Abstract
Leukocyte chemotaxis is deemed instrumental in initiation and progression of atherosclerosis. It is mediated by G-protein-coupled receptors (e.g., CCR2 and CCR5), the activity of which is controlled by G-protein-coupled receptor kinases (GRKs). In this study, we analyzed the effect of hematopoietic deficiency of a potent regulator kinase of chemotaxis (GRK2) on atherogenesis. LDL receptor-deficient (LDLr−/−) mice with heterozygous hematopoietic GRK2 deficiency, generated by bone marrow transplantation (n=15), displayed a dramatic attenuation of plaque development, with 79% reduction in necrotic core and increased macrophage content. Circulating monocytes decreased and granulocytes increased in GRK2+/− chimeras, which could be attributed to diminished granulocyte colony-forming units in bone marrow. Collectively, these data pointed to myeloid cells as major mediators of the impaired atherogenic response in GRK2+/− chimeras. LDLr−/− mice with macrophage/granulocyte-specific GRK2 deficiency (LysM-Cre GRK2flox/flox; n=8) failed to mimic the aforementioned phenotype, acquitting these cells as major responsible subsets for GRK2 deficiency-associated atheroprotection. To conclude, even partial hematopoietic GRK2 deficiency prevents atherosclerotic lesion progression beyond the fatty streak stage, identifying hematopoietic GRK2 as a potential target for intervention in atherosclerosis.—Otten, J. J. T., de Jager, S. C. A., Kavelaars, A., Seijkens, T., Bot, I., Wijnands, E., Beckers, L., Westra, M. M., Bot, M., Busch, M., Bermudez, B., van Berkel, T. J. C., Heijnen, C. J., Biessen, E. A. L. Hematopoietic G-protein-coupled receptor kinase 2 deficiency decreases atherosclerotic lesion formation in LDL receptor-knockout mice.
Keywords: mouse model of atherosclerosis, chemotaxis, leukocytes
Leukocyte migration toward atherosclerotic lesions is deemed instrumental in atherosclerosis initiation and progression (1). By releasing cytokines (2) and proteolytic enzymes, infiltrated leukocytes may also promote plaque destabilization, a process generally preceding thrombotic rupture and subsequent acute cardiovascular events. Because leukocyte migration may be a potentially attractive target for the prevention or control of plaque destabilization and ischemic disease, this research focuses on the pivotal role of leukocyte migration in atherosclerosis.
Chemokines, a family of cytokines with strong chemotactic capacity (3, 4), are key regulators of leukocyte transmigration into the vessel wall during atherogenesis (5, 6). Chemokines are inducible and control cellular recruitment, especially to inflammatory sites (7). In response to inflammatory stimuli, chemokines can be released by various cell types relevant to atherosclerosis, including endothelial cells, platelets, and leukocytes (8–11). They act by binding to dedicated receptors of the G-protein-coupled receptor (GPCR) family. GPCRs are coupled to heterotrimeric G proteins and induce, after ligand binding, activation of downstream signaling cascades (12, 13).
The expression and activity of GPCRs is regulated not only at the mRNA or protein level but also functionally. One such mechanism is receptor desensitization, which dampens the response to prolonged or repeated stimuli (14, 15). Dedicated GPCR kinases (GRKs) can induce receptor desensitization by phosphorylation of the ligand-occupied receptor, thereby enhancing its affinity for arrestin proteins. Binding of arrestins to the phosphorylated receptor will result in uncoupling and internalization of the receptor (16, 17). The GRK family comprises 7 serine/threonine kinases (18, 19). They regulate a range of processes, including cell migration (20, 21), neuronal pathways (22), and cell survival (23). Dysfunction of GRKs, particularly GRK2, has been implicated in various human pathologies, such as multiple sclerosis (24), rheumatoid arthritis (25), chronic pain (26), and heart failure (27), whereas GRK2 overexpression induces hypertension (28, 29). Recently, GRK5 was shown to be involved in atherogenesis in mice through smooth muscle cells and endothelial cells. However, monocyte migration and function were also affected by GRK5 deficiency (30). Because key chemokine receptors in atherosclerosis [CC-motif chemokine receptor (CCR) 2 and CCR5] are targeted by GRK2 (31), decreased levels of this receptor kinase conceivably result in excessive migration of inflammatory cells toward atherosclerotic lesions. Therefore, the aim of this study was to assess the role of leukocyte-specific GRK2 deficiency on the development of atherosclerosis in LDL receptor (LDLr)-deficient mice.
MATERIALS AND METHODS
Animals
Female LDLr−/− mice (32) were obtained from the animal breeding facility at Maastricht University (Maastricht, The Netherlands). Mice were fed a regular chow diet (RM3; Special Diet Services, Essex, UK) or a Western-type diet (WTD; 0.25% cholesterol; Special Diet Services). Drinking water and food were provided ad libitum. Experiments were performed at the Maastricht University animal facility. Male heterozygous GRK2 (GRK2+/−) mice (33), LysM-Cre GRK2flox/flox mice (34, 35), and littermate controls were used as donors for bone marrow transplantations and in vitro assays. Donor mice were maintained at the Utrecht University Medical Center (Utrecht, The Netherlands) animal facilities. All experimental protocols were approved by the Maastricht University ethics committee for animal experiments.
Bone marrow transplantation
Female LDLr−/− mice were lethally irradiated with a single dose (9 Gy, 0.5 Gy/min, MU 15 F/225 kV; Philips Healthcare, Best, The Netherlands) total-body irradiation 1 d before transplantation. On the next day, irradiated recipients received an intravenous tail vein injection of 5 × 106 bone marrow cells. Bone marrow was isolated from GRK2+/− and littermate controls or LysM-Cre GRK2flox/flox and littermate controls by flushing femurs and tibiae. Drinking water supplemented with antibiotics [60 U/ml polymyxin B sulfate (Sigma-Aldrich, St. Louis, MO, USA) and 100 μg/ml neomycin (Sigma-Aldrich)] was provided starting 1 wk before the bone marrow transplantation. After 6 wk of recovery, mice were fed a WTD for 12 wk and subsequently euthanized.
In vivo migration and proliferation
Female wild-type (WT) controls and GRK2+/− chimeras (n=4/5) were intraperitoneally injected 24 h before euthanasia with PBS or CC-motif chemokine ligand (CCL) 5 [RANTES; 200 ng/mouse; Peprotech, London, UK] to stimulate cell migration. At euthanasia, leukocytes were isolated by peritoneal lavage (sterile PBS), and lavages were subsequently analyzed for leukocyte content on an automated differential cell counter (XT-2000i; Sysmex, Norderstedt, Germany).
Splenocytes were isolated from spleen, after the tissue was gently homogenized over a 70-μm cell strainer (BD Biosciences, San Diego, CA, USA). To remove erythrocytes, cell suspensions were incubated in ice-cold erythrocyte lysis buffer (155 mM NH4Cl in 10 mM Tris/HCl, pH 7.2) for 3 min. Splenocytes (n=6/group) were then resuspended in RPMI 1640 medium (Invitrogen, Paisley, UK) supplemented with l-glutamine, 100 U/ml streptomycin/penicillin, and 10% FCS and cultured for 48 h in quadruplicate in a 96-well round-bottom plate (2×105cells/well; Corning, Lowell, MA, USA). Cells were stimulated with CCL5 (RANTES; 100 ng/ml). Concanavalin A (ConA; 8 μg/ml; Sigma-Aldrich) was used as a positive control. Proliferation was determined after addition of [3H]thymidine (0.5 μCi/well; Amersham Biosciences, Roosendaal, The Netherlands), incubation of the cells for the last 16 h, and measurement of cell-associated (incorporated) [3H]thymidine by a liquid scintillation analyzer (Tri-Carb 2900R; PerkinElmer, Waltham, MA, USA).
Histological analysis
For the GRK2+/− study, aortic roots were isolated, and 10-μm cryostat sections were stained with Oil Red O. Total plaque area and necrotic core size were determined in 7 sections/animal. Corresponding sections on separate slides were stained immunohistochemically with a macrophage-specific antibody (MOMA-2; Serotec, Oxford, UK). For the LysM-Cre GRK2flox/flox study, aortic root sections were collected, formalin-fixed, and paraffin-embedded. Subsequently, 4-μm-thick sections were stained with hematoxylin and eosin. Lesion size and necrotic core area were determined in 3-7 sections/animal. Corresponding sections on separate slides were stained immunohistochemically with a macrophage-specific antibody (MAC-3; BD, Franklin Lakes, NJ, USA). Movat's pentachrome stain was used to visualize vascular smooth muscle cells and elastin and to obtain insight into plaque progression stage and stability. Plaque collagen content was quantified based on Sirius Red staining and subsequent analysis of the plaques under polarized light. Vascular smooth muscle cells were analyzed more in depth by α-smooth muscle cell-specific actin (Sigma-Aldrich) staining. T-lymphocyte infiltration was quantified based on staining with a CD3ε-specific antibody (CD3ε; Dako, Glostrup, Denmark). Apoptosis was visualized using a TUNEL kit (Roche, Woerden, The Netherlands). Apoptotic cell content was determined by assessment of the TUNEL-positive area per section. Histological analyses were performed in a blinded manner by an independent operator using Quantimet (Leica, Wetzler, Germany) with QWin3 quantification software (Leica).
In vitro macrophage culture
Bone marrow cells were isolated from WT and GRK2+/− mice, and cells were pooled and cultured in RPMI 1640 medium supplemented with fetal calf serum and L929 conditioned medium to generate bone marrow-derived macrophages (BMDMs) as described previously (36). Medium was replaced every 3 d, and differentiated BMDMs were used for in vitro assays after 7 d.
Macrophage foam cell formation assay
BMDMs were cultured and plated in 24-well cell cluster plates (Corning). Macrophages were incubated for 24 h with VLDL (25 μg/ml) to induce foam cell formation. Subsequently, Oil Red O staining was performed to visualize the cellular lipid accumulation. Pictures were taken using a DM IL LED fluorescence microscope (Leica) with QWin3 software, and foam cell formation was scored in a blinded manner by an independent operator, calculating the number of positive Oil Red O-stained cells, as well as the staining intensity at a per-cell basis in ≥3 fields/condition (×100 view).
Macrophage apoptosis and phagocytosis assay
BMDMs were cultured and plated in 48-well cell cluster plates (Corning). Macrophages were incubated for 18 h in plain medium or medium supplemented with camptothecin (CPT; 500 μM) or oxidized LDL (oxLDL; 50 μg/ml). oxLDL was produced as described previously (37). Cells were labeled with annexin V-Alexa 488 (Invitrogen) and stained with propidium iodide to detect apoptosis and necrosis, respectively. Pictures were taken using a DM IL LED fluorescence microscope with Qwin3 software. Overlays were analyzed with ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA) to quantify the number of apoptotic and necrotic cells per field.
To investigate phagocytic capacity, cultured BMDMs were labeled with Cell Tracker Red (Invitrogen). Jurkat T cells labeled with Cell Tracker Green (Invitrogen) were exposed to a UV light source (UVS-26, 6-W bulb, 0.02 J/s/cm2; UVP, Upland, CA, USA) for 20 min and incubated (37°C with 5% CO2) for 45 min to induce apoptosis. Apoptotic Jurkat cells were added to the BMDMs at a ratio of 5:1 and incubated for 50 min. Macrophages were fixed with 1% paraformaldehyde and counterstained with DAPI (Invitrogen). Pictures were taken (DM IL LED fluorescence microscope), and overlays were analyzed with ImageJ.
Flow cytometry
At euthanasia, blood, spleen, and peritoneal leukocytes were collected. Blood and peritoneal leukocytes were analyzed for cellular composition on an automated differential cell counter (XT-2000i). Single-cell suspensions were made from spleen by crushing the tissue over a 70-μm cell strainer (BD) after treatment with DNase and Liberase (Roche). Erythrocytes in blood and spleen were removed by incubation with hypotonic lysis buffer (8.4 g of NH4Cl and 0.84 g of NaHCO3 per liter of distilled water). Nonspecific Fc receptor binding was blocked by the addition of anti-CD16/CD32 antibody (eBioscience, San Diego, CA, USA). To determine lymphocyte subsets, cells were labeled with CD3ϵ-FITC, CD8α-eFluor450, B220-PE-Cy7, CD25-APC (all eBioscience), and CD4-PerCP (BD). To detect monocytes and granulocytes, the cells were labeled with CD11b-PE-Cy7, Ly6G-PE (BD), and Ly6C-FITC (Miltenyi Biotec, Bergisch Gladbach, Germany). Dendritic cells were detected by labeling with CD3-PerCP-Cy5.5, CD19-PerCP-Cy5.5, MHC II-FITC, CD8a-eFluor450 (all eBioscience), CD11c-PE-Cy7, and CD4-APC-H7 (both BD) for resident dendritic cells. Chemokine receptor expression was determined by labeling peripheral blood leukocytes with CCR5-PE, CCR7-PerCP-Cy5.5, CD3e-eFluor450, NK1.1-APC (all eBioscience), CD11b-PE-Cy7, Ly6G-APC-Cy7, B220-V500 (all BD), and Ly6C-FITC (Miltenyi). After labeling, the samples were washed and analyzed on a FACSCanto II flow cytometer (BD). Samples and buffers were kept on ice throughout the experiment. Gating strategies used for the different flow cytometry stainings are shown in the supplemental data.
Colony-forming unit (CFU) assay
Bone marrow cells were isolated from 1 tibia and femur per mouse. WT, GRK2+/− and LysM-Cre GRK2flox/flox chimeras were used for the CFU assays. Cells were counted twice using a count chamber, and the concentration was calculated for each sample. A total of 10,000 bone marrow cells/well were added to 2 ml of methylcellulose medium with recombinant cytokines (MethoCult Medium; StemCell Technologies, Grenoble, France). After incubation for 7 d (37°C with 5% CO2), the total number of colonies was quantified by an independent operator, and granulocyte-macrophage CFU (GM-CFU), granulocyte CFU (G-CFU), and macrophage CFU (M-CFU) colonies were specified based on morphology of the individual cells and the colony as a whole.
Statistical analysis
Data are expressed as means ± se. To compare individual groups, a 2-tailed Student's t test was used; nonparametric data were analyzed using a Mann-Whitney U test. All analyses were performed using GraphPad Prism 5 software (GraphPad Software Inc., LA Jolla, CA, USA), and values of P < 0.05 were considered statistically significant.
RESULTS
Partial leukocyte GRK2 deficiency attenuates atherosclerosis
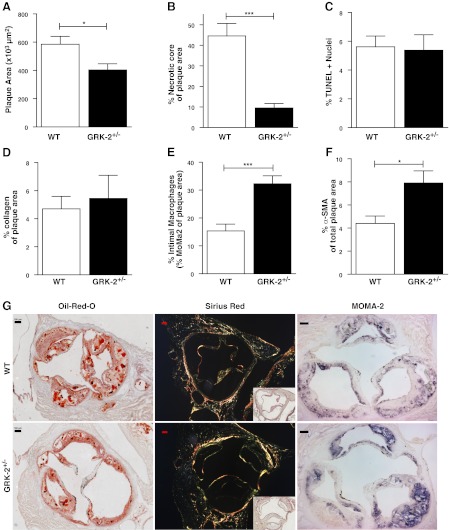
Partial hematopoietic GRK2 deficiency did not affect body weight and total cholesterol levels (Supplemental Fig. S1). Hematopoietic GRK2+/− chimeras in LDLr−/− mice (n=15) showed significantly decreased atherosclerotic lesion development in the aortic root compared with that of controls (403.0±43.8×103 vs. 585.0±56.4×103 μm2, P=0.017; Fig. 1A). The necrotic core size was >4-fold smaller in heterozygous GRK2 than in WT chimeras (9.5±2.3 vs. 44.6±6.1%, P<0.0001; Fig. 1B). The TUNEL-positive apoptotic cell burden (Fig. 1C) did not differ between GRK2 and WT chimeras. Further differentiation for TUNEL-positive cell density in the cap vs. the atheroma revealed that in both WT and GRK+/− chimeras >95% of TUNEL-positive cells were located in the atheroma (data not shown). Moreover, because we did not observe a shift in localization over these two compartments and because the atheroma mainly consists of macrophages (next to cell debris), we may infer that partial GRK2 deficiency has not affected macrophage apoptosis. Next, collagen content was not affected in GRK2+/− chimeras compared with WT chimeras (Fig. 1D). Surprisingly, intimal MOMA-2-positive macrophage content in heterozygous GRK2 chimeras was >2-fold higher than that of controls (32.3±2.9 vs. 15.3±2.4%, P=0.00015; Fig. 1E). Analysis of the lesions did not reveal significant differences in intimal (CD3ε+) lymphocyte numbers (data not shown). Intimal smooth muscle cell content, as assessed by smooth muscle cell-specific actin staining, was increased by heterozygous GRK2 deficiency (Fig. 1F). General assessment of plaque morphology, from Movat's pentachrome staining, showed a significant reduction of overall necrosis in lesions from GRK2+/− chimeras (Supplemental Fig. S2A). In line with the results obtained based on MOMA-2 staining, foam cell macrophage content was significantly increased in GRK2+/− lesions (Supplemental Fig. S2B). For neutrophil infiltration, it was observed that the number of granulocytes per lesion were very low, which was confirmed by the virtual absence of positive Ly6G staining (data not shown), whereas intraplaque/adventitial neutrophil numbers tended to be decreased in heterozygous GRK2 animals, based on morphological scoring of the lesions (Supplemental Fig. S2C). We did, however, observe a significant decrease in the amount of cells adhering to the luminal side of the atherosclerotic lesions in the GRK2+/− chimeras (Supplemental Fig. S2D), which could not be further attributed to a specific cell type. Overall, atherosclerotic lesions in GRK2+/− chimera were considered less advanced and more stable than those of controls, despite unchanged collagen levels (Supplemental Fig. S2E), based on plaque morphology and composition.
Figure 1.
Atherosclerotic plaque development is attenuated in GRK2+/− chimeras. A, B) Plaque area (A) and necrotic core size (B) were determined based on Oil Red O staining. C) Relative number of apoptotic cells was determined by TUNEL staining. D) Collagen content was measured by analysis of Sirius Red staining under polarized light. E) Macrophage content was measured in sections stained for the monocyte/macrophage marker MOMA-2. F) Smooth muscle cell content was measured by staining against α-smooth muscle cell actin (α-SMA). G) Representative pictures of Oil Red O, Sirius Red (polarized light; inset: bright field image), and MOMA-2 staining are shown for WT and GRK2+/− chimeras. Scale bars = 100 μm. Open bars, WT; solid bars, GRK2+/− chimeras. *P < 0.05, ***P < 0.001.
Macrophage GRK2 deficiency does not affect phagocytosis, apoptosis, or foam cell formation
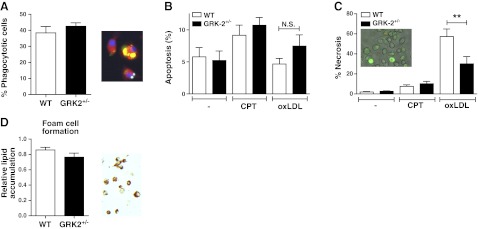
The major differences in plaque composition, regarding necrotic core and intimal macrophage content, prompted us to study the effect of GRK2 deficiency on macrophage function. Reduced GRK2 expression did not affect phagocytosis of apoptotic Jurkat T cells by BMDMs (Fig. 2A), rendering it unlikely that the reduced necrotic core size is attributable to increased phagocytosis.
Figure 2.
Phagocytosis, apoptosis, and macrophage-derived foam cell formation of BMDMs are not affected in vitro. A) The phagocytic capacity of BMDMs for apoptotic Jurkat T cells was determined in vitro. Phagocytosis was determined by scoring yellow staining in overlays from macrophages (Cell Tracker Red), apoptotic Jurkat T cells (Cell Tracker Green) and nuclei (DAPI; blue). B, C) In vitro apoptosis (B) and necrosis of BMDMs (C) was measured at baseline (—) and with CPT or oxLDL exposure. Cell death was quantified based on annexin V-Alexa 488 (apoptosis; green) and propidium iodide (necrosis; red/orange) staining. D) In vitro foam cell formation was measured with VLDL exposure for 24 h using Oil Red O staining (red; ×400). Open bars, WT; solid bars, GRK2+/− chimeras. **P < 0.01.
In line with the unchanged TUNEL+ cell density observed in plaques from WT and GRK2+/− chimeras, in vitro we observed no effects of GRK2 heterozygosity (n=9) on the susceptibility for apoptosis of BMDMs at baseline or in the presence of CPT (Fig. 2B), nor did we observe differences in necrosis between BMDMs at baseline and after CPT treatment (Fig. 2C). Notably, oxLDL-induced necrosis was markedly attenuated in GRK2+/− compared with WT BMDMs (30.1±7.2 vs. 57.2±7.6%, P=0.019; Fig. 2C). Thus, partial GRK2 deficiency was seen to reduce oxLDL-induced necrosis, which could have contributed to the phenotypic differences in necrotic core size in vivo.
Because macrophage and foam cell macrophage content was significantly increased in the GRK2 lesions compared with the WT lesions, we investigated in vitro whether GRK2 heterozygosity by itself influences foam cell formation. However, we were unable to detect any effects on the number of macrophage-derived foam cells nor on the level of lipid accumulation per cell after VLDL incubation (Fig. 2D), indicating that the observed plaque foam cell effects are not caused by GRK2 deficiency-associated changes in macrophage lipid handling.
Circulating leukocytes are affected by GRK2 deficiency
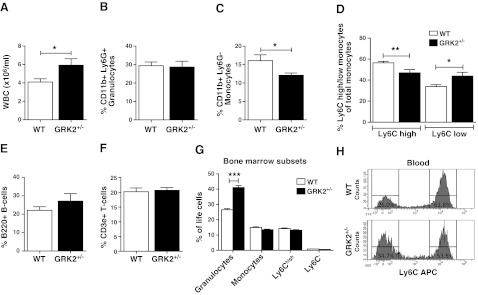
Peripheral blood analysis revealed increased circulating leukocyte numbers in GRK2+/− chimeras (5.9±0.6×106 vs. 4.1±0.4×106 cells/ml, P=0.03; Fig. 3A). In-depth analysis of peripheral blood samples from WT and GRK2+/− chimeras fed a WTD (0.25% cholesterol) by flow cytometry indicated that there was no relative difference in granulocyte (CD11b+/Ly6G+) abundance (Fig. 3B). Circulating monocyte (CD11b+/Ly6G−) numbers were significantly lowered in GRK2+/− compared with those in WT chimeras (12.09±0.61 vs. 16.09±1.58% monocytes, P=0.039; Fig. 3C) and displayed a decreased Ly6Chigh/Ly6Clow ratio (Fig. 3D). No genotype-related differences were observed in B cells (B220+; Fig. 3E) and T cells (CD3ε+; Fig. 3F) or in T-cell subsets (CD4+/CD8+; data not shown). Spleen resident dendritic cell content (CD11c+) and resident dendritic cell composition (CD4+/CD8+ double-negative; data not shown) did not reveal any differences between WT and GRK2+/− chimeras. In spleen, we did not observe any significant differences other than a relative decrease in the number of granulocytes in the GRK2+/− chimeras compared with that in WT controls (3.30±0.32 vs. 2.15±0.21% granulocytes; Supplemental Fig. S3A). B and T cells did not show any differences in spleen (Supplemental Fig. S3B, C), while splenic monocytes, and in particular the Ly6Chigh subset, showed a tendency toward reduced numbers, as observed in peripheral blood (Supplemental Fig. S3D, E). In bone marrow, on the other hand, we observed no differences in monocytes or monocyte subsets (Fig. 3G). This result also indicates that impaired egress might be underlying the observed monocytopenia. Granulocytes are accumulating in the bone marrow of GRK2-deficient mice (Fig. 3G), but this does not translate into any effects on the atherosclerotic lesion. In short, these findings indicate that the effects of GRK2 deficiency on plaque formation and on circulating leukocytes mainly affect the myeloid lineage, monocytes and granulocytes, in blood, spleen, and bone marrow.
Figure 3.
GRK2+/− chimeras have perturbed leukocyte patterns in circulation. A) Absolute number of white blood cells (WBC) was measured using a Sysmex differential cell counter. Relative fractions of leukocyte subsets were measured using flow cytometry analysis on a FACSCanto II. B) Granulocytes. C) Monocytes. D) Monocyte Ly6C subsets. E) B cells. F) T cells were measured in the circulation of WT control (open bars) and GRK2+/− chimeras (solid bars). G) Myeloid subsets were measured in bone marrow of donor mice. H) Representative histograms of Ly6Chigh and Ly6Clow distribution in blood of WT and GRK2+/− chimeras. *P < 0.05, **P < 0.01, ***P < 0.001.
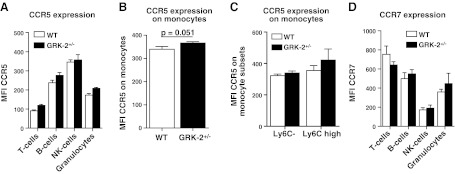
The reported role of GRK2 in chemokine receptor desensitization led us to investigate effects of GRK2+/− deficiency on leukocyte expression of relevant chemokine receptors such as CCR5 and CCR7 by flow cytometry. No statistically significant increase in CCR5 expression could be observed in any of the leukocyte subsets at baseline (Fig. 4A). However, CCL5-stimulated monocytes and, in particular, the proinflammatory Ly6Chigh subset, showed borderline significance toward increased CCR5 expression in GRK2+/− chimeras compared with WT controls (P=0.051; Fig. 4B, C). For CCR7 we could not detect any significant differences in expression for any leukocyte subset tested (Fig. 4D).
Figure 4.
Chemokine receptor expression on leukocytes from WT and GRK2+/− chimeras. Chemokine receptor (CCR5 and CCR7) expression was measured on circulating leukocytes from WT control (open bars) and GRK2+/− chimeras (solid bars). A, B) CCR5 expression on T cells, B cells, NK cells, and granulocytes (A) and monocytes (B). C) Ly6C monocyte subsets showed differential CCR5 expression. D) CCR7 expression on T cells, B cells, NK cells, and granulocytes.
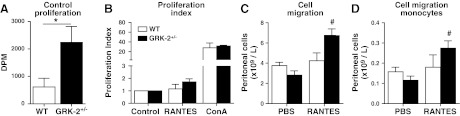
Baseline proliferation and CCL5-mediated migration are increased in GRK2+/− animals
Proliferation was measured by [3H]thymidine incorporation in total splenocytes under baseline conditions (PBS) or after stimulation for 16 h with CCL5 (RANTES) or with the general mitogen ConA. It was observed that baseline proliferation (control) was increased in GRK2+/− splenocytes compared with that in WT controls (P<0.05; Fig. 5A). However, the proliferation index in CCL5- or ConA-stimulated cells, corrected for baseline proliferation, was unaffected in GRK2+/− splenocytes (Fig. 5B).
Figure 5.
Baseline proliferation and migration on CCL5 stimulation is increased in GRK2+/− chimeras. A) Baseline proliferation (unstimulated) was determined in WT and GRK2+/− splenocytes. B) Proliferation index (corrected proliferation for control condition) was determined for CCL5 (RANTES; 100 ng/ml) or ConA (100 ng/ml) stimulation. C, D) Total cell migration (C) and monocyte migration (D) in WT control and GRK2+/− chimeras on intraperitoneal CCL5 (RANTES; 200 ng) challenge. *P < 0.05, #P < 0.05 vs. GRK2+/− PBS-treated.
Peritonitis is considered an established model to study leukocyte chemotaxis and activation and expansion outside of the atherosclerotic lesion. We opted to use CCL5-dependent peritonitis, because CCL5 is one of the chemokines desensitized by GRK2, which at the same time is considered to be relevant in atherosclerosis. In vivo migration was measured after intraperitoneal injection of PBS (control) or CCL5 (RANTES; 200 ng/mouse). CCL5 challenge led to increased peritoneal leukocyte presence compared with that in PBS-treated animals for GRK2+/− chimeras (P<0.05) but not for WT animals (Fig. 5C). In-depth analysis of monocyte migration into the peritoneal cavity of GRK2+/− chimeras showed a significant increased migration in CCL5-challenged animals compared with that in PBS-treated animals (Fig. 5D).
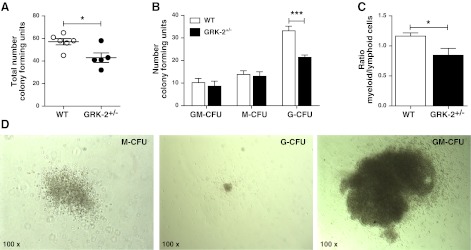
GRK2 deficiency decreases myeloid hematopoiesis
The observed effects of decreased GRK2 levels on the myeloid lineage and in particular on granulocytes and macrophages could reflect altered myelopoiesis. CFU assays with bone marrow cells from WT (n=6) and GRK2+/− mice (n=5) showed that bone marrow from GRK2+/− mice contained fewer CFUs (43.0±4.2 vs. 57.2±2.8 CFUs, P=0.017; Fig. 6A). This result was solely caused by a decreased number of G-CFUs in GRK2+/− chimeras (21.4±1.0 vs. 33.2±2.1 G-CFUs, P<0.001; Fig. 6B); GM-CFUs and M-CFUs were unaffected. Thus, GRK2+/− bone marrow cells show an attenuated G-colony stimulating factor (CSF) response, explaining the decreased myeloid (monocytes/granulocytes) over lymphoid (B cells/T cells/NK cells) cell ratio in the circulation (Fig. 6C).
Figure 6.
Myeloid progenitor cells are decreased in GRK2+/− bone marrow; which is associated with a reduced myeloid/lymphoid cell ratio. CFU assays were performed on bone marrow from WT and GRK2+/− donors. A) Total number of colonies in WT control compared with heterozygous GRK2+/− chimeras. Based on morphological analysis, GM-CFUs, M-CFUs, and G-CFUs were differentiated C) Myeloid (granulocytes+monocytes) over lymphoid (B cells+T cells) ratio in the circulation of GRK2+/− mice compared with controls. D) Representative images showing M-CFU, G-CFU, and GM-CFU colonies (×100). Open bars, WT; solid bars, GRK2+/− chimeras. *P < 0.05, ***P < 0.001.
Granulocyte and macrophage GRK2 deficiency does not explain phenotype
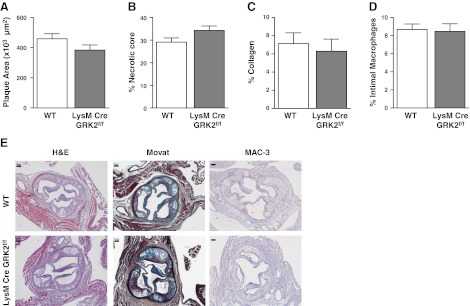
Because our results pointed to GRK2-deficient myeloid cells as the underlying cause for the phenotypic changes observed in the atherosclerotic lesion in GRK2+/− chimeras, we studied the effect of granulocyte- and macrophage-specific GRK2 deficiency in a LysM-Cre GRK2flox/flox bone marrow transplant model in LDLr−/− mice (n=8/9). To our surprise, no difference was observed in total plaque area between LysM-Cre GRK2flox/flox and WT chimeras (Fig. 7A). In addition, necrotic core size was unchanged in these myeloid cell-specific GRK2-deficient chimeras compared with that in controls (Fig. 7B), as were plaque collagen levels (Fig. 7C). Because the LysM-Cre GRK2flox/flox model results in lowered macrophage GRK2 levels, we quantified intimal macrophage content in the aortic root lesions, revealing no differences between groups (Fig. 7D).
Figure 7.
Atherosclerotic plaque development and intimal macrophage accumulation are unaffected in LysM-Cre GRK2flox/flox chimeras. A, B) Plaque area (A) and necrotic core size (B) were determined based on hematoxylin and eosin (H&E) staining. C) Collagen content was measured by analysis of Sirius Red staining under polarized light. D) Macrophage content was measured in sections stained for the monocyte/macrophage marker MAC-3. Open bars, WT; solid bars, LysM-Cre GRK2flox/flox chimeras. E) Representative images of H&E, Movat's pentachrome, and MAC-3 staining are shown for WT and LysM-Cre GRK2flox/flox chimeras. Top panels: WT; bottom panels, LysM-Cre GRK2flox/flox. Scale bars = 100 μm.
In analogy with the GRK2+/− chimera study, flow cytometry of peripheral blood showed marked changes in granulocytes and Ly6C monocyte subsets in the granulocyte- and macrophage-specific GRK2-deficient chimeras (Table 1). These differences did not translate to plaque phenotypic effects. It appears that granulocyte- and macrophage-specific GRK2 deficiency does not affect atherogenesis, despite perturbed leukocyte patterns in circulation. The involvement of GRK2-deficient leukocytes, other than monocytes, cannot be excluded. However, total monocyte counts were not affected in LysM-Cre GRK2flox/flox chimeras but were decreased in GRK2+/− chimeras, indicating that monocytes, rather than granulocytes or macrophages, were probably the cell type responsible for the atheroprotective phenotype in hematopoietic GRK2 deficiency.
Table 1.
Blood leukocyte composition is affected in macrophage- and granulocyte-specific GRK2 deficiency
| Population | WT | LysM-Cre GRK2 flox/flox |
|---|---|---|
| T cells | 20.2 ± 1.3 | 18.6 ± 0.9 |
| CD4+ T cells | 67.9 ± 0.5 | 69.4 ± 0.6 |
| CD8+ T cells | 25.7 ± 0.6 | 24.7 ± 0.2 |
| B cells | 22.1 ± 2 | 31 ± 1.3* |
| Granulocytes | 29.4 ± 2 | 24.5 ± 0.8* |
| Monocytes | 16.1 ± 1.6 | 15.8 ± 0.5 |
| Ly6Chigh monocytes | 56.5 ± 1.3 | 46.2 ± 1.2* |
| Ly6Clow monocytes | 33.8 ± 1.9 | 44.2 ± 0.9* |
Leukocyte populations were determined in the blood from WT and LysM-Cre GRK2flox/flox chimeras. Numbers shown are the relative percentages of living cells, except for CD4 and CD8 (% of CD3e+ cells) and Ly6Chigh/Ly6Clow monocytes (% of monocytes).
P < 0.05 vs. WT.
DISCUSSION
A role of GRK2 in atherosclerosis has not yet been documented but could be inferred from its key regulatory role in CCL2 (monocyte chemotactic protein-1) and CCL5 (RANTES) function, which are instrumental in leukocyte chemotaxis and transmigration during atherogenesis (21, 31, 38). Moreover, GRK2 levels were shown to be decreased in rheumatoid arthritis, an inflammatory disease sharing many features with atherosclerosis. Further, GRK2 expression was shown to be responsive to atherogenic cytokines such as IFN-γ and IL-6 (25, 39). Our data identify a role for hematopoietic GRK2 in atherogenesis, excluding GRK2 deficiency in granulocytes and macrophages being responsible for the observed effects.
First, we mapped the effect of heterozygous hematopoietic GRK2 deficiency on atherosclerosis in LDLr−/− mice. The donor mice were on a C57/BL6 background but were LDLr+/+. LDLr expression in hematopoietic cells did not affect either repopulation of the hematopoietic lineage or atherosclerosis development and is widely accepted as the donor genotype in atherosclerosis research (40). The GRK2+/− genotype results in an ∼50% reduction in GRK2 protein levels as shown before (41). Homozygous GRK2-deficient mice could not be used in these experiments because GRK2 deficiency is embryonically lethal, and embryos do not survive beyond gestational day 15.5. Most likely the in utero mortality is caused by cardiac dysfunction (33). Partial GRK2 deficiency resulted in reduced plaque burden and delayed plaque progression. In fact, GRK2+/− plaques did not progress beyond a fatty streak stage and contained more macrophages and intimal smooth muscle cells but showed profoundly diminished necrotic core formation. The latter pointed to a direct effect of GRK2 deficiency on macrophage phagocytosis or apoptosis. Indeed, GRK2 was previously shown to influence cytoskeleton function (42, 43), thereby potentially modifying macrophage phagocytic capacity. As shown previously, disrupted apoptotic cell clearance can accelerate atherosclerosis in mice (44). However, analysis did not support the notion that the GRK2+/−-associated reduction in necrotic core size is due to increased phagocytosis, because apoptotic cell handling by macrophages was not affected.
Impaired necrotic core expansion is not caused by altered apoptosis of plaque macrophages. However, GRK2+/− macrophage necrosis in vitro was sharply attenuated after oxLDL exposure. The actual affect of macrophage death on atherosclerosis is still a matter of debate. Several studies have shown that in early and intermediate plaques, enhanced macrophage apoptosis is beneficial (45, 46), albeit others suggested otherwise (47). In advanced atherosclerosis, the consensus is that macrophage death promotes necrotic core expansion and plaque destabilization (48, 49).
Enhanced plaque macrophage content and reduced lesion burden in GRK2+/− chimeras could reflect changes in peripheral inflammation. GRK2+/− chimeras showed decreased monocyte counts. Total leukocyte numbers were increased, an effect that was mainly attributable to augmented granulocyte counts in circulation. Myelopoiesis, assessed by CFU assay, was attenuated in GRK2+/− bone marrow, and G-CFUs, in particular, were decreased. Although it remains to be established how GRK2 exactly interferes with G-CSF response at a molecular level, this process may be linked to granulocyte release or life span because granulocyte numbers also increased in the bone marrow. At a functional level, mobilization of macrophages to inflammatory sites seemed to be increased with partial GRK2 deficiency, which is compatible with the desensitizing effect of GRK2 on chemokine receptor activity (50). Indeed, we could show that CCR5 chemokine receptor expression was increased on peripheral monocytes from GRK2+/− chimeras. At a functional level, this increase was accompanied by increased monocyte migration in response to a CCL5 (RANTES) challenge, as shown in a model of chemokine-induced peritonitis. However, the roles of other cell types such as T lymphocytes, dendritic cells, and macrophages cannot be excluded, and they might contribute to the increased monocyte migration indirectly, because relevant, potentially GRK2-responsive chemokine receptors (CCR1, CCR2, and CCR7) are also expressed by lymphocyte subsets (51, 52).
The involvement of the chemokine receptor and ligand axis in regulating stromal release of Ly6Chigh monocytes from the bone marrow has already been shown for the CCL2/CCR2 axis (53). However, the decreased Ly6Chigh monocyte levels in circulation and spleen are not likely to be due to GRK2-dependent tuning of CCR2 egress because in that case increased monocyte levels would be expected. We did, however, observe that mobilization of monocytes is affected at some level because there are no differences observed in the bone marrow. On the other hand, mice deficient for CCL2 or CX3CR1 had decreased atherogenesis. Inhibition of the CCL5/CCR5 axis by treatment with a CCR5 antagonist (Met-CCL5) resulted in an even more dramatic reduction of atherogenesis (54). A similar reduction in atherosclerosis development was observed in this study, indicating that GRK2-dependent processes other than receptor desensitization may be responsible for the phenotype observed.
Major effects of GRK2 deficiency on myeloid differentiation and mobilization have prompted us to address the contribution of granulocyte and macrophage GRK2 deficiency to the atheroprotection. The LysM-Cre GRK2flox/flox transgenic mouse was previously shown to confer effective deletion efficiency in macrophages and granulocytes (55, 56). Contrary to our expectations, the LysM-Cre GRK2flox/flox bone marrow chimeras did not mirror the previously observed GRK2+/− phenotype. In fact, LysM-Cre GRK2flox/flox chimeras completely lacked a plaque phenotype, disqualifying granulocyte and macrophage GRK2 deficiency as the major culprit, although the possibility that the phenotype of homozygous GRK2 deficiency differs from that of GRK2+/− cannot be excluded. On the other hand, in sepsis it was recently shown that macrophage- and granulocyte-specific deficiency resulted in no clear phenotype attributable to these cells (57). Flow cytometry of the LysM-Cre GRK2flox/flox chimeras and controls revealed a reduction in relative granulocyte abundance in blood of the GRK2flox/flox animals and in keeping the granulocyte- and macrophage-specific deletion, monocyte levels in circulation were not affected in the LysM-Cre GRK2flox/flox model. It has previously been shown that the LysM expression in circulating monocytes is not sufficient to get the CRE levels necessary for effective deletion of the floxed gene, in this case GRK2 (58). In contrast to this ineffective deletion in F4/80-negative immature macrophages or monocytes, the construct is highly efficient in the deletion of the floxed genes in F4/80-positive macrophages and in granulocytes (59, 60). Taken together, these data point to monocytes or potentially even T cells (albeit we did not observe overt effects of GRK2 deficiency on the number of circulating or plaque T cells) as responsible factors in the profound GRK2+/− plaque phenotype.
Overall, our data indicate an equally complex and critical role of GRK2 in monocyte homeostasis and mobilization in the context of atherosclerosis. Partial hematopoietic GRK2 deficiency appeared to have a profound effect on atherogenesis. Whereas all data seem to pinpoint the myeloid lineage as the major effectors, reduced GRK2 levels in granulocytes and macrophages cannot be held directly accountable for this effect. Cumulatively, these data lead us to propose a major role for monocyte GRK2 in the observed phenotypic changes. It should be noted, however, that although we did not observe any major effects of GRK2+/− on other leukocyte subsets known to affect atherogenesis, such as T lymphocytes and dendritic cells, the possibility that these subsets may nevertheless have modified the atherogenic response in an indirect manner cannot be excluded. Because even partial inhibition of GRK2 function already suffices to halt plaque progression at a fatty streak stage, our studies warrant further investigation into the perspective of GRK2 antagonists in atherosclerosis treatment and in determining the cell type responsible for effects on atherosclerosis development.
Supplementary Material
Acknowledgments
The authors thank Dr. M. Gijbels for her help in plaque morphology scoring. E.B. is the recipient of an Established Investigator Fellowship (grant 2003T201) of The Netherlands Heart Foundation.
This work was supported in part by the U.S. National Institutes of Health (grants R01-NS003939 and NS074999 to A.K.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BMDM
- bone marrow-derived macrophage
- CCL
- CC-motif chemokine ligand
- CCR
- CC-motif chemokine receptor
- CFU
- colony-forming unit
- ConA
- concanavalin A
- CPT
- camptothecin
- CSF
- colony-stimulating factor
- G-CFU
- granulocyte colony-forming unit
- GM-CFU
- granulocyte-macrophage colony-forming unit
- GPCR
- G-protein-coupled receptor
- GRK
- G-protein-coupled receptor kinase
- LDLr
- LDL receptor
- M-CFU
- macrophage colony-forming unit
- oxLDL
- oxidized LDL
- WT
- wild type
- WTD
- Western-type diet
REFERENCES
- 1. Lusis A. J. (2000) Atherosclerosis. Nature 407, 233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braunersreuther V., Zernecke A., Arnaud C., Liehn E. A., Steffens S., Shagdarsuren E., Bidzhekov K., Burger F., Pelli G., Luckow B., Mach F., Weber C. (2007) Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 27, 373–379 [DOI] [PubMed] [Google Scholar]
- 3. Rossi D., Zlotnik A. (2000) The biology of chemokines and their receptors. Annu. Rev. Immunol. 18, 217–242 [DOI] [PubMed] [Google Scholar]
- 4. Bazan J. F., Bacon K. B., Hardiman G., Wang W., Soo K., Rossi D., Greaves D. R., Zlotnik A., Schall T. J. (1997) A new class of membrane-bound chemokine with a CX3C motif. Nature 385, 640–644 [DOI] [PubMed] [Google Scholar]
- 5. Weber C., Schober A., Zernecke A. (2004) Chemokines: key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arterioscler. Thromb. Vasc. Biol. 24, 1997–2008 [DOI] [PubMed] [Google Scholar]
- 6. Liehn E. A., Zernecke A., Postea O., Weber C. (2006) Chemokines: inflammatory mediators of atherosclerosis. Arch. Physiol. Biochem. 112, 229–238 [DOI] [PubMed] [Google Scholar]
- 7. Moser B., Willimann K. (2004) Chemokines: role in inflammation and immune surveillance. Ann. Rheum. Dis. 63(Suppl. 2), ii84–ii89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burke-Gaffney A., Brooks A. V., Bogle R. G. (2002) Regulation of chemokine expression in atherosclerosis. Vasc. Pharmacol. 38, 283–292 [DOI] [PubMed] [Google Scholar]
- 9. Weber C. (2005) Platelets and chemokines in atherosclerosis: partners in crime. Circ. Res. 96, 612–616 [DOI] [PubMed] [Google Scholar]
- 10. Reape T. J., Groot P. H. (1999) Chemokines and atherosclerosis. Atherosclerosis 147, 213–225 [DOI] [PubMed] [Google Scholar]
- 11. Lukacs N. W., Strieter R. M., Chensue S. W., Kunkel S. L. (1996) Activation and regulation of chemokines in allergic airway inflammation. J. Leukoc. Biol. 59, 13–17 [DOI] [PubMed] [Google Scholar]
- 12. Thelen M. (2001) Dancing to the tune of chemokines. Nat. Immunol. 2, 129–134 [DOI] [PubMed] [Google Scholar]
- 13. Arai H., Charo I. F. (1996) Differential regulation of G-protein-mediated signaling by chemokine receptors. J. Biol. Chem. 271, 21814–21819 [DOI] [PubMed] [Google Scholar]
- 14. Muller S., Lohse M. J. (1995) The role of G-protein βγ subunits in signal transduction. Biochem. Soc. Trans. 23, 141–148 [DOI] [PubMed] [Google Scholar]
- 15. Hausdorff W. P., Caron M. G., Lefkowitz R. J. (1990) Turning off the signal: desensitization of β-adrenergic receptor function. FASEB J. 4, 2881–2889 [PubMed] [Google Scholar]
- 16. Arriza J. L., Dawson T. M., Simerly R. B., Martin L. J., Caron M. G., Snyder S. H., Lefkowitz R. J. (1992) The G-protein-coupled receptor kinases βARK1 and βARK2 are widely distributed at synapses in rat brain. J. Neurosci. 12, 4045–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Attramadal H., Arriza J. L., Aoki C., Dawson T. M., Codina J., Kwatra M. M., Snyder S. H., Caron M. G., Lefkowitz R. J. (1992) β-Arrestin2, a novel member of the arrestin/β-arrestin gene family. J. Biol. Chem. 267, 17882–17890 [PubMed] [Google Scholar]
- 18. Premont R. T., Inglese J., Lefkowitz R. J. (1995) Protein kinases that phosphorylate activated G protein-coupled receptors. FASEB J. 9, 175–182 [DOI] [PubMed] [Google Scholar]
- 19. Freedman N. J., Lefkowitz R. J. (1996) Desensitization of G protein-coupled receptors. Recent Prog. Horm. Res. 51, 319–351; discussion 352–353 [PubMed] [Google Scholar]
- 20. Penela P., Ribas C., Aymerich I., Eijkelkamp N., Barreiro O., Heijnen C. J., Kavelaars A., Sanchez-Madrid F., Mayor F., Jr. (2008) G protein-coupled receptor kinase 2 positively regulates epithelial cell migration. EMBO J. 27, 1206–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vroon A., Heijnen C. J., Lombardi M. S., Cobelens P. M., Mayor F., Jr., Caron M. G., Kavelaars A. (2004) Reduced GRK2 level in T cells potentiates chemotaxis and signaling in response to CCL4. J. Leukoc. Biol. 75, 901–909 [DOI] [PubMed] [Google Scholar]
- 22. Weiss E. R., Ducceschi M. H., Horner T. J., Li A., Craft C. M., Osawa S. (2001) Species-specific differences in expression of G-protein-coupled receptor kinase (GRK) 7 and GRK1 in mammalian cone photoreceptor cells: implications for cone cell phototransduction. J. Neurosci. 21, 9175–9184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen X., Zhu H., Yuan M., Fu J., Zhou Y., Ma L. (2010) G-protein-coupled receptor kinase 5 phosphorylates p53 and inhibits DNA damage-induced apoptosis. J. Biol. Chem. 285, 12823–12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vroon A., Kavelaars A., Limmroth V., Lombardi M. S., Goebel M. U., Van Dam A. M., Caron M. G., Schedlowski M., Heijnen C. J. (2005) G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Immunol. 174, 4400–4406 [DOI] [PubMed] [Google Scholar]
- 25. Lombardi M. S., Kavelaars A., Schedlowski M., Bijlsma J. W., Okihara K. L., Van de Pol M., Ochsmann S., Pawlak C., Schmidt R. E., Heijnen C. J. (1999) Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J. 13, 715–725 [DOI] [PubMed] [Google Scholar]
- 26. Kavelaars A., Eijkelkamp N., Willemen H. L., Wang H., Carbajal A. G., Heijnen C. J. (2011) Microglial GRK2: a novel regulator of transition from acute to chronic pain. Brain Behav. Immun. 25, 1055–1060 [DOI] [PubMed] [Google Scholar]
- 27. Raake P. W., Vinge L. E., Gao E., Boucher M., Rengo G., Chen X., DeGeorge B. R., Jr., Matkovich S., Houser S. R., Most P., Eckhart A. D., Dorn G. W., 2nd, Koch W. J. (2008) G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ. Res. 103, 413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dinudom A., Fotia A. B., Lefkowitz R. J., Young J. A., Kumar S., Cook D. I. (2004) The kinase Grk2 regulates Nedd4/Nedd4-2-dependent control of epithelial Na+ channels. Proc. Natl. Acad. Sci. U. S. A. 101, 11886–11890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu S., Premont R. T., Kontos C. D., Zhu S., Rockey D. C. (2005) A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat. Med. 11, 952–958 [DOI] [PubMed] [Google Scholar]
- 30. Wu J. H., Zhang L., Fanaroff A. C., Cai X., Sharma K. C., Brian L., Exum S. T., Shenoy S. K., Peppel K., Freedman N. J. (2012) G protein-coupled receptor kinase-5 attenuates atherosclerosis by regulating receptor tyrosine kinases and 7-transmembrane receptors. Arterioscler. Thromb. Vasc. Biol. 32, 308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oppermann M., Mack M., Proudfoot A. E., Olbrich H. (1999) Differential effects of CC chemokines on CC chemokine receptor 5 (CCR5) phosphorylation and identification of phosphorylation sites on the CCR5 carboxyl terminus. J. Biol. Chem. 274, 8875–8885 [DOI] [PubMed] [Google Scholar]
- 32. Ishibashi S., Brown M. S., Goldstein J. L., Gerard R. D., Hammer R. E., Herz J. (1993) Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 92, 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaber M., Koch W. J., Rockman H., Smith B., Bond R. A., Sulik K. K., Ross J., Jr., Lefkowitz R. J., Caron M. G., Giros B. (1996) Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc. Natl. Acad. Sci. U. S. A. 93, 12974–12979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matkovich S. J., Diwan A., Klanke J. L., Hammer D. J., Marreez Y., Odley A. M., Brunskill E. W., Koch W. J., Schwartz R. J., Dorn G. W., 2nd (2006) Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and β-adrenergic signaling. Circ. Res. 99, 996–1003 [DOI] [PubMed] [Google Scholar]
- 35. Nijboer C. H., Heijnen C. J., Willemen H. L., Groenendaal F., Dorn G. W., 2nd, van Bel F., Kavelaars A. (2010) Cell-specific roles of GRK2 in onset and severity of hypoxic-ischemic brain damage in neonatal mice. Brain Behav. Immun. 24, 420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kanters E., Pasparakis M., Gijbels M. J., Vergouwe M. N., Partouns-Hendriks I., Fijneman R. J., Clausen B. E., Forster I., Kockx M. M., Rajewsky K., Kraal G., Hofker M. H., de Winther M. P. (2003) Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 112, 1176–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Rijke Y. B., Biessen E. A., Vogelezang C. J., van Berkel T. J. (1994) Binding characteristics of scavenger receptors on liver endothelial and Kupffer cells for modified low-density lipoproteins. Biochem. J. 304, 69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aragay A. M., Mellado M., Frade J. M., Martin A. M., Jimenez-Sainz M. C., Martinez A. C., Mayor F., Jr. (1998) Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2. Proc. Natl. Acad. Sci. U. S. A. 95, 2985–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levine J. D., Coderre T. J., Helms C., Basbaum A. I. (1988) Beta 2-adrenergic mechanisms in experimental arthritis. Proc. Natl. Acad. Sci. U. S. A. 85, 4553–4556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Herijgers N., Van Eck M., Groot P. H., Hoogerbrugge P. M., Van Berkel T. J. (1997) Effect of bone marrow transplantation on lipoprotein metabolism and atherosclerosis in LDL receptor-knockout mice. Arterioscler. Thromb. Vasc. Biol. 17, 1995–2003 [DOI] [PubMed] [Google Scholar]
- 41. Eijkelkamp N., Heijnen C. J., Willemen H. L., Deumens R., Joosten E. A., Kleibeuker W., den Hartog I. J., van Velthoven C. T., Nijboer C., Nassar M. A., Dorn G. W., 2nd, Wood J. N., Kavelaars A. (2010) GRK2: a novel cell-specific regulator of severity and duration of inflammatory pain. J. Neurosci. 30, 2138–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carman C. V., Som T., Kim C. M., Benovic J. L. (1998) Binding and phosphorylation of tubulin by G protein-coupled receptor kinases. J. Biol. Chem. 273, 20308–20316 [DOI] [PubMed] [Google Scholar]
- 43. Cant S. H., Pitcher J. A. (2005) G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol. Biol. Cell 16, 3088–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ait-Oufella H., Kinugawa K., Zoll J., Simon T., Boddaert J., Heeneman S., Blanc-Brude O., Barateau V., Potteaux S., Merval R., Esposito B., Teissier E., Daemen M. J., Leseche G., Boulanger C., Tedgui A., Mallat Z. (2007) Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 115, 2168–2177 [DOI] [PubMed] [Google Scholar]
- 45. Arai S., Shelton J. M., Chen M., Bradley M. N., Castrillo A., Bookout A. L., Mak P. A., Edwards P. A., Mangelsdorf D. J., Tontonoz P., Miyazaki T. (2005) A role for the apoptosis inhibitory factor AIM/Spα/Api6 in atherosclerosis development. Cell Metab. 1, 201–213 [DOI] [PubMed] [Google Scholar]
- 46. Liu J., Thewke D. P., Su Y. R., Linton M. F., Fazio S., Sinensky M. S. (2005) Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 25, 174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Geng Y. J., Libby P. (2002) Progression of atheroma: a struggle between death and procreation. Arterioscler. Thromb. Vasc. Biol. 22, 1370–1380 [DOI] [PubMed] [Google Scholar]
- 48. Bot I., de Jager S. C., Zernecke A., Lindstedt K. A., van Berkel T. J., Weber C., Biessen E. A. (2007) Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation 115, 2516–2525 [DOI] [PubMed] [Google Scholar]
- 49. Han S., Liang C. P., DeVries-Seimon T., Ranalletta M., Welch C. L., Collins-Fletcher K., Accili D., Tabas I., Tall A. R. (2006) Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 3, 257–266 [DOI] [PubMed] [Google Scholar]
- 50. Jimenez-Sainz M. C., Murga C., Kavelaars A., Jurado-Pueyo M., Krakstad B. F., Heijnen C. J., Mayor F., Jr., Aragay A. M. (2006) G protein-coupled receptor kinase 2 negatively regulates chemokine signaling at a level downstream from G protein subunits. Mol. Biol. Cell 17, 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Loetscher P., Uguccioni M., Bordoli L., Baggiolini M., Moser B., Chizzolini C., Dayer J. M. (1998) CCR5 is characteristic of Th1 lymphocytes. Nature 391, 344–345 [DOI] [PubMed] [Google Scholar]
- 52. Hall S. E., Mao A., Nicolaidou V., Finelli M., Wise E. L., Nedjai B., Kanjanapangka J., Harirchian P., Chen D., Selchau V., Ribeiro S., Schyler S., Pease J. E., Horuk R., Vaidehi N. (2009) Elucidation of binding sites of dual antagonists in the human chemokine receptors CCR2 and CCR5. Mol. Pharmacol. 75, 1325–1336 [DOI] [PubMed] [Google Scholar]
- 53. Serbina N. V., Pamer E. G. (2006) Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 [DOI] [PubMed] [Google Scholar]
- 54. Combadiere C., Potteaux S., Rodero M., Simon T., Pezard A., Esposito B., Merval R., Proudfoot A., Tedgui A., Mallat Z. (2008) Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117, 1649–1657 [DOI] [PubMed] [Google Scholar]
- 55. Willemen H. L., Eijkelkamp N., Wang H., Dantzer R., Dorn G. W., 2nd, Kelley K. W., Heijnen C. J., Kavelaars A. (2010) Microglial/macrophage GRK2 determines duration of peripheral IL-1β-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain 150, 550–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Patial S., Saini Y., Parvataneni S., Appledorn D. M., Dorn G. W., 2nd, Lapres J. J., Amalfitano A., Senagore P., Parameswaran N. (2011) Myeloid-specific GPCR kinase-2 negatively regulates NF-κB1p105-ERK pathway and limits endotoxemic shock in mice. J. Cell. Physiol. 226, 627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Parvataneni S., Gonipeta B., Packiriswamy N., Lee T., Durairaj H., Parameswaran N. (2011) Role of myeloid-specific G-protein coupled receptor kinase-2 in sepsis. Int. J. Clin. Exp. Med. 4, 320–330 [PMC free article] [PubMed] [Google Scholar]
- 58. Goren I., Allmann N., Yogev N., Schurmann C., Linke A., Holdener M., Waisman A., Pfeilschifter J., Frank S. (2009) A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am. J. Pathol. 175, 132–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cross M., Mangelsdorf I., Wedel A., Renkawitz R. (1988) Mouse lysozyme M gene: isolation, characterization, and expression studies. Proc. Natl. Acad. Sci. U. S. A. 85, 6232–6236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clausen B. E., Burkhardt C., Reith W., Renkawitz R., Forster I. (1999) Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.