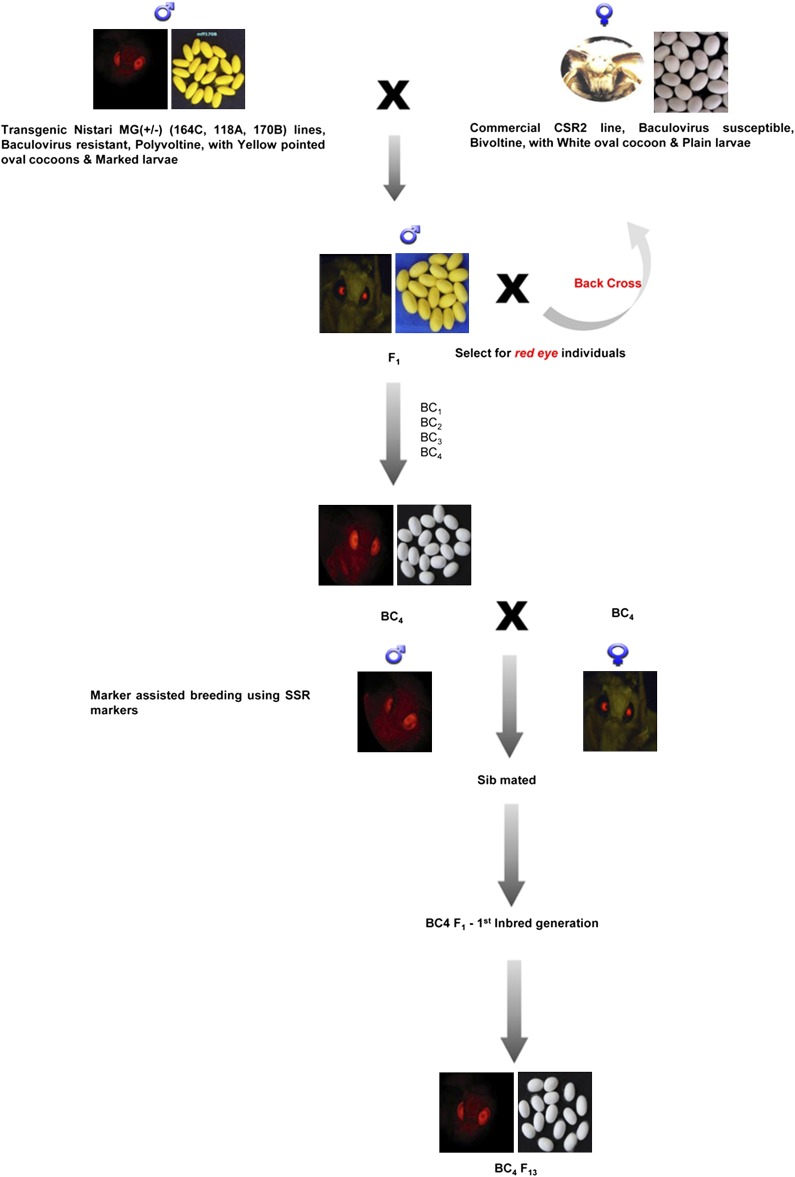
The transgene from the Nistari genetic background was transferred to baculovirus-susceptible and high-yielding commercial strain, CSR2, by recurrent backcrosses followed by inbreeding (Figure 5). Males from three MG(+/−) Nistari transgenic lines were crossed to CSR2 nontransgenic line to raise F1 hybrids. For each of the crosses, two replications were made (a replication refers to the whole silkworm population derived from eggs of a mated female moth). The F1 offspring (males) were backcrossed with the recurrent parent, CSR2, female to generate BC1 population. The performance of BC1 generation was recorded under normal and BmNPV inoculated conditions. The backcross offspring that carried the transgene as indicated by DsRed eye color were selected as donors of the antiviral trait, for further breeding. BC1 generation was carried forward to BC4 on the basis of survival rate upon BmNPV infection as well as on the basis of DsRed eye and other cocoon characteristics of the recurrent parent. BC4 males and females carrying the transgene were sib-mated and advanced to BC4F13.
Figure 5 .
Schematic showing introduction of transgene (dsRNA for multiple essential baculoviral genes) from the transgenic Nistari lines to high-yielding CSR2 strain.
Marker-assisted selection
DNA isolation from MG(+/−) transgenic moths was carried out as mentioned above. A total of ten simple sequence repeats (SSRs) that were polymorphic between donor (Nistari) and recurrent (CSR2) parents were employed to screen the backcross offspring at each generation. Only those offspring that carried CSR2-specific alleles and DsRed eye were selected as parents of the next generation. DNA samples from BC4F6 were analyzed by PCR using primers specific for the 10 SSR markers and the number of CSR2-specific alleles across all the SSR loci was scored to estimate the extent of CSR2 genome in the offspring. The PCR conditions for each of the 10 SSR loci along with the flanking primer sequences are given in Table S6.
Cocoon and silk characters
The transgenic, backcross, and control lines were studied for the silk yield characters: (1) cocoon weight (in grams), (2) cocoon shell weight (in grams), and (3) cocoon shell ratio (the ratio of cocoon shell weight to the cocoon weight expressed in percentage). The silk technological characters, namely, silk filament length (meters) and raw silk (percentage), were obtained from the silk-reeling properties of cocoons measured using standard procedures.
Comparison of the infectivity of OBs generated in transgenic and nontransgenic lines
We chose the transgenic CSR2 (164C) line and the nontransgenic CSR2 and infected with the OBs derived from wild-type BmNPV. The OBs were then harvested separately from the infected transgenic and nontransgenic larvae at fifth instar before pupation of the larvae. The two types of OBs were administered per os separately to transgenic as well as nontransgenic CSR2 larvae. Eight doses of 1000, 2000, 4000, 6000, 8000, 10,000, 20,000, and 40,000 were made from a stock of 4 × 106 OBs/ml for infection studies. A 1-cm2 piece of mulberry leaf was smeared with the suspension of OBs of specified dose at 10 µl per piece and infected per os to each larva in a paper boat on day 1 of the fourth instar. A total of 30 larvae were used for each dosage. The larval mortality was recorded from day 1 of postinfection until pupation. The percentage mortality values were converted to probit values by reading the corresponding probit units from the probit table. The probit values were plotted against log doses, and the LD50 values, standard error, and fiducial limits were calculated with the help of GraphPad Prism 6.
Statistical analyses
The transgenic lines were reared with appropriate controls from the year 2007 to 2011. The data presented is an average of two replications with 50–500 larvae per replicate. To improve homogeneity of variances, the data on the percentage mortality and viral proliferation count were subjected to arcsin or logarithmic transformations, respectively. A two-way analysis of variance (ANOVA) was used to estimate variation sources as well as their statistical significance between control and BmNPV inoculated groups using StatPlus 2007 Professional v. 4.9. The pairwise comparisons for various parameters among groups was performed after post-hoc analysis using Tukey’s multiple comparison test of the corresponding treatment factor using main effects in a one-way ANOVA. The significance of each group was calculated as a P-value. The difference with P < 0.05 was considered significant.
Results
Establishment of transgenic silkworm lines with potent antiviral traits
We introduced transgenes that carried concatenated sequences from the four baculovirus genes ie1, lef1, lef2, and p74. MG(+) and MG(−) refer to transgenes expressing the sense and the antisense strands, while MG(+/−) encodes double-strand RNA of the viral gene sequences. Germline transgenesis was carried out as described earlier (Tamura et al. 2000; Royer et al. 2005) by co-injecting a piggyBac vector and helper plasmid to freshly laid eggs of the Nistari strain (Table S2). Based on the higher intensity of the eye color three MG(+) (2G, 58L, and 244E), two MG(−) (126C and 239A), and eight MG(+/−) (118A, 118B, 154C, 154D, 164B, 164C, 170A, and 170B) transgenic lines were retained. Accurate chromosomal insertion sites were mapped for the MG(+/−) lines (see Materials and Methods).
Crosses were also made between the three MG(+) and the two MG(−) transgenic lines to drive the expression of the sense and antisense strands in the same cells. A total of 18 homozygous, single insertion lines were constructed by sib-mating for three successive generations. Together with the four viral gene sequences, the transgenes carried an entire GFP coding sequence in the same orientation (Figure 1). This drove the MG(+) lines to express an intense GFP fluorescence in the intestine cells driven by the cytoplasmic actin A3 gene promoter. This fluorescence was abolished in the MG(+) × MG(−) hybrids, reflecting the effectiveness of the RNAi process in the transgenic silkworm lines.
BmNPV challenges show reduced mortality in transgenics
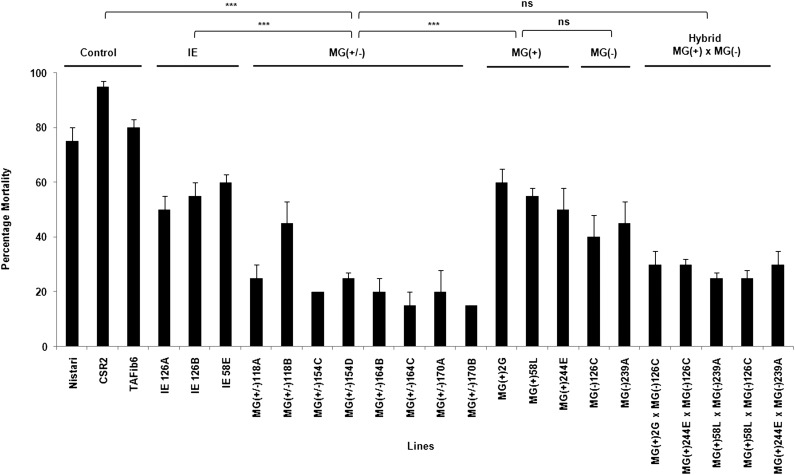
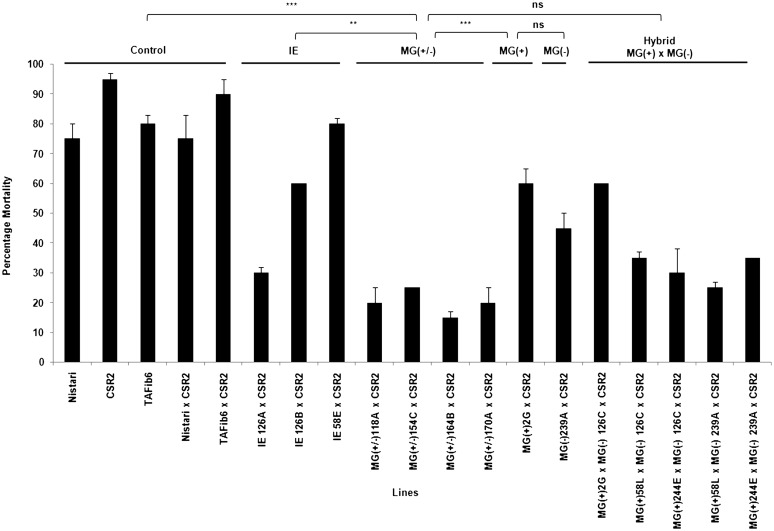
We analyzed the baculovirus infectivity by monitoring the mortality of the infected larvae. Figure 2 shows the virus effects on the mortality according to the status, transgenic or not, of the tested lines. A two-way ANOVA revealed highly significant differences between control and BmNPV treatment (P < 0.0001) as well as between the different groups of transgenic and nontransgenic lines (P < 0.0001) (Table 1). Significant treatment × lines interaction demonstrated that the lines responded differently under BmNPV-inoculated conditions. We observed the highest mortality in control lines, Nistari (75%) and CSR2 (95%), while the MG (+/−) lines, on an average, showed only ∼20% mortality upon viral inoculation. The pairwise comparisons revealed that the mortality rate after viral infection in MG(+/−) lines was significantly less than IE (P < 0.0001), MG(+) (P < 0.0001), and MG(−) (P < 0.0008) lines (Table S4). We found no difference in the survivability upon viral infection between MG(+/−) and MG(+) × MG(-) lines (P < 0.0848). In comparison to transgenic lines targeting single viral gene previously generated by us (Kanginakudru et al. 2007), all the multigene transgenics recorded significantly (P < 0.0001) higher survivability against viral infection indicating potential benefit of multigene targeting.
Figure 2 .
Transgenics show reduced mortality upon BmNPV infection. The histogram shows percentage mortality in the transgenic Nistari, nontransgenic, and control lines. TAFib6, transgenic line that expresses nontarget dsRNA; IE, transgenic line with single viral gene target; MG(+), transgenic line with segments of four viral target genes transcribed in sense orientation; MG(−), transgenic line with segments of four viral genes transcribed in antisense orientation; MG (+/−), transgenic line with four essential baculoviral target genes expressing as a stem-loop structure to induce RNAi; and MG(+) × MG(-), transgenic lines obtained by crossing MG(+) and MG(−) lines. Third-instar larvae were infected per os at a dose of 6000 OBs/larva. Mortality was scored as number of individuals succumbed to viral infection by counting the live moths eclosed from the infected larvae. Mortality was reduced significantly in lines having the multiviral genes. Bar indicates SD, N = 48. (***) P < 0.001; ns, not significant.
Table 1 . A two-way ANOVA for the percentage mortality of the transgenic and control lines under normal and BmNPV infected conditions.
| Source of variation | Sum of squares | d.f. | Mean squares | F ratio | P |
|---|---|---|---|---|---|
| Lines | 4738.72 | 5 | 947.74 | 41.25*** | 0.0001 |
| Treatments | 14120.53 | 1 | 14120.53 | 614.67*** | 0.0001 |
| Lines × treatments | 3285.97 | 5 | 657.19 | 28.60*** | 0.0001 |
| Within | 1929.68 | 84 | 22.97 | ||
| Total | 24074.92 | 95 |
A two-way analysis of variance for the percentage mortality of the IE, MG(+), MG(−), MG(±), MG(+) × MG(−) and control lines under normal and BmNPV infected conditions. Degrees of freedom (d.f.) for each source of variation, treatment, lines, and interactions between these is shown with sum of squares. The average numbers of three experiments, N = 96. ***P < 0.001.
Viral accumulation is reduced in multigene transgenic lines
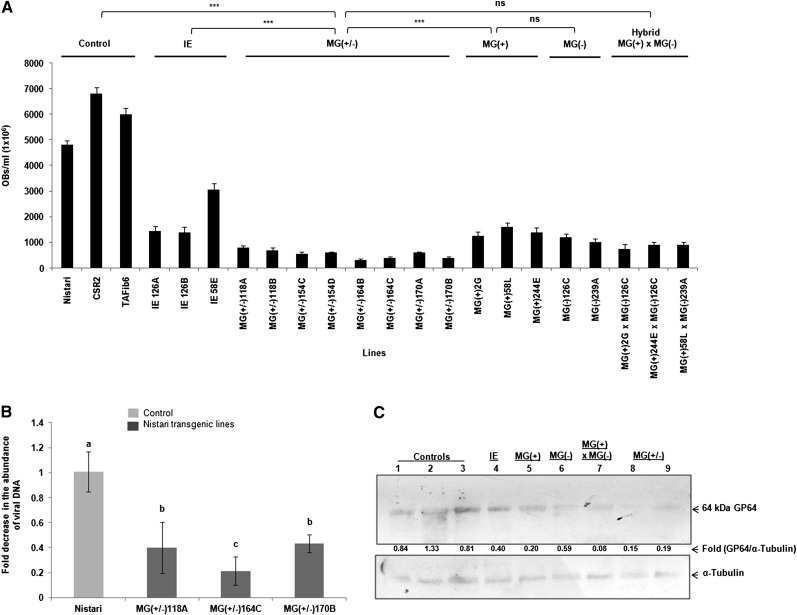
The baculovirus accumulation was compared among the transgenic lines. Results of one-way ANOVA showed significant differences (P < 0.0001) in viral accumulation among BmNPV-inoculated transgenic lines (Table 2). Of all the transgenics, MG(+/−) lines showed lowest viral accumulation and differed significantly from IE (P < 0.0001), MG(+) (P < 0.0001) and MG(−) (P < 0.0029) lines (Figure 3A and Table S5). The viral accumulation in MG(+/−) lines was comparable to MG(+) × MG(−) (P < 0.6732) lines.
Table 2 . A one-way ANOVA for the rate of viral proliferation of the transgenic and control lines under BmNPV-infected conditions.
| Source of variation | Sum of squares | d.f. | Mean squares | F ratio | P |
|---|---|---|---|---|---|
| Between lines | 5.61 | 5 | 1.12 | 43.81*** | 0.0001 |
| Within lines | 1.08 | 42 | 0.03 |
A one-way analysis of variance for the rate of viral proliferation of the IE, MG(+), MG(−), MG(±), MG(+) × MG(−) and control lines under BmNPV-infected conditions. Degrees of freedom (d.f.) and sum of squares for each source of variation is shown. N = 48. ***P < 0.001.
Figure 3 .
Viral accumulation as measured by OBs titer in transgenic and nontransgenic lines. (A) Virus load was determined by scoring the OBs from hemolymph of BmNPV-infected larvae. The number of OBs is much lower in transgenic lines in comparison to nontransgenic control lines. Bar indicates SD, N = 36. (***) P < 0.001; ns, not significant. (B) Quantitative PCR analysis of lef3 gene of BmNPV to determine the viral load in the hemolymph of infected transgenic and nontransgenic larvae. Three independent experiments were carried out in duplicates, with a set of five larvae and the results were normalized against endogenous 18S rRNA gene. The bars denoted by different letters differ significantly (P < 0.001). (C) Western blot showing reduced expression of viral coat protein in transgenics compared to nontransgenic moths. Western analysis was performed using anti-GP64 antibody. Lane 1, control CSR2 line; lane 2, TAFib, a transgenic line that expresses nontarget dsRNA; lane 3, control Nistari line; lane 4, transgenic line (IE 126A) with single viral gene target; lane 5, transgenic line MG(+)58L with segments of four viral target genes transcribed in sense orientation; lane 6, transgenic line MG(−)126C with segments of four viral genes transcribed in antisense orientation; lane 7, transgenic hybrid line MG(+)58L × MG(−)126C obtained by crossing MG (+) and MG (−) lines; lanes 8 and 9, transgenic lines MG(+/−) (164C and 170A) lines with four essential baculoviral target genes expressing in sense and antisense orientation.
The viral titer was determined by quantifying the viral DNA levels using the lef3 gene of BmNPV. The viral load, as determined by qPCR, showed significant (P < 0.001) reduction in the Nistari transgenic lines (118A, 170B, and 164C) as compared to Nistari nontransgenic lines. Overall, there was >2.5-fold decrease in viral load in the Nistari transgenics as compared to the nontransgenic line (Figure 3B).
Reduction in viral proliferation was further confirmed by Western blot experiments using antibody against GP64, an envelope fusion protein of budded viruses required for cell-to-cell transmission of infection (Monsma et al. 1996). Western blot experiments demonstrated that the accumulation of GP64 was lowest in the MG(+/−) lines as compared to all other transgenic or nontransgenic lines (Figure 3C). Taken together, our results confirm that the viral accumulation is significantly reduced in the transgenic lines in comparison to control lines and that the MG(+/−) lines are the most robust lines that could resist baculoviral infection.
Viral resistance in the hybrids of transgenic and nontransgenic lines
Crossbreeding is extensively used as a means of exploiting heterosis in the silkworm, and only hybrids are reared for large-scale silk production (Nagaraju et al. 1996). To test whether the protective effect of transgenic lines could be observed in the hybrids of transgenic and nontransgenic lines, we infected hybrids with BmNPV. Results of two-way ANOVA revealed significant difference (P < 0.0001) between infected and control lines as well as between transgenic and nontransgenic F1 hybrids. The ability of the transgene to suppress mortality was higher in hybrids made with MG(+/−) lines (Figure 4). The pairwise comparisons revealed that the CSR2 hybrids with MG(+/−) showed higher survivability and differed significantly from IE (P < 0.0017), MG(+) (P < 0.0001), and MG(−) (P < 0.0018) lines (Table S4). There was no difference in the survivability upon viral infection between CSR2 hybrids of MG(+/−) and MG(+) × MG(−) lines (P < 0.0758). Our data support that the RNAi-mediated suppression of baculoviral infection could be achieved in the hybrids involving transgenic lines MG(+/−) or MG(+) × MG(−) as one of the parental lines.
Figure 4 .
Histogram showing percentage mortality in the hybrids of transgenic Nistari and nontransgenic CSR2 lines. TAFib6, transgenic line that expresses nontarget dsRNA; IE, transgenic line with single viral gene target; MG(+), transgenic lines with segments of four viral target genes transcribed in sense orientation; MG(−), transgenic lines with segments of four viral genes transcribed in antisense orientation; MG(+/−), transgenic line with four essential baculoviral target genes expressing as a stem-loop structure to induce RNAi; and MG(+) × MG (−) transgenic lines obtained by crossing MG(+) and MG(−) lines. Third-instar larvae were infected per os at a dose of 6000 OBs/larva. Mortality was scored as number of moths succumbed to viral infection by counting the live moths eclosed from the infected larvae. Mortality was reduced significantly in lines having the multiviral genes. Bar indicates SD, N = 48. (**) P < 0.01; (***) P < 0.001; ns, not significant.
Transfer of transgenes from Nistari genetic background to nontransgenic CSR2 strain
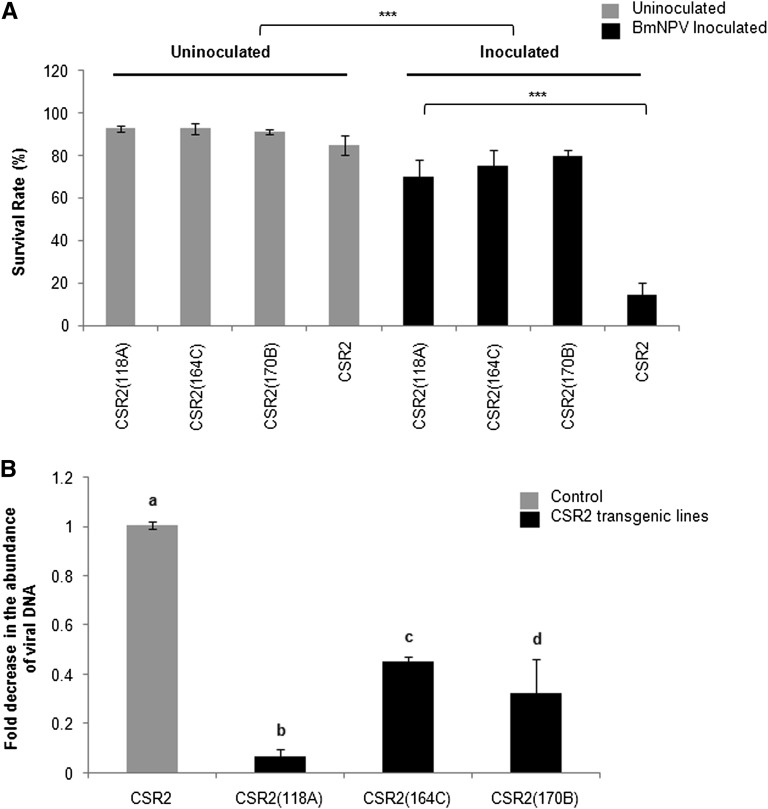
We aimed at combining the RNAi-aided antiviral trait with high-yield characteristics of strains used in sericulture. We selected the strain CSR2, which is currently being exploited in Indian sericulture for its high silk productive values, but highly susceptible to baculovirus infection. We introduced the multigene inverted-repeat transgene from the Nistari genetic background into the CSR2 line by recurrent backcross strategy followed by inbreeding as shown in Figure 5. A total of four backcrosses were made followed by 13 generations of sib-mating. The CSR2 line carrying the transgene showed significantly lower mortality (<15%) as compared to >75% mortality observed in nontransgenic CSR2, when administered with OBs per orally (Figure 6A). Consistent with this result, the viral DNA, isolated from the larvae infected with BmNPV and quantified by qPCR using primers for viral lef3 gene, showed significant (P < 0.001) reduction in the CSR2 transgenic lines (118A, 170B, and 164C) as compared to the nontransgenic lines. Overall, there was more than threefold reduction in viral load in the CSR2 transgenic lines as compared with the nontransgenic lines (Figure 6B).
Figure 6 .
Performance of CSR2 transgenic MG(+/−) (118A, 164C, and 170B) lines obtained from recurrent backcross population (BC4F13 batch). (A) Survival rate as compared to BmNPV-infected lines. Statistical significance (P) was calculated by Student’s t-test. Bar indicates SD, N = 24. (***) P < 0.001. (B) Quantitative PCR analysis of BmNPV using lef3 as a target gene to determine the viral load in the CSR2 transgenic and nontransgenic lines infected with 6000 OBs/larva. Three independent experiments were carried out in duplicates with a set of five larvae each, and the results were normalized against endogenous 18S rRNA gene. The bars denoted by different letters differ significantly (P < 0.001).
In addition to the transgene marker (DsRed eye color), 10 SSR markers located on different chromosomes polymorphic between Nistari transgenic line and CSR2 line were employed at each generation to select backcross offspring that carried CSR2-specific allele. Results show that in the BC4F6 progeny, 86.7, 86.9, and 81.2% of CSR2 genome was found to be incorporated in MG(+/−)118A, MG(+/−)164C, and MG(+/−)170B lines, respectively, as estimated by CSR2-specific SSR alleles at 10 loci (Figure S4 and Table S6).
Cocoon and silk properties in the transgene incorporated CSR2 lines
The cocoon and silk characters such as cocoon weight, cocoon shell weight, cocoon shell ratio, filament length, and raw silk percentage were measured in the CSR2 transgenic lines obtained through recurrent backcrosses coupled with selection for the transgene and compared with that of the recurrent parent, nontransgenic CSR2. The silk characters of the progeny at BC4F13 generation are given in Table 3. These results suggest that the CSR2 line incorporated with the transgene has commercial silk traits similar to the nontransgenic CSR2 line.
Table 3 . Cocoon and silk characteristics of transgenic CSR2 and nontransgenic CSR2 (Control) lines.
| Lines BC4-F13 | Cocoon weight (g) | Shell weight (g) | Shell ratio(%) | Filament length (m) | Raw silk (%) |
|---|---|---|---|---|---|
| CSR2 118A | 1.3124 ± 0.0233 | 0.2390 ± 0.0088 | 18.14 ± 0.5094 | 816.87 ± 27.73 | 14.08 ± 0.7957 |
| CSR2 164C | 1.3102 ± 0.0720 | 0.2407 ± 0.0226 | 18.48 ± 0.8143 | 823.50 ± 59.97 | 14.59 ± 1.4637 |
| CSR2 170B | 1.4312 ± 0.1411 | 0.2587 ± 0.0096 | 18.02 ± 1.1307 | 773.75 ± 24.75 | 13.59 ± 0.2970 |
| CSR2(Control) | 1.4405 ± 0.0100 | 0.2795 ± 0.0100 | 19.37 ± 0.0100 | 924.50 ± 87.38 | 17.11 ± 2.8072 |
Cocoon weight (grams), Shell weight (grams), Shell ratio (percentage), Filament length (meters) and Raw silk (percentage) are given as mean ± SD.
Per os infectivity of the OBs derived from the transgenic and nontransgenic lines
To test whether the knockdown of P74 by the dsRNA in the transgenic silkworms resulted in less virulent virus particles, we carried out infection experiments with the OBs derived from the infected transgenic and nontransgenic CSR2 lines and compared the LD50 values in these two lines. LD50 value for the OBs derived from the transgenic CSR2 larvae when fed to nontransgenic larvae of the same line was found to be >4500 (Table 4) as compared to the LD50 value of 1600 for OBs obtained from the infected nontransgenic CSR2 larvae administered per os to nontransgenic CSR2 line. Our results indicate >2.5-fold decrease in the infectivity of the OBs obtained from the transgenic line as compared to that of OBs from the nontransgenic line. When the OBs derived from the infected transgenic and nontransgenic CSR2 lines were used for infection per os of transgenic CSR2 line, there was 4-fold decrease in the infectivity of the OBs obtained from the transgenic line. The comparative mortality in the two groups was determined by subjecting the mortality data to probit analysis, and the infectivity of OBs was assessed from the LD50 values as presented in Table 4. The LD50 values between the two types of OBs differered significantly (P < 0.05) inferring a considerable difference in the infectivity of the OBs from the transgenic and nontransgenic lines.
Table 4 . Comparative infectivity of OBs obtained from transgenic CSR2 and nontransgenic CSR2 larvae.
| 95% fiducial limit |
|||||||
|---|---|---|---|---|---|---|---|
| Type of OBs used | Test strain | LD50 | Upper | Lower | 1/slope | Regression equation | Standard error |
| OBs from transgenics | Nontransgenics | 4786 | 3801 | 6025 | 1.804 | Y = 0.5542X + 2.962 | 0.1013 |
| OBs from nontransgenics | Nontransgenics | 1698 | 1258 | 2398 | 1.146 | Y = 0.8728X + 2.176 | 0.1320 |
| OBs from transgenics | Transgenics | 21877 | 18197 | 25703 | 1.948 | Y = 0.5134X + 2.773 | 0.0747 |
| OBs from nontransgenic | Transgenics | 2570 | 2238 | 2951 | 1.822 | Y = 0.5488X + 3.127 | 0.0573 |
Day 1 fourth instar transgenic and nontransgenic CSR2 lines were fed per os with doses of OBs ranging from 1000 to 40,000 per larva. Thirty larvae per dose per line were used. The mortality was recorded from day 1 postinfection until pupation. The probit values were plotted against log doses for calculation of LD50 using GraphPad Prism 6 software. LD50 values of the transgenic and nontransgenic lines infected either with transgenic- or nontransgenic-derived OBs differed significantly (P < 0.05).
Discussion
BmNPV are arthropod viruses that kill their hosts in a biphasic infection cycle with a systemic primary phase involved in multiplication of so-called BVs that establish infection within the host and a second phase where ODVs propagate the infection from host to host. Silkworm rearing suffers heavily from BmNPV infection, which accounts for more than half of the loss in cocoon production. Although a long-lasting question, no enduring therapy exists to combat nucleopolyhedrosis.
Recently, Jiang et al. (2012) revealed an anti-BmNPV activity in the gut juice of transgenic silkworm that could be enhanced by 33% by overexpressing the Bmlipase-1 which is shown to have antiviral activity. Modifying the host gene could help developing control strategies, but, at the moment, the effects are measurable only at sublethal doses of virus.
Recent developments suggest that RNA interference-based anti-pathogen strategies are efficient in blocking the activity of genes crucial to the accomplishment of the viral cycle, when there is a lack of other effective methods (Tan and Yin 2004; Mueller et al. 2010; Davidson and McCray 2011). The first report demonstrating the use of RNAi to inhibit Autographa californica NPV proliferation showed that delivering dsRNA derived from gp64 or ie1, two genes essential for baculovirus propagation, resulted in transitory inhibition of viral infection in vitro (Spodoptera frugiperda Sf21 cells) and in vivo (injection into Tenebrio molitor larvae) (Valdes et al. 2003). Combining RNAi and B. mori germline transformation (Tamura et al. 2000), Isobe et al. (2004) generated silkworms expressing BmNPV lef1 dsRNA and observed a moderate inhibition of viral replication that, however, did not alleviate baculovirus-rendered larval mortality substantially. Also, we targeted the baculoviral ie1 gene in both cultured cells and in the transgenic silkworm (Kanginakudru et al. 2007). While we noted a strong viral repression at early stages of infection, the viral proliferation recovered subsequently. All these attempts demonstrated the validity of the RNAi approach, but targeting a single gene to block baculovirus proliferation resulted in limited success. Hence, in the present study, we assayed the efficiency of the simultaneous inhibition of several key viral genes. To our knowledge, this is the first report where multiple genes of the same pathogen are being simultaneously used for effective RNAi mediated suppression of virus replication/infectivity in insects.
Indeed, we selected three baculoviral genes ie1, lef1, and lef3 that are essential for DNA replication and transcription and another gene p74 necessary for per os infectivity. We constructed piggyBac-derived vectors based on the concatenation of partial sequences from the four genes in the sense, the antisense strand, or inverted repeat arrangement. Transgenic lines were generated with the three types of transgenes. We also combined transgenes encoding the sense and the antisense strands from two distinct loci by crossing the corresponding parental lines. We assayed the capacity of the transgenes to inhibit the completion of the viral cycle by infecting the third-instar larvae with high doses of the OBs per orally, the natural mode of contamination that occurs in farmers’ silkworm rearing facilities. With this mode of infection, mortality mainly results from the dissemination of the BV in the organism impairing vital functions of host cells and tissues.
We observed that the various transgenic lines displayed different levels of resistance against BmNPV infection, accounted for by the type of transgenes and likely, within one group of lines, the chromosomal environment of the inserted sequences. The less efficient protection was noted in the lines expressing either the sense or the antisense strand alone. Significantly, the combination of both conferred a high level of protection against the virus, which suggested that the two single-strand RNAs form duplexes that are further processed by the RNAi machinery. The transgenic lines that showed the lowest mortality rates upon infection were those expressed the long stem-loop double-strand RNA. The resistance that we measured was mostly the consequence of the coincident inhibition of the IE1, LEF1, and LEF3 accumulation, which affected viral replication. This is demonstrated by the almost disappearance of the GP64 envelop protein in the MG(+/−) lines, which recorded the highest level of resistance against baculoviral infection.
The drop in the OBs titer in hemolymph of the infected transgenic larvae further demonstrated that the constraints on DNA replication strongly impaired the formation of occluded viruses. Furthermore, we speculated that the inhibition of the per os infectivity factor P74 generates flawed viruses unable to propagate the infection in the population. Indeed, in the present study, we observed a significant decrease in the infectivity of the OBs derived from the transgenic lines as compared to that of the OBs derived from the nontransgenic lines. These results support the earlier findings that deletion of P74, a virion envelop protein involved in per os infectivity in most of the baculoviruses, and its deletion results in significant loss of infectivity in Autographa californica nucleopolyhedrovirus (Faulkner et al. 1997). Haas-Stapleton et al. (2004) showed that P74 of AcMNPV is critical for oral infection of Trichoplusia ni larvae. The study also provided evidence that P74 facilitates binding of AcMNPV ODV to a specific receptor within the larval midgut epithelia of another host species, Heliothis virescens. P74-deficient ODV failed to compete effectively with wild-type ODV binding, and as a result the overall binding level of the mutant ODV was one-third of the wild-type ODV. Wang et al. (2009) have shown that P74 of AcMNPV has a role in virion occlusion, as individual gene deletion of either p26 or p10 could not abolish virion occlusion, but the deletion of p74 along with these two genes resulted in few or no virions in OBs. Peng et al. (2011) reported threefold reduction in host-cell-binding efficiency of the P74 mutant virus as compared to the wild-type ones. Taken together, our studies demonstrate that abrogation of P74 by the corresponding transgene-derived dsRNA resulted in more than twofold reduction in BmNPV infectivity. This is the first evidence to show the role of P74 of BmNPV in per os infectivity of OBs.
As a whole, the systemic infection as well as the horizontal (host-to-host) transmission is considerably impaired in transgenic silkworms to the point of conferring high level of refractoriness to the virus in the best lines. BmNPV belongs to the large virus family of baculoviridae for which 57 genome sequences are known (Miele et al. 2011). The comparative sequence analysis shows that the core genes lef1 and p74 that we targeted are widely conserved, suggesting that the protection may extend to other baculovirus genus or isolates for which Bombyx may be sensitive.
To commercially exploit the benefit of transgene-mediated baculoviral suppression, we introduced the RNAi-producing transgenes to the high-yielding, baculovirus-susceptible commercial strain, CSR2 which is currently in use in sericulture, through recurrent backcross breeding coupled with transgene marker selection (eye color), cocoon phenotype, and SSR marker-aided selection of the recurrent parent. In the BC4F6 progeny >85% CSR2 genome was found to be incorporated as screened by SSR markers. The resultant backcross lines (BC4F13) were evaluated for survival rate and other silk cocoon quality traits. Almost all the nontransgenic CSR2 lines succumbed to infection before reaching cocoon stage whereas >75% of the backcross-derived transgenic CSR2 lines survived and reproduced successfully. The cocoons, silk yield, and all other properties were similar to the control CSR2 line (Table 3). Although we deal with genetically transformed silkworms, this is reminiscent of the first in-field technology transfer developed by Hunter et al. (2010) whereby feeding bees (Apis mellifera) with dsRNA of Israeli acute paralysis virus protects them from colony collapse disorder.
In conclusion, we succeeded in obtaining high-yielding silkworm lines protected from the baculovirus infection that could be harnessed for the benefit of sericulture industry. The contained multilocational trials of the baculovirus-resistant transgenic silkworms are currently underway.
Supplementary Material
Acknowledgments
We thank P. J. Raju and Chandrashekaraiah of Andhra Pradesh State Sericulture Research and Development Institute (APSSRDI) and Basavaraja of the Centre for DNA Fingerprinting and Diagnostics (CDFD) for their help in establishing the breeding parameters and viral assays for transgenic strains, at different stages of the project. This work was supported by the Centre of Excellence (CoE) grant to J.N. by the Department of Biotechnology (DBT), Government of India, New Delhi. The work on transgenic silkworm was initiated by a grant from Indo-French Centre for the Promotion of Advanced Research (IFCPAR) to J.N. and P.C. E.V.S. and S.K. were the recipients of Senior Research Fellowship from the Council of Scientific and Industrial research (CSIR), New Delhi.
Footnotes
Communicating editor: M. P. Colaiácovo
Literature Cited
- Bonfim K., Faria J. C., Nogueira E. O., Mendes E. A., Aragao F. J., 2007. RNAi-mediated resistance to Bean golden mosaic virus in genetically engineered common bean (Phaseolus vulgaris). Mol. Plant Microbe Interact. 20: 717–726 [DOI] [PubMed] [Google Scholar]
- Bucher E., Lohuis D., van Poppel P. M., Geerts-Dimitriadou C., Goldbach R., et al. , 2006. Multiple virus resistance at a high frequency using a single transgene construct. J. Gen. Virol. 87: 3697–3701 [DOI] [PubMed] [Google Scholar]
- Davidson B. L., McCray P. B., Jr, 2011. Current prospects for RNA interference-based therapies. Nat. Rev. Genet. 12: 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. T., Leisy D. J., Rohrmann G. F., 1997. Characterization of the interaction between the baculovirus replication factors LEF-1 and LEF-2. J. Virol. 71: 3114–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner P., Kuzio J., Williams G. V., Wilson J. A., 1997. Analysis of p74, a PDV envelope protein of Autographa californica nucleopolyhedrovirus required for occlusion body infectivity in vivo. J. Gen. Virol. 78: 3091–3100 [DOI] [PubMed] [Google Scholar]
- Gandhe A. S., John S. H., Nagaraju J., 2007. Noduler, a novel immune up-regulated protein mediates nodulation response in insects. J. Immunol. 179: 6943–6951 [DOI] [PubMed] [Google Scholar]
- Haas-Stapleton E. J., Washburn J. O., Volkman L. E., 2004. P74 mediates specific binding of Autographa californica M nucleopolyhedrovirus occlusion-derived virus to primary cellular targets in the midgut epithelia of Heliothis virescens larvae. J. Virol. 78: 6786–6791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes R. R., Jr., Rohrmann G. F., 1991. In vitro transcription of baculovirus immediate early genes: accurate mRNA initiation by nuclear extracts from both insect and human cells. Proc. Natl. Acad. Sci. USA 88: 4513–4517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh N. E., Weaver R. F., 1990a Categorizing some early and late transcripts directed by the Autographa californica nuclear polyhedrosis virus. J. Gen. Virol. 71: 2195–2200 [DOI] [PubMed] [Google Scholar]
- Huh N. E., Weaver R. F., 1990b Identifying the RNA polymerases that synthesize specific transcripts of the Autographa californica nuclear polyhedrosis virus. J. Gen. Virol. 71: 195–201 [DOI] [PubMed] [Google Scholar]
- Hunter W., Ellis J., Vanengelsdorp D., Hayes J., Westervelt D., et al. , 2010. Large-scale field application of RNAi technology reducing Israeli acute paralysis virus disease in honey bees (Apis mellifera, Hymenoptera: Apidae). PLoS Pathog. 6: e1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe R., Kojima K., Matsuyama T., Quan G. X., Kanda T., et al. , 2004. Use of RNAi technology to confer enhanced resistance to BmNPV on transgenic silkworms. Arch. Virol. 149: 1931–1940 [DOI] [PubMed] [Google Scholar]
- Jiang L., Wang G., Cheng T., Yang Q., Jin S., et al. , 2012. Resistance to Bombyx mori nucleopolyhedrovirus via overexpression of an endogenous antiviral gene in transgenic silkworms. Arch. Virol. 157: 1323–1328 [DOI] [PubMed] [Google Scholar]
- Kanginakudru S., Royer C., Edupalli S. V., Jalabert A., Mauchamp B., et al. , 2007. Targeting ie-1 gene by RNAi induces baculoviral resistance in lepidopteran cell lines and in transgenic silkworms. Insect Mol. Biol. 16: 635–644 [DOI] [PubMed] [Google Scholar]
- Khurad A. M., Kanginakudru S., Qureshi S. O., Rathod M. K., Rai M. M., et al. , 2006. A new Bombyx mori larval ovarian cell line highly susceptible to nucleopolyhedrovirus. J. Invertebr. Pathol. 92: 59–65 [DOI] [PubMed] [Google Scholar]
- Kuzio J., Jaques R., Faulkner P., 1989. Identification of p74, a gene essential for virulence of baculovirus occlusion bodies. Virology 173: 759–763 [DOI] [PubMed] [Google Scholar]
- Leonard J. N., Schaffer D. V., 2006. Antiviral RNAi therapy: emerging approaches for hitting a moving target. Gene Ther. 13: 532–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele S. A., Garavaglia M. J., Belaich M. N., Ghiringhelli P. D., 2011. Baculovirus: molecular insights on their diversity and conservation. Int. J. Evol. Biol. 2011: 379424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov V. S., Rohrmann G. F., 2002. Baculovirus replication factor LEF-1 is a DNA primase. J. Virol. 76: 2287–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma S. A., Oomens A. G., Blissard G. W., 1996. The GP64 envelope fusion protein is an essential baculovirus protein required for cell-to-cell transmission of infection. J. Virol. 70: 4607–4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrinal N., Nagaraju J., 2010. Dynamic repositioning of dorsal to two different kappaB motifs controls its autoregulation during immune response in Drosophila. J. Biol. Chem. 285: 24206–24216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S., Gausson V., Vodovar N., Deddouche S., Troxler L., et al. , 2010. RNAi-mediated immunity provides strong protection against the negative-strand RNA vesicular stomatitis virus in Drosophila. Proc. Natl. Acad. Sci. USA 107: 19390–19395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraju J., Raje U., Datta R. K., 1996. Crossbreeding and heterosis in the silkworm, Bombyx mori: a review. Sericologia 36: 1–20 [Google Scholar]
- Peng K., van Lent J. W., Vlak J. M., Hu Z., van Oers M. M., 2011. In situ cleavage of baculovirus occlusion-derived virus receptor binding protein P74 in the peroral infectivity complex. J. Virol. 85: 10710–10718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K., van Lent J. W., Boeren S., Fang M., Theilmann D. A., et al. , 2012. Characterization of novel components of the baculovirus per os infectivity factor complex. J. Virol. 86: 4981–4988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer C., Jalabert A., Da Rocha M., Grenier A. M., Mauchamp B., et al. , 2005. Biosynthesis and cocoon-export of a recombinant globular protein in transgenic silkworms. Transgenic Res. 14: 463–472 [DOI] [PubMed] [Google Scholar]
- Shukla J. N., Nagaraju J., 2010. Two female-specific DSX proteins are encoded by the sex-specific transcripts of dsx, and are required for female sexual differentiation in two wild silkmoth species, Antheraea assama and Antheraea mylitta (Lepidoptera, Saturniidae). Insect Biochem. Mol. Biol. 40: 672–682 [DOI] [PubMed] [Google Scholar]
- Tamura T., Thibert C., Royer C., Kanda T., Abraham E., et al. , 2000. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat. Biotechnol. 18: 81–84 [DOI] [PubMed] [Google Scholar]
- Tan F. L., Yin J. Q., 2004. RNAi, a new therapeutic strategy against viral infection. Cell Res. 14: 460–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenius O., Papanicolaou A., Garbutt J. S., Eleftherianos I., Huvenne H., et al. , 2011. RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J. Insect Physiol. 57: 231–245 [DOI] [PubMed] [Google Scholar]
- Thomas J. L., Da Rocha M., Besse A., Mauchamp B., Chavancy G., 2002. 3xP3-EGFP marker facilitates screening for transgenic silkworm Bombyx mori L. from the embryonic stage onwards. Insect Biochem. Mol. Biol. 32: 247–253 [DOI] [PubMed] [Google Scholar]
- Tomita M., 2011. Transgenic silkworms that weave recombinant proteins into silk cocoons. Biotechnol. Lett. 33: 645–654 [DOI] [PubMed] [Google Scholar]
- Uchino K., Imamura M., Sezutsu H., Kobayashi I., Kojima K., et al. , 2006. Evaluating promoter sequences for trapping an enhancer activity in the silkworm Bombyx mori. J. Insect Biotechnol. Sericology 75: 89–97 [Google Scholar]
- Valdes V. J., Sampieri A., Sepulveda J., Vaca L., 2003. Using double-stranded RNA to prevent in vitro and in vivo viral infections by recombinant baculovirus. J. Biol. Chem. 278: 19317–19324 [DOI] [PubMed] [Google Scholar]
- van der Linden A. M., Plasterk R. H., 2004. Shotgun cloning of transposon insertions in the genome of Caenorhabditis elegans. Comp. Funct. Genomics 5: 225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Salem T. Z., Campbell D. J., Turney C. M., Kumar C. M., et al. , 2009. Characterization of a virion occlusion-defective Autographa californica multiple nucleopolyhedrovirus mutant lacking the p26, p10 and p74 genes. J. Gen. Virol. 90: 1641–1648 [DOI] [PubMed] [Google Scholar]
- Wang X. H., Aliyari R., Li W. X., Li H. W., Kim K., et al. , 2006. RNA interference directs innate immunity against viruses in adult Drosophila. Science 312: 452–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. Y., Fu F. L., Gou L., Wang H. G., Li W. C., 2010. RNA interference-based transgenic maize resistant to maize dwarf mosaic virus. J. Plant Biol. 53: 297–305 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.