Abstract
Reactive oxygen species play a key role in the response of plants to abiotic stress conditions. Their level is controlled in Arabidopsis thaliana by a large network of genes that includes the H2O2-scavenging enzymes cytosolic ascorbate peroxidase (APX) 1 and 2. Although the function of APX1 has been established under different growth conditions, genetic evidence for APX2 function, as well as for the mode of cooperation between APX1 and APX2, is very limited. This study characterized the response of Arabidopsis mutants deficient in APX1, APX2, and APX1/APX2 to heat, salinity, light, and oxidative stresses. The findings reveal that deficiency in APX2 resulted in a decreased tolerance to light stress, as well as an enhanced tolerance to salinity and oxidative stresses. Interestingly, plants lacking APX2 were more sensitive to heat stress at the seedling stage, but more tolerant to heat stress at the reproductive stage. Cooperation between APX1 and APX2 was evident during oxidative stress, but not during light, salinity, or heat stress. The findings demonstrate a role for APX2 in the response of plants to light, heat, salinity, and oxidative stresses. The finding that plants lacking APX2 produced more seeds under prolonged heat stress conditions suggests that redundant mechanisms activated in APX2-deficient plants during heat stress play a key role in the protection of reproductive tissues from heat-related damage. This finding is very important because heat-associated damage to reproductive tissues in different crops is a major cause for yield loss in agriculture production worldwide.
Key words: Abiotic stress, ascorbate peroxidase, cytosol, heat stress, hydrogen peroxide, reactive oxygen species
Introduction
Despite their toxic potential, reactive oxygen species (ROS) play a key role as signalling molecules that regulate growth, development, pathogen defence pathways, and abiotic stress acclimation (Torres and Dangl, 2005; Asada, 2006; Mittler et al., 2011; Suzuki et al., 2011). Plants possess a large network of ROS-producing and-scavenging pathways essential for the maintenance of ROS levels and the regulation of ROS signals (Mittler et al., 2004, 2011; Bailey-Serres and Mittler, 2006). This network is composed of over 150 genes encoding ROS-producing proteins such as NADPH oxidases and ROS-scavenging enzymes such as superoxide dismutases (SODs), catalases (CATs), and ascorbate peroxidases (APXs; Mittler et al., 2004).
Ascorbate peroxidases play important roles in the regulation of cellular ROS levels and are central components of the hydrogen peroxide-scavenging networks. Previous studies revealed that cytosolic APX1 is involved in a broad range of biological processes (At1g07890; Pnueli et al., 2003; Davletova et al., 2005a). It is constitutively expressed in roots, leaves, stems, and many other plant tissues, and its expression is significantly upregulated in response to a large number of biotic and abiotic stresses (Zimmermann et al., 2004). Arabidopsis thaliana plants deficient in cytosolic APX1 were characterized by growth suppression, altered stomatal responses, higher sensitivity to oxidative stress, accumulation of high H2O2 levels under different growth conditions, and high accumulation of oxidized proteins under light stress (Pnueli et al., 2003; Davletova et al., 2005a). Cytosolic APX1 was also shown to be essential for the protection of thylakoid and stromal/mitochondrial APXs (Davletova et al., 2005a) as well as the protection of nuclear DNA during light stress (Vanderauwera et al., 2011). These results suggest that the function of cytosolic APX1 is tightly linked to ROS signalling pathways in different cellular compartments and could be involved in the regulation of ROS levels in the entire cell.
A second cytosolic APX isozyme, namely APX2 (also called APX1B in Arabidopsis; At3g09640), is also involved in the response of plants to abiotic stress. Expression of APX2 is almost undetected in many plant tissues and is significantly upregulated in roots in response to wounding and oxidative stress, in roots and shoots in response to salinity and osmotic stress, and in roots, shoots, leaves, and pollen in response to heat stress (Zimmermann et al., 2004; Frank et al., 2009). Expression of APX2 was also shown to be restricted to vascular and bundle sheath cells in response to high light stress and regulated by H2O2, abscisic acid, and leaf water status (Fryer et al., 2003; Bechtold et al., 2008; Galvez-Valdivieso et al., 2009). In addition, a mutant with constitutively higher expression of APX2 showed enhanced tolerance to drought and high abscisic acid levels (Rossel et al., 2006). APX2 is highly responsive to heat stress and might play important roles in the regulation of heat tolerance in plants (Shi et al., 2001; Panchuk et al., 2002; Larkindale and Huang, 2004; Schramm et al., 2006). Expression of APX2 was induced by heat stress in an HSF3-dependent manner corresponding with the appearance of a particular isoform of APX required for heat tolerance (Panchuk et al., 2002). Expression of a tomato cytosolic APX, homologous to APX2 in Arabidopsis, was also significantly upregulated in pollen during heat stress (Frank et al., 2009). These results suggest that APX2 plays a key role in the regulation of heat stress responses in a wide range of different developmental stages in plants. Nevertheless, genetic loss-of-function evidence for APX2 role in the acclimation of plants to abiotic stress conditions is very limited.
A complex mode of crosstalk between different ROS-scavenging systems has been revealed by recent analyses of double mutants lacking one or more ROS-scavenging systems (Rizhsky et al., 2002; Miller et al., 2007; Rosa et al., 2010; Vanderauwera et al., 2011). Double mutants lacking two different ROS-scavenging enzymes showed specific phenotypes that were not observed in single mutants. For example, double antisense tobacco plants lacking APX and CAT were less sensitive to oxidative stress compared to single antisense plants lacking APX or CAT (Rizhsky et al., 2002). In Arabidopsis, a double mutant deficient in cytosolic and thylakoid APX exhibited specific phenotypes that were characterized by late flowering, low protein oxidation during light stress, and enhanced accumulation of anthocyanins (Miller et al., 2007). More recently, Vanderauwera et al. (2011) demonstrated that a combined deficiency in cytosolic APX1 and peroxisomal catalase 2 (CAT2) triggered a unique signalling pathway that activated an acclimation response involving DNA repair, regulation of cell cycle, and antiprogrammed cell death mechanisms and rendered double mutants more tolerant than wild type or single mutants to light or oxidative stress. Transgenic rice silenced for cytosolic APX1 or APX2 displayed a semi-dwarf phenotype. In contrast, transgenic rice silenced for both cytosolic APX1 and APX2 showed normal growth and development (Rosa et al., 2010). These results suggest that specific signals triggered by crosstalk between different ROS-scavenging pathways could trigger the activation of unique acclimation responses.
To study how signals regulated by different ROS-scavenging enzymes in the same cellular compartment are initiated and coordinated in cells, this study generated double mutants lacking two cytosolic ROS-scavenging enzymes, APX1 and APX2 (apx1/apx2) in Arabidopsis and analysed them. APX1 and APX2 are likely to be co-expressed in leaf vascular and bundle sheath cells, pollen, and different root and shoot cells during stress (Zimmermann et al., 2004; Frank et al., 2009). The analysis of the double and single mutants for apx1 and apx2 revealed that signals associated with cytosolic APX1 are distinct from those associated with APX2 under abiotic stress. In addition, this study found that signals generated in apx2 plants might play a key role in the protection of reproductive tissues against heat stress, and identified a role for APX2 in the protection of plants against light, oxidative, heat, and salinity stresses.
Materials and methods
Plant material, growth conditions, and molecular analysis
A. thaliana plants (cv. Columbia-0) were grown on peat pellets (Jiffy-7, Shippagan) under controlled conditions: 21 °C, 16/8 light/dark cycle, 50 µmol m–2 s–1, and 70% relative humidity (E-30, AR-66, Percival Scientific). Knockout plants deficient in cytosolic APX1 (SALK_000249; apx1) or APX2 (SALK_091880; apx2) were obtained through the SIGnAL project (http://signal.salk.edu/tabout.html), backcrossed, and screened in homozygous form as described in Rizhsky et al. (2004) and Davletova et al. (2005a,b). To generate double-knockout plants deficient in both APX1 and APX2 (apx1/apx2), apx1 plants were crossed with apx2 plants, selfed, and tested for homozygous genotype from F2 population by PCR analysis and RNA blots. F3 or F4 populations of apx1/apx2 plants were used in all experiments. RNA was isolated and analysed by RNA blot as previously described (Pnueli et al., 2003; Rizhsky et al., 2004; Davletova et al., 2005a). cDNA probes corresponding to the following genes were used for RNA blots: Zat7, At3g46080; Zat10, At1g27730; Zat12, At5g59820; APX1, At1g07890; APX2, At3g09640. RNA staining or a ribosomal 18S rRNA probe were used to control for RNA loading.
Stress assays
To analyse the response of plants to light stress, 6-day-old plants grown on peat pellets under controlled conditions were transferred to constant light condition (1000 µmol m–2 s–1) and grown for 14 days. Control plants were kept under controlled conditions with constant light (50 µmol m–2 s–1). All other growth conditions were maintained constant. Plants were then photographed under a dissecting microscope (Leica M80) and measured. Anthocyanin content was determined according to Bariola et al. (1999). For the analysis of transcript accumulation in response to light stress, 21-day-old plants grown under controlled conditions were subjected to light stress (1000 µmol m–2 s–1) and sampled at 0, 0.5, 1, and 3h. For the analysis of abiotic stress tolerance, seeds were surface-sterilized with bleach and placed in rows on 1% agar [0.5 × Murashige and Skoog (MS) medium] containing different concentrations of paraquat or NaCl as described by Luhua et al. (2008). Five- or six-day-old seedlings were then scored for root length as described by Luhua et al. (2008). Five- or six-day-old seedlings grown on 0.5 × MS agar were also subjected to heat stress (38 °C; 6h), recovered at 21 °C for 4 days and scored for root length. Basal and acquired thermotolerance assays were performed as described by Suzuki et al. (2008). To image H2O2 accumulation, 5- or 6-day-old seedlings grown on 0.5 × MS agar in the presence or absence of paraquat or NaCl were treated with 0.2 µM Amplex Red (Molecular Probes) for 20min and imaged with a Kodak 2000MM image station (Davletova et al., 2005b; Luhua et al., 2008). To study the heat stress response of reproductive tissues, 25-day-old plants grown under controlled conditions as described above were transferred to a growth chamber with the following temperature cycle: 06:00–09:00, 21 °C; 09:00–10:00, 25 °C; 10:00–12:00, 42 or 45 °C; 12:00–13:00, 25 °C; 13:00–06:00, 20 °C. Plants were grown for 28 days under these temperature conditions. The 16h light period was imposed from 06:00 to 22:00. Control 25-day-old plants grown under controlled conditions as described above were maintained in parallel under controlled growth conditions for 21–28 days. Siliques sampled from control or heat-stressed plants were soaked in 80% ethanol overnight to remove chlorophyll and cleared siliques were scored for length and number of seeds under a dissecting microscope (Leica M80). Eight to ten siliques were sampled from each plant. Ten plants grown under each condition were used for the analysis. All experiments were performed in triplicate and repeated at least three times. Statistical analysis was performed as described by Suzuki et al. (2005) and Davletova et al. (2005a), and results are presented as mean±SD.
Results and discussion
Generation of double-knockout plants deficient in cytosolic APX1 and APX2
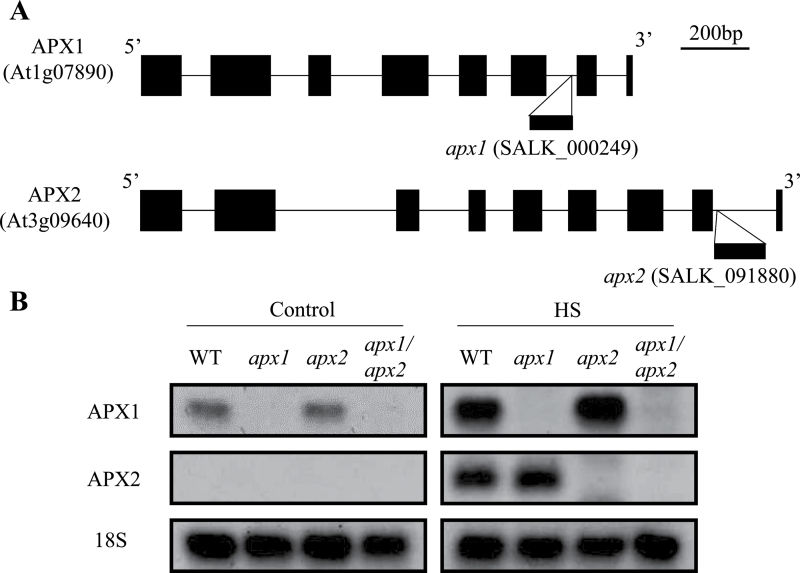
This study obtained knockout plants deficient in cytosolic APX1 (apx1; SALK_000249; Columbia-0 background) or APX2 (apx2; SALK_091880; Columbia-0 background) through the SIGnAL project. As shown in Fig. 1A, apx1 or apx2 plants contained a T-DNA insertion in the 6th or 8th intron, respectively. To generate double-knockout plants deficient both in APX1 and APX2 (apx1/apx2), apx1 plants were crossed with apx2 plants. Progenies of the crosses were selfed, tested for homozygous genotype of double-knockout plants, and bulked. To further confirm deficiency in cytosolic APXs in each mutant, accumulation of APX1 and APX2 transcripts was tested in 21-day-old plants that were subjected to heat stress (38 °C for 1h; Fig. 1B), or grown under controlled growth conditions. RNA blot analysis confirmed the absence of APX1 in apx1 and apx1/apx2 plants, as well as the absence of APX2 in apx2 and apx1/apx2 plants. Expression of APX1 or APX2 transcript was upregulated to the level detected in wild-type plants during heat stress in apx2 or apx1 plants, respectively. In contrast to the knockout APX1 line in the Wassilewskija background that showed late flowering and suppressed growth in a previous study (Pnueli et al., 2003), all single-and double-knockout Columbia-0 plants in this study did not display any visible phenotypes under controlled growth conditions (data not shown). Such differences in growth phenotypes between different ecotypes lacking APX1 might be due to differences in genetic background or growth conditions.
Fig. 1.
Gene structure and expression of APX1 and APX2 in wild-type plants and the different mutants used in this study. (A) A gene map showing the site of T-DNA insertion into the APX1 or APX2 genes in apx1 or apx2 plants. (B) Expression of APX1 and APX2 in wild-type (WT), apx1, apx2, and apx1/apx2 plants grown under controlled growth conditions (control: 21 °C) or subjected to heat stress (HS: 38 °C, 1h).
Response of single-and double-knockout plants to light stress
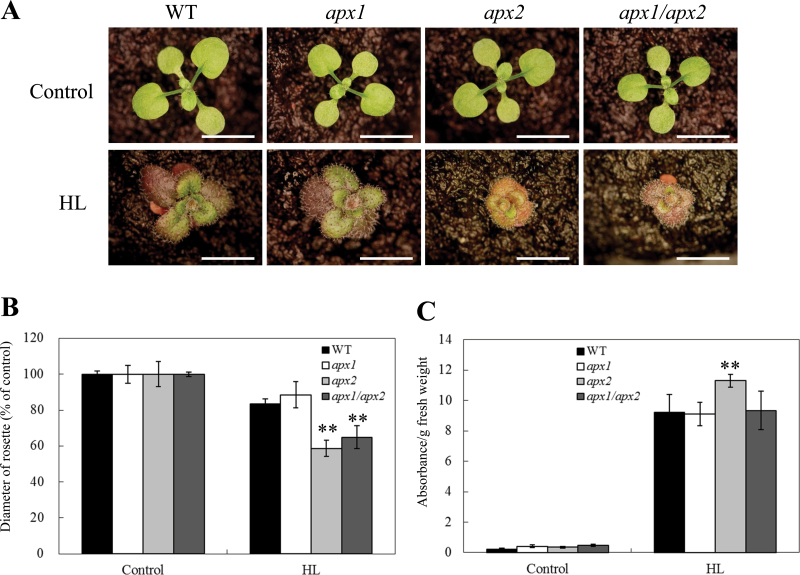
To examine how single-or double-knockout plants respond to light stress, 6-day-old wild-type, apx1, apx2, and apx1/apx2 plants were subjected to light stress (1000 µmol m–2 s–1) for 14 days and assayed for plant diameter and anthocyanin accumulation (Fig. 2). Plant diameter of apx1 plants was comparable to that of wild-type plants under light stress. In contrast, apx2 and apx1/apx2 plants showed a significantly smaller plant diameter compared to wild-type plants (Fig. 2A, B). This result suggested that similar growth inhibiting signals might be generated in apx2 and apx1/apx2 plants under light stress. Anthocyanin accumulation was nevertheless enhanced only in apx2 plants under the same condition (Fig. 1C). Anthocyanin synthesis might therefore be regulated by signals that are specifically activated in apx2 plants, but are suppressed in apx1/apx2 mutants. Anthocyanin accumulation was previously associated with ROS removal under light stress conditions in a light-sensitive mutant deficient in CAT2 activity (Vanderauwera et al., 2005). However, the present results indicate that a deficiency in APX2 has an opposite effect to that of CAT2 with respect to anthocyanin accumulation, suggesting that H2O2 scavenging in the cytosol by APX2, or in peroxisomes by CAT2, can have a significantly different effect on anthocyanin accumulation during light stress.
Fig. 2.
Long-term exposure of wild-type (WT), apx1, apx2, and apx1/apx2 plants to light stress. (A) Photographs of plants grown under controlled growth conditions (control: 50 µmol m–2 s–1) or light stress (HL: 1000 µmol m–2 s–1). Six-day-old plants were subjected to light stress for 14 days. Bar = 5mm. (B) Diameter of plants grown under controlled conditions or subjected to light stress as described in A. (C) Anthocyanin accumulation in plants grown under controlled conditions or subjected to light stress as described in A. Anthocyanin levels were determined from absorbance at 530nm (A530) and 657nm (A657), and calculated as (A530 – 0.25×A657)/fresh weight. Stress treatments and anthocyanin extraction were performed as described in Materials and methods. **, Student’s t-test significant at P < 0.01.
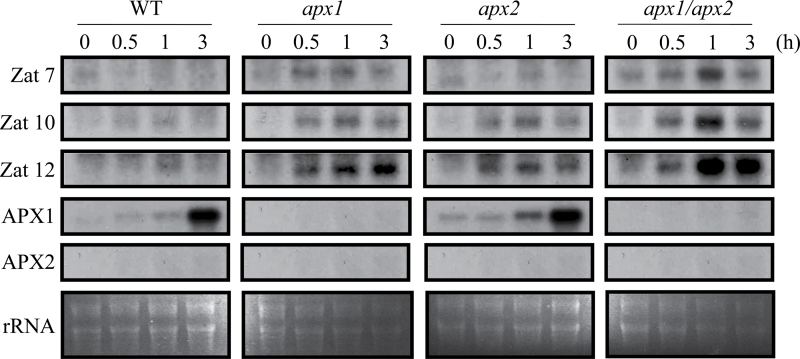
A short (0, 0.5, 1, and 3h) RNA blot time-course analysis conducted on wild-type, apx1, apx2, and apx1/apx2 plants subjected to light stress revealed that light stress resulted in an enhanced accumulation of transcripts encoding the oxidative stress-response zinc finger proteins Zat10 and Zat12 in apx1, apx2, and apx1/apx2 plants (Fig. 3). Compared to wild-type plants, apx1 and apx1/apx2 plants also showed higher accumulation of transcripts encoding another zinc finger protein, Zat7, under the same condition. Interestingly, the expression of Zat10, Zat12, and Zat7 was higher in apx1/apx2 plants compared to wild-type, apx1, or apx2 plants. These results could indicate that apx1/apx2 plants are subjected to a higher level of oxidative stress during light stress compared to wild-type, apx1, or apx2 plants.
Fig. 3.
Steady-state level of transcripts encoding different abiotic stress acclimation and ROS-response proteins in control (WT), apx1, apx2, and apx1/apx2 plants grown under controlled growth conditions (time 0), or subjected to light stress (1000 µmol m–2 s–1) for 0.5, 1, or 3h. Steady-state level of transcripts was determined by RNA gel blots as described in Materials and methods.
The involvement of APX2 in the response of plants to light stress was indicated by altered growth and anthocyanin accumulation in plants grown under light stress conditions for 14 days (Fig. 2). In contrast, the steady-state level of APX2 transcripts was not detected by RNA blots in wild-type and apx1 plants subjected to a short (0–3h) light stress of similar intensity (Fig. 3). Induction of APX2 under light stress was demonstrated in previous studies (Fryer et al., 2003; Rossel et al., 2006; Galvez-Valdivieso et al., 2009). It is possible, however, that the duration of light stress used for the assays shown in Fig. 3 was not long enough to upregulate APX2 expression. Another possibility is that the level of APX2 expression during light stress is too low to be detected by RNA blot analysis (compared to the detecting of APX2 expression in response to heat stress shown in Fig. 1B). At least with respect to growth suppression under light stress, the signals activated in apx2 mutants appeared to be dominant to those activated in apx1 mutants. This finding could indicate that signalling events occurring in vascular and bundle sheath cells that express APX2 during high light stress play an important role in the acclimation of plants to these stresses (Galvez-Valdivieso et al., 2009). Because the expression pattern of APX1 and APX2 is complex in leaves (both are expressed in vascular and bundle sheath cells, but only APX1 is expressed in mesophyll and epidermis cells), this study focused the analysis on roots and reproductive tissues that include pollen in which both APX1 and APX2 are likely to be co-expressed in different cells during oxidative, salinity, and heat stresses.
Response of single and double mutants to oxidative stress
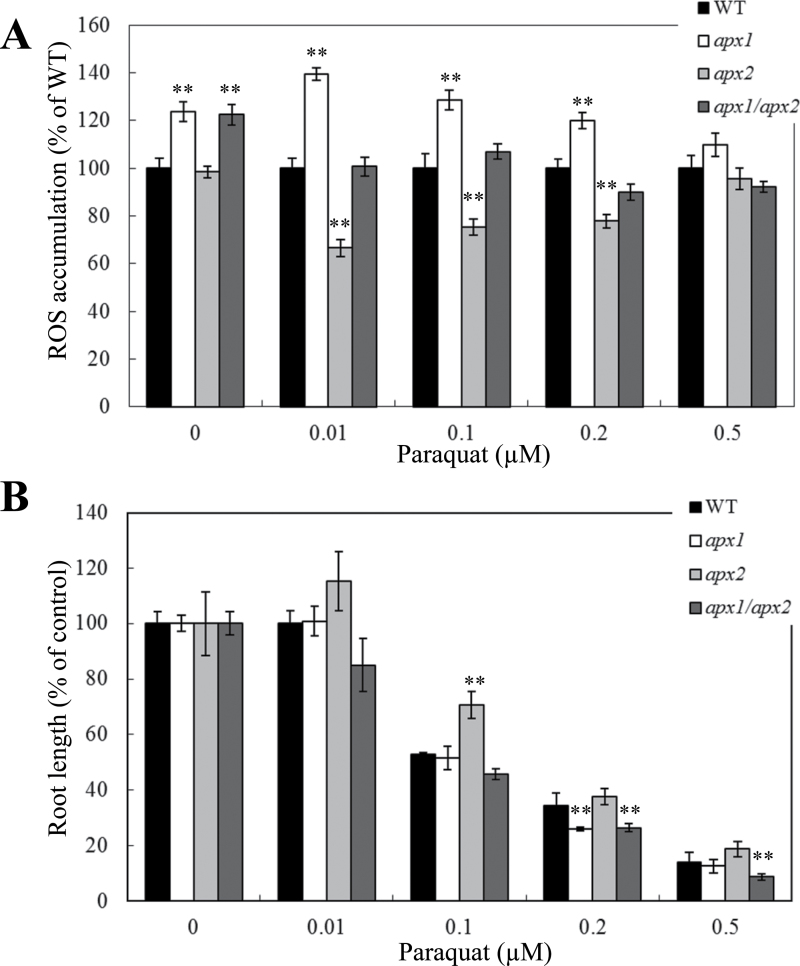
As shown in Fig. 4A, disruption in cytosolic APX1 resulted in enhanced ROS accumulation in roots of Arabidopsis seedlings under both controlled and oxidative stress (paraquat) conditions. Aside from one paraquat concentration (0.2 µM), the tolerance of apx1 to oxidative stress was nevertheless comparable with that of wild-type plants (Fig. 4B), implicating the existence of redundant ROS-response pathways that might complement for a deficiency in APX1. In contrast to apx1 plants, apx2 plants showed reduced ROS accumulation (Fig. 4A) and enhanced tolerance to oxidative stress imposed by paraquat treatment (Fig. 4B). It is likely that signals generated by the disruption in APX2 might activate redundant ROS-scavenging pathways reducing ROS accumulation in cells and promoting root growth under oxidative stress conditions. Interestingly, the enhanced tolerance and reduced ROS accumulation of apx2 mutants during oxidative stress was abolished in the double apx1/apx2 mutants (Fig. 4), suggesting that APX1 is an important component of the redundant mechanisms activated in roots of apx2 mutants. At least with respect to the mechanisms or signals activated in apx1 or apx2 during oxidative stress induced by paraquat in roots, it appears that the mechanisms/signals triggered in the apx1 mutant are dominant to those activated in the apx2 mutant (Fig. 4).
Fig. 4.
Effect of oxidative stress on H2O2 accumulation and root growth in seedlings of control (WT), apx1, apx2, and apx1/apx2 plants. (A) Accumulation of H2O2 in WT, apx1, apx2, and apx1/apx2 seedlings in the presence or absence of different concentrations of the superoxide-generating compound paraquat. H2O2 was detected using Amplex Red as described in Materials and methods. (B) Root growth of WT, apx1, apx2, and apx1/apx2 seedlings subjected to the same oxidative stress treatment as in A. **, Student’s t-test significant at P < 0.01.
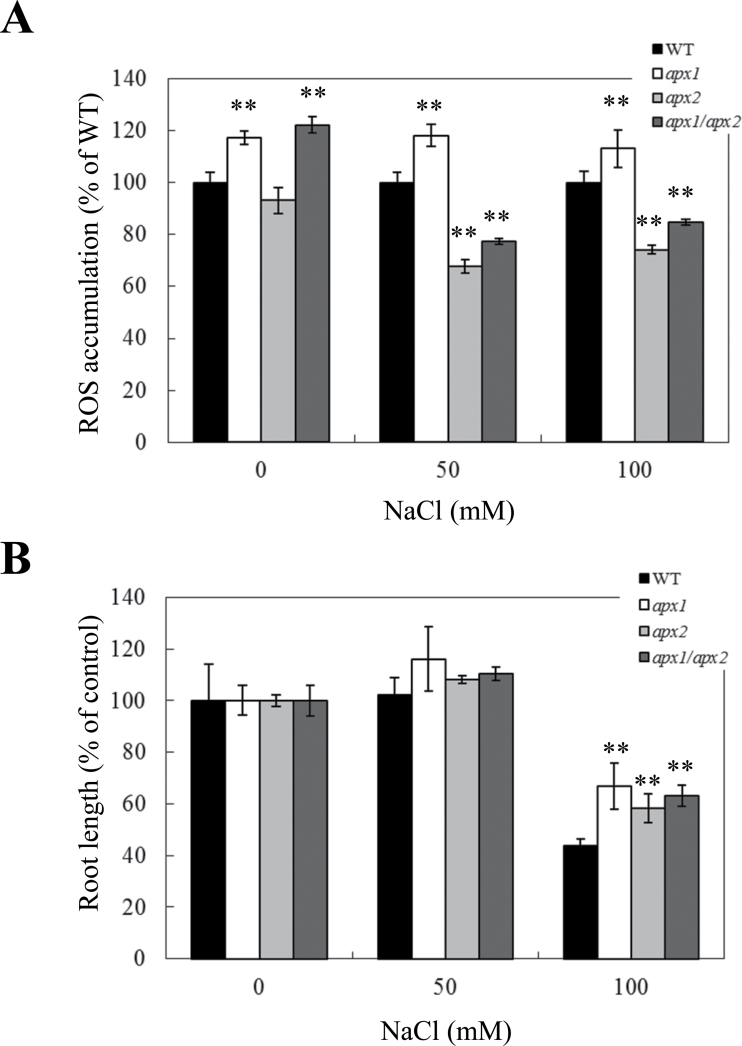
Disruption of cytosolic APXs enhances the tolerance of plants to salt stress
As shown in Fig. 5A, apx1 plants showed higher ROS accumulation in roots compared to wild-type plants under controlled growth conditions and salt stress. In contrast, ROS accumulation was suppressed in apx2 and apx1/apx2 plants under the same condition. Despite these differences in the patterns of ROS accumulation during salt stress, all mutants lacking one or both cytosolic APXs showed enhanced tolerance to salinity (Fig. 5B). The enhanced tolerance of apx1 plants to salt stress appeared to be associated with ROS accumulation whereas the enhanced tolerance of apx2 and apx1/apx2 plants appeared to be associated with lower levels of ROS accumulation. Because ROS levels were suppressed under salt stress in the apx1/apx2 double mutant (similar to the apx2 mutant), the mechanisms/signals triggered in roots of the apx2 background appear to be dominant to those activated in the apx1 mutant during salt stress. This observation is similar to the relationship observed during light stress (Fig. 2), but is in sharp contrast to that observed with oxidative stress (Fig. 4).
Fig. 5.
Effect of salinity stress on H2O2 accumulation and root growth of control (WT), apx1, apx2, and apx1/apx2 plants. (A) Accumulation of H2O2 in WT, apx1, apx2, and apx1/apx2 seedlings in the presence or absence of different concentrations NaCl. H2O2 was detected using Amplex Red as described in Materials and methods. (B) Root growth of WT, apx1, apx2, and apx1/apx2 seedlings subjected to salinity stress. **, Student’s t-test significant at P < 0.01.
Altered tolerance of apx2 and apx1/apx2 plants to heat stress in seedlings and during plant reproduction
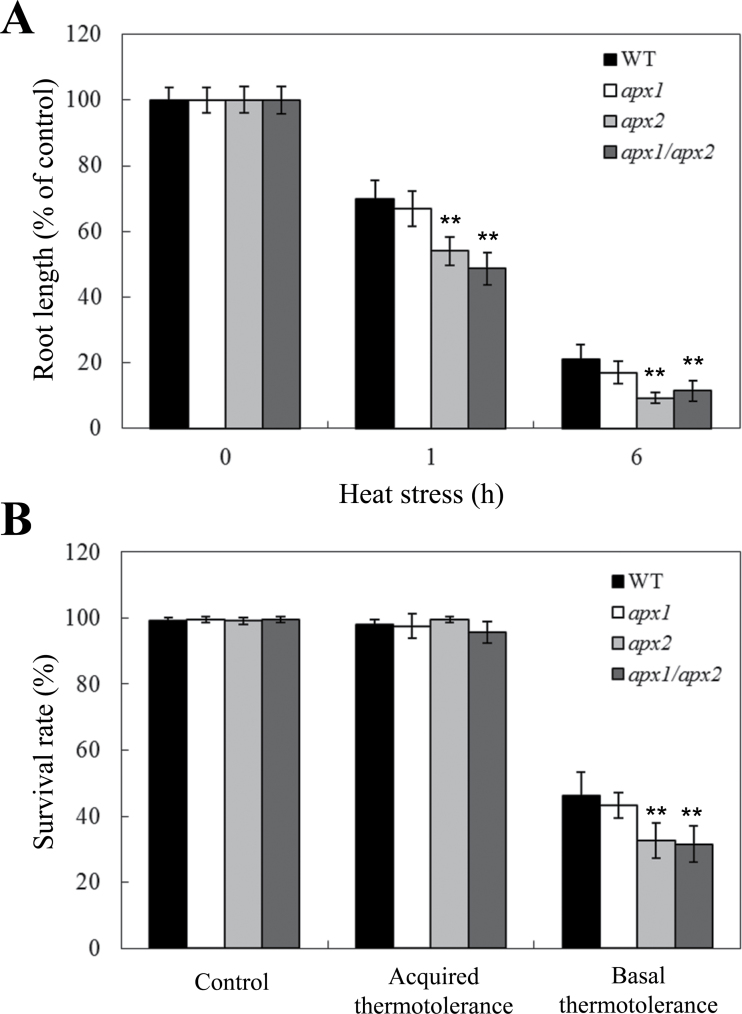
Previous studies demonstrating the involvement of ROS signalling and cytosolic APXs in the heat stress response of plants (Panchuk et al., 2002; Suzuki and Mittler, 2006; Larkindale and Vierling, 2008) prompted this study to investigate how a deficiency in both cytosolic APXs would affect thermotolerance in Arabidopsis. As shown in Fig. 6, root growth of apx2 and apx1/apx2 seedlings was more sensitive to heat stress compared to wild-type or apx1 plants. In contrast, this study could not detect significant differences between apx1 and wild-type plants in root growth during heat stress. To examine whether the deficiency in heat tolerance in the apx2 and apx1/apx2 mutants could be compensated by a prior heat acclimation, the basal and acquired thermotolerance (Suzuki et al., 2008) of the different lines was compared. As shown in Fig. 6B, prior acclimation of plants to heat stress (acquired thermotolerance) could compensate for the deficiency in apx2 and apx1/apx2.
Fig. 6.
Effect of heat stress on control (WT), apx1, apx2, and apx1/apx2 seedlings. (A) Root growth of WT, apx1, apx2, and apx1/apx2 seedlings under heat stress (38 °C). Five- to six-day-old plants were subjected to 1 or 6h heat stress, followed by a 4-day recovery period. (B) Acquired and basal thermotolerance of WT, apx1, apx2, and apx1/apx2 seedlings. Five-day-old seedlings were directly subjected to heat stress (45 °C) for 2h to measure basal thermotolerance, or treated at 38 °C for 1.5h, allowed to recover for 1h at 21 °C, and subjected to 45 °C for 2h to measure acquired thermotolerance.
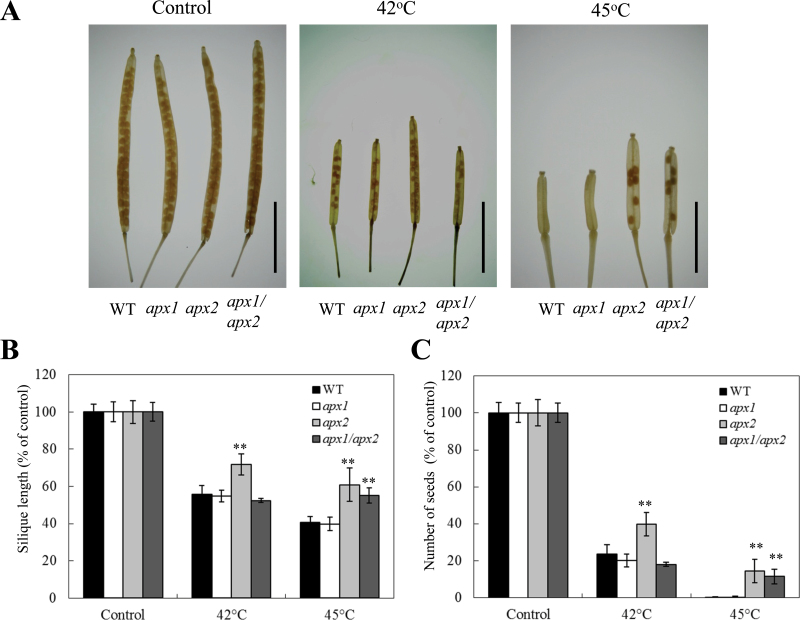
To study the role of cytosolic APXs in the heat stress response of reproductive tissues, 25-day-old plants were subjected to a daily 2-h heat stress period (42 or 45 °C) over 28 days and scored for silique length and number of seeds (Fig. 7). Interestingly, apx2 plants showed longer siliques and a higher number of seeds per silique compared to the other lines in response to the 42 °C treatment (Fig. 7). In contrast, apx1/apx2 plants as well as apx2 plants produced more seeds per silique and had longer siliques under the stronger heat stress treatment (45 °C). Under all experimental conditions tested, mutants deficient in apx1 were similar to wild-type plants in seed production and silique length (Fig. 7). These results suggest that mechanisms and signals generated during heat stress by the disruption in APX2 protect reproductive tissues against heat stress. These signals/mechanisms may be stage or tissue specific or may be a result of an overall higher fitness of apx2 plants during temperature stress. The finding that seedlings deficient in APX2 are more sensitive to heat stress (Fig. 6) could suggest that APX2-deficient plants have an overall lower fitness to heat thereby indicating that the enhanced protection of reproductive tissue (Fig. 7) is stage or tissue specific. Nevertheless, more studies are required to address this point. The expression of APX2 under controlled growth conditions is almost undetectable in all vegetative and reproductive tissues of Arabidopsis including pollen (Genevestigator; Zimmermann et al., 2004). In tomato, expression of a cytosolic APX, homologous to Arabidopsis APX2, was significantly upregulated in pollen under heat stress (Frank et al., 2009). Taken together, it is possible that signals associated with APX2 deficiency are specifically activated in reproductive tissues under heat stress conditions and play a key role in the regulation of plant productivity during heat stress. In contrast, signals associated with APX1 during heat stress might not play a significant role in the heat stress response of reproductive tissue. When it comes to heat stress, signals activated in roots and reproductive tissues of Arabidopsis, due to a deficiency in APX2, appear to be dominant to signals activated by a deficiency in APX1 (Figs. 6 and 7).
Fig. 7.
Effect of heat stress on seed production and silique length in control (WT), apx1, apx2, and apx1/apx2 plants. (A) Photographs of representative cleared siliques from WT, apx1, apx2, and apx1/apx2 plants grown under controlled growth conditions (control), or subjected to periodic heat stress at 42 or 45 °C. Bar=5mm (B and C) Graphs showing silique length (B), and number of seeds per silique (C), from WT, apx1, apx2, and apx1/apx2 plants grown under controlled growth conditions (control), or subjected to periodic heat stress at 42 or 45 °C. Siliques were obtained from plants subjected to different temperatures of heat stress (42 and 45 °C) and treated with 80% ethanol to remove chlorophyll as described in Materials and methods. **, Student’s t-test significant at P < 0.01.
Conclusions
In contrast to this study group’s previous work with tobacco or Arabidopsis plants deficient in APX1 and CAT2 (Rizhsky et al., 2002; Vanderauwera et al., 2011), the mechanisms and signals activated in response to the combined deficiency in APX1 and APX2 in roots or reproductive tissues of Arabidopsis plants in this work did not provide double mutants with a clear advantage over the wild type or single mutants. Although both APX1 and APX2 are co-expressed in wild-type plants in roots in response to oxidative, salinity, and heat stresses, and in pollen in response to heat (Zimmermann et al., 2004; Frank et al., 2009), the present findings indicate that the mode of cooperation between them is different from that between APX1 and CAT2, perhaps due to their co-localization to the cytosol. However, further analysis of cell type-specific gene expression in roots of Arabidopsis in response to heat is needed to address this question.
Disruption in APX1 or APX2 demonstrated the existence of different signals with respect to the regulation of ROS production in roots. Disruption in APX1 resulted in enhanced ROS accumulation in plants, suggesting that APX1 is a central component of the ROS-scavenging system of Arabidopsis. In contrast, disruption in APX2 appeared to result in the generation of signals that suppressed ROS accumulation during stress, perhaps via the activation of different redundant systems for ROS scavenging or the suppression of ROS production. However, it is unknown whether these signals function in the same roots cells or in different cell types (in leaves both APX1 and APX2 are expressed in vascular and bundle sheath cells, but only APX1 is expressed in mesophyll cells during light stress). At least during oxidative stress (Fig. 4), APX1-dependent signals in roots could be part of the ROS suppression mechanisms activated in apx2 plants. Further studies are required to uncover ROS-scavenging pathways activated in apx2 plants in the different cells types.
Deficiency in APX2 caused reduced heat stress tolerance (at the seedling stage) and light stress tolerance (Figs. 2 and 6), as well as enhanced tolerance to oxidative stress, heat (at the reproductive stage), and salinity stresses (Figs. 4, 5, and 7), demonstrating a role for APX2 in the response of plants to these abiotic stresses. This finding is important because genetic evidence for a role for APX2 in abiotic stress tolerance is very limited. Deficiency in APX1 appeared to only be required for tolerance to oxidative stress in the double apx1/apx2 mutant. In contrast, during salinity, light, and heat stresses, APX1 did not appear to compensate or be required for the mechanisms triggered by APX2 deficiency.
Because APX2 expression is almost undetectable in plants grown under controlled growth conditions, it is likely that the mechanisms activated in APX2 deficient plants are triggered only during the stress period. A similar behaviour is not expected with APX1 because this enzyme is always present in cells and its deficiency will likely trigger compensation mechanisms in the absence of stress. The present results suggest that, at least in cells in which these two enzymes are co-expressed, these compensation mechanisms do not involve APX2 or pathways triggered by APX2 deficiency. The only clue found for enhanced oxidative stress in the apx1/apx2 double mutants is the enhanced expression of Zat10, 12, and 7 during light stress, which appeared to be stronger in the double mutants compared to the single mutants and wild type (Fig. 3).
Perhaps the most notable phenotype of plants deficient in APX2 is their enhanced seed production when subjected to a prolonged treatment of periodic heat stresses (Fig. 7). Sensitivity of reproductive tissues to heat stress is a major problem in many different crops worldwide and results in losses to agricultural productivity estimated at billions of dollars annually (Zinn et al., 2010; Mittler and Blumwald, 2011; Mittler et al., 2012). Pathways triggered in APX2-deficient plants during heat stress should therefore be studied in whole plants and reproductive tissues because of their potential to enhance seed productivity under heat stress conditions. Once identified, these pathways could be used to enhance the heat stress tolerance of reproductive tissues of different crops, mitigating heat-related damage to agriculture production and preventing yield losses under heat stress (Zinn et al., 2010; Mittler and Blumwald, 2011).
Acknowledgements
This work was supported by the National Science Foundation (IBN-0420033, NSF-0431327, IOS-0639964, and IOS-0743954), University of North Texas College of Arts and Sciences, and University of Nevada at Reno College of Agriculture, Biotechnology and Natural Resources.
References
- Asada K. 2006. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology 141, 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Mittler R. 2006. The roles of reactive oxygen species in plant cells. Plant Physiology 141, 311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariola PA, MacIntosh GC, Green PJ. 1999. Regulation of S-like ribonuclease levels in Arabidopsis. Antisense inhibition of RNS1 or RNS2 elevates anthocyanin accumulation. Plant Physiology 119, 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold U, Richard O, Zamboni A, Gapper C, Geisler M, Pogson B, Karpinski S, Mullineaux PM. 2008. Impact of chloroplastic- and extracellular-sourced ROS on high light-responsive gene expression in Arabidopsis . Journal of Experimental Botany 59, 121–133 [DOI] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. 2005a. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis . The Plant Cell 17, 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Schlauch K, Coutu J, Mittler R. 2005b. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis . Plant Physiology 139, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G, Pressman E, Ophir R, Althan L, Shaked R, Freedman M, Shen S, Firon N. 2009. Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. Journal of Experimental Botany 60, 3891–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MJ, Ball L, Oxborough K, Karpinski S, Mullineaux PM, Baker NR. 2003. Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. The Plant Journal 33, 691–705 [DOI] [PubMed] [Google Scholar]
- Galvez-Valdivieso G, Fryer MJ, Lawson T, Slattery K, et al. 2009. The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. The Plant Cell 21, 2143–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Huang B. 2004. Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. Journal of Plant Physiology 161, 405–413 [DOI] [PubMed] [Google Scholar]
- Larkindale J, Vierling E. 2008. Core genome responses involved in acclimation to high temperature. Plant Physiology 146, 748–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhua S, Ciftci-Yilmaz S, Harper J, Cushman J, Mittler R. 2008. Enhanced tolerance to oxidative stress in transgenic Arabidopsis plants expressing proteins of unknown function. Plant Physiology 148, 280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Rizhsky L, Hegie A, Koussevitzky S, Mittler R. 2007. Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiology 144, 1777–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Blumwald E. 2011. Genetic engineering for modern agriculture: challenges and perspectives. Annual Review of Plant Biology 61, 443–462 [DOI] [PubMed] [Google Scholar]
- Mittler R, Finka A, Goloubinoff P. 2012. How do plants feel the heat? Trends in Biochemical Sciences 37, 118–125 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. 2004. Reactive oxygen gene network of plants. Trends in Plant Science 9, 490–498 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. 2011. ROS signaling: the new wave? Trends in Plant Science 16, 300–309 [DOI] [PubMed] [Google Scholar]
- Panchuk II, Volkov RA, Schöffl F. 2002. Heat stress- and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis . Plant Physiology 129, 838–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli L, Liang H, Rozenberg M, Mittler R. 2003. Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. The Plant Journal 34, 187–203 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Davletova S, Liang H, Mittler R. 2004. The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis . Journal of Biological Chemistry 279, 11736–11743 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE, Rodermel S, Inzé D, Mittler R. 2002. Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. The Plant Journal 32, 329–342 [DOI] [PubMed] [Google Scholar]
- Rosa SB, Caverzan A, Teixeira FK, Lazzarotto F, Silveira JA, Ferreira-Silva SL, Abreu-Neto J, Margis R, Margis-Pinheiro M. 2010. Cytosolic APx knockdown indicates an ambiguous redox responses in rice. Phytochemistry 71, 548–558 [DOI] [PubMed] [Google Scholar]
- Rossel JB, Walter PB, Hendrickson L, Chow WS, Poole A, Mullineaux PM, Pogson BJ. 2006. A mutation affecting ASCORBATE PEROXIDASE 2 gene expression reveals a link between responses to high light and drought tolerance. Plant, Cell and Environment 29, 269–281 [DOI] [PubMed] [Google Scholar]
- Schramm F, Ganguli A, Kiehlmann E, Englich G, Walch D, von Koskull-Döring P. 2006. The heat stress transcription factor HsfA2 serves as a regulatory amplifier of a subset of genes in the heat stress response in Arabidopsis . Plant Molecular Biology 60, 759–772 [DOI] [PubMed] [Google Scholar]
- Shi WM, Muramoto Y, Ueda A, Takabe T. 2001. Cloning of peroxisomal ascorbate peroxidase gene from barley and enhanced thermotolerance by overexpressing in Arabidopsis thaliana . Gene 273, 23–27 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Bajad S, Shuman J, Shulaev V, Mittler R. 2008. The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana . Journal of Biological Chemistry 283, 9269–9275 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. 2011. Respiratory burst oxidases: the engines of ROS signaling. Current Opinion in Plant Biology 14, 691–699 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Mittler R. 2006. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiologia Plantarum 126, 45–51 [Google Scholar]
- Suzuki N, Rizhsky L, Liang H, Shuman J, Shulaev V, Mittler R. 2005. Enhanced tolerance to environmental stress in transgenic plants expressing the transcriptional coactivator multiprotein bridging factor 1c. Plant Physiology 139, 1313–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL. 2005. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Current Opinion in Plant Biology 8, 397–403 [DOI] [PubMed] [Google Scholar]
- Vanderauwera S, Suzuki N, Miller G, et al. 2011. Extranuclear protection of chromosomal DNA from oxidative stress. Proceedings of the National Academy of Sciences, USA 108, 1711–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderauwera S, Zimmermann P, Rombauts S, Vandenabeele S, Langebartels C, Gruissem W, Inzé D, Van Breusegem F. 2005. Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiology 139, 806–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. 2004. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox.. Plant Physiology 136, 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn KE, Tunc-Ozdemir M, Harper JF. 2010. Temperature stress and plant sexual reproduction: uncovering the weakest links. Journal of Experimental Botany 61, 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]