Abstract
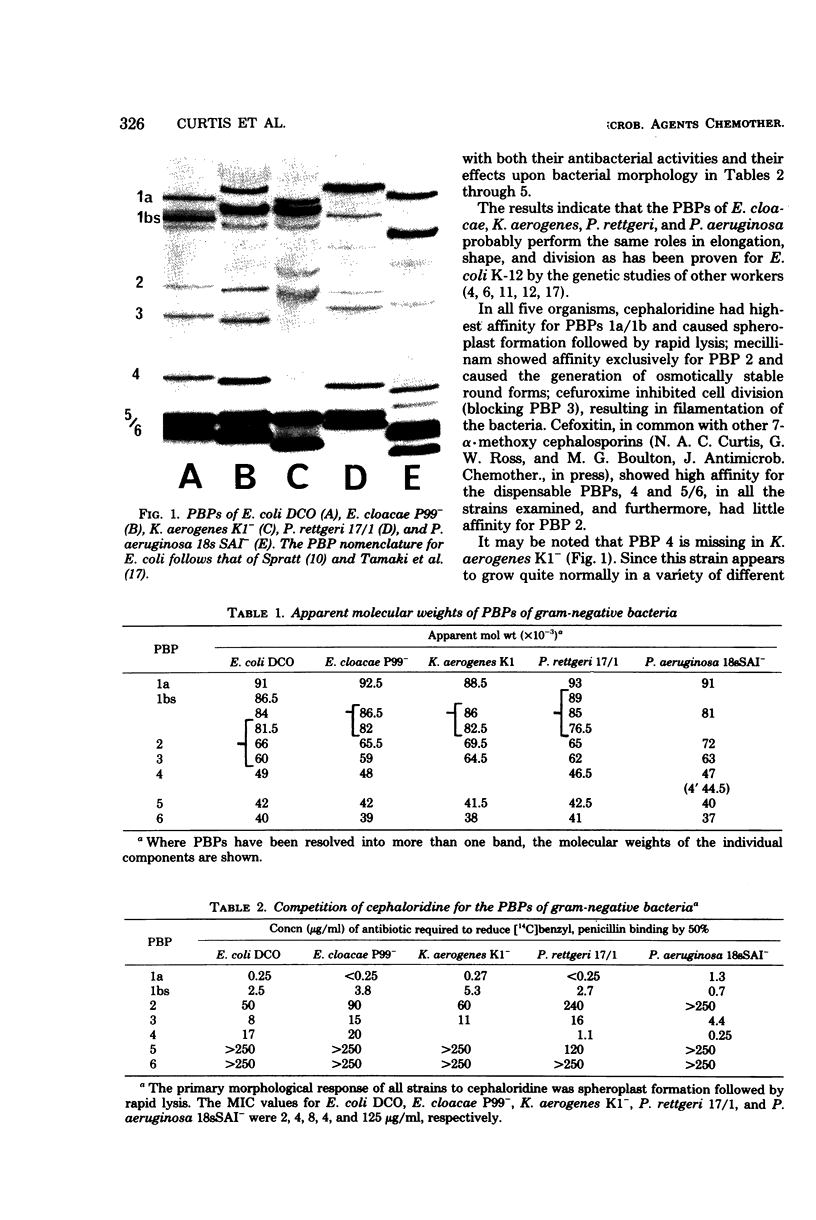
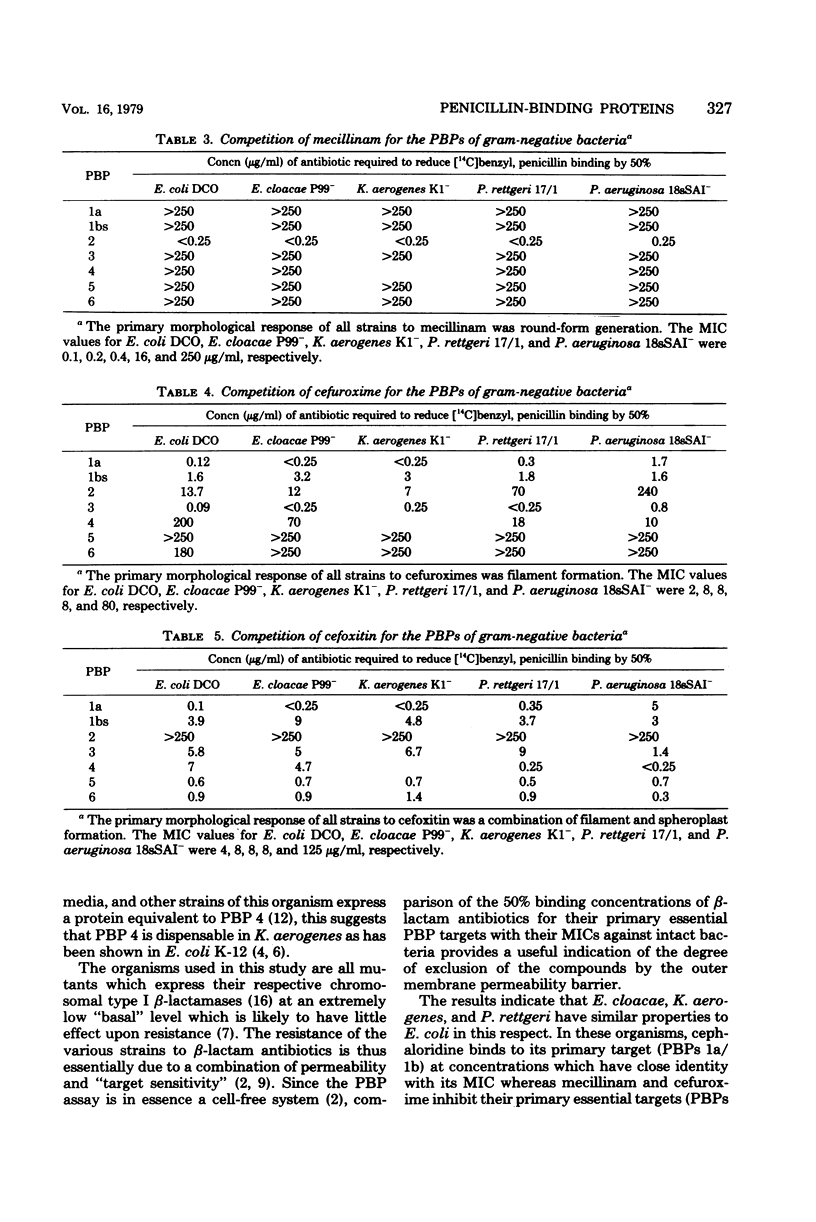
The competition of a number of beta-lactam morphogenic probes for the penicillin-binding proteins (PBPs) of Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella aerogenes, Proteus rettgeri, and Escherichia coli has been studied. The results indicate that the various gram-negative bacteria have similar, but not identical, PBP patterns and that the individual proteins probably perform similar morphogenic functions as in E. coli K-12. Comparison of the 50% binding concentrations of the compounds for the various PBPs of the five strains with their antibacterial activity indicates that the different antibiotics are excluded to a greater or lesser degree by the outer membrane permeability barrier and that the exclusion is most pronounced in P. aeruginosa.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curtis N. A., Brown C., Boxall M., Boulton M. G. Inhibition of Escherichia coli K-12 by beta-lactam antibiotics with poor antibacterial activity: interaction of permeability and intrinsic activity against penicillin-binding proteins. Antimicrob Agents Chemother. 1979 Mar;15(3):332–336. doi: 10.1128/aac.15.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Brown C., Boxall M., Boulton M. G. Modified peptidoglycan transpeptidase activity in a carbenicillin-resistant mutant of Pseudomonas aeruginosa 18s. Antimicrob Agents Chemother. 1978 Aug;14(2):246–251. doi: 10.1128/aac.14.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldner M., Glass D. G., Fleming P. C. Spontaneous mutant with loss of beta-lactamase in Aerobacter cloacae. J Bacteriol. 1969 Feb;97(2):961–961. doi: 10.1128/jb.97.2.961-.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya M., Strominger J. L. Simultaneous deletion of D-alanine carboxypeptidase IB-C and penicillin-binding component IV in a mutant of Escherichia coli K12. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2980–2984. doi: 10.1073/pnas.74.7.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. J., Ross G. W., Chanter K. V., Harris A. M. Comparison of the substrate specificities of the -lactamases from Klebsiella aerogenes 1082E and Enterobacter cloacae P99. Appl Microbiol. 1972 Apr;23(4):765–769. doi: 10.1128/am.23.4.765-769.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Takagaki Y., Maruyama I. N., Tamaki S., Nishimura Y., Suzuki H., Ogino U., Hirota Y. Mutants of Escherichia coli lacking in highly penicillin-sensitive D-alanine carboxypeptidase activity. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2976–2979. doi: 10.1073/pnas.74.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Distèche M., Pollock J. J., Ghuysen J. M., Puig J., Reynolds P., Perkins H. R., Coyette J., Salton M. R. Sensitivity to ampicillin and cephalothin of enzymes involved in wall peptide crosslinking in Escherichia coli K12, strain 44. Eur J Biochem. 1974 Feb 1;41(3):457–463. doi: 10.1111/j.1432-1033.1974.tb03287.x. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol. 1977 Jul;131(1):293–305. doi: 10.1128/jb.131.1.293-305.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. The mechanism of action of mecillinam. J Antimicrob Chemother. 1977 Jul;3 (Suppl B):13–19. doi: 10.1093/jac/3.suppl_b.13. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]



