Abstract
We developed a selective competitive enzyme-linked immunosorbent assay (ELISA) to monitor environmental and human exposure to polybrominated diphenyl ether BDE-47 that is used as a flame retardant. 2,2’,4,4’-Tetrabromodiphenyl ether (BDE-47) a dominant PBDE congener of toxicological concern, was the target analyte. To achieve effective hapten presentation on the carrier protein for antibody production, immunizing haptens with a rigid double-bonded hydrocarbon linker introduced at different positions on the target molecule were synthesized as well as coating haptens that mimic a characteristic fragment of the molecule. Rabbit antisera produced against each immunizing antigen were screened against competitive hapten coating antigens. Under optimized competitive indirect ELISA conditions, the linear detection range in the assay buffer that includes 50% dimethyl sulfoxide was 0.35 - 8.50 μg/L with an IC50 value of 1.75 μg/L for BDE-47. Little or no cross-reactivity (< 6%) was observed to related PBDE congeners containing the BDE-47 moiety and other halogenated compounds. Using a magnetic particle-based competitive direct ELISA increased the sensitivity by 10-fold over the indirect ELISA. The ELISA provided quantitative results when performed on small volume/weight samples such as dust, furniture foam, and blood/serum following sample preparation, suggesting a convenient screening tool.
INTRODUCTION
Polybrominated diphenyl ethers (PBDEs) are flame retardants that are added into consumer products. PBDEs in those products contain predominantly penta- (also including tetra-), octa-, and decaBDE congeners. The U. S. market used about 8,000 metric tons in 1999, which is about 98% of the global production of pentaBDE (1). PentaBDEs (predominantly BDE-47, -99, -100) were used in polyurethane foam in furniture and some building materials whereas BDE-209 was used in electronic plastic products (2), and fabric back coatings. The concentration of other polyhalogenated chemicals such as polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxin (PCDDs), polychlorinated dibenzofurans (PCDFs) and DDT (3) are declining as a result of reduction policies, whereas PBDEs in the environment are rapidly rising worldwide (4). PBDEs accumulate in house dust, sewage sludge, biosolids, wildlife/pets, and humans (5-9), especially BDE-47 and BDE-99 accumulate to a greater degree than the higher halogenated BDEs (1, 10). The PBDE levels of North Americans are among the highest in the world (6). Aside from workers in electronic-recycling facilities that experience high PBDE exposure (11), a major route of exposure to PBDEs is ingestion of indoor dusts (12).
PBDEs exhibit a wide spectrum of toxicity in laboratory animals, primarily developmental and neurological in nature (13-14). PBDEs are also structurally similar to thyroid hormones and may act as endocrine disruptors by altering thyroid hormone function (15-16), thus there is increasing concern for women of childbearing age.
Analytical methods for detection of PBDEs in biological samples such as milk and adipose tissues use sample preparation steps including solvent extraction, liquid-liquid extraction, and column chromatographic clean-up methods combined with gas chromatography and electron capture detection (GC-ECD) or GC with mass spectrometry (GC-MS) (17-18). Although sensitive, the methods are time-consuming and expensive. An X-ray fluorescence (XRF) analyzer measures total bromine levels of brominated flame retardants present in consumer products nondestructively and in real time (19). Alternatively, immunoassays are rapid, sensitive and selective analytical tools that have been used to determine trace chemicals of interest such as agrochemicals and their degradation products (21-23) and industrial contaminants such as PCBs, dioxins and nonylphenol (20, 24-27). Use of an immunoassay would provide more selective detection of PBDEs than XRF, and is less time consuming than typical instrumental methods.
Because of its abundance and toxicity, BDE-47 can be an indicator of exposure to lower brominated PBDEs and so was selected as the target for immunoassay development. Shelver et al. (28) developed a heterologous competitive indirect immunoassay using a rabbit antiserum generated against an immunizing hapten synthesized by introducing a butyric acid spacer to 5-hydroxy-BDE-47. It detected both BDE-47 and BDE-99 and was later developed into a magnetic particle-based immunoassay (29). Generally it is more difficult to develop an assay for lipophilic than polar compounds. However, we have taken several approaches to successfully develop selective assays for compounds such as dioxins and pyrethroid insecticides (26, 30). On the basis of this experience, an immunoassay for PBDEs was developed, optimized, characterized, and applied to diverse matrices including furniture foam, house dust and whole blood/serum using sample sizes as low as 10 μL or 10 mg.
EXPERIMENTAL SECTION
Chemicals and Instruments
The hapten coupling reagents, bovine serum albumin (BSA), keyhole limpet hemocyanin (KLH), goat anti-rabbit IgG peroxidase conjugate (GAR-HRP), Tween 20, and 3,3’,5,5’-tetramethylbenzidine (TMB) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Horseradish peroxidase (HRP) was purchased from Thermo Fisher Scientific Inc. (Rockford, IL). ELISA was performed on 96-well microtiter plates (Nunc MaxiSorp, Roskilde, Denmark) and read spectrophotometrically with a microplate reader (Molecular Devices, Menlo Park, CA) in dual wavelength mode (450–650 nm).
Hapten Synthesis
Because BDE-47 is of small molecular weight, it requires conjugation to carrier proteins in order to be immunogenic. BDE-47 and its analogs (haptens) containing a carboxylic acid group were designed (Supporting Information, Figure S1) and synthesized (Figure 1). The main reactions to prepare haptens containing a brominated diphenyl ether moiety and a double-bonded hydrocarbon linker were based on methods previously described (26, 31-32). The synthesis is detailed in the Supporting Information.
FIGURE 1.
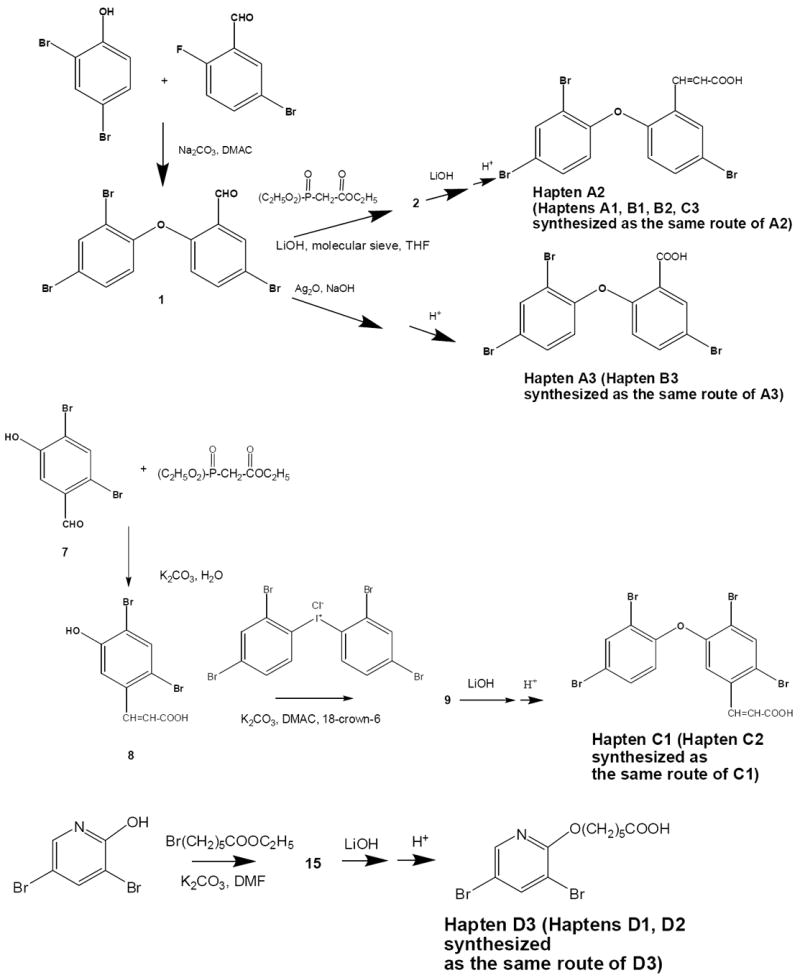
Synthetic routes of four different types of haptens A, B, C, and D. Linkers are attached at the 2’ (Type A), 4’ (Type B) or 5’ (Type C) position on the BDE-47 molecule. Haptens within a type (A1, A2, A3) vary in linker length or structure.
Preparation of Immunogen and Coating Antigens
Haptens containing carboxylic acids were activated by the mixed anhydride method (26) or the carbodiimide method (33). For immunogens, Type A-C haptens (Figure 1) were conjugated to KLH. Type A-D haptens were conjugated to BSA for coating antigens. For the magnetic particle-based ELISA, hapten D3 was coupled to HRP by the carbodiimide method (33). Complete details may be found in Supporting Information.
Immunization and Antiserum Preparation
The immunization procedure followed the protocol reported previously (33, 34). Two female New Zealand white rabbits were immunized for each immunogen (Rabbits 1304/1305, Hapten A2-KLH; Rabbits 1306/1307, Hapten A1-KLH; Rabbits 1308/1309, Hapten B2-KLH; Rabbits 1310/1311, Hapten B1-KLH; Rabbits 1312/1313, Hapten C1-KLH; and Rabbits 1314/1315, Hapten C2-KLH). Final serum was collected 5 months following the first immunization. Antiserum was obtained by centrifugation, stored at -80 °C and used without purification.
Competitive Indirect ELISA
The preparation of the buffers and the procedure for the indirect competitive ELISA was previously described (33). We typically use the indirect method because it is more conservative of haptens that are in short supply from complex synthetic methods; we have found hapten-BSA conjugates to be more stable than hapten-enzyme conjugates, and the indirect method is less susceptible to matrix effects. The IC50 value, an expression of the sensitivity of immunoassay and the limit of detection (LOD) defined as the IC10 value were obtained from a four parameter logistic equation. Borosilicate glass tubes were used to prepare standard and sample solutions to minimize the surface adsorption of BDE-47.
Immunological Analysis of Industrial, Environmental, and Biological Samples
Specific details are found in Supporting Information. House Dust. Dust from the vacuum cleaner bag collected from single family homes in Northern California was sieved to 150 μm with a stainless steel screen. A 100 mg dust sample was extracted using microwave-assisted solvent extraction (35). The hexane layer was collected and washed with concentrated H2SO4, then deionized water, and dried with anhydrous Na2SO4. After evaporation the extract was redissolved in DMSO then diluted 2 to 16 times prior to the immunoassay. Each sample (n=17) was analyzed in triplicate. Because PBDE-free dusts were not available for the recovery study, a Standard Reference Material 2585 house dust (National Institute of Standards and Technology, Gaithersburg, MD) having a certified value of 497±46 μg/kg of BDE-47 was tested.
Furniture Foam
Polyurethane foam samples (~10 mg) obtained from houses and local furniture stores were shaken with DMSO on an orbital shaker (Lab-line, Melrose Park, IL) for 24 hrs at room temperature. An aliquot of the DMSO extract was diluted 400 to 6400 times prior to the immunoassay. Each sample was analyzed in triplicate. For the recovery study, 10 mg of furniture foam that did not contain brominated flame retardants as measured by XRF was spiked at 0.1-3% BDE-47 and extracted as described above. Each sample (n=13) was analyzed in triplicate. Results are expressed as percent since levels of PBDEs are added to furniture foam in amounts of 3-6%.
Human Whole Blood/Serum
Calf serum (Invitrogen, Carlsbad, CA) was denatured as previously described (36). BDE-47 was extracted by liquid-liquid extraction (LLE) with CH2Cl2 in hexane. Concentrated H2SO4 was added to the extract to remove lipids. The organic layer was washed with NaOH followed by distilled water, then dehydrated through anhydrous Na2SO4 and evaporated. The residue was reconstituted with 50% DMSO in PBST. For the recovery study, BDE-47 was spiked at 1-5 ng/mL into 0.5 mL of calf serum. The samples were extracted as described above and analyzed in triplicate.
Human whole blood (10 μL) or a commercial human serum (Sigma, Milwaukee, WI) was mixed with distilled water, extracted with ethyl acetate, evaporated to dryness, and dissolved in 50 μL of DMSO, followed by addition of 50 μL of PBS prior to the immunoassay. Final dilution was 10-fold in the assay buffer. For the recovery study, BDE-47 dissolved in acetone was spiked at 10-100 ng/mL into 10 μL of the calf serum or whole blood. The samples were extracted as described above and analyzed in triplicate.
GC/MS Analysis of Dust
Dust samples (0.1 or 0.5 g) were spiked with surrogate recovery standards (SRSs; 25 ng each of BDE-126 and 13C12 BDE-209), extracted in 1:1 hexane:CH2Cl2 by ultrasonication, and cleaned up using bulk acidic silica and an alumina SPE cartridge. Extracts were analyzed using GC/MS with methane negative ion chemical ionization (Agilent/HP 6890 GC and 5973 MSD). The chromatography included separation on a DB-5 ms column. BDE-47 was quantified using the internal standard method (dibromobiphenyl as the internal standard). SRS recoveries were: 102 ± 23% for BDE-126; 93 ± 28% for 13C12 BDE-209. Spiked recoveries of BDEs in one representative house dust ranged from 78-109%. The average relative percent difference in replicate samples was less than 24%.
XRF Analysis of Furniture Foam
A portable handheld XRF analyzer (Innov-X Systems, Inc., Woburn, MA) was used to measure total bromine concentration in furniture foam samples. The analyzer was held against a large piece of foam in a couch cushion in situ. Each sample had one reading at 50 seconds per reading. Standard reading times are 30 seconds as recommended by the manufacturer. The extended measuring time was used for accurate data acquisition without repetition. A sample of at least one square inch was then removed from each product for further ELISA testing. Although the sample was not taken from the exact same site as the XRF reading, multiple XRF readings from the same foam cushion showed good agreement (data not shown). A limit of detection was set conservatively at 0.1% (w/w). The XRF results were compared with ELISA measurements. Although, XRF cannot distinguish the chemical form of the bromine, we utilized the XRF method for comparisons because it is being used for on site assessment of flame retardants in furniture foam. Gas chromatographic methods were not performed for this study.
RESULTS AND DISCUSSION
Hapten Synthesis
Since synthesis and production of antibodies is time- and cost-consuming, the hapten chemistry should be thoroughly researched to develop the most sensitive and selective immunoassay (20, 37). BDE-47 is of small molecular weight and requires conjugation to carrier proteins to be immunogenic. Antibodies are generally formed to the part of the molecule that is most distal to the point of attachment to the carrier protein. Potential haptens with a functional group (-COOH) for conjugation were designed (Figure S1). We prepared haptens that mimic the whole BDE-47 molecule and contained a rigid linker with at least one double-bonded hydrocarbon and a carboxylic reactive group so that the hapten would extend from the surface of the carrier-protein during antibody formation. The number of carbons in the linker ranged between 1 and 5 and the linkers were attached at the 2’ (Type A), 4’ (Type B), or 5’ (Type C) position of the BDE-47 molecule in order to expose different parts of the PBDE molecule for antibody recognition. Due to the lipophilicity of PBDEs, a long side chain could allow the lipophilic hapten to fold back into the hydrophobic interior of the carrier protein and decrease the affinity of the resulting antibodies. A rigid linker, such as a carbon chain with one or more double bonds, can generate sensitive and selective antibodies for lipophilic molecules like dioxins (26). A rigid spacer was introduced into the dibromophenoxy-bromobenzaldehyde (compound 1) by enylation with phosphonoacetate or phosphonocrotonate using LiOH and molecular sieve by the Wittig or Horner-Wadsworth-Emmons reaction (Figure 1, 32). The aldehyde intermediates were oxidized by Ag2O to obtain acid haptens A3 and B3. In contrast to haptens type A-C, type D haptens that only mimic the dibromophenoxy were designed as coating antigens to enhance the affinity of BDE-47 to the antibody and to increase assay sensitivity. The haptens were synthesized to obtain different lengths of a single-bonded hydrocarbon linker on a phenyl or pyridine ring. Ethyl bromobutyrate or ethyl bromohexanoate was used for the carbon linker attachment on the dibromophenol or 3,5-dibromo-2-hydroxypyridine.
Antibody Characterization
The antisera collected after each boost were subjected to titration by the homologous indirect ELISA. All of the antisera showed relatively constant high titers after the fifth immunization (data not shown) and no significant affinity for BSA alone. All twelve antisera were screened against nine coating antigens at the two concentrations (5 and 500 μg/L) of BDE-47 (data not shown). The combinations of antibody and coating antigen that had over 80% inhibition at 500 μg/L and with over 20% inhibition at 5 μg/L, were again screened using 10 concentrations ranging from 0.003 to 5000 μg/L. The antisera against hapten type A consistently had the highest IC50s, while antisera against hapten type C had mixed results depending upon the coating antigen used. The antisera with the lowest IC50 were mainly raised against immunizing hapten B2 and B1 (Supporting Information, Table S1), suggesting that these immunizing haptens containing a double-bonded carbon linker at the 4’-position are the closest mimics of BDE-47 among the synthesized haptens. Antibody 1309 generated against the immunizing hapten B2 was selected for further immunoassay development due to both high maximum signals and low IC50 values.
Molecular Modeling
Structural modeling of haptens is a useful tool to select haptens that best mimic the target. Since PBDEs including BDE-47 have a non-coplanar conformation and exist in a twist between the two phenyl moieties (38), the hapten with the same molecular geometry as that of BDE-47 is desired to produce specific antibodies. After the optimization of the molecular geometry of haptens and other PBDE congeners, their molecular geometries were overlaid on that of BDE-47. The order of RMS values, which indicate the error in molecular geometry between the structures tested and BDE-47, is as follows: hapten C1 > hapten C2 > hapten C3 > hapten A1 > hapten B3 > BDE-153 > hapten A3 > hapten A2 > BDE-99 > hapten B2 > hapten B1 > BDE-47 (Table S2). Because the RMS values of haptens B1 (RMS=0.0475) and B2 (RMS=0.0832) are closest to that of BDE-47 (RMS=0.0000), the antibodies generated against them provided relatively highly sensitive assays (Table S1). Antibody 1309 was generated against hapten B2 which was the optimal hapten via molecular modeling. Although hapten A3 containing a short spacer also had a low RMS value (0.2188), haptens with longer (C-3 to -5) carbon linker were selected as immunizing haptens. Haptens with a short spacer (C-1; RMS=1.3871) may not provide maximum presentation of the unique structural features of the analyte to the immune system, because of steric hindrance.
Enhancement of Assay Sensitivity using Heterologous Coating Haptens
As shown in Table S3, heterologous formats were far more sensitive than the homologous one. The highest sensitivity is obtained when the binding affinity of the antibody to the target analyte is much stronger than to the coating antigen. The heterologous coating hapten C3, which imitates the whole structure of the BDE-47, but presents different molecular geometry, showed more sensitivity than the homologous coating hapten. The type D haptens that imitate one ring of BDE-47 provided more sensitivity than the whole structure mimic hapten C3. The longer spacer of coating hapten D2 than hapten D1 exhibited better assay sensitivity. Finally, a pyridine-like coating hapten D3 with the same length of linker as hapten D2 provided the most sensitive assay. Due to the contribution of the extra electron cloud of N in the ring, D3 is more structurally different from the immunizing hapten mimicking BDE-47, and thus decreases the affinity to antibody 1309 and increases the assay sensitivity remarkably.
Assay Optimization
The optimum concentrations of coating antigen hapten D3-BSA and Ab 1309 were 5 μg/mL and 1:8000 dilution in the well, respectively. Since BDE-47 is highly lipophilic and adsorbs to glass, plastic or other particle surfaces, a co-solvent is important for consistent assay performance and sensitivity. Use of DMSO decreased background and increased sensitivity compared to 40% methanol in PBST. DMSO (50%) was selected to prepare standard or sample solutions due to the low IC50 value (1.8 μg/L) and maximum A/D ratio (Table S4). The IC50 value was not affected by higher ionic strength, although a decrease in Amax indicated that the binding interaction of antibody to antigen was affected. There were no significant effects of pH ranging from 6.5 to 9.5 in the buffer on the IC50 value, but, the maximum absorbances were variable at low concentrations of BDE-47 with all pHs tested (data not shown). Thus, the pH of PBS was maintained at 7.5. Ab 1309 was diluted in 0.2% BSA in PBS. Finally, the optimized ELISA used hapten D3-BSA coating antigen at a concentration of 5 μg/mL and Ab 1309 produced against hapten B2-KLH at a dilution of 1/8,000 in wells. The coated plate was blocked with 0.5% BSA. The assay buffer was 50% DMSO in 0.15 M PBS, pH 7.5. This heterologous assay had a linear range (IC20-80) of 0.35–8.50 μg/L in the buffer system and an IC50 value of 1.75 μg/L (Figure S3). The LOD in the buffer was defined as 0.2 μg/L, the IC10 value.
Magnetic Particle-Based ELISA
To improve speed and sensitivity, we developed an IgG magnetic particle-based competitive direct ELISA. Although this initial assay is currently characterized by high background (likely due to nonspecific binding of the hapten D3-HRP conjugate to the magnetic particles possessing relatively larger surface areas), compared with the coating antigen ELISA using a 96-well plate, this ELISA was 10-fold more sensitive (Figure S3). This increased sensitivity may result from a relatively well-oriented antibody on the enlarged surface area of the goat anti-rabbit IgG magnetic particles as well as from using a direct competitive hapten D3-HRP conjugate. Dispersive magnetic particle-based technology facilitates the separation of the desired immunocomplex in solution and can be easily automated (22). Although this direct competitive format is more sensitive, it was not further investigated because it used large quantities of antibody compared to the plate assay and high throughput was not necessary for this study.
Cross-Reactivity (CR)
Various compounds structurally related to BDE-47 were evaluated for CR. The assay was highly selective for BDE-47, showing very low CRs (< 6%) to other PBDEs congeners including BDE-49, having the same number of Br atoms as BDE-47 (Table S5). The antibody binding pocket generated against a hapten containing a low number of bromines may preclude the binding of larger PBDEs containing more bromine substitutions. Although the RMS value of BDE-99 is close to those of BDE-47 and hapten-B2 (Table S2), the low CR (5%) indicates that the antibody produced binds less to BDE-99, likely due to differences in electrostatic potentials and steric hindrance caused by having five Br atoms. Little or no cross-reactivity was seen to other related halogenated compounds such as PCB, dioxin, dihalophenols, and triclosan. 3-Methoxy-BDE-47 showed relatively high CR (35%) among metabolites of BDE-47.
Matrix Effects
The standard curves in spiked calf serum and furniture foam samples were parallel to those of the BDE-47 standard curve prepared in assay buffer (Figure S2A and S2B) indicating no matrix effect. This clearly demonstrates the efficacy of each sample preparation method in removing interfering components resulting in quantitative measurement of BDE-47. Recoveries were 82-138% in serum/blood samples, 71-130% in furniture foam samples, and 105% in a certified house dust sample.
House dust is a complex mixture of biologically derived materials such as animal dander, fungal spores, dead skin cells, dirt, and mineral particles deposited from outdoors. A clean-up that used an alkaline (NaOH) solution during the microwave-assisted hexane extraction followed by a H2SO4 clean-up of the hexane extract, provided results comparable to GC/MSD analysis for house dust (Table 1). This clean-up process can be simplified for high throughput using a multilayer silica gel treated with H2SO4 and NaOH.
TABLE 1.
Selected Levels of BDE-47 in Different Matrices Determined by the ELISA and Instrumental Analysis
| Sample | BDE-47 equivalent measured by ELISA | BDE-47 measured by GC/MS or total Br by XRF | ||
|---|---|---|---|---|
|
| ||||
| Dilution of extract prior to immunoassay | Mean±SD | CV | Mean±SD | |
| House Dust | ||||
| Dust I | 2-16 | 3322±146 ng/g | 4 | 4366 ng/ga |
| Dust II | 2-16 | 523±114 ng/g | 22 | 497 ng/ga |
| Dust III | 2-16 | 251±61.2 ng/g | 24 | 140 ng/ga |
| Dust IV | 2-16 | 2818±102 ng/g | 4 | 2144 ng/ga |
| Dust V | 2-16 | 1333±100 ng/g | 7 | 693 ng/ga |
| Dust VI | 2-16 | 7050±218 ng/g | 3 | 4455 ng/ga |
| Commercial serum | ||||
| Serum I | 10 | < 10 ng/mL | 17 | -b |
| Serum II | 10 | 32.74±8.28 ng/mL | 25 | - |
| Whole blood | ||||
| Blood I | 10 | < 10 ng/mL | - | |
| Blood II | 10 | < 10 ng/mL | - | |
| Blood III | 10 | 12.83±2.04 ng/mL | 16 | - |
| Furniture foam | ||||
| Foam I | 800-3200 | < 0.03% | 4.29%c | |
| Foam II | 800-3200 | 1.78±0.19% | 11 | 3.47%c |
| Foam III | 800-3200 | < 0.03% | 4.37%c | |
| Foam IV | 800-3200 | 2.51±0.22% | 9 | 5.55%c |
| Foam V | 800-3200 | 3.58±0.57% | 16 | 6.39%c |
| Foam VI | 800-3200 | < 0.03% | < 0.1%c | |
| Foam VII | 800-3200 | < 0.03% | < 0.1%c | |
BDE-47 measured by GC/MS.
Not tested.
Total Br measured by XRF.
The values of furniture foam and house dust were average values of data calculated from 50-, 100-, 200- and 400-fold dilution factor of the extract, and from 800-, 1600- and 3200-fold dilution factor, respectively.
Sulfuric acid treatment removed lipophilic residues coextracted by the CH2Cl2/hexane mixture in serum improving the assay to as low as 1 μg/L (Table S6). However, the LLE method using 10-μL sample is a simple method for crude screening of BDE-47 in the blood/serum or milk as demonstrated by the good recoveries seen for human whole blood. The small sample volume (10 μL) and simple sample preparation coupled to the sensitive ELISA can provide relatively high-throughput analysis in 96-well plates. It may be feasible to develop miniaturized tools for sample preparation and apply those to automated analysis.
DMSO extraction of furniture foam using a 10-mg sample and dilution provided suitable monitoring for BDE-47. Although pentaBDE is currently banned in many but not all countries, this ELISA can be used to screen their occurrence in consumer products still in use.
Validation for Application to Real Samples
A relatively good correlation for selected house dust samples was exhibited between GC/MS data and ELISA (Table 1). The concentrations of BDE-47 equivalent in the dust samples, V and VI, determined by the ELISA were around 1.6-1.9 times higher than those determined by the instrumental analysis. Similarly, as shown in Figure 2A, there is a positive correlation (r2=0.78) between BDE-47 data in a representative collection of dust samples that includes the samples in Table 1 measured by the ELISA and GC/MS. The immunochemical method over-estimated the occurrence of BDE-47 compared to GC/MS. This higher concentration could result from other unidentified cross-reacting compounds and/or a nonspecific interference from this dust matrix. Although BDE-99 cross reacts at 6%, a mixture having a 5-fold higher concentration of BDE-99 than BDE-47 in a solution did not effect the ELISA response for the detection of BDE-47 nor did an equal concentration mixture of BDE-47, -99, and -153 (data not shown). For dust analysis, this ELISA may best be used as a screening tool or to rank samples for instrumental analysis.
FIGURE 2.

Validation for characterization of the ELISA. Correlations (A) between BDE-47 concentrations in dust samples measured by the ELISA and GC-MS; (B) BDE-47 concentrations in furniture samples measured by the ELISA and total bromine concentrations measured by XRF; (C) Measurement of BDE-47 spiked into diverse samples and measured by ELISA.
There was a moderate correlation (r2=0.38) between the concentration of BDE-47 equivalent in furniture foams measured by the ELISA and the total bromine occurrence measured by XRF (Figure 2B). This is not surprising since the ELISA selectively detects BDE-47 while the XRF is measuring total bromine that may be present from the use of other brominated flame retardants (39). The validation for application of this ELISA to real samples (total n=17) was achieved in diverse sample matrices including serum, whole blood, house dust, and furniture foam in a wide range of concentrations, which were spiked with BDE-47. The linear regression of the results showed a highly positive correlation (r2=0.998) with a slope of 0.75 (Figure 2C).
In conclusion, to develop a selective immunoassay for BDE-47, the introduction of a rigid double bonded hydrocarbon spacer in a near perfect molecular mimic of BDE-47 aids in the recognition of the BDE-47 hapten while an antibody is generated. A heterologous coating hapten having a brominated pyridine moiety that influences the affinity of the antibody for the analyte in the competitive assay results in the highest sensitivity assay. The two immunoassay formats developed for PBDEs are highly sensitive with IC50 values of 1.75 μg/L for BDE-47 in a competitive indirect format and 0.1 μg/L in a competitive direct format. Concentrated sulfuric acid treatment and a hexane extraction that removes interfering lipid materials was used as a common sample preparation method prior to the immunoassay providing quantitative measurement in serum and dust. Additionally, this immunoassay, using sample sizes as low as 10-μL or 10-mg can be adapted for relatively high-throughput analysis.
Supplementary Material
Acknowledgments
The authors thank Drs. Eun Kee Park and Pavel A. Aronov from the University of California Davis, and Mr. Damien Jenn (a student intern from ENSIACET, France) for their kind contributions to this study. This research was supported in part by the National Institute of Environmental Health Sciences Superfund Basic Research Program, P42 ES04699, NIEHS Center for Children’s Environmental Health & Disease Prevention, 1 P01 ES11269, and the UC Davis-Howard Hughes Medical Institute (UC Davis-HHMI) training program.
Footnotes
Supporting Information Available
There are additional methods and data as referenced in this article. This information is available free of charge via the Internet at http://pubs.acs.org.
LITERATURE CITED
- 1.Hale R, La Guardia M, Harvey E, Mainor M. Potential role of fire retardant-treated polyurethane foam as a source of brominated diphenyl ethers to the US environment. Chemosphere. 2002;46:729–735. doi: 10.1016/s0045-6535(01)00237-5. [DOI] [PubMed] [Google Scholar]
- 2.Betts KS. Unwelcome guest: PBDEs in indoor dust. Environ Health Perspect. 2008;116:A202–A208. doi: 10.1289/ehp.116-a202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure and toxicology. Environ Health Perspect. 2001;109:49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- 5.Hale RC, La Guardia MJ, Harvey EP, Gaylor MO, Mainor TM, Duff WH. Flame retardants. Persistent pollutants in land-applied sludges. Nature. 2001;412:140–141. doi: 10.1038/35084130. [DOI] [PubMed] [Google Scholar]
- 6.Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ Sci Technol. 2008;42:8158–8164. doi: 10.1021/es801792z. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton HM, Dodder NG, Kucklick JR, Reddy CM, Schantz MM, Becker PR, Gulland F, Porter BJ, Wise SA. Temporal trends in anthropogenic and naturally produced brominated compounds in California sea lions, 1993 to 2003. Mar Pollut Bull. 2006;52:522–531. doi: 10.1016/j.marpolbul.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 8.Dye JA, Venier M, Zhu L, Ward CR, Hites RA, Birnbaum LS. Elevated PBDE levels in pet cats: sentinels for humans? Environ Sci Technol. 2007;41:6350–6356. doi: 10.1021/es0708159. [DOI] [PubMed] [Google Scholar]
- 9.Sjödin A, Päpke O, McGahee E, Focant J, Jones RS, Pless-Mulloli T, Toms LL, Herrmann T, Müller J, Needham LL, Patterson DG., Jr Concentration of polybrominated diphenyl ethers (PBDEs) in household dust from various countries. Chemosphere. 2008;73(suppl):S131–136. doi: 10.1016/j.chemosphere.2007.08.075. [DOI] [PubMed] [Google Scholar]
- 10.WHO. Brominated Diphenyl Ethers Environmental Health Criteria. Vol. 162. WHO; Geneva, Switzerland: 1994. [Google Scholar]
- 11.Stapleton HM, Sjödin A, Jones RS, Niehüser S, Zhang Y, Patterson DG., Jr Serum levels of polybrominated diphenyl ethers (PBDEs) in foam recyclers and carpet installers working in the United States. Environ Sci Technol. 2008;42:3453–3458. doi: 10.1021/es7028813. [DOI] [PubMed] [Google Scholar]
- 12.Allen JG, McClean MD, Stapleton HM, Webster TF. Critical factors in assessing exposure to PBDEs via house dust. Environ Int. 2008;34:1085–1091. doi: 10.1016/j.envint.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Suvorov A, Girard S, Lachapelle S, Abdelouahab N, Sebire G, Takser L. Perinatal exposure to low-dose BDE-47, an emergent environmental contaminant, causes hyperactivity in rat offspring. Neonatology. 2009;95:203–209. doi: 10.1159/000155651. [DOI] [PubMed] [Google Scholar]
- 14.Gee JR, Moser VC. Acute postnatal exposure to brominated diphenylether 47 delays neuromotor ontogeny and alters motor activity in mice. Neurotoxicol Teratol. 2008;30:79–87. doi: 10.1016/j.ntt.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Abdelouahab N, Suvorov A, Pasquier JC, Langlois MF, Praud JP, Takser L. Thyroid disruption by low-dose BDE-47 in prenatally exposed lambs. Neonatology. 2009;96:120–124. doi: 10.1159/000209316. [DOI] [PubMed] [Google Scholar]
- 16.Turyk ME, Persky VW, Imm P, Knobeloch L, Chatterton R, Anderson HA. Hormone disruption by PBDEs in adult male sport fish consumers. Environ Health Perspect. 2008;116:1635–1641. doi: 10.1289/ehp.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petreas M, She J, Brown FR, Winkler J, Windham G, Rogers E, Zhao G, Bhatiz R, Charles MJ. High body burdens of 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47) in California women. Environ Health Perspect. 2003;111:1175–1179. doi: 10.1289/ehp.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lind Y, Darnerud PO, Atuma S, Aune M, Becker W, Bjerselius R, Cnattingius S, Glynn A. Analytical methods for the determination of alkylphenolic surfactants and polybrominated diphenyl ethers in wastewaters and sewage sludges. II Method development. Environ Technol. 2004;25:975–985. doi: 10.1080/09593332508618393. [DOI] [PubMed] [Google Scholar]
- 19.Allen JG, McClean MD, Stapleton HM, Webster TF. Linking PBDEs in house dust to consumer products using X-ray fluorescence. Environ Sci Technol. 2008;42:4222–4228. doi: 10.1021/es702964a. [DOI] [PubMed] [Google Scholar]
- 20.Harris AS, Lucas AD, Krämer PM, Marco M-P, Gee SJ, Hammock BD. Use of immunoassays for the detection of urinary biomarkers of exposure. In: Kurtz D, Stanker L, Skerritt J, editors. New Frontiers in Agrochemical Immunoanalysis. AOAC International; Arlington: 1995. pp. 217–236. [Google Scholar]
- 21.Leahey JP. Metabolism and environmental degradation. In: Leahey JP, editor. The Pyrethroid Insecticides. Taylor & Francis; London, United Kingdom: 1985. pp. 263–341. [Google Scholar]
- 22.Ahn KC, Lohstroh P, Gee SJ, Gee NA, Lasley B, Hammock BD. High-throughput automated luminescent magnetic particles-based immunoassay to monitor human exposure to pyrethroid insecticides. Anal Chem. 2007;79:8883–8890. doi: 10.1021/ac070675l. [DOI] [PubMed] [Google Scholar]
- 23.Lee K, Park EK, Stoecklin-Marois M, Koivunen ME, Gee SJ, Hammock BD, Beckett LA, Schenker MB. Occupational paraquat exposure of agricultural workers in large Costa Rican farms. Int Arch Occup Environ Health. 2009;82:455–462. doi: 10.1007/s00420-008-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mapes JP, McKenzie KD, Stewart TN, McClelland LR, Studabaker WB, Manning WB, Friedman SB. An on-site immunoassay for detecting PCB in soil. Bull Environ Contam Toxicol. 1993;50:219–225. doi: 10.1007/BF00191725. [DOI] [PubMed] [Google Scholar]
- 25.Johnson JC, Van Emon JM. Quantitative enzyme-linked immunosorbent assay for determination of polychlorinated biphenyls in environmental soil and sediment samples. Anal Chem. 1996;68:162–169. doi: 10.1021/ac950410j. [DOI] [PubMed] [Google Scholar]
- 26.Sanborn JR, Gee SJ, Gilman SD, Sugawara Y, Jones AD, Rogers J, Szurdoki F, Stanker LH, Stoutamire DW, Hammock BD. Hapten synthesis and antibody development for polychlorinated dibenzo-p-dioxin immunoassays. J Agric Food Chem. 1998;46:2407–2416. [Google Scholar]
- 27.Samsonova JV, Rubtsova MY, Franek M. Determination of 4-n-nonylphenol in water by enzyme immunoassay. Anal Bioanal Chem. 2003;375:1017–1019. doi: 10.1007/s00216-003-1815-3. [DOI] [PubMed] [Google Scholar]
- 28.Shelver WL, Keum Y-S, Kim H-J, Rutherford DH, Hakk H, Bergman Å, Li QX. Hapten syntheses and antibody generation for the development of a polybrominated flame retardant ELISA. J Agric Food Chem. 2005;53:3840–3847. doi: 10.1021/jf047863m. [DOI] [PubMed] [Google Scholar]
- 29.Rubio F, Parrotta C, Li Q, Shelver W. Development of sensitive magnetic particle immunoassay for polybrominated diphenyl ethers. Organohalogen Compd. 2005;67:27–30. doi: 10.1016/j.chemosphere.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 30.Shan G, Leeman WR, Stoutamire DW, Gee SJ, Chang DPY, Hammock BD. Enzyme-linked immunosorbent assay for the pyrethroid permethrin. J Agric Food Chem. 2000;48:4032–4040. doi: 10.1021/jf000351x. [DOI] [PubMed] [Google Scholar]
- 31.Marsh G, Stenutz R, Bergman Å. Synthesis of hydroxylated and methoxylated polybrominated diphenyl ethers: natural products and potential polybrominated diphenyl ether metabolites. Eur J Org Chem. 2003;14:2566–2576. [Google Scholar]
- 32.Takacs JM, Jaber MR, Clement F, Walters C. A useful procedure for the preparation of (E,E)-2,4-dienoates: LiOH-promoted dienylation by 4-phosphono-crotonate. J Org Chem. 1998;63:6757–6760. [Google Scholar]
- 33.Lee JK, Ahn KC, Park OS, Ko YK, Kim DW. Development of an immunoassay for the residues of the herbicide bensulfuron-methyl. J Agric Food Chem. 2002;50:1791–1803. doi: 10.1021/jf011150b. [DOI] [PubMed] [Google Scholar]
- 34.Voller A, Bidwell DE, Bartlett A. Enzyme immunoassay in diagnostic medicine: theory and practice. Bull WHO. 1976;53:55–65. [PMC free article] [PubMed] [Google Scholar]
- 35.Regueiro J, Llompart M, Garcia-Jares C, Cela R. Factorial-design optimization of gas chromatographic analysis of tetrabrominated to decabrominated diphenyl ethers. Application to domestic dust. Anal Bioanal Chem. 2007;388:1095–1107. doi: 10.1007/s00216-007-1350-8. [DOI] [PubMed] [Google Scholar]
- 36.Sandau CD, Sjödin A, Davis MD, Barr JR, Maggio VL, Waterman AL, Preston KE, Preau JL, Jr, Barr DB, Needham LL, Patterson DG., Jr Comprehensive solid-phase persistent chlorinated pesticides. Anal Chem. 2003;75:71–77. doi: 10.1021/ac026121u. [DOI] [PubMed] [Google Scholar]
- 37.Skerritt JH, Lee N. Approaches to the synthesis of haptens for immunoassay of organophosphate and synthetic pyrethroid insecticides. In: Beier RC, Stanker LH, editors. Immunoassays for Residue Analysis. American Chemical Society; Washington, D.C.: 1996. pp. 124–149. [Google Scholar]
- 38.Eriksson L, Hu J. 2,3,4,5,6-Pentabromophenyl phenyl ether. Acta Crystallogr Sect E. 2002;E58:794–796. [Google Scholar]
- 39.Shi T, Chen S-J, Luo X-J, Zhang X-L, Tang C-M, Luo Y, Ma Y-J, Wu J-P, Peng X-Z, Mai B-X. Occurrence of brominated flame retardants other than polybrominated diphenyl ethers in environmental and biota samples from southern China. Chemosphere. 2009;74:910–916. doi: 10.1016/j.chemosphere.2008.10.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


