Abstract
Drought stress is a major factor limiting nitrogen fixation (NF) in crop production. However, the regulatory mechanism involved and the origin of the inhibition, whether local or systemic, is still controversial and so far scarcely studied in temperate forage legumes. Medicago truncatula plants were symbiotically grown with a split-root system and exposed to gradual water deprivation. Physiological parameters, NF activity, and amino acid content were measured. The partial drought treatment inhibited NF in the nodules directly exposed to drought stress. Concomitantly, in the droughted below-ground organs, amino acids accumulated prior to any drop in evapotranspiration (ET). It is concluded that drought exerts a local inhibition of NF and drives an overall accumulation of amino acids in diverse plant organs which is independent of the decrease in ET. The general increase in the majority of single amino acids in the whole plant questions the commonly accepted concept of a single amino acid acting as an N-feedback signal.
Key words: Amino acids, drought, local regulation, Medicago truncatula, N-feedback inhibition, nitrogen fixation, transpiration.
Introduction
Forage legumes such as Medicago truncatula are important in agricultural systems as feed sources for livestock and raw food materials for humans (Graham and Vance, 2003). In addition, M. truncatula, considered as a model legume, is phylogenetically related to some of the most economically important European legume crops. One of the main characteristics of legumes is their ability to establish symbiosis with nitrogen-fixing soil bacteria. However, legumes show inconsistent production rates, mostly due to abiotic factors and particularly drought (Zahran, 1999).
Drought causes a reduction in the rates of nitrogen fixation (NF) in legumes. Despite considerable research effort in the last decades, the molecular mechanism(s) responsible for this inhibition remains largely unknown. Several factors have been proposed to be involved in this inhibition: oxygen limitation, carbon shortage, and regulation by nitrogen metabolism. Although drought, like other abiotic stresses, does cause an increase in nodular oxygen diffusion resistance (Durand et al., 1987), increasing the oxygen concentration around the rhizosphere of drought-stressed nodules does not fully restore NF rates, suggesting that other factors are also involved (Del Castillo et al., 1994; Del Castillo and Layzell, 1995). Several studies carried out in grain legumes (i.e. pea, soybean, and common bean) subjected to drought showed that the reduction of NF rates was related to a concurrent inhibition of sucrose synthase activity, followed by an accumulation of sucrose and a decrease in malate to fuel bacteroid respiration (Gonzalez et al., 1995, 1998; Gordon et al., 1997; Ramos et al., 1999). Reactive oxygen species might also play a role in the regulation of sucrose synthase under moderate oxidative stress (Marino et al., 2006). Nevertheless, recent work in the forage legumes Medicago sativa (Naya et al., 2007) and M. truncatula (R. Ladrera, C. Arrese-Igor, and E.M. González, unpublished results) suggested a different cause, since NF inhibition occurred before any measurable decline in either the activity rate or concentration of nodule carbon metabolism enzymes (Larrainzar et al., 2009).
The plant N status and/or some N compounds have been hypothesized to act as signals regulating NF by a feedback mechanism upon the application of inorganic N to N2-fixing plants. Several compounds have been associated with the inhibition of NF, namely glutamine (Gln) (Neo and Layzell, 1997), ureides (Serraj et al., 1999), asparagine (Asn) (Oti-Boateng and Silsbury, 1993; Bacanamwo and Harper, 1997; Sulieman et al., 2010), or a combination of these (Vadez et al., 2000). In most cases, results of both N stress and drought experiments are assumed to be similar, as if both stresses shared a common regulation mechanism. Furthermore, experimental data suggesting an N-feedback mechanism governing NF under drought stress have been mainly obtained in soybean (Glycine max L. Merr), a ureide-exporter grain legume (Serraj et al., 1999; Sinclair and Serraj, 1995; deSilva et al., 1996; Purcell et al., 1998; Vadez and Sinclair, 2000, 2001; King and Purcell, 2005; Ladrera et al., 2007), and they do not necessarily reflect what occurs in amide-exporter plants such as Medicago and pea (Pisum sativum L.), among others. Serraj et al. (2001) refined the N-feedback regulation model further by proposing two possible origins for the inhibition under drought stress: (i) a direct feedback inhibition within nodules; or (ii) an indirect feedback process due to N signals coming from the aerial part. One of the few studies of the changes in amino acid levels in amide-exporting legumes under drought stressed used a broad metabolomic approach, not specifically targeted to test the N-feedback hypothesis (Larrainzar et al., 2009). As far as is known, this is the first study in which N-feedback inhibition is tested in the amide-exporter model legume M. truncatula subjected to water deprivation.
Serraj et al. (2001) suggested that the accumulation of N compounds in nodules under drought stress is a consequence of decreased xylem transport leading to impaired amino acid export, implying that changes in plant transpiration rates may also have an effect in this regulation process. Walsh (1990) stated that the rates of N-compound export from nodules were determined by the phloem water input to them. Thus, it is hypothesized that a reduction in the phloem supply to nodules, as may be the case under water deficit conditions, would result in a diminution in the export of N compounds with a subsequent accumulation of these compounds in nodules.
The aim of the present work is to test whether regulation by an N-feedback process, related to changes in transpiration and/or accumulation of N compounds in different plant organs, is responsible for the drought-induced decline of NF in M. truncatula. As far as is known, this is the first time that the N-feedback inhibition under drought stress is tested in an amide-exporter temperate forage legume. To do so, a split-root system (SRS) was employed, which allows the specific discrimination between local and systemic regulatory mechanisms. A preliminary report with this system has been published (Gil-Quintana et al., 2009). The use of this experimental system may help to determine the origin of the inhibition of NF and test the possible N-feedback caused by amino acid accumulation in the model legume M. truncatula.
Materials and methods
Plant growth conditions and drought stress treatment
Medicago truncatula Gaertn. cv. Jemalong A17 plants were grown in 1 litre pots containing a mixture of perlite:vermiculite (2:5, v/v) and grown under controlled environmental conditions (14h photoperiod; 400 µmol m–2 s–1 light intensity; 22 ºC/16 ºC day/night temperature; 60–70% relative humidity) for 4 weeks. Seedlings were inoculated with the efficient N-fixing strain Ensifer meliloti 2011 once a week for the first 3 weeks of growth (Larrainzar et al., 2007, 2009). In order to produce SRS plants, 4-week-old plants were transferred into a double pot (600ml each) with the same substrate mixture, distributing the root system into two equal parts. Plants were watered to field capacity three times a week with Evans nutrient solution (Evans, 1981). For the first 3 weeks, nutrient solution was supplemented with 0.25mM NH4NO3 to improve plant performance at the initial stage of nodule development. For the rest of the growth period, the nutrient solution was N free and optimum plant growth relied on symbiotic NF.
Twelve-week-old plants were randomly separated into three sets containing six biological replicates each. Controls (C) were supplied daily with nutrient solution to field capacity on both sides of the SRS, drought treatment (D) was achieved by withholding water/nutrients from both sides, and partial drought plants (PD) were irrigated to field capacity on one side of the SRS (PDC) while water/nutrients were withheld from the other side (PDD). A schematic representation of the experimental set-up is shown in Fig. 1A. Water-stressed plants and their corresponding controls were harvested at days 2, 4, and 7 after the onset of drought. By using this timing, leaf water potential declined gradually from control values, reaching progressively a stage of moderate (approximately –1.4MPa) and severe (approximately –2.7MPa) drought, as recorded in previous studies (Antolin et al., 1995; Larrainzar et al., 2009). Four types of nodule and root samples (C, PDC, PDD, and D) and three types of leaf samples (C, PD, and D) were harvested, immediately frozen in liquid nitrogen, and stored at -80 ºC for analytical determinations. For dry weight (DW) measurements, samples from leaf, root, and nodule tissue were collected and desiccated for 48h at 80 ºC.
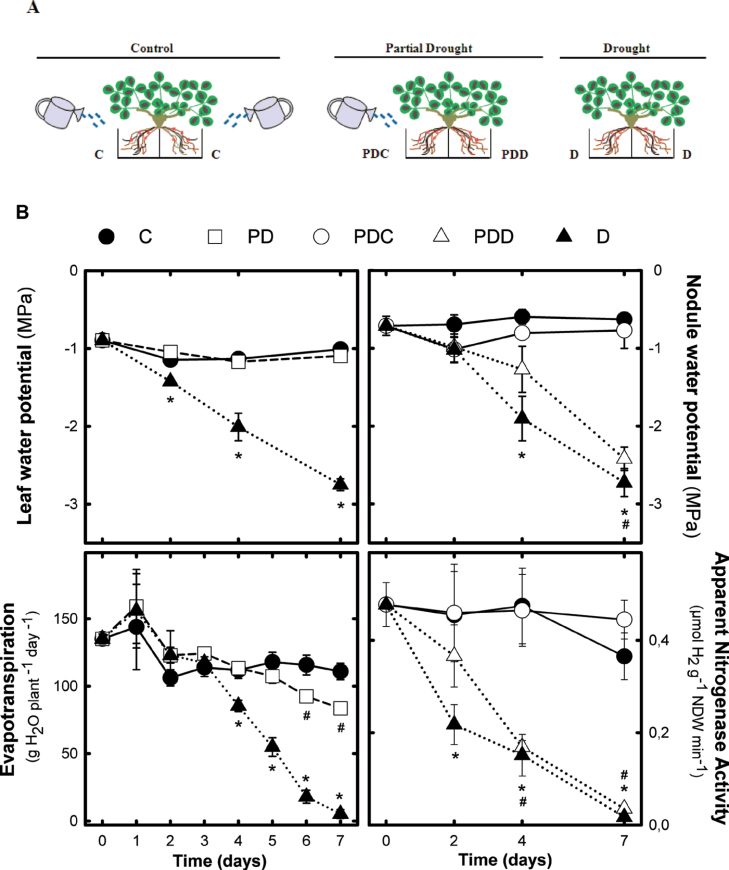
Fig. 1.
Schematic representation of the SRS experimental set-up (A) and the effect of partial drought on Ψleaf, Ψnodule, ET, and NF (B). In (A), 12-week-old M. truncatula nodulated plants were randomly separated into three groups. Control plants, C, were watered to field capacity and, in drought plants, D, water/nutrient solution was withheld. In partial drought plants, PD, one side of the root system was watered to field capacity, PDC, while in the other side water/nutrient solution was withdrawn, PDD. Water deprivation was prolonged during 7 d and harvest was carried out on days 2, 4, and 7 after the onset of the treatment. In (B), values represent means ±SE (n=12 for Ψleaf; n=6 for Ψnodule; n=8 for ET; and n=6 for ANA). An asterisk (*) represents significant differences (P ≤ 0.05) between D and C treatments, and differences between C and PD or PDD are denoted with a hash sign (#).
Water relations
Evapotranspiration (ET) was gravimetrically determined on a daily basis. Leaf water potential (Ψleaf) was measured in the first fully expanded leaf 2h after the beginning of the photoperiod using a pressure chamber (Soil Moisture Equipment, Santa Barbara, CA, USA) as earlier described (Scholander et al., 1965). Nodule water potential (Ψnodule) was measured in C52 sample chambers coupled to a Wescor HR-33T Dew Point Microvoltmeter (Wescor, Logan, UT, USA). Approximately four nodules per treatment were collected and confined in each chamber for at least 1h until temperature and vapour equilibration was reached.
Carbon isotopic composition (δ13C)
Aliquots (3–4mg) of ground dried leaf and root material were analysed in a stable isotope mass spectrometer Delta Plus (ThermoQuest, Bremen, Germany) coupled to an elemental analyser NC 2500 (Carlo Erba, Milan, Italy). δ13C measures the deviation of the isotopic composition of plant material from the carbon dioxide generated from a fossil belemnite from the Pee Dee Formation taken as standard. δ13C is a tool for understanding the relationship between photosynthesis and water use in plants (Farquhar et al., 1989).
Nitrogen fixation determination
NF was measured as apparent nitrogenase activity (ANA). H2 evolution from sealed root systems was measured in an open flow-through system under N2:O2 (79%:21%, v/v) according to Witty and Minchin (1998) using an electrochemical H2 sensor (Qubit System, Canada). The H2 sensor was calibrated with high purity gases (Praxair) using a gas mixer (Air Liquide, Spain) flowing at the same rate as the sampling system (500ml min–1).
Protein determination, protease activity and western blot analysis
Frozen leaves [200mg fresh weight (FW)], roots (200mg FW), and nodules (100mg FW) were homogenized using a mortar and pestle with 500–600 µl of extraction buffer (50mM MOPS, 5mM MgCl2, 20mM KCl, 1mM Na2EDTA, pH 7) where 1.5mg ml–1 dithiothreitol (DTT) and 0.7 µl ml–1 β-mercaptoethanol were freshly added. Homogenates were centrifuged at 20500 g and 4 ºC for 20min. Supernatants were then collected and used for soluble protein quantification and protease activity.
Protein was quantified using a Bradford-based dye-binding assay (Bio-Rad) employing bovine serum albumin as standard.
Protease activity was measured with a protease fluorescent detection kit (Sigma-Aldrich) using trypsin as standard. The measurement is based on the fluorescence emitted by the cleavage of fluorescein isothiocyanate (FITC)-labelled casein substrate by the protease activity of the sample (Twining, 1984).
For the immunodetection of 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase (DAHPS; EC 4.1.2.15), soluble protein extracts (50 µg per lane) were separated by SDS–PAGE according to Laemmli (1970) and blotted onto polyvinylidene difluouride (PVDF) membranes. Membranes were incubated for 1h using specific antibodies against DAHPS (1:1000 dilution; Biogenes, Berlin, Germany) and immunoreacting bands detected as previously described (Larrainzar et al., 2007).
Amino acid determination
Frozen nodules (100mg FW), leaves (200mg FW), and roots (200mg FW) were ground to powder under liquid N2 and subsequently homogenized using a mortar and pestle with 3ml (for roots and leaves) or 5ml (for nodules) of 1M HCl. Homogenates were transferred to glass tubes and incubated on ice for 10min. Subsequently, extracts were centrifuged at 20000 g and 4 ºC for 10min. Supernatants were neutralized using NaOH, and internal standards norvaline and homoglutamic acid were spiked. Samples were then derivatized with 1mM FITC dissolved in acetone at room temperature for 15h in 20mM borate buffer (pH 10). The content of free amino acids was determined using a Beckman Coulter capillary electrophoresis PA-800 (Beckman Coulter Inc., USA) coupled to laser-induced fluorescence detection (argon laser at 488nm), as described in Arlt et al. (2001) and Takizawa and Nakamura (1998), with minor modifications. A fused-silica capillary, 43/53.2cm long and 50 µm internal diameter (Beckman Coulter Inc., USA), was employed. For amino acid separation, 45mM α-cyclodextrin in 80mM borax buffer (pH 9.2) was used. Analyses were performed at 20 ºC and at a voltage of 20kV. Total amino acid content is presented as the summation of single amino acids for each sample and expressed on a DW basis.
Statistical analysis
One-way analysis of variance (ANOVA) was performed after checking the normal distribution of the samples via the Shapiro–Wilk test and the homogeneity of variances via Levene’s test. If differences in means were obtained, then comparisons between each treatment and the control were performed using the least significant difference (LSD) test. Differences were considered to be significant at P ≤ 0.05.
Results
Characterisation of SRS water status and NF
SRS experiments were conducted with the aim of investigating the response of one side of the root system to the deprivation of water on the other side, as schematized in Fig. 1A. Drought significantly reduced the dry weight of PDD roots only on day 7, while there were no treatment differences in shoot and nodule dry weight (Supplementary Fig. S1B available at JXB online). Plant water status was monitored by measuring leaf and nodule water potentials (Fig. 1B). Ψleaf was stable in C and PD treatments, whereas D plants showed a gradual decrease from the second day of the experiment, reaching values of –2.7MPa on the last day (Fig. 1B). Similarly, C plants maintained stable ψnodule and D plants diminished their Ψnodule from day 4 onwards (Fig. 1B). Ψnodule in PD treatment presented a distinct behaviour depending on the side of the SRS; the watered part (PDC) maintained control Ψnodule values, while the droughted fraction (PDD) started a slight decline of Ψnodule at day 4, falling significantly on the last day of the assay to reach values similar to D plants.
δ13C was measured on the last day of the experiment. C and PD leaves had similar δ13C values (–31.38 ‰ and –31.33‰, respectively), whereas D presented a significantly higher (less negative) δ13C value (Supplementary Fig. S2A at JXB online). In roots, only the total drought (D) plants showed significantly higher δ13C values as a consequence of the leaf δ13C changes observed in this treatment (Supplementary Fig. S2B).
ET was determined gravimetrically on a daily basis throughout the experiment (Fig. 1B). C plants maintained stable ET values, ~110g H2O plant–1 d–1, during the whole study period. PD plants kept control ET values until day 6, when a significant (20%) decrease in ET was observed. However, ET only decreased to 84g H2O plant–1 d–1 at the end of the study. In contrast, ET of D plants gradually declined from day 4 and was almost completely inhibited on day 7 (Fig. 1B).
ANA was determined as a measure of NF (Fig. 1B). C and PDC nodules maintained steady ANA rates (between 0.36 and 0.47 µmol H2 g–1 NDW min–1), while a gradual fall was observed in D plants from day 2 until values close to zero were reached at the most intense drought level. A similar trend, with a 2 d delay compared with D, was observed in PDD nodules. On the last day of the experiment, ANA expressed on a per-plant basis, showed a significant increase in PDC treatment (0.030±0.003 µmol H2 plant min–1) when compared with C plants (0.018±0.004 µmol H2 plant min–1), while PDD and D nodules had minimum ANA values in both treatments.
Drought-induced changes in plant N status
The levels of total free amino acid, protein content, and protease activity were determined in leaf, root, and nodule tissue (Fig. 2). Under C conditions, nodules presented the highest amino acid content (220 µmol g–1 DW), roots showed the lowest values (18 µmol g–1 DW), and leaves had an intermediate content (50 µmol g–1 DW). This pattern of relative amino acid content is also observed in the levels of protein. Nodules were the organs with the highest protein content (340mg protein g–1 DW), followed by leaves (200mg protein g–1 DW) and roots (12mg protein g–1 DW).
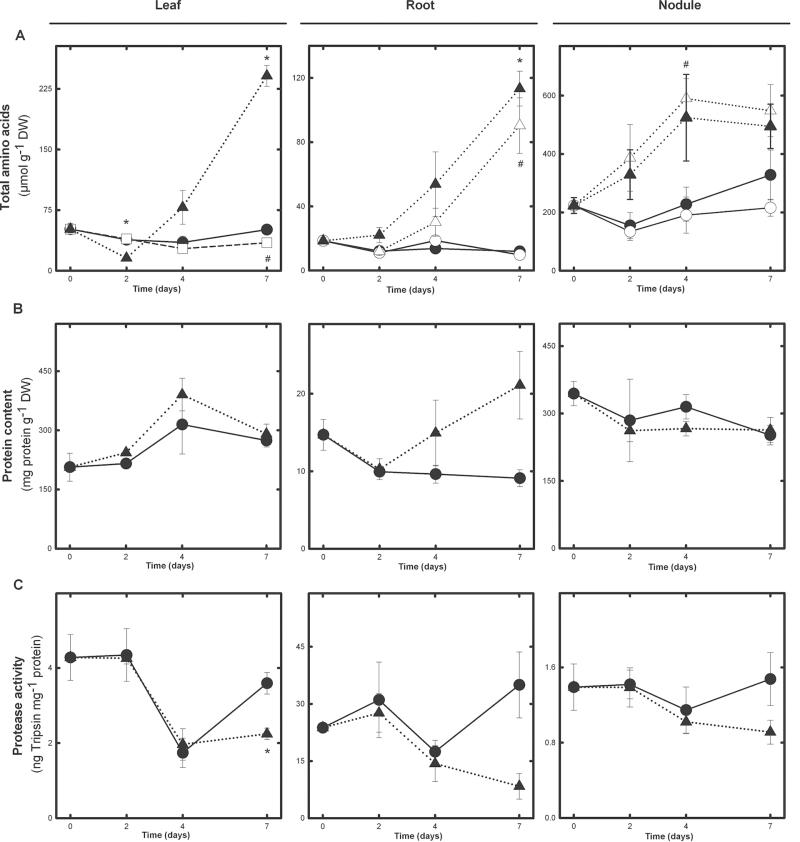
Fig. 2.
Effect of partial drought on total amino acid content (A), protein content (B), and protease activity (C) in leaf, root, and nodule tissue. For (A), symbols are as in Fig. 1B. For (B) and (C), only C and D treatments are presented. Significant differences (P ≤ 0.05) between C and D are marked with an asterisk (*), and differences between C and PD or PDD are denoted with a hash sign (#). Values represent means ±SE [for (A) n=6; for (B, C) n=4].
After the onset of drought treatment, a general rise in the levels of total amino acid was observed in D and PDD samples (Fig. 2A). This increase was first observed in nodule and root tissue after 2 d of treatment, but was only significant on day 4 and 7 in nodules and roots, respectively. The accumulation of amino acid in leaves was only significant on day 7 of drought (Fig. 2A).
Comparing the levels of free amino acids at the most severe drought stage, nodules showed a non-significant >2-fold increase, while the levels increased ~7.5- and 4.8-fold in drought-stressed root and leaf samples, respectively. Neither PD leaves nor roots and nodules corresponding to the irrigated root system (PDC) showed an accumulation of free amino acids, with values close to well-irrigated (C) plants. In contrast, both tissues corresponding to the non-irrigated root fraction (PDD) and D plants presented a significant accumulation of free amino acids (Fig. 2A).
Protein concentration was not significantly affected by drought in any plant organ throughout the experiment (Fig. 2B). Similarly, no differences were observed in the protein content of different tissues in PD plants on day 7 (data not shown).
To test whether the observed amino acid accumulation was related to increased proteolysis, total protease activity was determined (Fig. 2C). Protease activity detected in a tissue appeared to be inversely related to its soluble protein and amino acid content: roots, the organ with the lowest amino acid and soluble protein levels, exhibited the highest protease activity (30ng trypsin mg–1 protein), a value >20-fold that of nodules and >8-fold greater than that observed in leaves. Under the conditions tested, however, drought stress did not have any clear effect on total protease activity, and the only significant change observed was a partial reduction in leaf protease activity on day 7 of D compared with C samples (Fig. 2C).
Drought induces an overall accumulation of individual amino acids in M. truncatula
The levels of the 20 standard amino acids synthesized by plants, together with the non-proteogenic γ-aminobutyric acid (GABA), were measured in leaves, roots, and nodules of M. truncatula plants.
Among the amino acids involved in primary N assimilation [glutamate (Glu), Gln, aspartate (Asp), and Asn], Asn was the most abundant in all organs tested, representing >70% of total amino acids in nodules (196 µmol g–1 DW), 47% in roots (11.7 µmol g–1 DW), and 38% in leaves (23.5 µmol g–1 DW). In nodules the sum of Asn, Gln, Asp, and Glu accounted for 83% of the total amino acids. The second most abundant amino acid in leaves and nodules in control conditions was Glu (22% and 9% of the total, respectively), while in roots the content of GABA was 9% of the total amino acid under control conditions (Supplementary Fig. S3 at JXB online).
In order to elucidate patterns in the drought-induced changes in the content of individual amino acids, graphs have been grouped within the context of the metabolic pathway from which they are synthesized. Figures 3 and 4 illustrate the changes in the levels of 18 amino acids during the experimental time course. Levels of glycine (Gly), serine (Ser), and cysteine (Cys) did not show significant variations during the drought treatment and are, therefore, not shown.
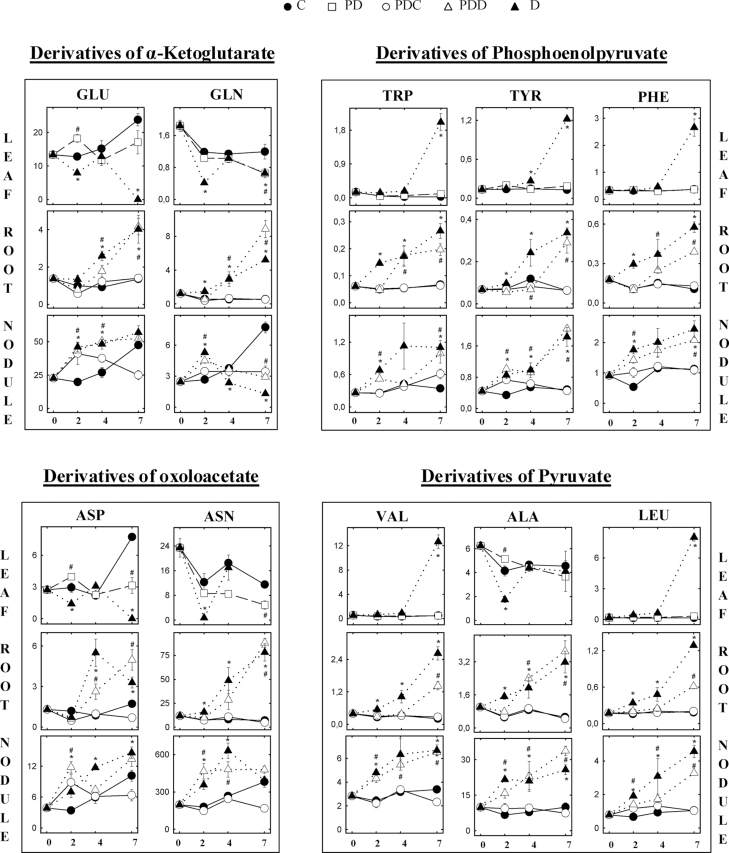
Fig. 3.
Effect of partial drought on the content of single amino acids in leaves, root, and nodules. Symbols are as in Fig. 1B. Values in µmol g–1 DW represent means ±SE (n=6).
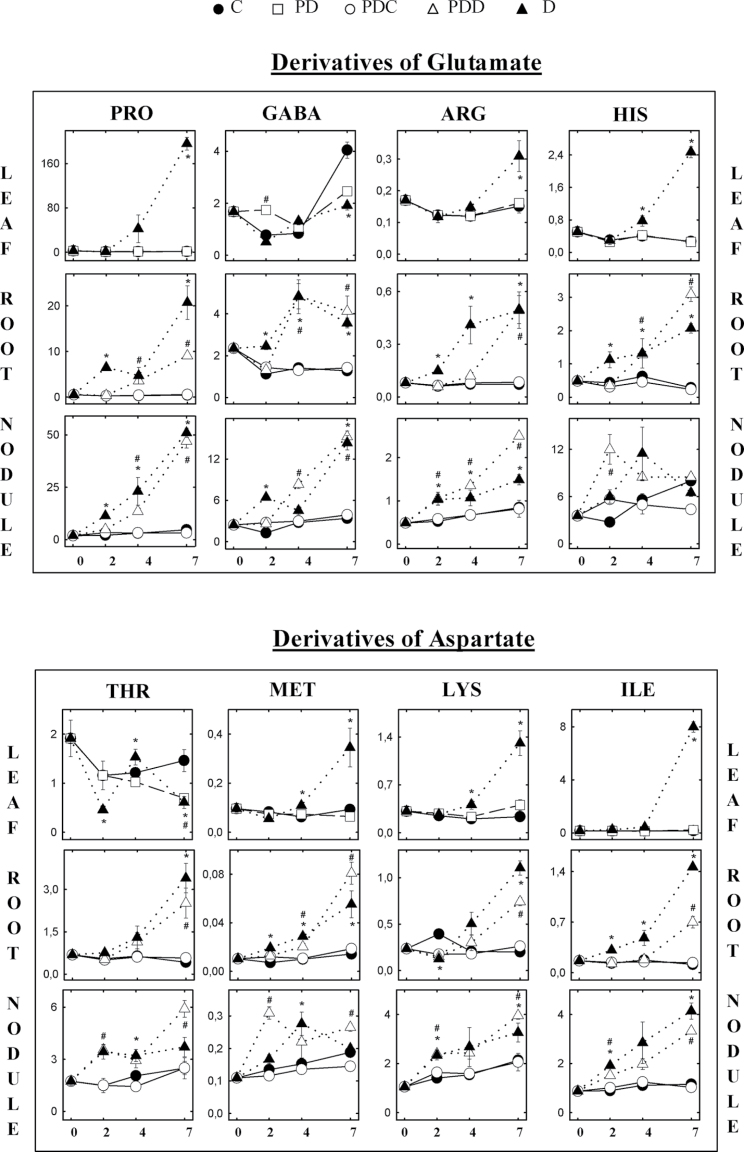
Fig. 4.
Effect of partial drought on single amino acid content in leaves, root, and nodules. Symbols are as in Fig. 1B. Values in µmol g–1 DW represent means ±SE (n=6).
Under C conditions, the level of the individual amino acids remained stable in all the organs tested throughout the study period (Figs. 3, 4). The unique exception to this pattern was observed in nodules, where an increasing trend was found in the levels of Asn, Asp, Gln, and Glu (Fig. 3) and their derived amino acids (Fig. 4). This pattern could be associated with the active NF occurring in this organ under C conditions.
When water deprivation was imposed, a general accumulation of individual amino acids was observed in all the plant organs tested (Figs. 3, 4). This accumulation was observed in roots and nodules from day 2 in D plants. However, in leaves of D plants, the main accumulation was found at the most intense drought stage (day 7), with some exceptions: alanine (Ala) content remained stable, and the levels of threonine (Thr), Glu, Gln, Asp, and GABA either decreased or remained close to control values instead of accumulating at the end of the study period. This second group shared a common pattern, in which there was a marked decline at day 2 and a subsequent restoration of values close to those of C plants. Asn could not be accurately quantified in D leaves at day 7 due to the 140-fold increase of proline (Pro) in these samples. However, the significant decline observed in PD leaves, together with the absence of a shoulder in the Pro peak of the electropherogram, suggests a decline of Asn in leaves of D plants, rather than any accumulation.
As a general observation, PD shoots did not accumulate amino acids with the same pattern as D plants. However, and similar to the pattern observed in D leaves, Gln, Asp, Asn, Thr, and GABA levels decreased in PD leaves.
The gradual accumulation of amino acids observed in leaves of D plants was also found in roots. Most of them showed significant differences at day 2, while Glu and Asp, and lysine (Lys) and Thr accumulated significantly only after day 4 and 7, respectively. Similarly, PDD roots showed increased levels in the content of the majority of amino acids, although this accumulation was slightly delayed compared with D plants. Glu, Gln, tryptophan (Trp), phenylalanine (Phe), Asp, Ala, Pro, GABA, histidine (His), and methionine (Met) accumulated significantly after day 4, whereas the remaining amino acids accumulated only at day 7. In contrast, the half of the root kept under well-irrigated conditions (PDC) did not accumulate any amino acid, maintaining levels close to those of C roots throughout the time course.
The trend of amino acid accumulation in nodules was very similar to the above description for roots in D, PDD, and PDC treatments. D nodules showed a significant accumulation of the majority of amino acids from day 2, which was accentuated with the more severe stress. In PDD nodules, the majority of the amino acids showed a gradual accumulation from day 2, with some amino acids reaching higher levels in PDD than in D nodules on day 7 [arginine (Arg), Thr, Met, Lys, Ala, and Asn]. Gln and Asn accumulated significantly in nodules of D plants during the moderate drought stress period (days 2 and 4) but declined to C levels (Asn) or even below C levels (Gln) under severe drought (day 7). In PDD nodules, Gln showed no increase at the end of the study period, and Asn levels were stable from day 4, but significantly higher than those of PDC nodules.
In order to investigate whether the accumulation of aromatic amino acids could be related to increased levels of their biosynthesis, immunodetection of the first enzyme in the Shikimate pathway, DAHPS, was carried out (Supplementary Fig. S4 at JXB online). No significant differences were observed in the relative amount of the protein detected.
Discussion
Local inhibition of NF in M. truncatula plants under drought stress
Although it is well known that symbiotic NF is rapidly inhibited by drought stress (Zahran, 1999), it remains to be understood whether this inhibition occurs at the nodule level (local control) or is driven by a systemic signal(s) from the aerial part in forage legumes. To respond to this question, plants were grown using an SRS so that one half of the root system of a plant remained fully hydrated (PDC), whereas the other half experienced progressive water deficit (PDD), with both halves sharing the same aerial part (PD). Thus, NF inhibition in the well-watered part would be indicative of a systemic regulation of the process. The same experimental system has been employed previously to show a local regulation of NF mediated by carbon metabolism in pea plants (Marino et al., 2007).
In the present work, several lines of evidence suggest that NF inhibition in drought-stressed M. truncatula occurred at the local level. First, a clear correlation between NF activity in plants subjected to partial drought and Ψnodule in PDD and PDC roots was established. ANA and Ψnodule values in the well-irrigated section of roots (PDC) showed no significant differences throughout the experiment, while roots subjected to drought (PDD) showed reductions in Ψnodule and a clear inhibition of NF (Fig. 1B). Secondly, a local inhibition mechanism can be inferred when considering that amino acids did not accumulate in D leaves until day 7 of drought, while a decline in ANA rates was already observed as early as day 2. This local inhibition of NF is in agreement with previous works in pea (Marino et al., 2007) and soybean (Ladrera et al., 2007). In the latter study, ureides accumulated in nodules, but not in leaves, in agreement with the hypothesis that it is an increase in N compounds in nodules that is responsible for NF inhibition under drought stress (Vadez et al., 2000; King and Purcell, 2005).
Nodule amino acid accumulation occurring under drought stress is independent of shoot water relations
Earlier studies, mostly carried out with tropical legumes, have shown an accumulation of N compounds in legume nodules when subjected to water deprivation (see King and Purcell, 2005, and references therein). One of the proposed explanations for this event is impairment of the export of N compounds derived from NF provoked by a reduction in water supply (Pate et al., 1969; Walsh, 1990). The maintenance in PD of Ψleaf and δ13C values in the same range of C plants indicated that half of the root system was able to maintain the shoot water status of PD plants (Fig. 1B; Supplementary Fig. S2A at JXB online). This, together with the slight decrease in ET in PD plants (20%) during the last 2 d of the experiment (Fig. 1B), shows that an adequate water status was maintained in shoots. In contrast, in the root fraction of PD plants, drought provoked a marked difference between PDC and PDD Ψnodule (Fig. 1B). While PDC nodules maintained stable values parallel with C nodules, Ψnodule of PDD dropped to the values exhibited by D nodules. Based on studies developed under optimal water status conditions, a model was proposed in which nodule water requirements were met by either phloem input or diffusion across the root cortex (Walsh et al., 1989a , b ; Walsh, 1990). The present study demonstrates that phloem supply was insufficient to maintain PDD nodule water status even though the aerial part was under optimal water conditions. This suggests that phloem flow is affected when the exogenous water supply is limited. However, the nature of this disturbance is not presently understood.
Other authors suggested that ET was the main cause for the accumulation of N compounds in pea nodules when transpiration rates were lowered by incubating plants in a high humidity atmosphere (Devisser and Poorter, 1984). A similar conclusion was reached by Minchin and Pate (1974) when examining nodule functioning through a day/night time course. However, none of these studies reported the nodule water status under the different conditions assayed. Results obtained in the current work show that the accumulation of the majority of single amino acids in D underground organs precedes any measurable drop in ET rates and, more interestingly, it also occurred in PDD roots, even when PD plants presented ET rates comparable with C values. Additionally, PDC nodules and roots did not show any accumulation of amino acids even at the stage when ET of these plants had significantly declined.
Amino acid accumulation does not appear to be related to increased proteolysis or de novo amino acid synthesis
Drought stress induces both a decline in the concentration of soluble protein (Irigoyen et al., 1992) and an increase in the level of protease activity in M. sativa nodules (Becana et al., 1986). In order to test whether the observed accumulations of amino acids could be explained by increased proteolytic activity, total protease activity (comprising Ser, Asp, Cys, and metalloproteases) was measured in different plant organs. No significant differences were observed between C and D samples under the conditions tested (Fig. 2C).
In other plant systems, a positive correlation between the level of tolerance to drought stress and the protease activity of different cultivars has been established (de Carvalho et al., 2001; Mosolov and Valueva, 2011). Furthermore, given the various structural and functional classes of proteases and their specific regulatory mechanisms, it is difficult to generalize their role in a process, with certain drought-tolerant plants exhibiting up-regulation of some proteases and down-regulation of others (Kohli et al., 2012). In the present case, the fact that there is not a clear shift in total protease activity is in agreement with the relatively stable measurements of total soluble protein (Fig. 2B), and suggests that this may not be the main mechanism behind the observed accumulation of amino acids.
It could then be hypothesized that amino acid accumulation could be related to increased biosynthesis, as shown for Pro and branched chain amino acids in plants subjected to drought stress (Rhodes et al., 1986; Shen et al., 1989; Girousse et al., 1996; Yoshiba et al., 1997; Nambara et al., 1998). As far as the levels of N assimilation enzymes (Asn synthetase, Gln synthetase, and Asp aminotransferase) are concerned, this possibility is not the most plausible. Results from absolute quantification of their levels in nodules of drought-stressed M. truncatula plants showed that the content of these enzymes is actually reduced by stress (Larrainzar et al., 2009). Furthermore, immunodetection of the first enzyme in the Shikimate biosynthetic pathway, DAHPS, responsible for the biosynthesis of aromatic amino acids (Trp, Tyr, and Phe) did not show any clear increase in the levels of this protein in nodules (Supplementary Fig. S4 at JXB online). Therefore, it seems that there is a complex regulation process controlling the amino acid content in plant cells under drought, with some of them (e.g. Pro) requiring the activation of their biosynthetic enzymes, while others are independent of these pathways (Good and Zaplachinski, 1994).
Taking these data together, the observed accumulation of amino acids in different organs of drought-stressed M. truncatula plants does not seem to be related to either increased proteolytic activity or higher de novo biosynthesis. Protein biosynthesis is known to be affected during the first drought stages, with decreases of water potential of <0.5MPa with respect to control plants (Hsiao, 1973). A reduction in the rates of protein biosynthesis, and, thus, a decline in the rates of incorporation of amino acids into proteins when the cell growth rate slows down seems to be the most plausible hypothesis to explain this accumulation of amino acids occurring at the whole-plant level.
N signal regulating NF in M. truncatula plants under drought stress
The water status alteration by drought stress provoked a profound variation in the total amino acid content in different plant tissues. None of the amino acids measured had a distinct accumulation pattern to nominate it as a candidate N signal. Results obtained at early stages of drought (day 2) would be the first place to look in order to detect this hypothetical N signalling molecule. However, at this stage, most of the amino acids were found to accumulate in both roots and nodules of D and PDD treatments. The general increase in the majority of single amino acids in the whole plant opens up a new scenario to question the commonly accepted concept of a single amino acid acting as an N-feedback signal.
The role of Asn, alone or in combination with ureides, as the putative N signal controlling NF under water deficit conditions has been suggested before (Vadez et al., 2000). In the present study, Asn, the most abundant amino acid in nodules, showed a significant accumulation on day 2 when NF was inhibited in D plants. Thus, the accumulation of Asn preceded the inhibition of NF, which might support a role for this amino acid in NF regulation. However, at this early stage, the increase in Asn was even more marked in PDD nodules, where NF was not significantly inhibited. This lack of correlation between Asn content and NF rates is in agreement with previous studies, in which drought induced a 2-fold increase in the levels of Asn in soybean and continued at those levels after rewatering, when NF had already reached control values (King and Purcell, 2005).
The involvement of Asp in the feedback inhibition of NF has also been suggested (King and Purcell, 2005), but, again, the present results do not support this hypothesis. Asp, similarly to the majority of the amino acids, accumulated after 2 d of treatment in D and PDD nodules compared with C conditions. However, no significant differences were measured between PDC and PDD treatments either in Asp nodule content or in ANA, which suggests that Asp does not play a significant role in the inhibition of NF.
Aranjuelo et al. (2011) determined the concentration of six amino acids (Ala, Asp, Ser, Asn, Glu, and Pro) in nodules of M. sativa plants after 7 d of drought, and they found patterns of nodule accumulation for these amino acids highly comparable with the present results. In addition to the amino acid signal candidates suggested in the literature as implicated in NF inhibition, other amino acids such as Arg, Trp, isoleucine (Ile), Thr, and leucine (Thr) have been shown to accumulate in several non-fixing plant species under stress (Nambara et al., 1998; Lea et al., 2007; Nikiforova et al., 2006). This observation suggests that amino acid accumulation may be a more widespread plant stress response than initially envisaged.
In brief, the present work tests for the first time the N-feedback hypothesis in an amide-exporter legume subjected to drought stress. It can be concluded that a local inhibition of NF occurs in the forage legume M. truncatula. The overall accumulation of single amino acids in all the plant organs tested points to a more intricate N signal regulation than the suggested candidates Asn or Asp in the literature so far. The observed accumulation of a number of amino acids in different parts of the plant, irrespective of the NF rates, is believed to be a consequence of a general drought stress response that could not be directly related to NF. Given the main differences in nodule anatomy and N metabolism between the temperate legume M. truncatula and the tropical legume soybean, further research is needed to investigate the relevance of the N-feedback hypothesis under drought conditions.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Plant biomass after 7 days of partial drought treatment in shoots (A), roots (B), and nodules (C).
Figure S2. Effect of 7 days of partial drought on carbon isotopic composition in leaves (A) and roots (B).
Figure S3. Distribution of amino acid content in leaves, roots, and nodules of control plants.
Figure S4. Western immunoblots of DAHPS in control and drought nodules of M. truncatula.
Acknowledgements
We are grateful to Gustavo Garijo for technical assistance, Joseba Aldasoro for help in cultivation and material harvesting, and Dr Frank R. Minchin for critical reading and helpful comments on the manuscript. The DAHPS antibody was kindly provided by Dr Mercedes Royuela and Dr Ana Zabalza. This work was financed by the Spanish Ministry of Economy and Competitiveness (AGL 2011–23738 and AGL 2011-30386-C02-01). EG-Q is a holder of a PhD fellowship from the Public University of Navarre (735/2008). EL is a recipient of a Marie Curie International Outgoing Fellowship for Career Development (PIOF-GA-2009–253141).
Glossary
Abbreviations:
- ANA
apparent nitrogenase activity
- C
control
- D
drought
- DAHPS
3-deoxy-d-arabino-heptulosonate-7-phosphate synthase;
- ET
evapotranspiration
- FW
fresh weight
- FITC
fluorescein isothiocyanate
- (N)DW
(nodule) dry weight
- NF
nitrogen fixation
- PD
partial drought
- SRS
split-root system
- δ13C
carbon isotopic composition
- Ψleaf
leaf water potential
- Ψnodule
nodule water potential.
References
- Antolin MC, Yoller J, Sanchez–Diaz M. 1995. Effects of temporary drought on nitrate-fed and nitrogen-fixing alfalfa plants. Plant Science. 107, 159–165 [Google Scholar]
- Aranjuelo I, Molero G, Erice G, Avice J-C, Nogues S. 2011. Plant physiology and proteomics reveals the leaf response to drought in alfalfa (Medicago sativa L.). Journal of Experimental Botany. 62, 111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt K, Brandt S, Kehr J. 2001. Amino acid analysis in five pooled single plant cell samples using capillary electrophoresis coupled to laser-induced fluorescence detection. Journal of Chromatography A. 926, 319–325 [DOI] [PubMed] [Google Scholar]
- Bacanamwo M, Harper JE. 1997. The feedback mechanism of nitrate inhibition of nitrogenase activity in soybean may involve asparagine and/or products of its metabolism. Physiologia Plantarum. 100, 371–377 [Google Scholar]
- Becana M, Aparicio-Tejo PM, Sanchez-Diaz M. 1986. Nitrate metabolism in alfalfa root-nodules under water-stress. Journal of Experimental Botany. 37, 798–806 [Google Scholar]
- de Carvalho MHC, d’Arcy-Lameta A, Roy-Macauley H, Gareil M, El Maarouf H, Pham-Thi AT, Zuily-Fodil Y. 2001. Aspartic protease in leaves of common bean (Phaseolus vulgaris L.) and cowpea (Vigna unguiculata L. Walp): enzymatic activity, gene expression and relation to drought susceptibility. FEBS Letters. 492, 242–246 [DOI] [PubMed] [Google Scholar]
- Del Castillo LD, Hunt S, Layzell DB. 1994. The role of oxygen in the regulation of nitrogenase activity in drought-stressed soybean nodules. Plant Physiology. 106, 949–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo LD, Layzell DB. 1995. Drought stress, permeability to O2 diffusion, and the respiratory kinetics of soybean root nodules. Plant Physiology. 107, 1187–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deSilva M, Purcell LC, King CA. 1996. Soybean petiole ureide response to water deficits and decreased transpiration. Crop Science. 36, 611–616 [Google Scholar]
- Devisser R, Poorter H. 1984. Growth and root nodule nitrogenase activity of Pisum sativum as influenced by transpiration. Physiologia Plantarum. 61, 637–642 [Google Scholar]
- Durand JL, Sheehy JE, Minchin FR. 1987. Nitrogenase activity, photosynthesis and nodule water potential in soybean plants experiencing water-deprivation. Journal of Experimental Botany. 38, 311–321 [Google Scholar]
- Evans HJ. 1981. Symbiotic nitrogen fixation in legume nodules.. In: Moore TC, ed. Research experiences in plant physiology, Vol. 1, New York: Springer-Verlag; 294–310 [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. 1989. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 40, 503–537 [Google Scholar]
- Gil-Quintana E, Aldasoro J, Ladrera R, Arrese-Igor C, González EM. 2009. Transpiration rate and amino acid distribution in water stressed Medicago truncatula plants. Acta Horticulturae. 846, 339–344 [Google Scholar]
- Girousse C, Bournoville R, Bonnemain JL. 1996. Water deficit-induced changes in concentrations in proline and some other amino acids in the phloem sap of alfalfa. Plant Physiology. 111, 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EM, Aparicio-Tejo PM, Gordon AJ, Minchin FR, Royuela M, Arrese-Igor C. 1998. Water-deficit effects on carbon and nitrogen metabolism of pea nodules. Journal of Experimental Botany. 49, 1705–1714 [Google Scholar]
- Gonzalez EM, Gordon AJ, James C, Arrese-Igor C. 1995. The role of sucrose synthase in the response of soybean nodules to drought. Journal of Experimental Botany. 46, 1515–1523 [Google Scholar]
- Good AG, Zaplachinski ST. 1994. The effects of drought stress on free amino acid accumulation and protein synthesis in Brassica napus . Physiologia Plantarum. 90, 9–14 [Google Scholar]
- Gordon AJ, Minchin FR, Skot L, James CL. 1997. Stress-induced declines in soybean N2 fixation are related to nodule sucrose synthase activity. Plant Physiology. 114, 937–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham PH, Vance CP. 2003. Legumes: importance and constraints to greater use. Plant Physiology. 131, 872–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao TC. 1973. Plant responses to water stress. Annual Review of Plant Physiology and Plant Molecular Biology. 24, 519–570 [Google Scholar]
- Irigoyen JJ, Emerich DW, Sanchez-Diaz M. 1992. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiologia Plantarum. 84, 55–60 [Google Scholar]
- King CA, Purcell LC. 2005. Inhibition of N2 fixation in soybean is associated with elevated ureides and amino acids. Plant Physiology. 137, 1389–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli A, Narciso JO, Miro B, Raorane M. 2012. Root proteases: reinforced links between nitrogen uptake and mobilization and drought tolerance. Physiologia Plantarum. 145, 165–179 [DOI] [PubMed] [Google Scholar]
- Ladrera R, Marino D, Larrainzar E, Gonzalez EM, Arrese-Igor C. 2007. Reduced carbon availability to bacteroids and elevated ureides in nodules, but not in shoots, are involved in the nitrogen fixation response to early drought in soybean. Plant Physiology. 145, 539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 227, 680–685 [DOI] [PubMed] [Google Scholar]
- Larrainzar E, Wienkoop S, Scherling C, Kempa S, Ladrera R, Arrese-Igor C, Weckwerth W, Gonzalez EM. 2009. Carbon metabolism and bacteroid functioning are involved in the regulation of nitrogen fixation in Medicago truncatula under drought and recovery. Molecular Plant-Microbe Interactions. 22, 1565–1576 [DOI] [PubMed] [Google Scholar]
- Larrainzar E, Wienkoop S, Weckwerth W, Ladrera R, Arrese-Igor C, Gonzalez EM. 2007. Medicago truncatula root nodule proteome analysis reveals differential plant and bacteroid responses to drought stress. Plant Physiology. 144, 1495–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea PJ, Sodek L, Parry MAJ, Shewry R, Halford NG. 2007. Asparagine in plants. Annals of Applied Biology. 150, 1–26 [Google Scholar]
- Marino D, Frendo P, Ladrera R, Zabalza A, Puppo A, Arrese-Igor C, Gonzalez EM. 2007. Nitrogen fixation control under drought stress. Localized or systemic?. Plant Physiology. 143, 1968–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino D, Gonzalez EM, Arrese-Igor C. 2006. Drought effects on carbon and nitrogen metabolism of pea nodules can be mimicked by paraquat: evidence for the occurrence of two regulation pathways under oxidative stresses. Journal of Experimental Botany. 57, 665–673 [DOI] [PubMed] [Google Scholar]
- Minchin FR, Pate JS. 1974. Diurnal functioning of legume root nodule. Journal of Experimental Botany. 25, 295–308 [Google Scholar]
- Mosolov VV, Valueva TA. 2011. Inhibitors of proteolytic enzymes under abiotic stresses in plants. Applied Biochemistry and Microbiology. 47, 453–459 [PubMed] [Google Scholar]
- Nambara E, Kawaide H, Kamiya Y, Naito S. 1998. Characterization of an Arabidopsis thaliana mutant that has a defect in ABA accumulation: ABA-dependent and ABA-independent accumulation of free amino acids during dehydration. Plant and Cell Physiology. 39, 853–858 [DOI] [PubMed] [Google Scholar]
- Naya L, Ladrera R, Ramos J, Gonzalez EM, Arrese-Igor C, Minchin FR, Becana M. 2007. The response of carbon metabolism and antioxidant defenses of alfalfa nodules to drought stress and to the subsequent recovery of plants. Plant Physiology. 144, 1104–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neo HH, Layzell DB. 1997. Phloem glutamine and the regulation of O2 diffusion in legume nodules. Plant Physiology. 113, 259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforova VJ, Bielecka M, Gakiere B, Krueger S, Rinder J, Kempa S, Morcuende R, Scheible WR, Hesse H, Hoefgen R. 2006. Effect of sulfur availability on the integrity of amino acid biosynthesis in plants. Amino acids. 30, 173–183 [DOI] [PubMed] [Google Scholar]
- Oti-Boateng C, Silsbury JH. 1993. The effects of exogenous amino acid on acetylene reduction activity of Vicia faba L cv Fjord. Annals of Botany. 71, 71–74 [Google Scholar]
- Pate JS, Gunning BES, Briarty LG. 1969. Ultrastructure and functioning of transport system of leguminous root nodule. Planta. 85, 11–34 [DOI] [PubMed] [Google Scholar]
- Purcell LC, Serraj R, de Silva M, Sinclair TR, Bona S. 1998. Ureide concentration of field-grown soybean in response to drought and the relationship to nitrogen fixation. Journal of Plant Nutrition. 21, 949–966 [Google Scholar]
- Ramos MLG, Gordon AJ, Minchin FR, Sprent JI, Parsons R. 1999. Effect of water stress on nodule physiology and biochemistry of a drought tolerant cultivar of common bean (Phaseolus vulgaris L.). Annals of Botany. 83, 57–63 [Google Scholar]
- Rhodes D, Handa S, Bressan RA. 1986. Metabolic changes associated with adaptation of plant-cells to water stress. Plant Physiology. 82, 890–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA. 1965. Letters to the Editor. Science. 149, 920–922 [DOI] [PubMed] [Google Scholar]
- Serraj R, Vadez V, Denison RF, Sinclair TR. 1999. Involvement of ureides in nitrogen fixation inhibition in soybean. Plant Physiology. 119, 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraj R, Vadez V, Sinclair TR. 2001. Feedback regulation of symbiotic N2 fixation under drought stress. Agronomie. 21, 621–626 [Google Scholar]
- Shen L, Foster JG, Orcutt DM. 1989. Composition and distribution of free amino acids in flatpea (Lathyrus sylvestris L) as influenced by water deficit and plant age. Journal of Experimental Botany. 40, 71–79 [Google Scholar]
- Sinclair TR, Serraj R. 1995. Legume nitrogen fixation and drought. Nature. 378, 344–344 [Google Scholar]
- Sulieman S, Fischinger SA, Gresshoff PM, Schulze J. 2010. Asparagine as a major factor in the N-feedback regulation of N2 fixation in Medicago truncatula . Physiologia Plantarum. 140, 21–31 [DOI] [PubMed] [Google Scholar]
- Takizawa K, Nakamura H. 1998. Separation and determination of fluorescein isothiocyanate-labeled amino acids by capillary electrophoresis with laser-induced fluorescence detection. Analytical Sciences. 14, 925–928 [Google Scholar]
- Twining SS. 1984. Fluorescein isothiocyanate-labeled casein assay for proteolytic enzymes. Analytical Biochemistry. 143, 30–34 [DOI] [PubMed] [Google Scholar]
- Vadez V, Sinclair TR. 2000. Ureide degradation pathways in intact soybean leaves. Journal of Experimental Botany. 51, 1459–1465 [PubMed] [Google Scholar]
- Vadez V, Sinclair TR. 2001. Leaf ureide degradation and N2 fixation tolerance to water deficit in soybean. Journal of Experimental Botany. 52, 153–159 [PubMed] [Google Scholar]
- Vadez V, Sinclair T, Serraj R. 2000. Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiologia Plantarum. 110, 215–223 [Google Scholar]
- Walsh KB. 1990. Vascular transport and soybean nodule function III. Implications of a continual phloem supply of carbon and water. Plant, Cell and Environment. 13, 893–901 [Google Scholar]
- Walsh KB, Canny MJ, Layzell DB. 1989. a Vascular transport and soybean nodule function. II. A role for phloem supply in product export. Plant, Cell and Environment. 12, 713–723 [Google Scholar]
- Walsh KB, McCully ME, Canny MJ. 1989. b Vascular transport and soybean nodule function: nodule xylem is a blind alley, not a throughway. Plant, Cell and Environment. 12, 395–405 [Google Scholar]
- Witty JF, Minchin FR. 1998. Methods for the continuous measurement of O2 consumption and H2 production by nodulated legume root systems. Journal of Experimental Botany. 49, 1041–1047 [Google Scholar]
- Yoshiba Y, Kiyosue T, Nakashima K, YamaguchiShinozaki K, Shinozaki K. 1997. Regulation of levels of proline as an osmolyte in plants under water stress. Plant and Cell Physiology. 38, 1095–1102 [DOI] [PubMed] [Google Scholar]
- Zahran HH. 1999. Rhizobium–legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiology and Molecular Biology Reviews. 63, 968–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.