Abstract
Behavioral analysis is a high-end read-out of aging impact on an organism, and here, we have analyzed behaviors in 4-, 22-, and 28-month-old male C57BL/6J with a broad range of tests. For comparison, a group of 28-month-old males maintained on dietary restriction (DR) was included. The most conspicuous alteration was the decline in exploration activity with advancing age. Aging also affected other behaviors such as motor skill acquisition and grip strength, in contrast to latency to thermal stimuli and visual placement which were unchanged. Object recognition tests revealed intact working memory at 28 months while memory recollection was impaired already at 22 months. Comparison with female C57BL/6J (Fahlström et al., Neurobiol Aging 32:1868–1880, 2011) revealed that alterations in aged males and females are similar and that several of the behavioral indices correlate with age in both sexes. Moreover, we examined if behavioral indices in 22-month-old males could predict remaining life span as suggested in the study by Ingram and Reynolds (Exp Aging Res 12(3):155–162, 1986) and found that exploratory activity and motor skills accounted for up to 65% of the variance. Consistent with that a high level of exploratory activity and preserved motor capacity indicated a long post-test survival, 28-month-old males maintained on DR were more successful in such tests than ad libitum fed age-matched males. In summary, aged C57BL/6J males are marked by a reduced exploratory activity, an alteration that DR impedes. In light of recently published data, we discuss if a diminishing drive to explore may associate with aging-related impairment of central aminergic pathways.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-011-9320-7) contains supplementary material, which is available to authorized users.
Keywords: Sensorimotor, Memory, Cognition, Gender, Calorie restriction
Introduction
Rodents are used as models for human diseases, and during the twentieth century, a number of rodent mouse strain were introduced (Fox 1965; Irwin et al. 1968), many of which are still in use (http://ncrr.nih.gov/comparative_medicine/resource_directory/rodents.asp or http://research.jax.org/repository/index.html) (Bucan and Abel 2002; Chia et al. 2005; Taft et al. 2006; Mekada et al. 2009). Early-on efforts were made to characterize the behavior of rodent models by systematic testing and results from this work stressed inter-strain behavioral differences (Thompson 1953; Southwick and Clark 1968). The introduction of techniques to engineer targeted gene modifications led to a demand on a batch-like approach to speedily characterize phenotypic changes (caused by a genetic modification), including organism behavior. This impetus generated today widely accepted behavioral test batteries (e.g., SHIRPA and EMPReSS, http://empress.har.mrc.ac.uk/; Crawley and Paylor 1997; Rogers et al. 1997; Paigen and Eppig 2000; Moldin et al. 2001; Karl et al. 2003) which essentially capitalized on already existing behavioral testing protocols, arranged them into comprehensive batteries followed by validation across laboratories (idem). Because targeted gene modifications have been easier to do in the mouse, this is now the dominating rodent model of human diseases. Over the past decade, the accumulation of behavioral data generated for, in particular C57BL/6 substrains, has rapidly accelerated which among other things has re-emphasized (see above) the importance of using genetically well-defined mouse models (Rogers et al. 1999; Crabbe et al. 1999; Bothe et al. 2004; Bryant et al. 2008; Crusio et al. 2009; Crawley et al. 1997; Matsuo et al. 2010). However, most of the data generated so far concern adult mice and developmental aspects, while behavioral characterization of aging in mice is scarcer (Sprott and Eleftheriou 1974; Goodrick 1967, 1973, 1975; Dean et al. 1981; Ingram and Reynolds 1986; Ingram et al. 1982, 1981; Lau et al. 2008; see also Collier and Coleman 1991; Ingram 1988; Ingram and Jucker 1999). Rodent models have proven to be very important in aging research, and combined with work on simpler organism models, our insights have greatly expanded (over the past decades) concerning mechanisms governing life span, aging-related impairments, and the biology of normal aging. In a recent report on behavioral changes in aging female C57BL/6 (Fahlström et al. 2011), we concluded that the decline in drive to explore is a key factor underlying many aspects of reduced behavioral performance during aging. Although a gradual decline in exploratory activity was noted in several earlier publications (Goodrick 1967, 1973, 1975; Sprott and Eleftheriou 1974; Dean et al. 1981; Ingram et al. 1981, 1982; Ingram and Reynolds 1986; Forster et al. 1996; Lau et al. 2008), this conspicuous alteration in mouse behavior during aging has attracted only modest attention. The present study was initiated to answer (1) if aging modulates behaviors in male as it does in female C57BL/6, if (2) behavioral indices in early aging can predict remaining life span, and (3) to characterize differences in behaviors of ad libitum fed and dietary restricted (70% of ad libitum) aged male C57BL/6.
Materials and methods
Animals
All animals used were offsprings to pregnant C57BL/6J (http://jaxmice.jax.org/strain/005304.html) delivered from Charles River, Germany, during 2007–2009. All animals were purchased as specified pathogen-free (SPF) animals and kept under standardized conditions in the animal facility at the Department of Neuroscience, Karolinska Institutet. Health monitoring, according to the FELASA recommendations (http://www.felasa.org/recommendations.htm), showed that the animals were free of pathogens with the exceptions of Helicobacter spp. The animal facility is under the supervision of a laboratory animal veterinarian. Postpartum, offsprings were cared for by their mothers until weaning at days 20–23; post-weaning male offsprings were arranged in sibling groups and kept in a Makrolon™, M3, cages (Techniplast, Buguggiate, Varese, Italy), provided with woodchip standard bedding material (Tapvei, Kortteinen, Finland), a card board house, and nesting material (paper). Cages were cleaned once a week, and the animals were inspected on a daily basis. Room temperature was kept at 21 ± 0.2°C and relative humidity at 50 ± 5% (variance estimates based on continuous records during 1 month). A 12:12-h light/dark cycle was used with a dawn and dusk system of 0.5 h each. A radio was used as background noise.
Food and water were served ad libitum (AL) and changed once a week at about 10 AM. All animals were fed commercially available food pellets (Lactamin R34, Lantmannen, Sweden). Animals were weighed at regular intervals (every other week or once a month). Animals maintained on a dietary restriction (DR) received 70% of the intake recorded for AL-fed animals. Animals on DR were drawn from the same colony as those fed AL and were maintained on this regime from the age of 3 months. To avoid single housing, the food was served once a day, a regime that allows all animals to fed and brings down the animal-to-animal body weight variation among cage litter members (Altun et al. 2007). In C57Bl/6J, there appears to be no difference in life-extending effect of DR between group-held and single-housed animals (Ikeno et al. 2005).
We used a cross-sectional age cohort design (Table 1), with two groups of young adult, one group in “early aging” at 22 months and one group at advanced age, 28 months. In addition, one group of 28-month-old males maintained on DR was included. When behavioral testing begun, whole body weight was recorded for the animals in the different groups, see Table 1. Following completion of the testing, the 22-month mice had their death recorded by the date until all had died (by December 2010).
Table 1.
Compilation of mice used, cross-sectional age group arrangement, and animal body weight
| Age group (months) | Number | Whole body weight (g) |
|---|---|---|
| 4 | 10 | 31.2 ± 1.7 |
| 4 | 10 | 31.7 ± 2.8 |
| 22 | 13 | 38.7 ± 5.2 |
| 28 | 11 | 37.0 ± 2.6 |
| 28 DR | 8 | 25.2 ± 2.5 |
DR dietary restricted, animals fed with 70% of the daily ad libitum intake
Behavioral testing
Animals were allowed to acclimatize to the behavior laboratory for at least 90 min prior to testing. Test sessions occurred during day time (9 AM–5 PM), i.e., during the light period of the 24-h cycle, and each test occurred at the same time of the day with only small variations across cohorts. The same two observers were present throughout all sessions. The observers were placed in standard positions on both sides of the test platform. Sessions were video-recorded for post hoc analysis or verification of manually recorded data. The different tests were imposed in a sequence with interludes as indicated in Table 2 (McIlwain et al. 2001). All tests are standard test briefly described below and in greater detail in literature cited. All age groups were tested in sequence in the following order: 4-, 22-, 28- (AL), 28- (DR), and 4-month-old mice. The purpose with starting and ending with a 4-month-old mice group was to control the consistency across the test period.
Table 2.
Run list for the different components of the test battery used
| Age group | Number | Week 1 | Week 2 | Week 3 | ||||
|---|---|---|---|---|---|---|---|---|
| EPM | D&L | OF | OR1 | Rotarod | OR2 | SMT | ||
| 4 | 10 | 10 | 9b | 10 | 10 | 8c | 8d | 10 |
| 4 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 22 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 |
| 28 | 11 | 11 | 10 | 11 | 11 | 10 | 10 | 10 |
| 28 DR | 8 | 7 (8)a | 7a | 7a | 7a | 7a | 7a | 7a |
EPM elevated plus maze, D&L dark and light arena maze, OF Open field and locomotor activity, OR1 object recognition test 1 (<5-min delay), OR2 object recognition test 2 (24-h delay), SMT test battery of sensorimotor functions
aOne animal died during the testing period
bProtocol error; one case not included
cTwo animals jumped off the rotarod and were omitted from this test
dTwo animals omitted because objects placed wrong in probe trial
Elevated plus maze
Elevated plus maze (EPM) relies on the animal’s preference for dark and enclosed spaces over bright, exposed spaces and involves a conflict between the desire to explore and the anxiety of exposure and height (Lau et al. 2008). The EPM has a centrally placed open platform (height above floor 50 cm) from which four 30-cm-long arms extend, two open (i.e., without walls) and two closed (i.e., with 30-cm-high walls) (Lister 1987; Montgomery 1958; Pellow et al. 1985; Walf and Frye 2007; Fahlström et al. 2011). The following behaviors were recorded during 300 s: time spent in closed arms (CA) and open arms (OA), respectively; ambulation into the CA and OA
Open field and assessment of mobility, exploration, and habituation (Actimot detection system, TSE, Germany)
This apparatus was used to assess behavior including horizontal locomotor activity and rears (vertical movements), enabling examination of exploration (exploration track; Fig. S1) as well as habituation (Diaz Heijtz et al. 2004). The first 3 min was used to assess the behavior in a novel open field (OF) while the extended recording (60 min) was used to assess locomotion and pattern of habituation (locomotor activity).
Dark and light arena transition test (TSE, Germany)
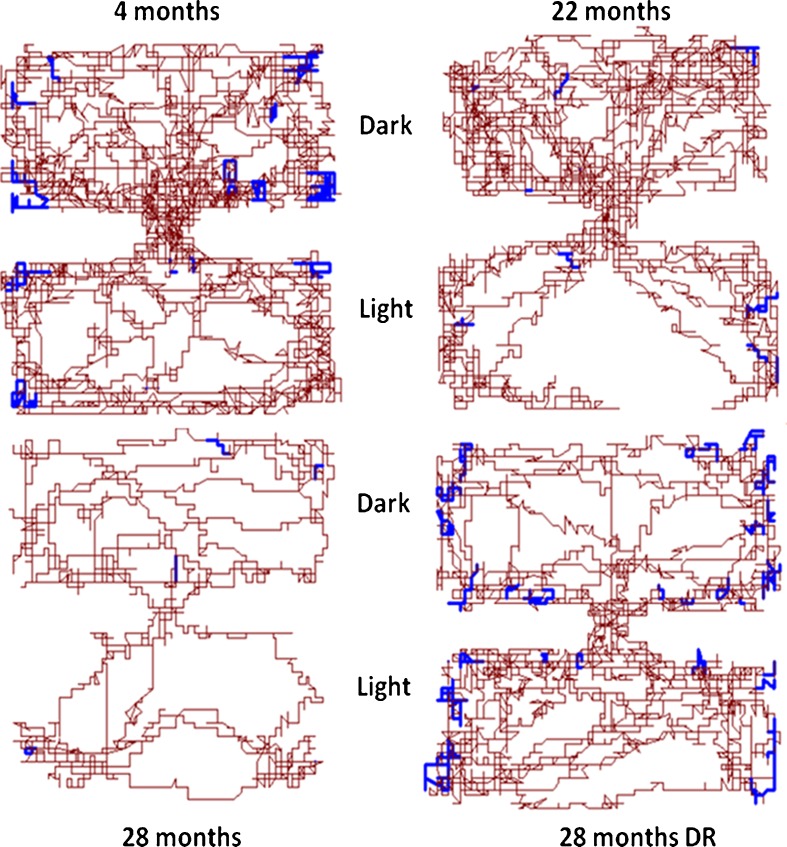
The same detection system was used as above, but the arena consisted of two Plexiglas chambers connected by a small opening (2 × 2 in.). One part of the arena was dark (<10 lux, stray light from the illuminated arena), while the light arena was illuminated by 400 lux. The mice were placed in the light arena and the following indices were recorded during 300 s: latency to enter the dark arena, exploration track (Fig. 3), and locomotor activity in respective arena.
Fig. 3.
Individual exploration trajectories and rears in dark and light arena (300 s). Representative individual trajectories showing the change in exploratory activity across adult life span. Brown lines indicate locomotion (horizontal movements) while blue indicates rears (vertical movements). Exploratory activity declines with age and more time is spent in the light arena. Aged mice maintained on DR explore more than ad libitum fed age-matched controls. In accord with younger mice, aged DR mice explore the full arena including all corners
Object recognition
Two related versions of object recognition (OR) were used, one designed to evaluate working memory (OR1) (Ennaceur and Delacour 1988) and one to evaluate memory consolidation (OR2) (Fernandez et al. 2008). Following habituation and pretrial (see Fahlström et al. 2009), the time and number of visits to the two objects were recorded, and the preference for the novel object was calculated as (N − F/(F + N)). Latency between pretrial and probe trial was 1 min. One week later, the age groups were tested with the OR2. In this OR test, following habituation and pretrial (Fahlström et al. 2011), the animal was allowed to explore the objects until 10 s had been spent at the objects. In the OR2 test, the latency between pretrial and probe trial was 24 h. The cutoff time was set at 15 min. Object preference was derived directly from the fraction of the 10 s spent at each object.
Rotarod (LE8200, LSI Letica, Scientific Instruments, Spain)
In the rotarod (RR) experiments, we followed the protocol available at http://empress.har.mrc.ac.uk/ with the exception that routinely a fourth test round was included (Carter et al. 2001). Time to fall-off and speed at fall-off was recorded. Two 4-month-old animals that jumped off were discarded in this test.
Beam balance
Beam balance was used to assess motor coordination and balance as described in (Altun et al. 2007; Clifton et al. 1991). Speed and number of limb misplacements (slips) were recorded. In addition, the performance (beam score) on the beam was ranked 1 (best)–5 (worst) adopted from Clifton et al. (1991); all animals were given a score regardless if they failed or not in the beam walk test.
Grip strength (body suspension test)
Grip strength (body suspension test) as described at http://empress.har.mrc.ac.uk/ was used to assess forelimb muscle strength (Metz and Schwab 2004).
Hot plate
Hot plate test was used to assess nociceptive threshold (Altun et al. 2007; Espejo and Mir 1993; Langerman et al. 1995). The animal was placed on the heated surface (52°C) until it licked paws, stamped, jumped, or vocalized. The time lapse between placement and reaction was recorded as response latency. The cutoff time was set at 30 s to avoid tissue damage.
Visual placement (see http://empress.har.mrc.ac.uk/) and (Metz and Schwab 2004)
A score of 2, 1, or 0 was given depending on the reaction.
Statistical processing of data
Comparisons of experimental groups were carried out with analysis of variance (ANOVA) and Bonferroni’s post hoc test (multiple comparison of all groups; two-tailed test). Record series of repeated measurement were analyzed for differences between groups by ANOVA for repeated measurements. Changes within a group across trials were analyzed using Friedman’s ANOVA and Kendall’s concordance. Correlation of two parameters (interval scale) was accomplished using least square linear regression and calculation of the fraction of explained variance (r2) or by Spearman rank-order correlation (ordinal scale and interval scale). Values indicated in the running text and figure legends are mean ± SEM. Statistical significance levels were set to: * = p < 0.05; ** = p < 0.01; *** = p < 0.001. The statistics analyses described above were performed using Statistica 6.1 (StatSoft, Tulsa, OK, USA).
The capacity to predict remaining life span of behavioral indices recorded at 22 months was analyzed by repeated linear regression and calculation of the adjusted coefficient of determination (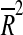 ) with α ≤ 0.01 as threshold (for further details, see Supplemented materials and Fig. S3). In the latter analysis, we used case-wise censoring if a data entry was missing in the behavioral analysis. Calculations to predict remaining life span were done using custom written software in Mathematica 8.0 (Wolfram Research, Champaign, IL, USA).
) with α ≤ 0.01 as threshold (for further details, see Supplemented materials and Fig. S3). In the latter analysis, we used case-wise censoring if a data entry was missing in the behavioral analysis. Calculations to predict remaining life span were done using custom written software in Mathematica 8.0 (Wolfram Research, Champaign, IL, USA).
Results
Elevated plus maze
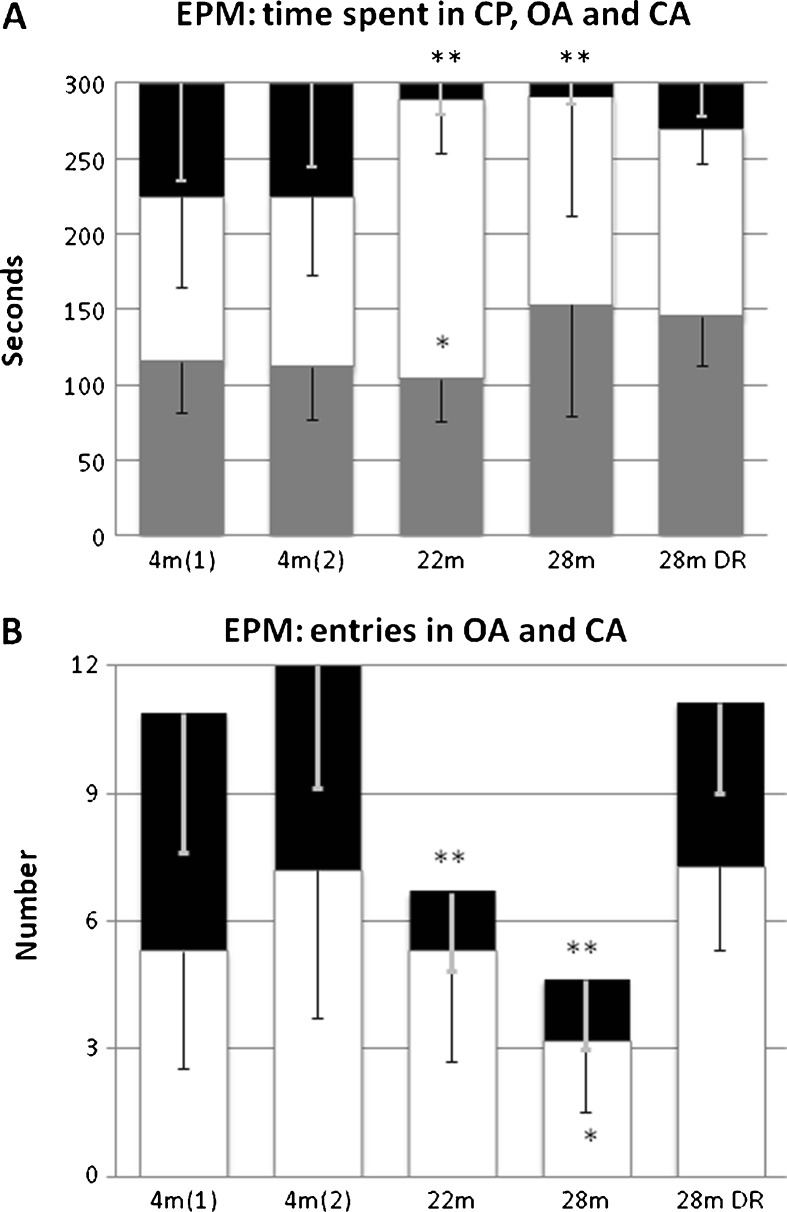
Four-month-old males spent about the same amount of time on the central platform, in the opened and the closed arms, respectively, and showed no clear preference to entry into the closed arms vs. open arms (Fig. 1a). In the 22- and 28-month-old mice groups, there was a dramatic reduction in exploratory activity, in particular, entries to and time spent in the open arms. In aged DR males, time spent and number of entries into the arms were not significantly different from young adults.
Fig. 1.
Elevated plus maze (EPM). Stacked columns showing a time spent in the different locations of the maze and b number of exploratory entries into the arms. In both early and advanced aging, there is a dramatic reduction in time spent in the open arm (OA). Also the number of entries into the OA declined in early and advanced aged mice (p < 0.01). While the overall exploratory activity assessed by total number of entries were significantly reduced in 28-month-old mice (p < 0.01), age-matched mice maintained on DR were not significantly different from adult controls. Black = OA, white = CA, and gray = central platform (CP). Values indicated are mean ± SEM. Statistical significance: *p < 0.05; **p < 0.01; ***p < 0.001
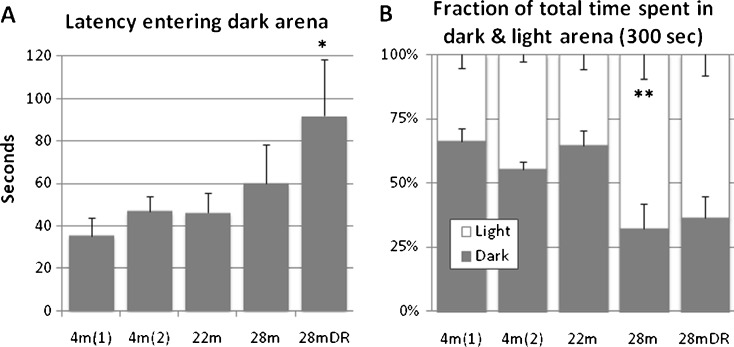
Dark and light arena transitions
Latency to enter the dark arena did not change significantly across adult life span, but aged DR males showed an increased latency (Fig. 2). Twenty-eight-month-old males maintained on DR or AL spent more time in the light than dark compartment; however, their behavior was quite different (Fig. 3). While aged DR males with one exception were almost as active as 4-month-old males and navigated the full dark and light arenas (all eight corners), aged AL males often failed to accomplish this and in two cases spent all 300 s in the light arena.
Fig. 2.
Exploration of dark and light arena. a Latency to entry of the dark arena and b time spent exploring the dark and light arena, respectively, during 300 s. While latency to enter dark area did not change significantly across adult life span, latency was increased in aged dietary restricted mice. Both ad libitum fed and DR fed aged mice spent more time exploring the light arena than adults and mice in early aging. Values indicated are mean ± SEM. Statistical significance: *p < 0.05; **p < 0.01; ***p < 0.001
Open field and locomotor activity (AM)
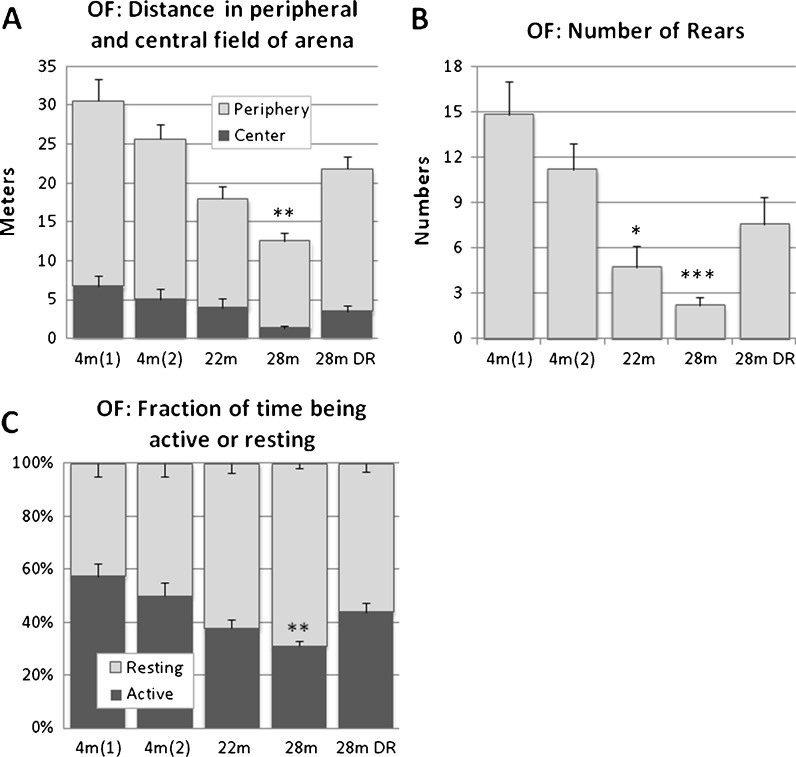
Exploratory activity was assessed during 180 s in an open field arena. Locomotion and rears decreased with age, and more time was spent as inactive (Fig. 4). Aged males maintained on DR and 22-month-old mice both showed an activity pattern in-between young adults and aged males fed AL.
Fig. 4.
Changes in exploration activities in the open field arena (180 s). a Stacked columns showing total locomotor distance and fraction of this occurring in the peripheral and central field, respectively. b Number of rears and c stacked columns showing fractions of the 180 s used to rest and move, respectively. Across adult life span locomotion and rearing decline while periods of rest increase. Values indicated are mean ± SEM. Statistical significance: *p < 0.05; **p < 0.01; ***p < 0.001
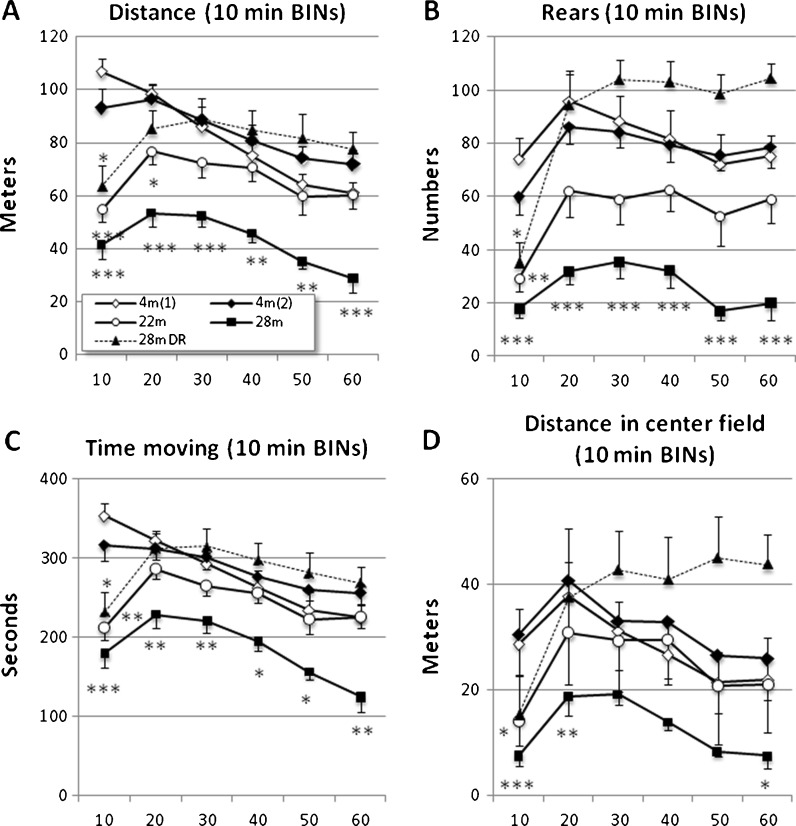
Locomotion, rears, and exploration tracks were also recorded over a 60-min period in the OF arena (Figs. 5 and S1). Initially all groups with aged mice deviated significantly from the 4-month-old mice. During the second 10-min period, aged DR males were indistinguishable from 4-month-old mice, and at later time points, both the frequency of rears and the distance covered in the central field of the arena tended to exceed those recorded for 4-month-old mice (Figs. 5 and S1). In contrast, 28-month-old AL mice were less active and diverted significantly from 4-month-old at almost every time point (Fig. 5). The behavioral indices of 22-month-old mice was in-between the two former age groups, suggesting that the decline in activity is a gradual process (Figs. 5 and S2). After the initial peak of activity, habituation set in and activity declined in all age groups but was less marked in the aged DR group (Fig. 5).
Fig. 5.
Changes in exploration activities in the open field arena (60 min). a Total locomotor distance arranged in consecutive 10 min BINs, b number of rears, c time moving in seconds, and d fraction of total locomotor distance occurring in the central field of the arena. Key to animal groups in a. Under the first 10 min, all groups of aged mice had a lower level of activity than adults (a–d). After this initial period, 28-month-old DR mice showed an exploratory activity similar to young adult mice. At later time points, they showed less habituation than young adults and continued to explore all regions of the arena (a–d; see also Fig. S1). The level of activity among 22-month-old mice was in-between adults and 28-month-old mice, while the latter group consistently showed less activity throughout the test period. Values indicated are mean ± SEM. Statistical significance: *p < 0.05; **p < 0.01; ***p < 0.001
Sensorimotor tests
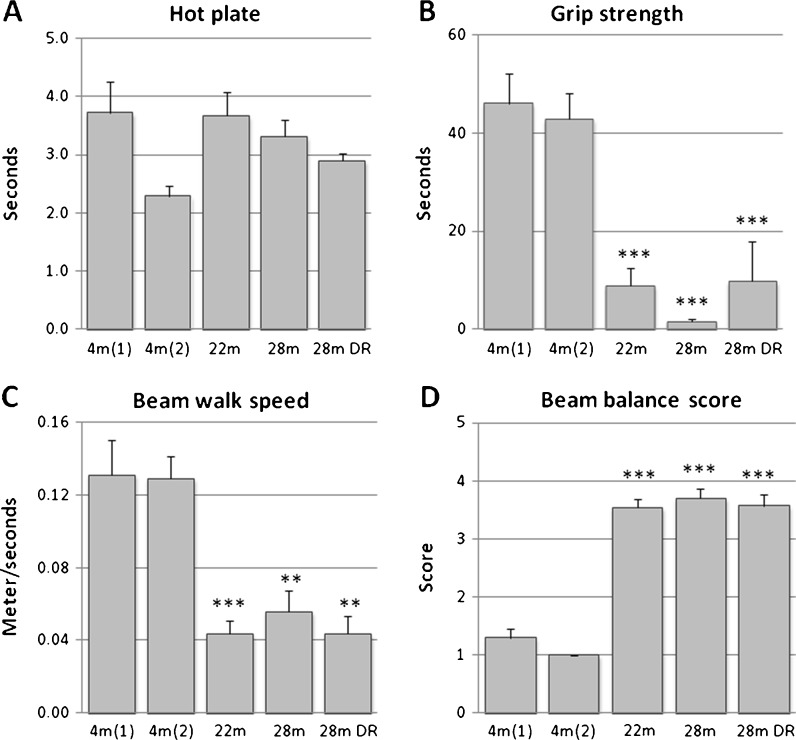
Visual placement reaction showed no impairment with advancing age (data not shown), and the response latency to noxious thermal stimulation (hot plate) did not differ significantly between age groups (Fig. 6a). Forelimb grip strength showed a marked decrease in all aged mice groups (Fig. 6b). The increase in whole body weight from 4 to 22 months (Table 1) probably explains some of this difference, but body weight cannot explain the low values recorded for aged DR mice nor the difference between 22- and 28-month-old AL mice. If body weight was accounted for (for details, see Supplemented materials, Fig. S5), the residual variances still showed that grip strength decreases significantly with age. However, with this analytical approach, the aged animals on DR performed the worst due to their low body weight (Table 1; see also “Discussion”). Beam walk speed was used to assess motor skills and coordination (Fig. 6c), and locomotion was significantly slower in all groups of aged mice including DR males. Two of the 28-month-old males failed entirely to walk on the beam. Consistent with the decline in speed, aged mice received poor beam balance score (Fig. 6d).
Fig. 6.
Changes across adult life span in sensorimotor behavior. a Thermal nociceptive threshold tested by “hot plate,” b forelimb grip strength assessed by the grip strength test, and c, d locomotor speed and quality score assessment of the behavior, respectively, on the beam. Values indicated are mean ± SEM. Statistical significance: *p < 0.05; **p < 0.01; ***p < 0.001
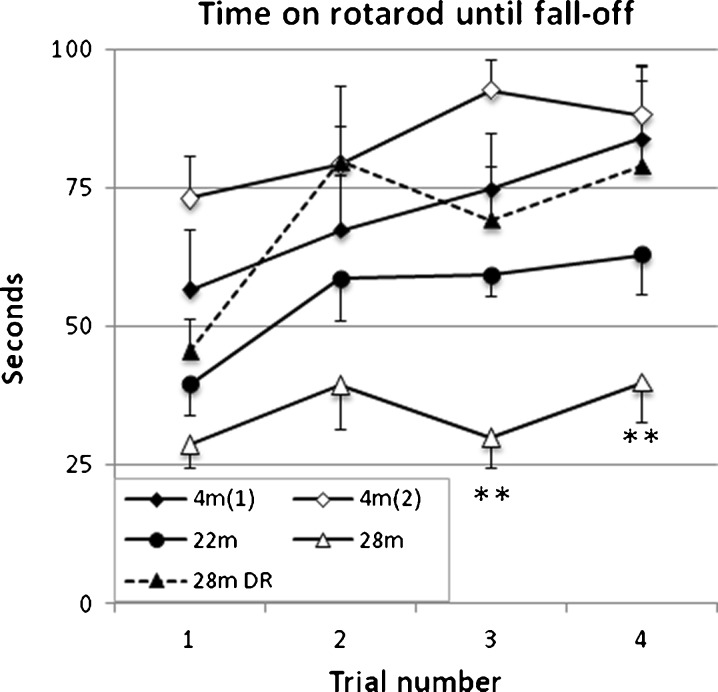
Motor coordination and motor skill acquisition were further examined with the RR (Fig. 7). All age groups except the 28-month-old AL males showed some consistent improvement across trials and after the first trial aged DR males performed similar to 4-month-old males. The performance of the 28-month-old AL males was significantly poorer than 4-month-old males in both trials 3 and 4 (Fig. 7). As in the grip-strength test analysis (above), we also examined the impact of body weight on the read-out of the RR test and obtained a result overall close to the original analysis (cf. Figs. 7 and S6).
Fig. 7.
Rotarod performance across adult life span. Key to age groups in graph. Data points were interconnected to ease tracing of each series. Young adult mice, 22 and 28 months old on DR all showed some improvement across trial. In the 28-month-old group of ad libitum fed mice, the improvement was minimal and this group performed significantly worse than young adults in trials 3 and 4. As indicated in Table 2, two 4-month-old mice jumped off the RR and were discarded from this test. Values indicated are mean ± SEM. Statistical significance: *p < 0.05; **p < 0.01; ***p < 0.001
Object recognition tests
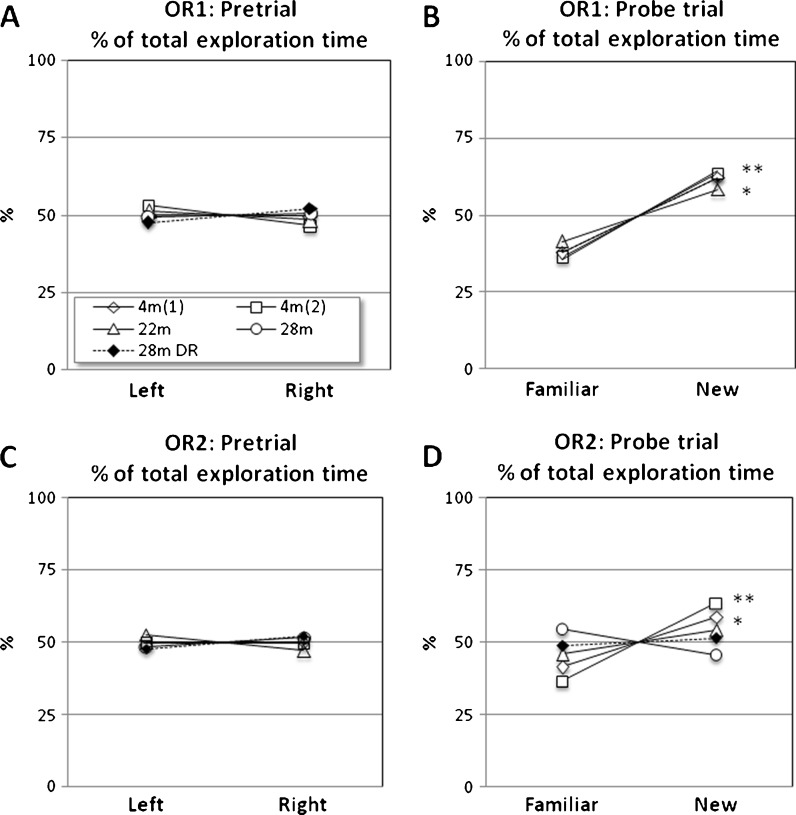
Working memory was assessed with an object recognition test (OR1), and the 28-month-old mice managed this test as well as 4-month-old did (Fig. 8a, b). The 22-month-old mice did also spend significantly more time at the novel object; however, the difference in object preference was not as large as in the other groups.
Fig. 8.
Working memory and memory consolidation. a Pretrial on working memory with two identical objects (left and right). Fraction of the time spent at each object on the ordinate, while object position is displayed on the abscissa. Key to age group in a. b Probe trial with familiar and novel object and all age groups show a preference for the novel object (p < 0.05 and p < 0.01). c The pretrial of the object memory consolidation test and d probe trial after 24 h disclosing that the aged mice were unsuccessful to retain the memory of the familiar object while 4-month-old male mice managed this task
A variant of the same test was used to analyze memory consolidation (OR2; 24-h delay). The OR2 revealed that both the 22- and the 28-month-old AL mice as well as the 28-month-old DR mice were unable to distinguish the novel object from the familiar object after a 24-h delay (Fig. 8c, d). In contrast, both groups of 4-month-old males showed a significant preference for the novel object.
Can behavioral indices recorded during aging predict remaining life span?
Inspired by an earlier report (Ingram and Reynolds 1986), we explored if behavioral indices recorded at 22 months of age could predict remaining life span. To this end, we recorded all deaths (post-testing) in the 22-month-old group by the date. The (post-testing) survival varied from 91 to 438 days, with a mean of 256 days. We then examined to what extent differences in test results or body weight (Table 1) could account for the variances in post-test survival using a multiple linear regression approach (further information is available in Supplemented materials and Fig. S5). Predictive power was assessed by calculation of the adjusted coefficient of determination, and in this iterative analytical process, we experienced very little gain by increasing the complexity beyond three variables (Fig. S3). The top results of this exercise using one (60–65% explained variance in survival), two (70–80% explained variance), or three variables (>90% explained variance) are listed in Table 3 (Fig. S4). It is interesting that models with the strongest predictive power all included test indices of exploration. Thus, large number of entries into the closed arm or a large total number of arm entries in the EPM at 22 months predict a long post-test survival. In contrast, sheltering in the closed arm correlated inversely with post-test survival. It seems therefore plausible to conclude that reduced exploratory behavior reflects biological aging in mice. As evident in Table 3, also motor skills and muscle strength above average predicted a longer post-test survival. The regression coefficients (β) seem remarkably consistent between the different models (Table 3). However, the positive effect of body weight appears to be conditioned to other positive features such as exploratory behavior and grip strength; in fact, 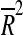 for weight alone was negative (see also “Discussion” and Ingram 1988; Ingram and Reynolds 1987).
for weight alone was negative (see also “Discussion” and Ingram 1988; Ingram and Reynolds 1987).
Table 3.
Adjusted coefficient of determination and regression coefficients for eight predictive models
| Variable 1 | Variable 2 | Variable 3 | 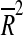 |
β1 | β2 | β3 | β0 | |
|---|---|---|---|---|---|---|---|---|
| The two best models with one variable | Rotarod trial 3 | 0.66 | 7.76a | −220g | ||||
| # entries CA, EPM | 0.6 | 28.8b | 86.8g | |||||
| The two best models with two variables | Total # arm entries, EPM | Rotarod trial 3 | 0.78 | 16.7b | 5.10a | −154g | ||
| Total # arm entries, EPM | Dist in peripheral field, OF | 0.77 | 23.8b | 4.69d | −307g | |||
| The two best models with three variables | # entries OA, EPM | Dist in peripheral field, OF | Grip strength | 0.93 | 60.3b | 5.16d | 6.16f | −338g |
| # entries OA, EPM | Time in peripheral field, Locomotion | Grip strength | 0.92 | 73.4b | 8.07d | 7.24f | −569g | |
| Models including weight | # entries CA, EPM | Weight | 0.75 | 30.2b | 9.63e | −306g | ||
| # rears, OF | Weight | Grip strength | 0.87 | 19.8c | 11.8e | 4.87f | −389g |
For each number of predictors (1, 2, and 3), the two best performing models are listed. All these models include at least one exploratory component. Combining exploratory variables with data of motor function increased the predictive ability. Several of the top performing models included weight as a positive predictor, and two weight models are also listed. The model with whole body weight and number of entries into the closed arm (EPM) is the third best model for k = 2, and the model with weight, number of rears in OF, and grip strength is the sixth best for k = 3. 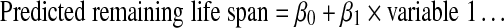
aDays per rotarod score (unit for the regression coefficient)
bDays per entry (unit for the regression coefficient)
cDays per rear (unit for the regression coefficient)
dDays per percentage (unit for the regression coefficient)
eDays per gram (unit for the regression coefficient)
fDays per second (unit for the regression coefficient)
gDays (unit for the regression coefficient)
Consistency of the testing across this study
The young adult mice were drawn from the same colony and split in two groups to serve as a build-in control of test consistency in this study (see “Material and methods”). Analysis of variance of these two groups across the test battery revealed no difference between groups greater than chance (multivariate ANOVA; Wilks p = 0.527). The difference recorded in the hot plate test (Fig. 6a) would have been significant had it been a single test situation. Thus, we conclude that the tests conditions and the tests used in this study were, perhaps with the exception of the hot plate test, robust.
Discussion
Decline in exploratory activity marks the aging C57BL/6J male mouse
Male C57BL/6J mice are active explorers in early adulthood, and the results of adult male mice in the test battery (recorded here) are in line with data published by Crabbe et al. (1999), Wahlsten et al. (2003), and Matsuo et al. (2010). The most conspicuous alteration in behavior during aging is the decline in exploratory activity (Figs. 1, 2, 3, 4, and 5), a change noted by several investigators (Goodrick 1967, 1973, 1975; Sprott and Eleftheriou 1974; Dean et al. 1981; Ingram et al. 1981; Ingram et al. 1982; Ingram and Reynolds 1986; Forster et al. 1996; Lau et al. 2008). The drive to explore is a fundamental behavior that likely impact other behaviors when affected (see below and Fahlström et al. 2011), and the decline is a gradual process clearly evident at 22 months that deteriorates further in advanced age. Although the functional domain of the brain responsible for decisions underlying exploration behavior has yet not been revealed, experimental work suggests that in mammals these processes reside in the prefrontal cortex and that (projections to the prefrontal cortex of) the ascending locus coeruleus–norepinephrine system modulates these processes (reviewed in Aston-Jones and Cohen 2005). Just recently, this concept gained further support from work in Caenorhabditis elegans (Bendesky et al. 2011) showing that the decision to explore (in a foraging choice situation) is governed by catecholamine signaling. This is of considerable interest since the central aminergic systems (at least) in rodents are vulnerable to aging with overt signs of axon terminal loss and neuroaxonal dystrophy (Johnson et al. 1993; Cowen et al. 2005; van Luijtelaar et al. 1988). As discussed below, high exploratory activity also correlates positively with long post-testing survival expectancy.
With advancing age, also other behaviors are affected. Skilled motor performance assessed in the beam and rotarod tests declines as do grip strength (Figs. 6b and 7) (see also Dean et al. 1981; Ingram 1988). However, some behaviors are well-preserved in advanced age as the placement reaction and hot plate latency, suggesting that sensorimotor mechanisms underlying these behaviors are intact. Furthermore, both 22- and 28-month-old males managed as well as young adults in the object recognition test of working memory (Fig. 8). The sparing of non-spatial working memory also in advanced age confirms and extends Benice et al. (2006) observation that memory function is intact in 18–20-month-old male and female C57BL/6J mice (see also Dean et al. 1981). In contrast, object memory consolidation was impaired in both 22- and 28-month-old male mice examined here (Fig. 8). The impairment of a delayed memory recall in aged C57BL/6J is in line with several reports using different test to probe memory recall function (Dean et al. 1981; Benice et al. 2006; Frick et al. 2000; Forster et al. 1996).
Dietary restriction modulates behavioral alterations seen in aging ad libitum fed mice
Reducing calorie intake by manipulation of diet formulas or food restriction (DR) is the most universal regime to challenge the process of aging (McCay et al. 1989; Stunkard 1983; see recent reviews by Anderson and Weindruch 2010; Baur et al. 2010). The detailed mechanism(s) by which food restriction modulates aging remains largely unknown, but it increases health span and life span by curbing morbidity and by modulating (basal) metabolism (reviewed in Guarente and Picard 2005). Food restriction impacts organismal behavior not only by preserving adult behaviors into advanced age (Ingram and Reynolds 1983, 1987; Idrobo et al. 1987) but apparently also by “setting” the level of basal physical activity already in young adulthood (Weed et al. 1997; McCarter et al. 1997; Holloszy and Schechtman 1991; Chen et al. 2005). We included a group of 28-month-old male mice maintained on a modest DR regime (30% reduction of ad libitum consumption) for comparison. In related work, we have shown that a 30% food restriction increases the (median) expected life span by about 20–25% (Altun et al. 2007; Fahlström et al., unpublished results), for C57BL/6 from about 30 to 36 months.
The 28-month-old DR mice were markedly different from the AL-fed age-matched controls in all tests with an exploratory component (Figs. 1, 2, 3, and 4) and in locomotor activity as well as adaptive motor performance (Figs. 5 and 7). In several aspects, they were indistinguishable from young adult male mice. However, on closer inspection, there were qualitative differences between young adult and aged DR males behaviors; for example, in the dark and light arena transition test, aged DR males covered a larger distance and used more time to explore the light compartment (Figs. 1 and 2), and in the locomotor test (Figs. 4 and S1–S2), DR animals were more active in the central field, thus did not show the same preference for the peripheral field as did young adults and aged AL-fed mice. Finally, aged males on DR did not show the same degree of habituation over time in the locomotion activity test (Fig. 5). In contrast to these distinct effects, the DR regime used here did not impede the devastating effects of aging on grip strength, beam balance, or delayed object memory recollection (OR2; Figs. 6b, d and 8). The beneficial effects of DR on memory-dependent behaviors remain somewhat controversial (Burger et al. 2010; Minor et al. 2008; Fontan-Lozano et al. 2007; Mattson 2010), and it cannot be excluded that particularities of different DR regimes alone or interacting with strain (genetic) differences account for these differences.
Correlations among tests within and across age groups
When using a range of tests to behaviorally characterize mice, cross-correlation analysis of the results is helpful to assess if tests are complementary or if they measure same underlying properties of an organism’s behavior (e.g., Ingram 1996; Ingram 1988; Wahlsten et al. 2003; Fahlström et al. 2011). Within age group, cross-correlation analysis of the mice studied here revealed that results from sensorimotor and rotarod tests did not correlate consistently with the outcome of the exploratory tests in young adult males (EPM, OF, dark–light transition, or OR). In contrast, among tests with an exploratory component, consistent cross-correlations were observed such as males that were active in the EPM and made multiple entries into the OA also were very active, including exploration of the central field, in the OF and locomotor tests (Supplementary Table S1–S2). Mice that spent more time in the CA and made fewer entries to the OA of the EPM also avoided the central field of the OF arena and showed a preference for the dark compartment in the dark and light transition test. Although the level of exploratory activity was significantly reduced in 22-month-old males (Figs. 1, 3, and S2), the correlations among exploratory indices were similar as in young adult mice (Table S2). However, some differences were noted, for example, the capacity to manage the rotarod in the first trial correlated positively with activity level in the EPM. This incipient change in cross-correlation pattern became overt in the 28-month-old AL-fed mice. In this age group, only few variables of the tests with an exploratory component cross-correlated, while fairly strong cross-correlation became evident between indices of the sensorimotor test and different exploratory indices (Table S2). In contrast, among aged males maintained on DR cross-correlations among test variables were in close agreement with those seen in young adult males
The EPM, light and dark arena transition test, and OF test, all share that they involve a conflict between the desire to explore and the anxiety of exposure or height (see “Materials and methods”). Among the young adult mice, there were individuals with a “daring” or an “anxious” behavioral profile as judged by the correlations among tests with explorative and anxiety components (see above), a pattern that was not as evident in the 28-month-old mice. A comparison between the 4- and 28-month-old mice across tests with an anxiety component is not conclusive because the results varied from test to test. For example, the EPM read-outs suggest an increased level of anxiety among the 28-month-old mice cf. 4 month old (Fig. 1); however, in the light and dark arena transition test, the latency to escape into the dark compartment tended to be longer in aged mice and, furthermore, aged mice spent more time to explore the light compartment (Fig. 3). Earlier studies on aging-related behavioral changes in rodents, using the number of deposits and/or urinations as indicators of stress/anxiety level during behavioral testing, showed that anxiety level did not change with advancing age (Edstrom and Ulfhake 2005; Altun et al. 2007).
Comparison of behavioral changes during aging in female and male C57BL/6
Behavioral analysis of adult C57BL/6J have mainly considered male mice; however, a few studies included both genders and reported no effect of sex in the tests used for behavioral phenotyping (Wahlsten et al. 2003; Lau et al. 2008). Corresponding data on possible gender differences during aging are even scarcer; Wax and Goodrick (1978) studied wheel running in males and females of two different mouse strains and concluded that although there were differences between strains, they saw no effect of gender within strains. Also in the study by Dean et al. (1981), no differences were noted between sexes. Comparison of the results obtained in male C57BL/6J here with recently published behavioral assessments of female C57BL/6 (Fahlström et al. 2011) reveal that alterations evident in 28-month-old male and female mice are very similar. In general, exploration activities decline progressively during aging and are conspicuous at 28 months in both sexes (cf. Figs. 4 and 5 of Fahlström et al. 2011). Many capacities assessed by the sensorimotor tests are reduced to a similar extent with advancing age in both genders (cf. Figs. 6 and 7 in this study with Figs. 7–9 in Fahlström et al. 2011) or not affected by aging in either sex (thermal nociceptive threshold). Thus, changes of several behavioral indices correlate with age in both male and female C57BL/6 (Table 4). Males and females showed no impairment of working memory at 28 months (OR1; cf. Fig. 8 with Fig. 10 in Fahlström et al. 2011) while at this age both genders failed in the object memory consolidation test (OR2; cf. Fig. 8 and Fig. 11 in Fahlström et al. 2011). The lack of a gender difference in object recognition tests appears to be at variance with the accumulating body of evidence suggesting a gender difference in cognitive decline and memory function in early senescence (22–24 months old), considered caused by the cessation of reproduction and decreased systemic levels of gonadal hormones in females (reviewed in Frick 2009). The current study and the previous study by Fahlström et al. (2011) were not designed specifically to address the issue of a gender difference in early aging and at advanced age studies agree that both sexes are affected. However, there are some gender differences among the animals used here and in the study by Fahlström et al. (2011) evident in results of the EPM (cf. Fig. 1 in this study with Fig. 4 in Fahlström et al. 2011). While total number of arm entries was similar in young adults of both genders, females showed a strong preference for the closed arms (∼80%) not evident in males (∼50%). The decline in arm entries with advancing age had the same magnitude in both genders (−60% to −70%) but affected entries into the OA the most in males. Thus, in the EPM, aged males are more similar to aged females than young adult males to age-matched females. This observation and earlier notification of differences in aggressive behavior between genders in adulthood (Van Loo et al. 2003) deserves further attention and suggests that a test capturing secondary manifestations of sexual differentiation should be included in a primary behavioral screen of genetically modified mice.
Table 4.
Correlation (r) between behavioral data and age as revealed by the Spearman rank-order test
| Parameter | Age | |
|---|---|---|
| Male | Femalea | |
| OF distance (m) | −0.659 | −0.614 |
| OF # rears | −0.751 | −0.817 |
| Locomotion (m) | −0.750 | −0.558 |
| # rears (locomotion) | −0.719 | −0.559 |
| Grip strength | −0.859 | −0.527 |
| Beam walk, m/s | −0.673 | −0.696 |
| Beam balance score | 0.864 | 0.803 |
| RR 4th trial | −0.619 | −0.332 |
| Entries CA, EPM | −0.427 | −0.650 |
| Entries OA, EPM | −0.600 | −0.058 |
| Time in OA, EPM | −0.662 | −0.058 |
| Total # arm entries, EPM | −0.600 | −0.467 |
All correlations except those indicated in italics are significant at p <0.05
aData on female C57BL/6 adopted from Fahlström et al. (2011) and unpublished observations (Fahlström et al., personal communication)
Biological and chronological age and prediction of remaining life span from behavioral indices in early senescence
The pace of aging and the expected life span varies among individuals (Ulfhake et al. 2002; Collier and Coleman 1991) also in inbred mice strains such as C57BL/6J (Ingram 1988). Individual trajectories of aging are shaped by the genetic makeup, the environment, and interactions on the epigenetic level, and as a consequence, biological age is in part dissociated from chronological age of an organism. The search for markers of biological age in chronologically age-matched populations has attracted considerable interest in research on human but also in rodent aging (Swindell et al. 2008 and references therein). Behavioral changes/impairments can be considered a high-end read-out of aging impacts on the organism, and a few studies have analyzed the capacity of behavioral indices to predict (remaining) life span (Wax and Goodrick 1978; Ingram et al. 1982; Ingram and Reynolds 1987). Ingram and Reynolds (1987) assessed predictive value of behavioral indices in aging C57BL/6J males and found linear relations between life span and several of the test indices (exploratory and locomotor activity and grip strength) which combined, using multiple regression analysis, accounted for about 40% of the differences in observed life span. Consistent with this, we found that tests holding an exploration component were somewhat more powerful to predict remaining life span (EPM > OF) than motor skills and muscle strength (RR > grip strength) (Table 3). The linear correlations obtained here were stronger than those seen among the mice analyzed by Ingram and Reynolds (1987), and another difference between studies was that RR (here executed according to the protocol of EMPReSS; but with four trials instead of three) in the present study showed a fairly high linear correlation with remaining life span. It cannot be excluded that this discrepancy was caused by differences in RR platform and protocol used in the two studies; this is in fact plausible since the two studies agree on that other indices of high locomotor activity correlate positively with life span. In the present study, the best single indices could explain 60–65% of the observed variances in life span, combining two indices (multivariate approach; see Supplemented materials) generated linear models that could account for 70–80% and models with three indices—92–93% of the variances in remaining life span (Table 3). An important observation made here was that the best models (based on one, two, or three indices) all held at least one exploratory component, suggesting that alterations in exploration activity reflect important underlying modulations caused by biological aging (see also above).
In this study, whole body weight (Table 1) at 22 months did not correlate with remaining life span (r2 = 0.108; p = 0.388) (cf. Ingram 1988; Ingram and Reynolds 1987); however, whole body weight was a useful measure to predict remaining life span when combined with behavioral indices (see “Results” and Table 3) in this age cohort. As evident in Tables S1 and S2, whole body weight did not correlate significantly with any of the recorded behavioral indices within age groups. The impact of whole body weight on behavioral indices such as grip strength and RR was limited and could not account for the pattern of changes observed across age groups (cf. Figs. 6b and S5, 7 and S6, respectively). Since whole body weight is a compounded measure, it may be ill-suited to use in this context. A measure which reflects changes in body composition across life span, such as lean body mass, muscle mass-to-whole body mass, or MRI/DXA, is probably better (see also Edstrom and Ulfhake 2005; Altun et al. 2007). Although the latter methods are more laborious or expensive, we strongly recommend such measures to be included in future studies.
Consistent with that individuals with a high level of spontaneous exploratory activity and relatively preserved capacity to manage motor tests such as RR and grip strength test were the most long-lived among the 22-month-old male C57BL/6J, 28-month-old males maintained on dietary restriction which challenge normal aging and extend expected survival (see above) performed much better than ad libitum fed age-matched males in these tests and, moreover, were almost indistinguishable from young adults in the EPM, locomotor test, and rotarod (Figs. 1, 5, 7, and S2). The only exemption was that 28-month-old males maintained on DR were not successful in the grip strength and beam balance tests (Fig. 6b–d).
Concluding remarks
Aged C57BL/6J males are marked by reduced explorative activity; the decline is gradual and as discussed above possibly associated with (pathological) aging-related changes in the central aminergic pathways. Dietary restriction impedes the emergency of many aging-related stigmata, and aged C57BL/6J males maintained on a modest food restriction were as active as young adult males in tests with an exploratory component. Aging also affects several other but not all behaviors examined here, and comparison with corresponding data recorded in female C57BL/6J indicates that the impact of aging on the behaviors in these tests are quite similar in males and females. Finally, the variance of behavioral changes evident in early aging (22 months old) can predict remaining life expectancy suggesting that these alterations reflect underlying biological aging.
Electronic supplementary materials
(DOC 3628 kb)
Acknowledgments
The use of laboratory animals was under the ethical permits N253/08 and N120/09 (to B. Ulfhake). The work was supported by grants from The Swedish Research Council (VR to B. Ulfhake).
References
- Altun M, Bergman E, Edstrom E, Johnson H, Ulfhake B. Behavioral impairments of the aging rat. Physiol Behav. 2007;92(5):911–923. doi: 10.1016/j.physbeh.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010;21(3):134–141. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Baur JA, Chen D, Chini EN, Chua K, Cohen HY, Cabo R, Deng C, Dimmeler S, Gius D, Guarente LP, Helfand SL, Imai S, Itoh H, Kadowaki T, Koya D, Leeuwenburgh C, McBurney M, Nabeshima Y, Neri C, Oberdoerffer P, Pestell RG, Rogina B, Sadoshima J, Sartorelli V, Serrano M, Sinclair DA, Steegborn C, Tatar M, Tissenbaum HA, Tong Q, Tsubota K, Vaquero A, Verdin E. Dietary restriction: standing up for sirtuins. Science. 2010;329(5995):1012–1013. doi: 10.1126/science.329.5995.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendesky A, Tsunozaki M, Rockman MV, Kruglyak L, Bargmann CI. Catecholamine receptor polymorphisms affect decision-making in C. elegans. Nature. 2011;472(7343):313–318. doi: 10.1038/nature09821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137(2):413–423. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav. 2004;3(3):149–157. doi: 10.1111/j.1601-183x.2004.00064.x. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, McRoberts JA. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J Neurogenet. 2008;22(4):315–331. doi: 10.1080/01677060802357388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucan M, Abel T. The mouse: genetics meets behaviour. Nat Rev Genet. 2002;3(2):114–123. doi: 10.1038/nrg728. [DOI] [PubMed] [Google Scholar]
- Burger JM, Buechel SD, Kawecki TJ. Dietary restriction affects lifespan but not cognitive aging in Drosophila melanogaster. Aging Cell. 2010;9(3):327–335. doi: 10.1111/j.1474-9726.2010.00560.x. [DOI] [PubMed] [Google Scholar]
- Carter RJ, Morton J, Dunnett SB (2001) Motor coordination and balance in rodents. Curr Protoc Neurosci Chapter 8:Unit 8 12 [DOI] [PubMed]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310(5754):1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Chia R, Achilli F, Festing MF, Fisher EM. The origins and uses of mouse outbred stocks. Nat Genet. 2005;37(11):1181–1186. doi: 10.1038/ng1665. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Jiang JY, Lyeth BG, Jenkins LW, Hamm RJ, Hayes RL. Marked protection by moderate hypothermia after experimental traumatic brain injury. J Cerebral Blood Flow and Metabolism. 1991;11:114–121. doi: 10.1038/jcbfm.1991.13. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Coleman PD. Divergence of biological and chronological aging: evidence from rodent studies. Neurobiol Aging. 1991;12(6):685–693. doi: 10.1016/0197-4580(91)90122-Z. [DOI] [PubMed] [Google Scholar]
- Cowen T, Ulfhake B, King RHM. Aging in the peripheral nervous system. In: Dyck PJ, Thomas PK, editors. Peripheral neuropathy. Philadelphia: Elsevier Saunders; 2005. pp. 483–507. [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284(5420):1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132(2):107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31(3):197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- Crusio WE, Goldowitz D, Holmes A, Wolfer D. Standards for the publication of mouse mutant studies. Genes Brain Behav. 2009;8(1):1–4. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- Dean RL, III, Scozzafava J, Goas JA, Regan B, Beer B, Bartus RT. Age-related differences in behavior across life span of the C57BL/6J mouse. Experimental Aging Res. 1981;7(4):427–451. doi: 10.1080/03610738108259823. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Scott L, Forssberg H. Alteration of dopamine D1 receptor-mediated motor inhibition and stimulation during development in rats is associated with distinct patterns of c-fos mRNA expression in the frontal–striatal circuitry. Eur J Neurosci. 2004;19(4):945–956. doi: 10.1111/j.0953-816X.2004.03154.x. [DOI] [PubMed] [Google Scholar]
- Edstrom E, Ulfhake B. Sarcopenia is not due to lack of regenerative drive in senescent skeletal muscle. Aging Cell. 2005;4(2):65–77. doi: 10.1111/j.1474-9728.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-X. [DOI] [PubMed] [Google Scholar]
- Espejo EF, Mir D. Structure of the rat’s behaviour in the hot plate test. Behavioural Brain Res. 1993;56(2):171–176. doi: 10.1016/0166-4328(93)90035-O. [DOI] [PubMed] [Google Scholar]
- Fahlström A, Yu Q, Ulfhake B (2011) Behavioral changes in aging female C57BL/6 mice. Neurobiol Aging 32(10):1868–1880 [DOI] [PubMed]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28(35):8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontan-Lozano A, Saez-Cassanelli JL, Inda MC, Santos-Arteaga M, Sierra-Dominguez SA, Lopez-Lluch G, Delgado-Garcia JM, Carrion AM. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J Neurosci. 2007;27(38):10185–10195. doi: 10.1523/JNEUROSCI.2757-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci USA. 1996;93(10):4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox WM. Reflex-ontogeny and behavioural development of the mouse. Anim Behav. 1965;13(2):234–241. doi: 10.1016/0003-3472(65)90041-2. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55(1):2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95(1):293–307. doi: 10.1016/S0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Goodrick CL. Behavioral characteristics of young and senescent inbred female mice of the C57BL-6J strain. J Gerontol. 1967;22(4):459–464. [PubMed] [Google Scholar]
- Goodrick CL. Exploration activity and emotionality of albino and pigmented mice: inheritance and effects of test illumination. J Comp Physiol Psychol. 1973;84(1):73–81. doi: 10.1037/h0035054. [DOI] [PubMed] [Google Scholar]
- Goodrick CL. Behavioral differences in young and aged mice: strain differences for activity measures, operant learning, sensory discrimination, and alcohol preference. Exp Aging Res. 1975;1(2):191–207. doi: 10.1080/03610737508257960. [DOI] [PubMed] [Google Scholar]
- Guarente L, Picard F. Calorie restriction—the SIR2 connection. Cell. 2005;120(4):473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Schechtman KB. Interaction between exercise and food restriction: effects on longevity of male rats. J Appl Physiol. 1991;70(4):1529–1535. doi: 10.1152/jappl.1991.70.4.1529. [DOI] [PubMed] [Google Scholar]
- Idrobo F, Nandy K, Mostofsky DI, Blatt L, Nandy L. Dietary restriction: effects on radial maze learning and lipofuscin pigment deposition in the hippocampus and frontal cortex. Arch Gerontol Geriatr. 1987;6(4):355–362. doi: 10.1016/0167-4943(87)90014-8. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Richardson A, Strong R, Diaz V, Nelson JF. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60(12):1510–1517. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Motor performance variability during aging in rodents assessment of reliability and validity of individual differences. Ann N Y Acad Sci. 1988;515:70–96. doi: 10.1111/j.1749-6632.1988.tb32969.x. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Brain-behavior linkages in aged rodent models: strategies for examining individual differences. Neurobiol Aging. 1996;17(3):497–499. doi: 10.1016/0197-4580(96)00003-6. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Archer JR, Harrison DE, Reynolds MA. Physiological and behavioral correlates of lifespan in aged C57BL/6J mice. Exp Gerontol. 1982;17(4):295–303. doi: 10.1016/0531-5565(82)90019-5. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Jucker M. Developing mouse models of aging: a consideration of strain differences in age-related behavioral and neural parameters. Neurobiol Aging. 1999;20(2):137–145. doi: 10.1016/S0197-4580(99)00033-0. [DOI] [PubMed] [Google Scholar]
- Ingram DK, London ED, Reynolds MA, Waller SB, Goodrick CL. Differential effects of age on motor performance in two mouse strains. Neurobiol Aging. 1981;2(3):221–227. doi: 10.1016/0197-4580(81)90025-7. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Reynolds MA. Effects of protein, dietary restriction, and exercise on survival in adult rats: a re-analysis of McCay, Maynard, Sperling, and Osgood [1941] Exp Aging Res. 1983;9(1):41–42. doi: 10.1080/03610738308258419. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Reynolds MA. Assessing the predictive validity of psychomotor tests as measures of biological age in mice. Exp Aging Res. 1986;12(3):155–162. doi: 10.1080/03610738608259454. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Reynolds MA. The relationship of body weight to longevity within laboratory rodent species. Basic Life Sci. 1987;42:247–282. doi: 10.1007/978-1-4613-1939-9_18. [DOI] [PubMed] [Google Scholar]
- Irwin S, Banuazizi A, Kalsner S, Curtis A. One trial learning in the mouse I. Its characteristics and modification by experimental–seasonal variables. Psychopharmacologia. 1968;12(4):286–302. doi: 10.1007/BF00401408. [DOI] [PubMed] [Google Scholar]
- Johnson H, Ulfhake B, Dagerlind A, Bennett GW, Fone KC TH. The serotoninergic bulbospinal system and brainstem-spinal cord content of serotonin-, TRH-, and substance P-like immunoreactivity in the aged rat with special reference to the spinal cord motor nucleus. Synapse. 1993;15:63–89. doi: 10.1002/syn.890150108. [DOI] [PubMed] [Google Scholar]
- Karl T, Pabst R, Horsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp Toxicol Pathol. 2003;55(1):69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- Langerman L, Zakowski MI, Piskoun B, Grant GJ. Hot plate versus tail flick: evaluation of acute tolerance to continuous morphine infusion in the rat model. J Pharmacol Toxicol Meth. 1995;34(1):23–27. doi: 10.1016/1056-8719(94)00077-H. [DOI] [PubMed] [Google Scholar]
- Lau AA, Crawley AC, Hopwood JJ, Hemsley KM. Open field locomotor activity and anxiety-related behaviors in mucopolysaccharidosis type IIIA mice. Behav Brain Res. 2008;191(1):130–136. doi: 10.1016/j.bbr.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92(2):180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. Behavioral profiles of three C57BL/6 substrains. Front Behav Neurosci. 2010;4:29. doi: 10.3389/fnbeh.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. The impact of dietary energy intake on cognitive aging. Front Aging Neurosci. 2010;2:5. doi: 10.3389/neuro.24.005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter RJ, Shimokawa I, Ikeno Y, Higami Y, Hubbard GB, Yu BP, McMahan CA. Physical activity as a factor in the action of dietary restriction on aging: effects in Fischer 344 rats. Aging (Milano) 1997;9(1–2):73–79. doi: 10.1007/BF03340130. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 1989;5(3):155–171. [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav. 2001;73(5):705–717. doi: 10.1016/S0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- Mekada K, Abe K, Murakami A, Nakamura S, Nakata H, Moriwaki K, Obata Y, Yoshiki A. Genetic differences among C57BL/6 substrains. Exp Anim. 2009;58(2):141–149. doi: 10.1538/expanim.58.141. [DOI] [PubMed] [Google Scholar]
- Metz GA, Schwab ME. Behavioral characterization in a comprehensive mouse test battery reveals motor and sensory impairments in growth-associated protein-43 null mutant mice. Neuroscience. 2004;129(3):563–574. doi: 10.1016/j.neuroscience.2004.07.053. [DOI] [PubMed] [Google Scholar]
- Minor RK, Villarreal J, McGraw M, Percival SS, Ingram DK, Cabo R. Calorie restriction alters physical performance but not cognition in two models of altered neuroendocrine signaling. Behav Brain Res. 2008;189(1):202–211. doi: 10.1016/j.bbr.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Moldin SO, Farmer ME, Chin HR, Battey JF., Jr Trans-NIH neuroscience initiatives on mouse phenotyping and mutagenesis. Mamm Genome. 2001;12(8):575–581. doi: 10.1007/s00335-001-4005-7. [DOI] [PubMed] [Google Scholar]
- Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1958;4(8):254–260. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- Paigen K, Eppig JT. A mouse phenome project. Mamm Genome. 2000;11(9):715–717. doi: 10.1007/s003350010152. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8(10):711–713. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Jones DN, Nelson PR, Jones CM, Quilter CA, Robinson TL, Hagan JJ. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res. 1999;105(2):207–217. doi: 10.1016/S0166-4328(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Southwick CH, Clark LH (1968) Interstrain differences in aggressive behavior and explorative activity of inbred mice. Communications in Behavioral Biology, Part A, 1, 49–59.
- Sprott RL, Eleftheriou BE. Open-field behavior in aging inbred mice. Gerontologia. 1974;20(3):155–162. doi: 10.1159/000212009. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ. Nutrition, aging and obesity: a critical review of a complex relationship. Int J Obes. 1983;7(3):201–220. [PubMed] [Google Scholar]
- Swindell WR, Harper JM, Miller RA. How long will my mouse live? Machine learning approaches for prediction of mouse life span. J Gerontol A Biol Sci Med Sci. 2008;63(9):895–906. doi: 10.1093/gerona/63.9.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft RA, Davisson M, Wiles MV. Know thy mouse. Trends Genet. 2006;22(12):649–653. doi: 10.1016/j.tig.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Thompson WR (1953) The inheritance of behaviour: behavioural differences in fifteen mouse strains. Can J Psychol 7(4):145–155 [DOI] [PubMed]
- Ulfhake B, Bergman E, Fundin BT. Impairment of peripheral sensory innervation in senescence. Auton Neurosci. 2002;96(1):43–49. doi: 10.1016/S1566-0702(01)00368-X. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene–environment interaction. J Neurobiol. 2003;54(1):283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo PL, Zutphen LF, Baumans V. Male management: coping with aggression problems in male laboratory mice. Lab Anim. 2003;37(4):300–313. doi: 10.1258/002367703322389870. [DOI] [PubMed] [Google Scholar]
- Luijtelaar MG, Steinbusch HW, Tonnaer JA. Aberrant morphology of serotonergic fibers in the forebrain of the aged rat. Neurosci Lett. 1988;95(1–3):93–96. doi: 10.1016/0304-3940(88)90638-6. [DOI] [PubMed] [Google Scholar]
- Wax TM, Goodrick CL. Nearness to death and wheelrunning behavior in mice. Exp Gerontol. 1978;13(3–4):233–236. doi: 10.1016/0531-5565(78)90017-7. [DOI] [PubMed] [Google Scholar]
- Weed JL, Lane MA, Roth GS, Speer DL, Ingram DK. Activity measures in rhesus monkeys on long-term calorie restriction. Physiol Behav. 1997;62(1):97–103. doi: 10.1016/S0031-9384(97)00147-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 3628 kb)