Abstract
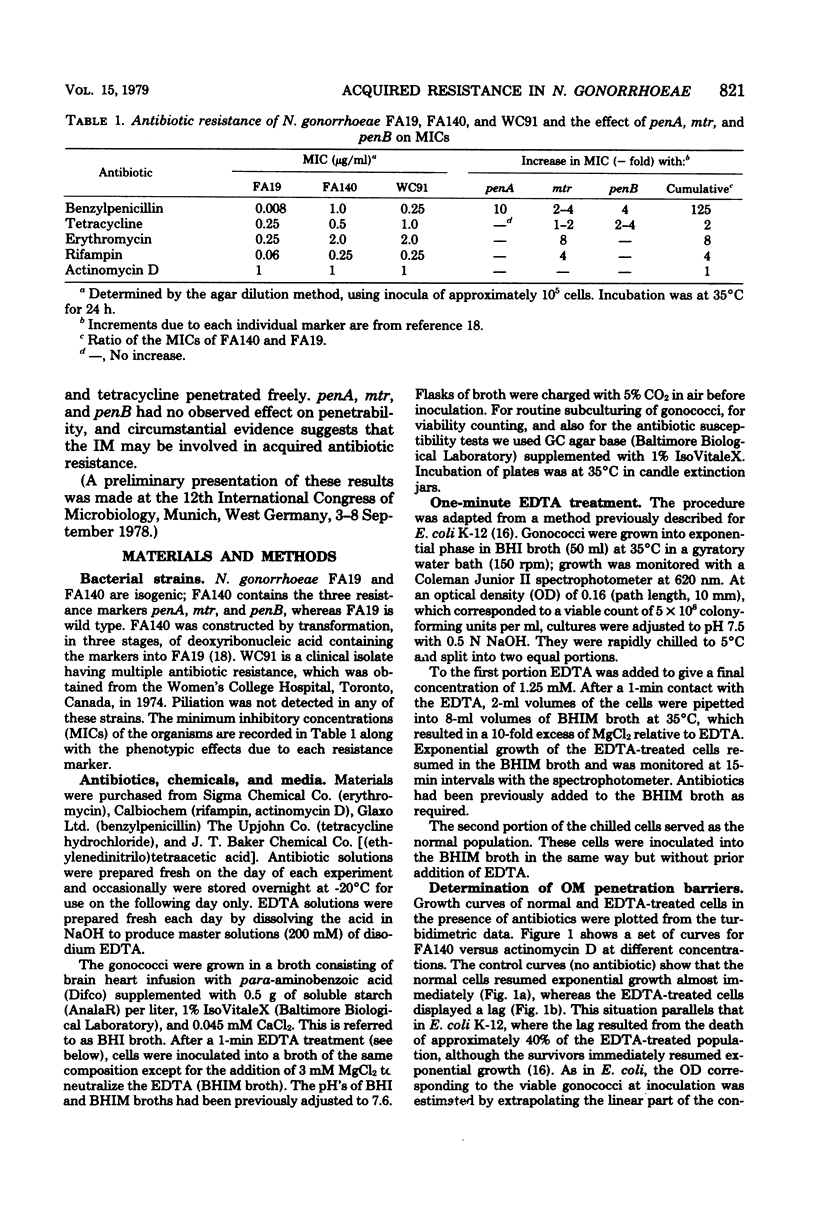
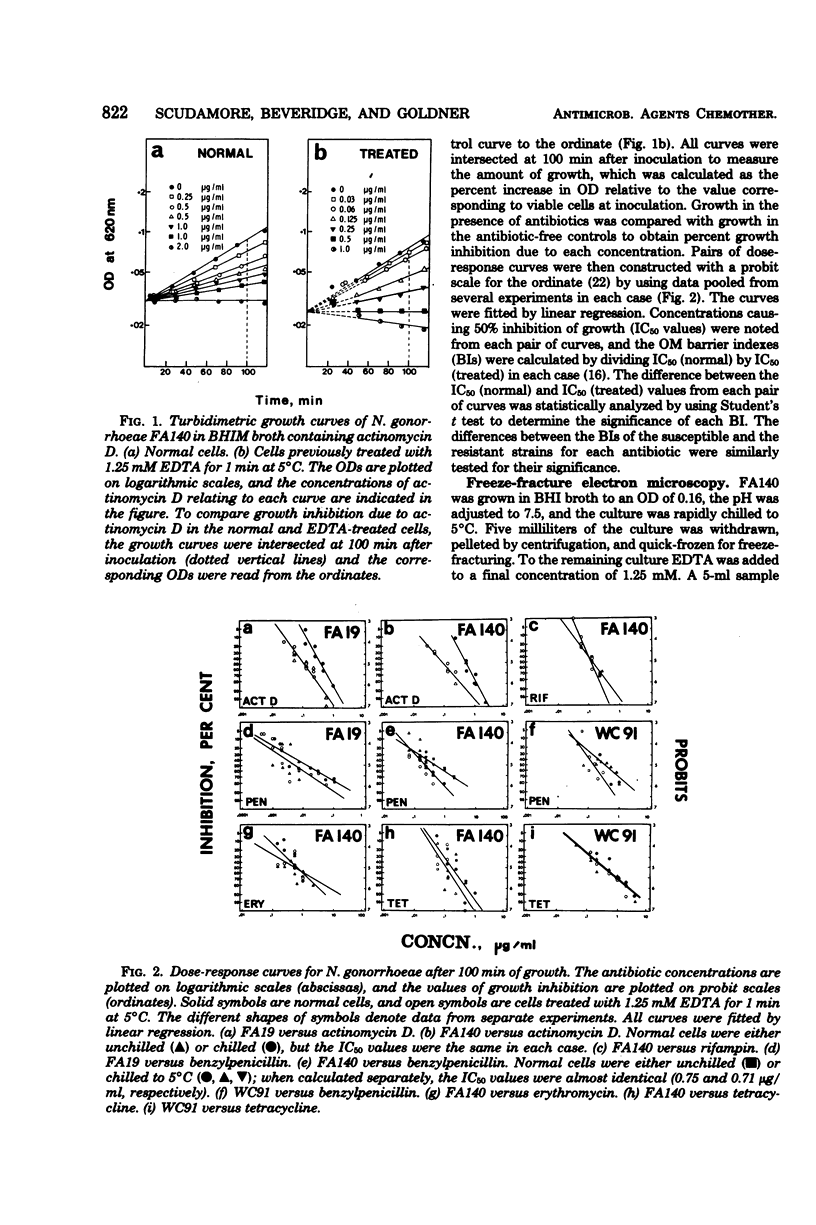
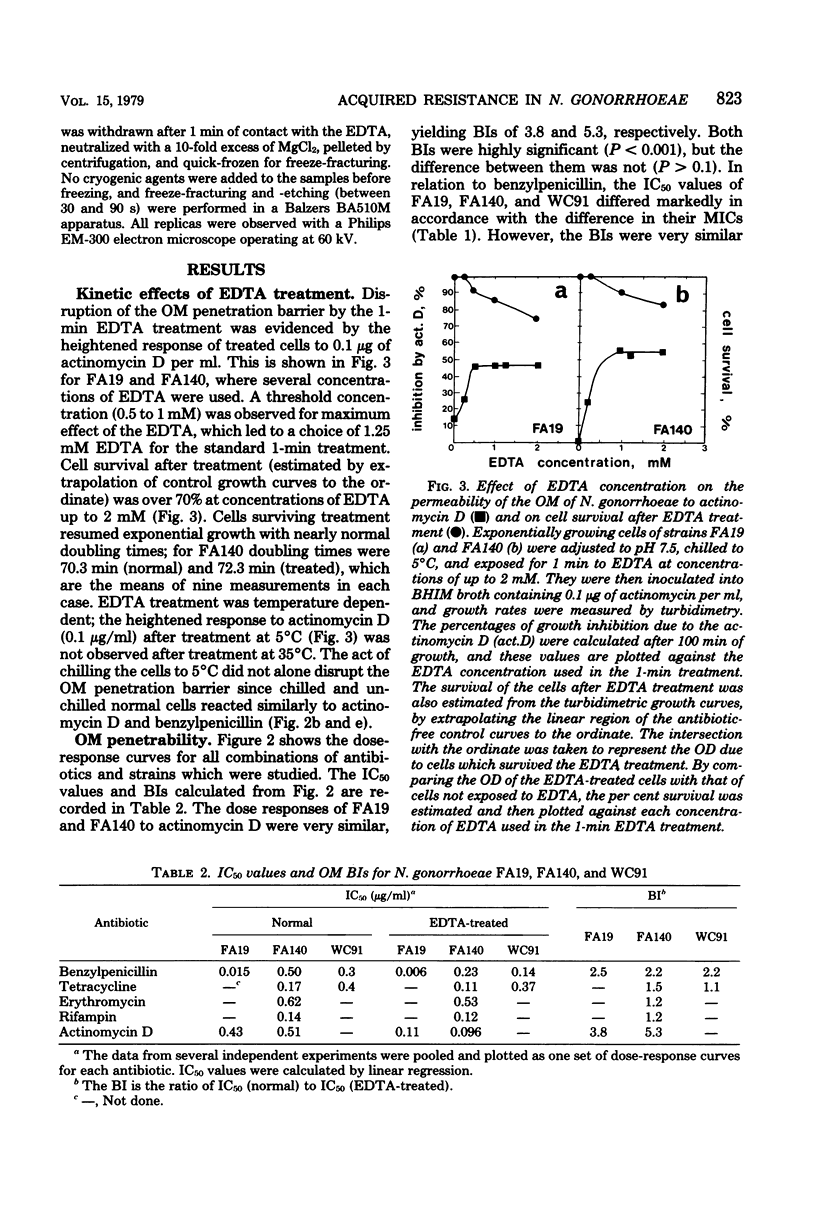
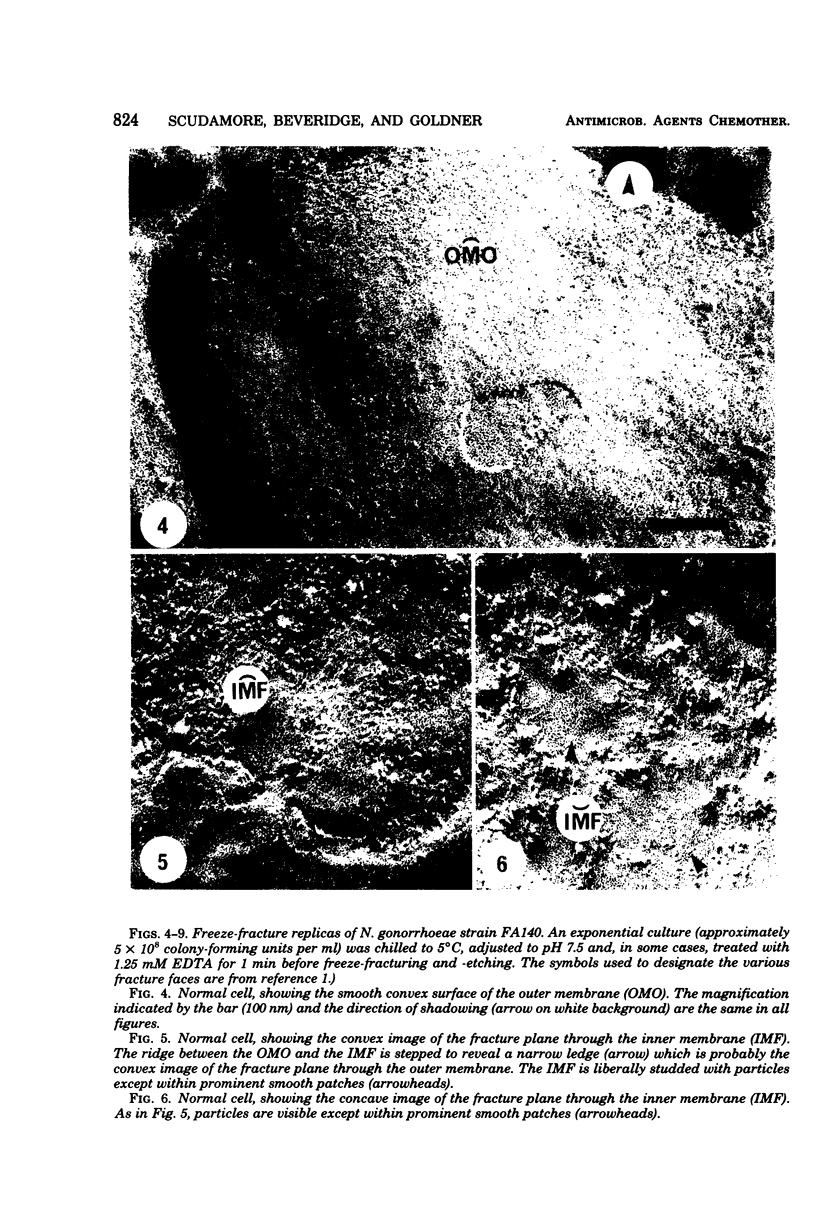
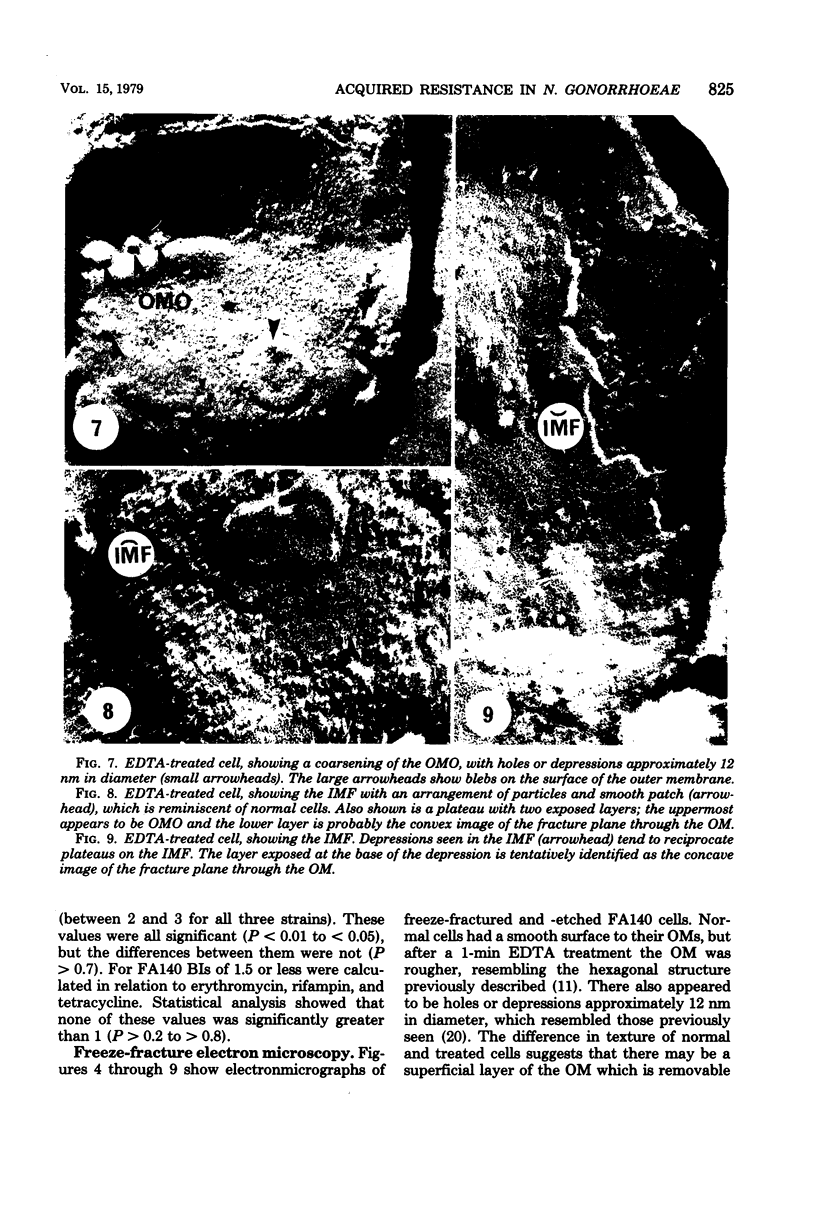
Acquired antibiotic resistance in Neisseria gonorrhoeae is principally associated with three genetic markers, penA, mtr, and penB. penA is a specific marker for penicillin resistance, whereas mtr and penB are nonspecific in conferring resistance to penicillin and several other antibiotics as well. It has been suggested that the nonspecific markers may cause a general decrease in the penetrability of the gonococcal outer membrane. To investigate this, antibiotic penetration of the outer membrane was studied in two isogenic strains—FA19 (susceptible parent) and FA140 (containing penA, mtr, and penB)—and also in a clinical isolate with multiple resistance. The method involved brief treatment of exponential cells with ethylenediaminetetraacetic acid at 5°C to disrupt the outer membrane barrier. The 50% inhibitory concentrations of antibiotics for treated and normal cells were measured turbidimetrically, and from their ratios outer membrane penetration barriers were calculated. Small barriers were observed for actinomycin D and benzylpenicillin, and these were very similar in the susceptible and resistant strains. Also, in FA140 no significant barriers for rifampin, erythromycin, and tetracycline were detected. These results suggest that mechanism(s) other than reduced outer membrane penetrability underlie acquired resistance due to penA, mtr, and penB.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Leive L. Effect of ethylenediaminetetraacetate upon the surface of Escherichia coli. J Bacteriol. 1977 Jun;130(3):1364–1381. doi: 10.1128/jb.130.3.1364-1381.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G., Comer S., Sparling P. F. Chromosomal location of antibiotic resistance genes in Neisseria gonorrhoeae. J Bacteriol. 1976 Mar;125(3):1207–1210. doi: 10.1128/jb.125.3.1207-1210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formanek H. A three dimensional model of the digestion of peptidoglycan by lysozyme. Biophys Struct Mech. 1977 Dec 27;4(1):1–14. doi: 10.1007/BF00538836. [DOI] [PubMed] [Google Scholar]
- Guymon L. F., Sparling P. F. Altered crystal violet permeability and lytic behavior in antibiotic-resistant and -sensitive mutants of Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):757–763. doi: 10.1128/jb.124.2.757-763.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Walstad D. L., Sparling P. F. Cell envelope alterations in antibiotic-sensitive and-resistant strains of Neisseria gonorrhoeae. J Bacteriol. 1978 Oct;136(1):391–401. doi: 10.1128/jb.136.1.391-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Novotny P., Short J. A., Walker P. D. An electron-microscope study of naturally occurring and cultured cells of Neisseria Gonorrhoeae. J Med Microbiol. 1975 Aug;8(3):413–427. doi: 10.1099/00222615-8-3-413. [DOI] [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. Size restriction on peptide utilization in Escherichia coli. J Biol Chem. 1968 Dec 10;243(23):6291–6299. [PubMed] [Google Scholar]
- Rodriguez W. J., Saz A. K. Differential binding of penicillin by membrane fractions from penicillin-susceptible and -resistant gonococci. Antimicrob Agents Chemother. 1978 Apr;13(4):589–597. doi: 10.1128/aac.13.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R., Owen P. Bacterial membrane structure. Annu Rev Microbiol. 1976;30:451–482. doi: 10.1146/annurev.mi.30.100176.002315. [DOI] [PubMed] [Google Scholar]
- Scudamore R. A., Beveridge T. J., Goldner M. Outer-membrane penetration barriers as components of intrinsic resistance to beta-lactam and other antibiotics in Escherichia coli K-12. Antimicrob Agents Chemother. 1979 Feb;15(2):182–189. doi: 10.1128/aac.15.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Sarubbi F. A., Jr, Blackman E. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):740–749. doi: 10.1128/jb.124.2.740-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Escherichia coli resistance to beta-lactam antibiotics through a decrease in the affinity of a target for lethality. Nature. 1978 Aug 17;274(5672):713–715. doi: 10.1038/274713a0. [DOI] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. II. Freeze-fracture, freeze-etch studies on gonocci. J Exp Med. 1972 Nov 1;136(5):1258–1271. doi: 10.1084/jem.136.5.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREFFERS H. P. The linear representation of dosage-response curves in microbial-antibiotic assays. J Bacteriol. 1956 Jul;72(1):108–114. doi: 10.1128/jb.72.1.108-114.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]