Abstract
Psychological stress has extreme adverse consequences on health. However, the molecular mechanisms that mediate and accelerate the process of aging due to stress hormone are not well defined. This review has focused on diverse molecular paths that come out in response to chronic psychological stress via releasing of excessive glucocorticoids (GCs), involved in the aging process. GCs suppress transcription of nuclear cell adhesion molecules which impair synaptic plasticity, memory formation, and cognitive ability. Again, GCs promote muscle atrophy by means of motivating ubiquitin proteasome system and can repress muscle protein synthesis by inhibition of PI3-kinase/Akt pathway. GCs also inhibit interleukin-2 synthesis through suppressing T cell receptor signal that leads to loss of T cell activation, proliferation, and B-cell activation. Moreover, GCs increase the expression of collagenase-3, RANK ligand, and colony stimulating factor-1 that induce bone resorption. In general, stress-induced GCs can play causal role for aging and age-related disorders.
Keywords: Chronic psychological stress, Glucocorticoid, Glucocorticoid receptor, Aging
Introduction
Aging is not a disease (Aubert and Lansdorp 2008) but the progressive decline of cellular functions, accompanied by complex phenotypes and life-long amassing of random damages in somatic cells/tissues (Csermely and Sőti 2006), which eventually results in mortality (Aubert and Lansdorp 2008). Among the many signs and symptoms, cognitive impairment (Hof and Morrison 2004), immune suppression (Kitajima et al. 1996), muscle atrophy (Schakman et al. 2009), and osteoporosis (Tamuraa et al. 2004) are some of the important features associated with aging (Nalla et al. 2004; Ager et al. 2006). Aubrey and de Grey (1999) defined aging as “deteriorative changes with time during post-maturational life that underlies an increasing vulnerability to challenges, thereby decreasing the ability of the organism to survive”. Regarding aging, numerous theories have been evolved to explain the phenomenon over the decades which are enlisted and briefly described in Box I. However, it is a common scenario that the metabolically tuned human body falls in an unpleasant state of emotional and physiological arousal provoked by environmental, psychological, or physiological stressors that he/she experiences in situations and perceives as threatening and risk to homeostasis (Sobhani et al. 2010). This event results from a long-term exposure of stimulus and precipitates a series of phenomena-activating physiologic responses which are generally non-adaptive and finally figured chronic state. In response to stress-like depression, anxiety; personality factors, social isolation, and sub-acute/chronic life stress etc. (Ho et al. 2010), hypothalamic–pituitary–adrenocortical axis (HPA) is stimulated to secrete glucocorticoids (GCs), a stress hormone (Slominski 2007) which play an important role in brain aging, suppression of immune system, and muscle protein degradation (Porter and Landfield 1998; Kitajima et al. 1996). Interestingly, the excessive release of GCs is encouraged by chronic psychological stresses that lead us to the highway of aging in which immune system, central nervous system, and endocrine system are influenced some how and concomitantly patronizing to get old (Ho et al. 2010).
| Box I. Major theories explain the phenomenon of aging |
| Evolutionary theory: Mutated and normal genes which are not harmful at younger age could be deteriorating to late life (Weinert and Timiras 2003). |
| Molecular theory: Accumulation of somatic mutation, changes in gene expression, amassing of abnormal proteins due to lack of efficiency of protein-synthetic apparatus, and impairment of translation process cause aging (Weinert and Timiras 2003). |
| Neuroendocrine control theory: Alterations in neuroendocrine control of homeostasis results in aging-related physiological changes (Timiras 2007). |
| Free-radical theory: Aging occurs as a cost of cellular damages such as lipid (membrane), protein, and DNA damage due to high reactive free radicals that produced during oxidative metabolism (Weinert and Timiras 2003; Timiras 2007; Volonte and Galbiati 2009). |
| Telomere theory: Cells reach at senescent state due to loss of telomere during gradual replication (Weinert and Timiras 2003; Timiras 2007). |
Perhaps, molecular mechanisms that arbitrate and hasten the process of aging due to psychological stress in relay of GCs are not well understood and frustrated many researchers for decades. Here, we are focusing on the role of GCs in the diverse molecular mechanisms in response to psychological stress that involved in aging and age-related disorders.
Stress and glucocorticoids
In stressful state, corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) are released from parvocellular neurons (Ströhle and Holsboer 2003) that project from the paraventricular nucleus to the median eminence (Samson et al. 2002). CRH and AVP travel from the hypothalamus via the hypophyseal–portal blood vessels to the anterior pituitary gland (Engelmann et al. 2004) where they act synergistically via type 1 CRH receptor and type 1b vasopressin receptor to trigger the release of adrenocorticotrophic hormone (ACTH) from the corticotrophs into the systemic circulation (Buckingham et al. 1996). In turn, ACTH acts on the adrenal cortex via type 2 melanocortin receptors to initiate the synthesis of glucocorticoids (Papadimitriou and Priftis 2009) immediately into the systemic circulation in a diffusive manner. On the other hand, the sensitivity of HPA to incoming stimuli is modulated by a GC-mediated negative feedback system (Noorlander et al. 2006) through which the sequential release of CRH/AVP and ACTH from the hypothalamus and anterior pituitary gland is suppressed (Buckingham 2006).
Aging and hippocampal complex
Cognitive decline, one of the most common health-related worries, is governed by the hippocampal complex (Woodruff-Pak 1988). In the aged hippocampal complex, events like neurotransmitter and receptor alterations, dendritic alterations, decreased synaptic connections, decreases in average neuron size/number, and inhibition of long-term potency had been reported by McLay et al. 1997. Koivisto et al. (1995) estimated memory impairment in 76.4% and clinically defined age-associated memory impairment in 53.8% from studying of 1,049 Finnish individuals older than 60 years. Furthermore, age-associated cognitive impairment has been also found in vivo on rats and macaque monkeys (Hof and Morrison 2004). Recently, experiments on Sprague–Dawley rats by Lau et al. (2007) revealed that raised glucocorticoid secretion inhibits cell proliferation and neurogenesis in the hippocampus and found a causal relationship with depression. It is well-known that the adult brain possesses substantial plasticity in order to learn new skills, establish new memories, and respond to injury throughout the life (Purves et al. 2004).
In vitro studies on nuclear cell adhesion molecules (NCAM) shows that presence of polysialic acid (PSA) on NCAM decreases NCAM-mediated cell adhesion and involves in the initial signaling events in synaptic plasticity (Ronn et al. 2000). In vivo study on rats by Hof and Morrison (2004) showed that the expression of PSA–NCAM is obvious for learning, and knockout mice study also demonstrated similar result (Murase and Schuman 1999). Furthermore, elimination of PSA from NCAM suggests that the balance between PSA–NCAM and NCAM is important factor for plasticity (Kiss et al. 2001). NCAM is considered to be a reliable marker of synaptic plasticity with its concentration reflecting the numbers of recently generated synapses. From this perspective, NCAM reductions would be regarded as a consequence of the structural changes. The specific reduction in the NCAM-140 isoform that was reported in chronically stressed rats which strongly indicates that glucocorticoid receptors (GRs) participate in the stress-induced modulation of cell adhesion molecules (Sandi 2004) via suppressing nuclear factor-κB (NF-κB) and activator protein-1 (AP-1). NF-κB, a potential site of NCAM promoter has been detected in the postsynaptic density in cortical and hippocampal neurons and plays important roles in neuronal survival as well as in neuronal plasticity (Simpson and Morris 2000). Transgenic mice with inhibited NF-κB in forebrain neurons showed significant neurodegeneration by treatment with neurotoxic agents such as FeSO4 and kainite in the hippocampal neurons, and knockout mice lacking p65 subunit of NF-κB have a deficit in the spatial learning is well documented (Takeuchi and Fukunaga 2004). Therefore, NCAM reduction might reflect the decreased synaptic density that is induced by chronic stress.
Suppression of NCAM gene expression by GCs
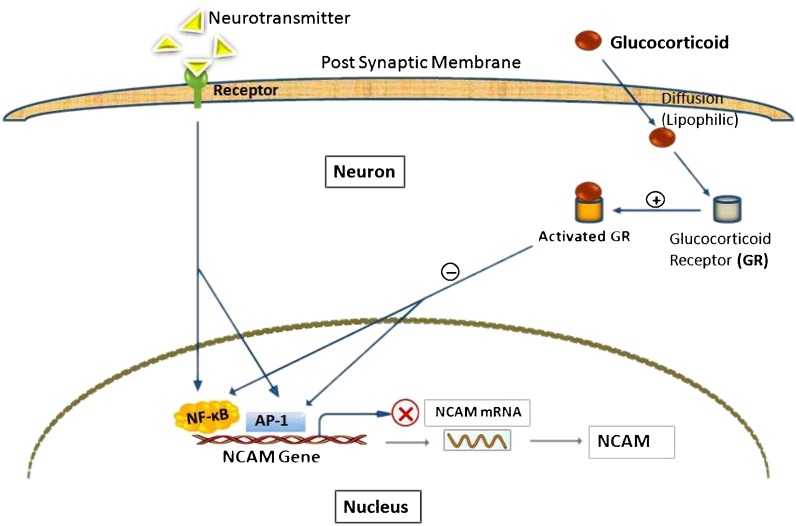
GCs are lipophilic steroids thus can readily enter the neuron to interact with the intracellular GR (Huchison et al. 1993) results in conformational changes in the GR molecule and induces a poorly understood process known as receptor activation (Beato et al. 1995; Fig. 1). Downregulation of NF-κB-driven NCAM genes result from an interaction between activated GR and p65 subunit of NF-κB. Direct interaction between p65 and activated GR modifies or changes in p65 conformation that masks the activation domain(s) of p65 which result in downregulation of NCAM genes transcription (Bosscher et al. 1997). In addition, a potential AP-1 recognition sequence is found very close to the apparent transcription start site on NCAM genes (Colwell et al. 1992) which might also be repressed by GR (Datson et al. 2001). The inhibition of both AP-1 and NF-κB activity has been demonstrated in vivo in the brain of rat (Vreugdenhil et al. 2001). The mechanism behind that is GR and AP-1 simply compete for a common coactivator complex containing CREB-binding protein (CBP) or p300. But it can only explain the transrepression of AP-1 activity by GR when the amount of CBP/p300 is limited. An interaction with the activated GR may induce AP-1 to recruit a corepressor complex instead of a coactivator (Karin and Chang 2001).
Fig. 1.
Suppression of NCAM expression by GCs. GCs triggers (+) GR to active state which negatively (−) regulated NF-κB and AP-1, consequently blocking (X) NCAM gene expression instead of normal membrane receptor mediated signaling pathways
Aging and immune system
Aging is associated with the immunosenescence of most of the molecular machines of immune system (Graham et al. 2006) like dysregulation of inflammatory processes (Cesari et al. 2004), impaired wound healing due to diminish ability of macrophages to produce proinflammatory cytokines (Gomez et al. 2005), and decreased ability of T cells to response when challenged with antigen, with large differences seen at age 60 and increasingly thereafter (Murasko et al. 1987). Another change seemingly inherent in normal aging is that B-lymphocytes impaired functionality with decrease in the antibody production (Castle 2000). One of the most conclusive evidence is in NK cells from the spleen and lymph nodes of older animals showed (in vitro) decreased immune functionality compared to younger rats (Graham et al. 2006).
Indeed, GCs have direct inhibitory actions on many inflammatory and structural cells involved in inflammation (Bhattacharyya et al. 2011). GCs slow down the release of inflammatory mediator’s viz. secretion of chemokines and proinflammatory cytokines from alveolar macrophages in vitro (Barnes 1998) and in vivo in patients with asthma (John et al. 1998). In addition, GCs have a direct inhibitory effect on mediators released from eosinophils (Borish et al. 1992). GCs therapy increases apoptosis (Owen et al. 1987), contributes to slowdown eosinophil circulation, and reduces plasma concentrations of eosinophil cationic protein (Wempe et al. 1992) and cytokine production (Evans et al. 1993). Furthermore, GCs have an indirect inhibitory effect on mediators released from mast cells (Cohan et al. 1989) which may be linked to the reduction in interleukin (IL)-3 and stem cell factor production. Mast cells can produce various cytokines (Kashiwakura et al. 2009), but whether those are inhibited by GCs is not yet certain.
GC-mediated T cell receptor signal inhibition
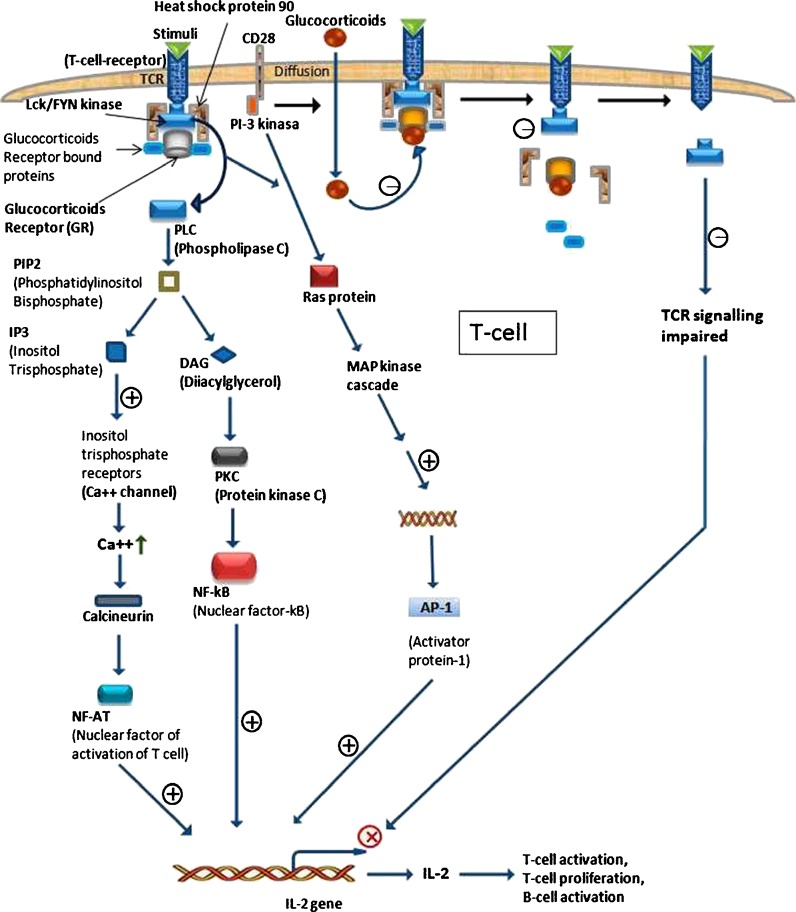
T cell activation, proliferation, survival, and release of lymphokines such as IL-2 and macrophage colony stimulating factor (GM-CSF; Hamilton 2008), which are likely to play an important role in the recruitment and survival of inflammatory cells but are very effectively inhibited by GCs (Barnes 1998). Here, we discuss the mechanism of the effects of GCs action in activated human CD4 T cells where T cell receptor (TCR) signaling pathway acts as a platform (Rentero et al. 2008; Fig. 2). It is well established that phosphorylation of lymphocyte-specific protein tyrosine kinase (Lck) and FYN, members of the sarcoma (SRC) family of nonreceptor tyrosine kinases, are proximal events in T cell activation (Palacios and Weiss 2004) and known to positively regulate the signaling initiated upon TCR stimulation through a variety of downstream pathways (Salmond et al. 2009). Conversely, reduced Lck and FYN kinase (a protein–tyrosine kinase) activities may have an important role in the fast immunosuppressive effects of GCs in immune cells (Stahna et al. 2007). In line with this notion, decreased activation of several signaling pathways downstream of the TCR upon dexamethasone (DEX; a synthetic GCs analog, inhibits Lck and FYN kinases in these immune cells) treatment has been observed, including suppression of PKB, PKC, ERK, JNK, and p38 MAPKs. The biochemical and functional responses to TCR ligands are largely determined by FYN–CD3 and Lck–CD4 associations. DEX treatment rapidly alters the cellular distribution of Lck and FYN which would likely result in decreased Lck/FYN kinase activities and suppressed TCR signaling (Löwenberg et al. 2005). TCR activation results in membrane translocation of Lck and FYN in an HSP90-dependent manner (Bijlmakers and Marsh 2000). Lck predominantly associates with CD 4 or CD 8 cell surface receptors (Artyomov et al. 2010) and FYN binds to CD3 coreceptors, resulting in Lck and FYN kinase activation (Lovatt et al. 2006). Once activated, Lck and FYN phosphorylate immune-receptor tyrosine-based activation motifs on the TCR, allowing downstream signal transduction to proliferate, differentiate T cells and consequently produce cytokines (Palacios and Weiss 2004). The classic mechanism of GCs action involves the GR and modulation of transcriptional and translational events (Rose et al. 2010). GR- and SRC-like kinases share a requirement for HSP90 for proper functioning, opening the possibility that GR act on Lck and FYN activities that are mediated by HSP90 (Nika et al. 2010). GCs cause disruption of TCR-associated multiprotein complexes containing GR, HSP90, Lck, and FYN, leading to reduce Lck/FYN enzymatic activities and impaired TCR signaling (Löwenberg et al. 2006).
Fig. 2.
Mechanism of immunosuppression of T cell activation by GCs. Schematic representation of TCR signaling process subdivided into two sides. Left sides present normal signaling through which IL-2 gene expressed. But, on right sides it is seen that GRs mediated TCR signaling where the intracellular membrane signal complex is negatively (−) regulated and consequently the tuned innermembrenal complex loosening their relation results in TCR signal impairment, through this IL-2 gene expression is retarded (X)
Aging and muscle
The age-associated changes in body composition result from lower levels of anabolic hormones, neuromuscular alterations, decline in muscle protein synthesis, and a gradual/selective loss of muscle fibers (Bross et al. 1999). Humorously, the reduction of myofibrillar protein synthesis in the elderly individual is not caused by a decline in the availability of mRNA encoding actin and myosin but alterations in post-translational events (Welle et al. 1996). However, GCs inhibit protein synthesis in skeletal muscles (McGrath and Goldspink 1982) and stimulate muscle protein degradation (Kayali et al. 1987) which is responsible for muscle atrophy. The stimulatory effect of GCs on muscle proteolysis results from the activation of ubiquitin–proteasome and lysosomal systems (Schakman et al. 2009). Increase in the components like ubiquitin, E2 enzyme, E3 enzyme, and 26S proteasome of the ubiquitin–proteasome pathway is synonymous with the activation of the pathway and interestingly, the ubiquitin–proteasome pathway is involved in the aging process muscles (Cai et al. 2004).
Inhibition of muscle protein synthesis by GCs
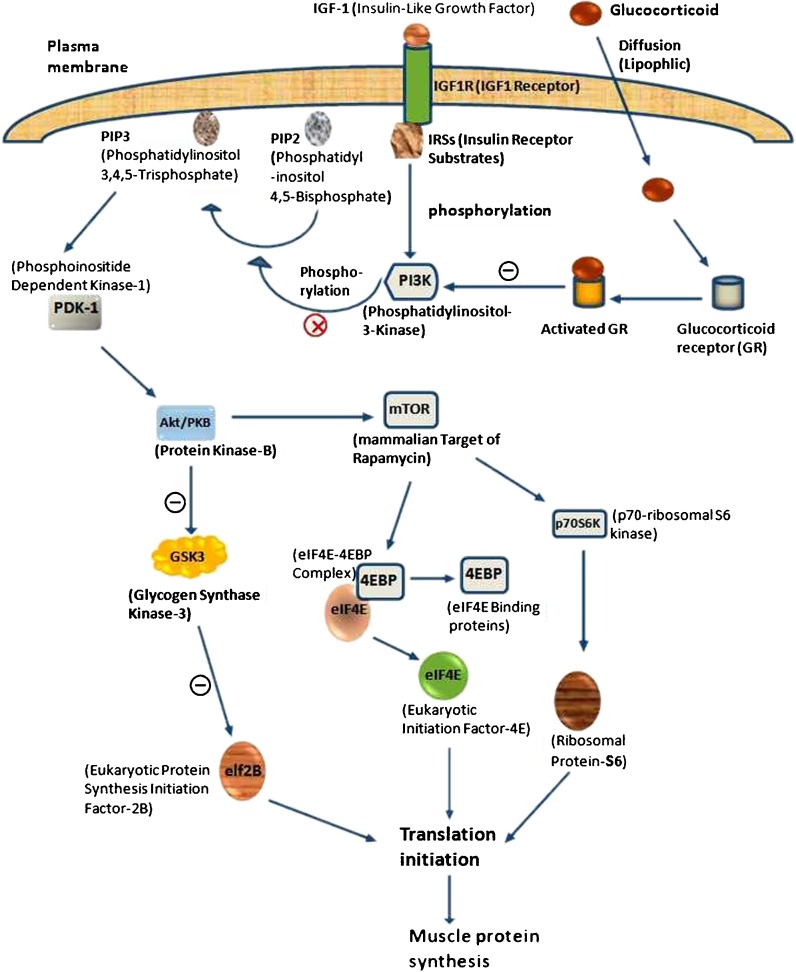
The Akt/PKB (serine/threonine protein kinase) signaling pathway is one of the prerequisite for muscle protein synthesis which consists of growth factor receptors, adapter proteins like insulin receptor substrates (IRS), class I phosphatidylinositol 3-kinase (PI3K) and Akt/PKB kinases (Plas and Thompson 2005). Insulin-like growth factor I receptor (IGF1R) signaling can prevent apoptosis and induce muscle protein synthesis which is mediated by PI3K (Fig. 3). The binding of IGF1 or IGF2 with IGF1R trigger the receptor’s intrinsic tyrosine kinase activities. Consequently, IRSs are phosphorylated (Tseng et al. 2002) and then interact with Src homology-2 domains of PI3K. Activated PI3K catalyzes the conversion of phosphatidylinositol 4, 5-bisphosphate to PIP3. After that, PIP3 binds to Pleckstrin homology domain of phosphatidylinositol-dependent kinase-1 which phosphorylates Thr308 residue on Akt (Mora et al. 2005). A further protective pathway activated by Akt involves inhibition of GSK3 (glycogen synthase kinase-3) which results from its phosphorylation at an N-terminal serine residue (Ser21 in GSK3Alpha and Ser9 in GSK3Beta; Goll et al. 1991; Scheid and Woodgett 2001). Ultimately, GSK3 catalyses the phosphorylation and inhibition of eIF2B (eukaryotic protein synthesis initiation factor-2B), thereby inhibits protein synthesis. Hence IGF1R, by inhibiting GSK3, stimulates the de-phosphorylation and activation of eIF2B, contributing to an increased rate of protein synthesis (Senthil et al. 2002). In relax state, instead of bindings with elF4G, eIF4E forms an inactive complex with any one of the eIF4E-binding proteins (4EBP1, 4EBP2, or 4EBP3) and that is why translation is downregulated (Anthony et al. 2001). In activate stage, phosphorylated mammalian target of rapamycin (mTOR), a substrate for Akt, promotes signaling events to phosphorylate 4EBP to dissociate from elF4E and result a marriage with elF4G to activate elF4F complex for promoting the initiation phase of protein translation by relieving 4EBP-mediated inhibition of eIF4E (Matsuo et al. 1997). At the moments of phosphorylation by mTOR, the ribosomal p70S6K and ribosomal protein-S6 becomes activated (Senthil et al. 2002) and that is required for biosynthesis of the cell’s translational apparatus, a critical component of cell growth and proliferation (Boylan et al. 2001). However, GCs can cause insulin resistance and leading to decrease PI3K activity in muscle (Saad et al. 1993) by activating the GR, showing the way to a competition with IRS-1 for association of PI3K subunits p110 and p85. The association of these subunits with the GR reduces IRS-1-associated PI3K (phosphorylation) activity and p-Akt (Hu et al. 2009).
Fig. 3.
Mechanism of muscle protein synthesis inhibition by GCs. Generally PI3K phosphorylates phosphatidylinositol 4, 5-bisphosphate (PIP2) to PIP3 which follow the Akt/PKB pathways for protein synthesis. But stress induced activated GRs negatively (−) regulate PI3K and block (X) the phosphorylation of PIP2. Consequently, the normal pathway falls down and depletion of protein synthesis
Role of GCs in muscle protein degradation
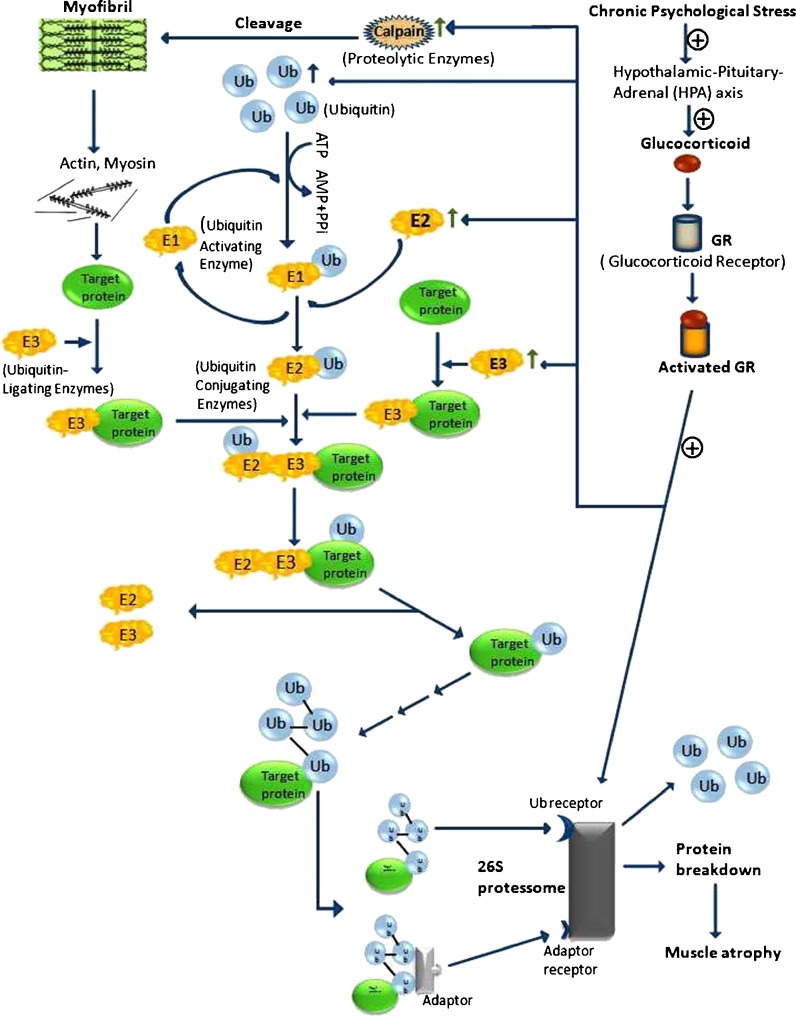
Muscle tissue contains sarcoplasmic, myofibrillar, and stroma proteins (Tan et al. 1988). The role of the calpain in muscle protein degradation is likely to involve principally the myofibrillar or the cytoskeletal proteins. Calpain rapidly cleaves titin, nebulin, and desmin that attach the Z-disk (Combaret et al. 2005) to release actin, myosin, and other polypeptide filaments (Goll et al. 1991). The released filaments can be reassembled or the polypeptide fragments produced by calpain degradation (Goll et al. 2008) and C-protein can be ubiquitinated and degraded to amino acids by the proteosome and cellular peptidases (Goll et al. 2003). Although calpain are unable to degrade actin and myosin, they seem to be involved in myofibrillar disassembly, with calpain-releasing myofibrils for degradation via ubiquitin–proteasome proteolytic pathway (Hasselgren et al. 2002; Fig. 4).
Fig. 4.
Protein degradation via UPS by GCs. Activated GRs positively (+) regulates and increases the activity of calpain, E2, E3 and 26S proteosome. As a results muscle proteins are degraded and ultimately fallout in muscle atrophy
Through a two-step catalytic process, ubiquitin is activated by E1. ATP hydrolysis results in the formation of acyl-adenylate intermediate linking AMP with the carboxy-terminal carboxyl group of glycine in ubiquitin. This adenylated carbonyl group then forms a thioester linkage with a cysteine residue of E1 (Lecker et al. 1999). The activated ubiquitin unit is subsequently shifted to the active site cysteine of a second enzyme E2 which can recognize E3 that determine the specificity of substrate ubiquitylation (Ravid and Hochstrasser 2008), and catalyze the transfer of ubiquitin from the E2 thioester intermediate to an isopeptide linkage with a selected substrate (Lecker et al. 1999). Progressive rounds of E3-promoted ubiquitin ligation result in the attachment of a polyubiquitin chain to the substrate (Bodine et al. 2001). Proteins tagged with polyubiquitin chains are recognized for degradation into small peptides by the 20S proteasome where the proteolytic sites lay on inner surface of the ß rings and found three types of proteolytic activity: chymotrypsin-like, trypsin-like, and caspase-like that work together to catalyze the complete digestion of proteins (Orlowski and Wilk 2003). Calpastatin is an endogenous specific inhibitor protein for calpain present in almost all mammals (Murachia 1983; Sato et al. 2011), and having the property of trimming down its mRNA concentrations if treated with DEX (Yeh et al. 1994). The stimulatory effect of GCs on muscle proteolysis results from the activation of ubiquitin proteasome system (UPS; Tisdale 2005), cathepsins, and calpain. GCs stimulate expression level of several components of the UPS either E2, E3 or proteasome (Schakman et al. 2008) which are guilty for protein degradation.
Effect of GCs on bone resorption
Age-related changes in mesenchymal stem cells include increases in senescent cells, loss of differentiation and proliferation potential, and loss of bone formation (Stolzing et al. 2008). The cell populations of bone osteoblasts and osteoclasts that participate in remodeling process are derived from different progenitor (Robling et al. 2006). The osteoblasts derive from mesenchymal stem cells are found in the bone marrow, periosteum, and soft tissues. They deposit osteoid and mineralize it; forming new bone (Lian and Stein 2001) involves spatiotemporal coordination of interaction among diverse endocrine, paracrine, and autocrine factors (Datta et al. 2008). For osteoclastogenesis, the TNF-related cytokine RANK ligand (RANKL) and the polypeptide growth factor CSF-1 are necessitated (Boyle et al. 2003) to induce expression of genes that characterize the osteoclast lineage, together with those encoding tartrate-resistant acid phosphatase (TRAP), cathepsin K (CATK), calcitonin receptor, and the β3-integrin, guiding to the development of mature osteoclasts (Lacey et al. 1998).
Acidification by secretion of protons directs to activate TRAP and CATK, which are responsible for the degradation of bone mineral and collagen matrices (Boyle et al. 2003). RANKL binds with RANKL-like osteoprotegerin (OPG; Khosla 2001) instead of receptor activator of NF-kB (RANK; Anderson et al. 1997) and neutralize RANKL to regulate both osteoclastogenesis and activation of mature osteoclasts negatively (Boyle et al. 2003). Collagenase 3 (MMP-13) secreted by human osteoblasts, the human chondrocytes that degrades type I collagen fibrils, is the major component of the bone matrix. Figure 5 shows that GCs trigger GCs-dependent cytosolic proteins that join to a selected area of the 3′ untranslated region of the collagenase 3 RNA to modulate the stability of other mRNAs and prolonged the half-life (Canalis and Delany 2002). GCs increase the expression of RANKL and decrease the expression of OPG at mRNA levels in stromal and osteoblastic cells linage (Hofbauer et al. 1999). Contrarily, GCs also enhance the expression of CSF-1 that induces osteoclastogenesis in presence of RANKL (Rubin et al. 1998). GCs have a direct inhibitory effect on osteoblasts mediated by three routes (Weinstein et al. 1998): (1) inhibition of the replication of osteoblastic lineage, (2) a decrease in the genesis of new osteoblastic cells, and (3) induction of osteoblastic cell death and/or apoptosis (Doga et al. 2004).
Fig. 5.
Mechanism of GCs induced osteoporosis. The illustration represents two ways viz. bone resorption and bone matrix break down as a process for osteoporosis. Here, activated GRs positively (+) regulated MMP-13 and increased ejection of collagenase 3 which is responsible for bone matrix break down. On the other side, RANKL and MCSF positively (+) regulated and OPG is negatively (−) regulated by GRs. The both regulation induce bone resorption
Summary
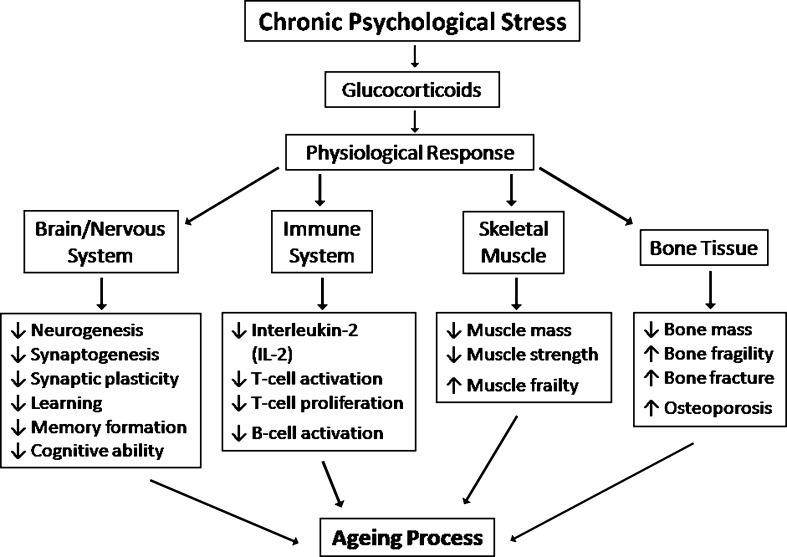
In response to psychological stress, hypothalamus secretes CRH and stimulates pituitary to emit ACTH which is transported to adrenal glands to release GCs for exerting numerous metabolic effects to contribute aging (Fig. 6). The hippocampus is more vulnerable due to the presence of highest density of GRs resulting GCs-mediated transcription–suppression of NCAM via the inhibition of NF-kB and AP-1. Activated GRs dissociates TCR linked multiprotein complex through which TCR-signaling is suppressed. As a consequence T cell activation, T cell proliferation and B-cell activation are impaired. Again, GCs-induced muscle atrophy by means of UPS is characterized by reduced myofibrillar protein content. The stimulation of UPS by GCs is mediated through the increased expression of atrogin-1 and MuRF-1 which encode two ubiquitin ligases those involved in the target protein degradation. Participation of calpain causes myofibrillar disaggregation and release of myofibrils for degradation by the ubiquitin–proteasome proteolytic pathway. Calpastatin inhibits calpain and GCs reduce calpastatin to facilate calpain’s proteolytic activities. GCs also exert an anti-anabolic action by blunting muscle protein synthesis which may result from changes in the IGF-I-induced signal transduction through the inhibition of PI3-kinase/Akt pathway. Moreover, GCs amplify expression of collagenase 3 (MMP-13) that degrades type I collagen fibrils, the major component of the bone matrix. Furthermore, GCs enhance CSF-1 expression to induce osteoclastogenesis in the presence of RANKL. As a result, bone mass is reduced with a consequent increase in bone fragility and susceptibility to fracture and eventually osteoporosis occurred; in turn, that is a part of aging process. From the overall studies, it can be concluded that the chronic psychological stress has deteriorating effects on most of the tissues of the body and contribute in the acceleration of aging (Fig. 6).
Fig. 6.
An overview of the role of chronic psychological stress leading to aging
Acknowledgements
The authors thank Dr. M. P. Mattson, National Institute of Ageing, Baltimore, MD, USA and Dr. Karl Riabowol, Dept. of Biochemistry and Molecular Biology and Oncology, Calgary University, Canada for critical reading and comments. The authors also like to acknowledge those of KUBIOTECH/MagBiotech group who supplied us hundreds of article on aging, GCs and psychological stress.
Contributor Information
K. M. Mehedi Hasan, FAX: +880-417-31244, Email: mdihsn@hotmail.com.
Md. Shaifur Rahman, FAX: +880-417-31244, Email: mdshaifur@gmail.com.
K. M. T. Arif, FAX: +880-417-31244, Email: taufiq0571@gmail.com
Mahbub E. Sobhani, FAX: +880-417-31244, Email: mahbubsobhani@yahoo.com.sg
References
- Ager JW, Balooch G, Ritchie RO. Fracture, aging, and disease in bone. J Mater Res. 2006;21:1878–1892. doi: 10.1557/jmr.2006.0242. [DOI] [Google Scholar]
- Anderson DM, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- Anthony TG, Anthony JC, Yoshizawa F, Kimball SR, Jefferson LS. Oral administration of leucine stimulates ribosomal protein mRNA translation but not global rates of protein synthesis in the liver of rats. J Nutr. 2001;133:1171–6. doi: 10.1093/jn/131.4.1171. [DOI] [PubMed] [Google Scholar]
- Artyomov MN, Lis M, Devadas S, Davis MM, Chakraborty AK. CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery. PNAS. 2010;107(39):16917. doi: 10.1073/pnas.1010568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci. 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Beato M, Herrlich P, Schutz G. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Zhao Y, Kay YWH, Muglia LJ. Glucocorticoids target suppressor of cytokine signaling 1 (SOCS1) and type 1 interferons to regulate Toll-like receptor-induced STAT1 activation. PNAS. 2011;108(23):9554–9559. doi: 10.1073/pnas.1017296108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlmakers MJ, Marsh M. Hsp90 is essential for the synthesis and subsequent membrane association, but not the maintenance, of the Src-kinase p56 (Lck) Mol Biol Cell. 2000;11:1585–1595. doi: 10.1091/mbc.11.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine SC, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Borish L, et al. Detection of alveolar macrophage-derived IL-1 beta in asthma. Inhibition with corticosteroids. J Immunol. 1992;149:3078–3082. [PubMed] [Google Scholar]
- Bosscher KD, et al. Glucocorticoid-mediated repression of nuclear factor-κB dependent transcription involves direct interference with transactivation. Proc Natl Acad Sci USA. 1997;94:13504–13509. doi: 10.1073/pnas.94.25.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan JM, Anand P, Gruppuso PA. Ribosomal protein S6 phosphorylation and function during late gestation liver development in the rat. J Biol Chem. 2001;276:44457–44463. doi: 10.1074/jbc.M103457200. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Bross R, Storer T, Bhasin S. Aging and muscle loss. Trends Endocrinol Metab. 1999;10(5):194–198. doi: 10.1016/S1043-2760(98)00143-X. [DOI] [PubMed] [Google Scholar]
- Buckingham JC. Glucocorticoids: exemplars of multi-tasking. Br J Pharmacol. 2006;147:S258–S268. doi: 10.1038/sj.bjp.0706456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham JC, Loxly HD, Christian HC, Philip JG. Activation of the hypothalamo-pituitary-adrenocortical axis by immuno insults: the role and interactions of cytokines, eicosanoids and glucocorticoids. Pharmacol Biochem Behav. 1996;54:285–298. doi: 10.1016/0091-3057(95)02127-2. [DOI] [PubMed] [Google Scholar]
- Cai D, Lee KKH, Li M, Tang MK, Chan KM. Ubiquitin expression is up-regulated in human and rat skeletal muscles during aging. Arch Biochem Biophys. 2004;425:42–50. doi: 10.1016/j.abb.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci. 2002;966:73–81. doi: 10.1111/j.1749-6632.2002.tb04204.x. [DOI] [PubMed] [Google Scholar]
- Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- Cesari M, et al. Inflammatory markers and physical performance in older persons: the in Chianti study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.M242. [DOI] [PubMed] [Google Scholar]
- Cohan VL, et al. Dexamethasone does not inhibit the release of mediators from human mast cells residing in airway, intestine, or skin. Am Rev Respir Dis. 1989;140:951–954. doi: 10.1164/ajrccm/140.4.951. [DOI] [PubMed] [Google Scholar]
- Colwell G, Lj B, Forrest D, Brackenbur R. Conserved regulatory elements in the promoter region of the N-CAM gene. Genomics. 1992;14:875–882. doi: 10.1016/S0888-7543(05)80108-9. [DOI] [PubMed] [Google Scholar]
- Combaret L, et al. USP19 is a ubiquitin-specific protease regulated in rat skeletal muscle during catabolic states. Am J Physiol Endocrinol Metab. 2005;288:E693–E700. doi: 10.1152/ajpendo.00281.2004. [DOI] [PubMed] [Google Scholar]
- Csermely P, Sőti C. Cellular networks and the aging process. Arch Physiol Biochem. 2006;112:60–64. doi: 10.1080/13813450600711243. [DOI] [PubMed] [Google Scholar]
- Datson NA, Perk JVD, Kloet ERD, Vreugdenhil E. Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. Eur J Neurosci. 2001;14:675–689. doi: 10.1046/j.0953-816x.2001.01685.x. [DOI] [PubMed] [Google Scholar]
- Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS. The cell biology of bone metabolism. J Clin Pathol. 2008;61:577–587. doi: 10.1136/jcp.2007.048868. [DOI] [PubMed] [Google Scholar]
- Doga M, Bonadonna S, Giustina A. Glucocorticoids and bone: cellular, metabolic and endocrine effects. Hormones. 2004;3:184–190. doi: 10.14310/horm.2002.11125. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic neurohypophysial system regulates the hypothalamic–pituitary–adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25(3–4):132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Evans PM, O’Connor BJ, Fuller RW, Barnes PJ, Chung KF. Effect of inhaled corticosteroids on peripheral blood eosinophil counts and density profiles in asthma. J Allergy Clin Immunol. 1993;91:643–650. doi: 10.1016/0091-6749(93)90270-P. [DOI] [PubMed] [Google Scholar]
- Goll DE, Dayton WR, Singh I, Robson RM. Studies of the alpha-actinin/actin interaction in the Z-Disk by using calpain. J Biol Chem. 1991;266:8501–8510. [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Goll DE, Neti G, Mares SW, Thompson VF. Myofibrillar protein turnover: the proteasome and the calpains. J Anim Sci. 2008;86:E19–E35. doi: 10.2527/jas.2007-0395. [DOI] [PubMed] [Google Scholar]
- Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Graham JE, Christian LM, Kiecolt-Glaser JK. Stress, age, and immune function: toward a lifespan approach. J Behavior Med. 2006;29:389–400. doi: 10.1007/s10865-006-9057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubrey DN, Grey J. Molecular biology intelligence unit 9 the mitochondrial free radical theory of aging. Austin, TX, USA: RG Landes; 1999. [Google Scholar]
- Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- Hasselgren PO, Wray C, Mammen J. Molecular regulation of muscle cachexia: it may be more than the proteasome. Biochem Biophys Res Commun. 2002;290:1–10. doi: 10.1006/bbrc.2001.5849. [DOI] [PubMed] [Google Scholar]
- Ho RC, Neo LF, Chua AN, Cheak AA, Mak A. Research on psychoneuroimmunology: does stress influence immunity and cause coronary artery disease? Ann Acad Med. 2010;39:191–196. [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. TRENDS Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanism of glucocorticoids-induced osteoporosis. Endocrinology. 1999;140:4382–4389. doi: 10.1210/en.140.10.4382. [DOI] [PubMed] [Google Scholar]
- Hu Z, Wang H, Lee IH, Du J, Mitch WE. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J Clin Investig. 2009;119:3059–3069. doi: 10.1172/JCI38770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchison KA, et al. Biochem. 1993;32:3953–3957. doi: 10.1021/bi00066a015. [DOI] [PubMed] [Google Scholar]
- John M, et al. Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1alpha, granulocyte-macrophage colony-stimulating factor, and interferon-gamma release from alveolar macrophages in asthma. Am J Respir Crit Care Med. 1998;157:256–262. doi: 10.1164/ajrccm.157.1.9703079. [DOI] [PubMed] [Google Scholar]
- Karin M, Chang L. AP-1–glucocorticoid receptor crosstalk taken to a higher level. J Endocrinol. 2001;169:447–451. doi: 10.1677/joe.0.1690447. [DOI] [PubMed] [Google Scholar]
- Kashiwakura J, Kawakami Y, Yuki K, Zajonc DM, Hasegawa S, Tomimori Y, Caplan B, Saito H, Furue M, Oettgen HC, Okayama Y, Kawakami T. Polyclonal IgE induces mast cell survival and cytokine production. Allergol Int. 2009;58:411–419. doi: 10.2332/allergolint.08-OA-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayali AG, Young VR, Goodman MN. Sensitivity of myofibrillar proteins to glucocorticoid-induced muscle proteolysis. Am J Physiol. 1987;252:E621–E626. doi: 10.1152/ajpendo.1987.252.5.E621. [DOI] [PubMed] [Google Scholar]
- Khosla S. The OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/en.142.12.5050. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Troncoso E, Djebbara Z, Vutskits L, Muller D. The role of neural cell adhesion molecules in plasticity and repair. Brain Res Rev. 2001;36:175–184. doi: 10.1016/S0165-0173(01)00093-5. [DOI] [PubMed] [Google Scholar]
- Kitajima T, Ariizumi K, Bergstresser PR, Takashima A. A novel mechanism of glucocorticoid-induced immune suppression: the inhibition of T cell-mediated terminal maturation of a murine dendritic cell line. J Clin Invest. 1996;98:142–147. doi: 10.1172/JCI118759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto K, Reinikainen KJ, Hamrinen T, Vanhanen M, HeIkaIa EL, Mykkarren L, Laakso M, Pyorala K, Riekkinen PJS. Prevalence of age-associated memory impairment in a randomly selected population from eastern Finland. Neurology. 1995;45:741–747. doi: 10.1212/WNL.45.4.741. [DOI] [PubMed] [Google Scholar]
- Lacey DL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- Lau WM, Qiu G, Helmeste DM, Lee TMC, Tang SW, So KF, Tange SW. Corticosteroid decreases subventricular zone cell proliferation, which could be reversed by paroxetine. Restor Neurol Neurosci. 2007;25:17–23. [PubMed] [Google Scholar]
- Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin–proteasome pathway in normal and disease states. J Nutr. 1999;129:227S–237S. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- Lovatt M, Filby A, Parravicini V, Werlen G, Palmer E, Zamoyska R. Lck regulates the threshold of activation in primary t cells, while both lck and fyn contribute to the magnitude of the extracellular signal-related kinase response. Mol Cell Biol. 2006;26(22):8655–8665. doi: 10.1128/MCB.00168-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwenberg M, et al. Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood. 2005;106:1703–1710. doi: 10.1182/blood-2004-12-4790. [DOI] [PubMed] [Google Scholar]
- Löwenberg M et al. (2006) Glucocorticoids cause rapid dissociation of a T-cell receptor-associated protein complex containing LCK and FYN. EMBO reports 7 [DOI] [PMC free article] [PubMed]
- Lian JB, Stein GS. Osteoblast biology. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. 2. San Diego, CA: Academic Press; 2001. pp. 21–71. [Google Scholar]
- Matsuo H, et al. Structure of translation factor eIF4E bound to m7GDP and interaction with 4E-binding protein. Nat Struct Biol. 1997;4:717–724. doi: 10.1038/nsb0997-717. [DOI] [PubMed] [Google Scholar]
- McGrath JA, Goldspink DF. Glucocorticoid action on protein synthesis and protein breakdown in isolated skeletal muscles. Biochem J. 1982;206:641–645. doi: 10.1042/bj2060641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay RN, Freeman SM, Harlin RE, Ide CF, Kastin AJ, Jadina JE. Aging in the hippocampus: interrelated actions of neurotrophins and glucocorticoids. Neurosci Biobehav Rev. 1997;21:615–629. doi: 10.1016/S0149-7634(96)00046-2. [DOI] [PubMed] [Google Scholar]
- Mora A, Sakamoto K, McManus EJ, Alessi DR. Role of the PDK1-PKB-GSK3 pathway in regulating glycogen synthase and glucose uptake in the heart. FEBS Lett. 2005;579:3632–3638. doi: 10.1016/j.febslet.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Murachia T. Calpain and calpastatin. Trends Biochem Sci. 1983;8:167–169. doi: 10.1016/0968-0004(83)90165-2. [DOI] [Google Scholar]
- Murase S, Schuman EM. The role of cell adhesion molecules in synaptic plasticity and memory. Curr Opin Cell Biol. 1999;11:549–553. doi: 10.1016/S0955-0674(99)00019-8. [DOI] [PubMed] [Google Scholar]
- Murasko DM, Weiner P, Kaye D. Decline in mitogen induced proliferation of lymphocytes with increasing age. Clin Exp Immunol. 1987;70:440–448. [PMC free article] [PubMed] [Google Scholar]
- Nalla RK, Kruzic JJ, Kinney JH, Ritchie RO. Effect of aging on the toughness of human cortical bone: evaluation by R-curves. Bone. 2004;35:1240–1246. doi: 10.1016/j.bone.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, Höfer T, Viola A, Acuto O. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32(6):766–777. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorlander CW, Graan PNE, Middeldorp J, Beers JJBC, Visser GHA. Ontogeny of hippocampal corticosteroid receprors: effects of antenatal glucocorticoids in human and mouse. J Comp Neurol. 2006;499:924–934. doi: 10.1002/cne.21162. [DOI] [PubMed] [Google Scholar]
- Orlowski M, Wilk S. Ubiquitin-independent proteolytic functions of the proteasome. Arch Biochem Biophys. 2003;415:1–5. doi: 10.1016/S0003-9861(03)00197-8. [DOI] [PubMed] [Google Scholar]
- Owen WFJ, et al. Regulation of human eosinophil viability, density, and function by granulocyte/macrophage colony-stimulating factor in the presence of 3T3 fibroblasts. J Exp Med. 1987;166:129–141. doi: 10.1084/jem.166.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- Papadimitriou A, Priftis KN. Regulation of the hypothalamic–pituitary–adrenal axis. Neuroimmunomodulation. 2009;16(5):265–271. doi: 10.1159/000216184. [DOI] [PubMed] [Google Scholar]
- Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- Porter NM, Landfield PW. Stress hormones and brain aging: adding injury to insult? Nat Neurosci. 1998;1:3–4. doi: 10.1038/196. [DOI] [PubMed] [Google Scholar]
- Purves D, et al. Neuroscience. 3. Sunderland, MA: Sinauer; 2004. pp. 575–582. [Google Scholar]
- Ravid T, Hochstrasser M. Diversity of degradation signals in the ubiquitin–proteasome system. Nature Rev Mol Cell Biol. 2008;9:679–689. doi: 10.1038/nrm2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentero C, Magenau A, Williamson D, Tedla N, Gaus K. Membrane domains as signaling centers in macrophages and T-cells: from concepts to experiments. Immun Endoc Metab Agents Med Chem. 2008;8:336–348. doi: 10.2174/187152208787169152. [DOI] [Google Scholar]
- Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- Ronn LCB, Berezin V, Bock E. The neural cell adhesion molecule in synaptic plasticity and ageing. Int J Dev Neurosci. 2000;18:193–199. doi: 10.1016/S0736-5748(99)00088-X. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Vegiopoulos A, Herzig S. Role of glucocorticoids and the glucocorticoid receptor in metabolism: insights from genetic manipulations. J Steroid Biochem Mol Biol. 2010;122(1–3):10–20. doi: 10.1016/j.jsbmb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Rubin J, et al. Dexamethasone promotes expression of membrane-bound macrophage colony-stimulating factor in murine osteoblast-like cells. Endocrinology. 1998;139:1006–1012. doi: 10.1210/en.139.3.1006. [DOI] [PubMed] [Google Scholar]
- Saad MJ, Folli F, Kahn JA, Kahn CR. Modulation of insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of dexamethasone-treated rats. J Clin Invest. 1993;92:2065–2072. doi: 10.1172/JCI116803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev. 2009;228(1):9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Samson WK, Taylor MM, Follwell M, Ferguson AV. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regulatory Peptides. 2002;104(1–3):97–103. doi: 10.1016/S0167-0115(01)00353-6. [DOI] [PubMed] [Google Scholar]
- Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- Sato K, Minegishi S, Takano J, Plattner F, Saito T, Asada A, Kawahara H, Iwata N, Saido TC, Hisanaga S. Calpastatin, an endogenous calpain-inhibitor protein, regulates the cleavage of the Cdk5 activator p35 to p25. J Neurochem. 2011;117(3):504–515. doi: 10.1111/j.1471-4159.2011.07222.x. [DOI] [PubMed] [Google Scholar]
- Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197:1–10. doi: 10.1677/JOE-07-0606. [DOI] [PubMed] [Google Scholar]
- Schakman O, Gilson H, Kalista S, Thissen JP. Mechanisms of muscle atrophy induced by glucocorticoids. Horm Res. 2009;72(Suppl 1):36–41. doi: 10.1159/000229762. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Woodgett JR. PKB/AKT: functional insights from genetic models. Nature Rev Mol Cell Biol. 2001;2:760–768. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- Senthil D, Choudhury GG, Abboud HE, Sonenberg N, Kasinath BS. Regulation of protein synthesis by IGF-I in proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2002;283:F1226–F1236. doi: 10.1152/ajprenal.00109.2002. [DOI] [PubMed] [Google Scholar]
- Simpson CS, Morris BJ. Regulation of neuronal cell adhesion molecule expression by NF-κB. J Biol Chem. 2000;275:16879–16884. doi: 10.1074/jbc.275.22.16879. [DOI] [PubMed] [Google Scholar]
- Slominski AA. Nervous breakdown in the skin: stress and the epidermal barrier. J Clin Invest. 2007;117:3166–3169. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani ME, Molla MAW, Rahman MS. A review on biomolecular basis of the role of psychological stress in the development and progression of cancer. Mag Euro Med Oncol. 2010;3:136–141. doi: 10.1007/s12254-010-0217-4. [DOI] [Google Scholar]
- Stahna C, Löwenberg M, Hommesc DW, Buttgereita F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007;275(1–2):71–78. doi: 10.1016/j.mce.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Ströhle A, Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36:207–214. doi: 10.1055/s-2003-45132. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Fukunaga K. Different activation of NF-κB by stimulation of dopamine D2L and D2S receptors through calcineurin activation. J Neurochem. 2004;90:155–163. doi: 10.1111/j.1471-4159.2004.02476.x. [DOI] [PubMed] [Google Scholar]
- Tamuraa Y, Okinagab H, Takamic H. Glucocorticoid-induced osteoporosis. Biomed Pharmacother. 2004;58:500–504. doi: 10.1016/j.biopha.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Tan FC, Goll DE, Otsuka Y. Some properties of the millimolar Ca2+-dependent proteinase from bovine cardiac muscle. J Mol Cell Cardiol. 1988;20:983–997. doi: 10.1016/0022-2828(88)90576-7. [DOI] [PubMed] [Google Scholar]
- Timiras PS. Physiological basis of aging and geriatrics. 4. New York: Informa Healthcare; 2007. pp. 40–64. [Google Scholar]
- Tisdale MJ. The ubiquitin–proteasome pathway as a therapeutic target for muscle wasting. J Support Oncol. 2005;3:209–217. [PubMed] [Google Scholar]
- Tseng YH, Ueki K, Kriauciunas KM, Kahn CR. Differential roles of insulin receptor substrates in the anti-apoptotic function of insulin-like growth factor-1 and insulin. J Biol Chem. 2002;277:31601–31611. doi: 10.1074/jbc.M202932200. [DOI] [PubMed] [Google Scholar]
- Volonte D, Galbiati F. Caveolin-1, cellular senescence and pulmonary emphysema. Aging. 2009;01:831–835. doi: 10.18632/aging.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil E, Kloet ERD, Schaaf M, Datson NA. Genetic dissection of corticosterone recepto rfunction in the rat hippocampus. Eur Neuropsychopharmacol. 2001;11:423–430. doi: 10.1016/S0924-977X(01)00119-5. [DOI] [PubMed] [Google Scholar]
- Weinert BT, Timiras PS. Physiology of aging invited review: theories of aging. J Appl Physiol. 2003;95:1706–1716. doi: 10.1152/japplphysiol.00288.2003. [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Bhatt K, Thornton C. Polyadenylated RNA, Actin mRNA, and myosin heavy chain mRNA in young and old human skeletal muscle. Am J Physiol. 1996;270:E224–E229. doi: 10.1152/ajpendo.1996.270.2.E224. [DOI] [PubMed] [Google Scholar]
- Wempe JB, et al. Blood eosinophil numbers and activity during 24 hours: effects of treatment with budesonide and bambuterol. J Allergy Clin Immunol. 1992;90:757–765. doi: 10.1016/0091-6749(92)90099-N. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS. Psychology and aging. Englewood Cliffs: Prentice-Hall; 1988. [Google Scholar]
- Yeh JY, Ou BR, Forsberg NE. Effects of dexamethasone on muscle protein homeostasis and on calpain and calpastatin activities and gene expression in rabbits. J Endocrinol. 1994;141:209–217. doi: 10.1677/joe.0.1410209. [DOI] [PubMed] [Google Scholar]