Abstract
Background
Six known steps are required for the circulating thyroid hormone (TH) to exert its action on target tissues. For three of these steps, human mutations and distinct phenotypes have been identified.
Scope of Review
The clinical, laboratory, genetic and molecular characteristics of these three defects of TH action are the subject of this review. The first defect, recognized 45 years ago, produces resistance to TH and carries the acronym, RTH. In the majority of cases it is caused by TH receptor β gene mutations. It has been found in over 3,000 individuals belonging to approximately 1,000 families. Two relatively novel syndromes presenting reduced sensitivity to TH involve membrane transport and metabolism of TH. One of them, caused by mutations in the TH cell-membrane transporter MCT8, produces severe psychomotor defects. It has been identified in more than 170 males from 90 families. A defect of the intracellular metabolism of TH in 10 individuals from 8 families is caused by mutations in the SECISBP2 gene required for the synthesis of selenoproteins, including TH deiodinases.
Major Conclusions
Defects at different steps along the pathway leading to TH action at cellular level can manifest as reduced sensitivity to TH.
General Significance
Knowledge of the molecular mechanisms involved in TH action allows the recognition of the phenotypes caused by defects of TH action. Once previously known defects have been ruled out, new molecular defects could be sought, thus opening the avenue for novel insights in thyroid physiology.
Keywords: thyroid hormone, nuclear receptor, transmembrane transporter, selenoprotein, deiodinases, metabolism, MCT8, RTH, SBP2, inherited defects
Introduction
The concept of resistance to thyroid hormone (RTH) was born with the observation of apparent high thyroid hormone (TH) levels in three siblings that did not present symptoms and findings of hormone excess, but rather hormone sufficiency and even deficiency [1, 2]. The identification of other cases supported the concept of RTH though proof had to await the cloning of the TH receptors (TRs) [3, 4]. In fact, 22 years later, a mutation in the TRβ gene (also known as THRB) was found in a subject with RTH [5] closing the circle of hypothesis and proof.
More recently, TH cell membrane transport defects (THCMTD) [6, 7] and TH metabolism defects (THMD) [8] were described. This led to the broadening of the definition of reduced sensitivity to TH to encompass all defects that can interfere with the biological activity of a chemically intact hormone, secreted in normal amounts. The term reduced sensitivity to TH or RSTH, to denote reduced effectiveness of TH in the broader sense, was accepted at the 8th International Workshop on Resistance to Thyroid Hormone, which took place on the Azorean Island of San Miguel, October 2007 [9].
The long sought TRα gene (also known as THRA) defect was identified and reported only this year [10, 11], making this review particularly timely. We begin with a brief outline of thyroid physiology, including the regulation of hormone synthesis, secretion, transport, metabolism and action. The description of each syndrome in terms of clinical, genetic and biochemical aspects follow this. All reported cases and those not published by the authors have been reviewed and are included in this review. Mechanisms are discussed in light of animal models when available. Some clinical and diagnostic comparisons are provided as well as a combined section on treatment.
Brief outline of thyroid physiology
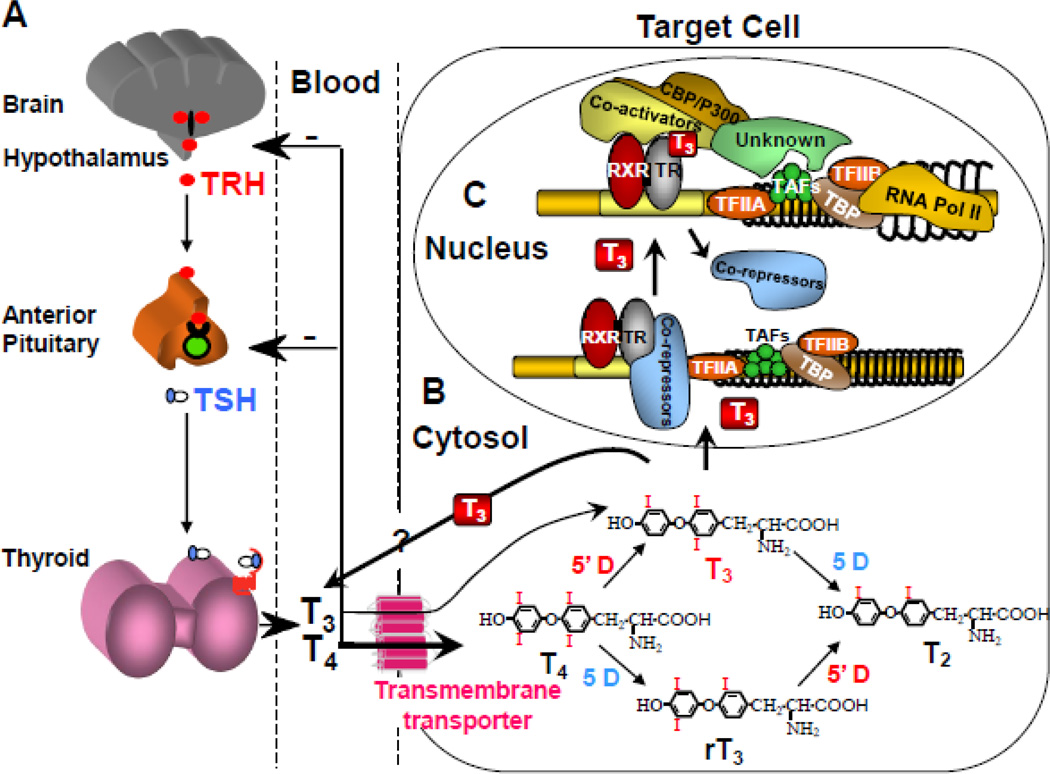
Supply of TH is insured by a feedback control mechanism involving the hypothalamus, pituitary, and thyroid gland (Fig. 1A). A decrease in the circulating TH concentration causes thyrotropin-releasing hormone (TRH) to be secreted into the portal system, reaching directly the anterior pituitary gland to stimulate the release of thyrotropin (TSH) into the systemic circulation. The latter stimulates the thyroid follicular cells to synthesize and secrete more hormone. TH excess, on the other hand, shuts down the system through the same pathway, until homeostasis is reinstated.
Figure 1.
Regulation of TH supply, metabolism and genomic action. (A) Central feedback control that regulates the amount of TH in blood. (B) Intracellular metabolism of TH, regulating TH bioactivity. (C) Genomic action of TH. For details see text.
CBP/P300, cAMP-binding protein/general transcription adaptor; TFIIA and TFIIB, transcription intermediary factor II, A and B; TBP, TATA-binding protein; TAF, TBP-associated factor. (Modified from Refetoff S, Dumitrescu AM. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab. 2007 Jun;21(2):277–305.)
This centrally regulated system does not respond to changes in TH requirements in a particular organ or cell. Local requirements in TH are adjusted by additional mechanisms. One is the control of TH entry into the cell through active transmembrane transporters [12]. Another is the activation of the hormone precursor thyroxine (T4) by removal of the outer ring iodine (5’-deiodination) to form 3,3’,5-triiodothyronine (T3) or, inactivate T4 and T3 by removal of the inner ring iodine (5-deiodination) to form 3,3’,5’-triiodothyronine (reverse T3; rT3) and T2, respectively (Fig. 1B). Modulating the concentrations of deiodinases at the cell level, provides additional means for the local regulation of hormone supply [13].
Finally, the abundance and the type of TRs, through which TH action is mediated, determine the nature and degree of hormonal response. TH action takes place in the nucleus with lesser direct effect in the cytosol [14]. The genomic effect of TRs has been most extensively studied [15, 16] (Fig. 1C). TRs are transcription factors that, together with co-regulators, bind to specific areas of DNA on genes whose expression they regulate.
From the foregoing it is obvious that proper TH action requires 1) the availability of an authentic TH, 2) its correct transport across cell membrane, 3) intracellular metabolism and hormone activation, 4) cytosolic and nuclear processing, 5) association with receptors and 6) interaction with co-regulators or other post receptor effects required for the expression of the TH effects.
How Thyroid Hormone Deficiency and Excess Coexist
TH deficiency and excess produce typical symptoms and signs reflecting the effects of global shortage and excess of the hormone, respectively, on all body tissues. A departure to this rule was recognized with the identification of the RTH syndrome. Usually caused by mutations in the TRβ gene, subjects have high TH levels without TSH suppression. This paradox is observed clinically and in biochemical tests some of which suggest TH deficiency and others sufficiency or excess, depending on the level of TRβ gene expression in various tissues [17]. The syndrome of TH cell membrane transport defect (THCMTD) presents a similar paradox, as subjects have high serum T3 concentration but the uptake of TH is not uniform in all tissues and cell types [18].
Resistance to thyroid hormone (RTH)
Intracellular TH action
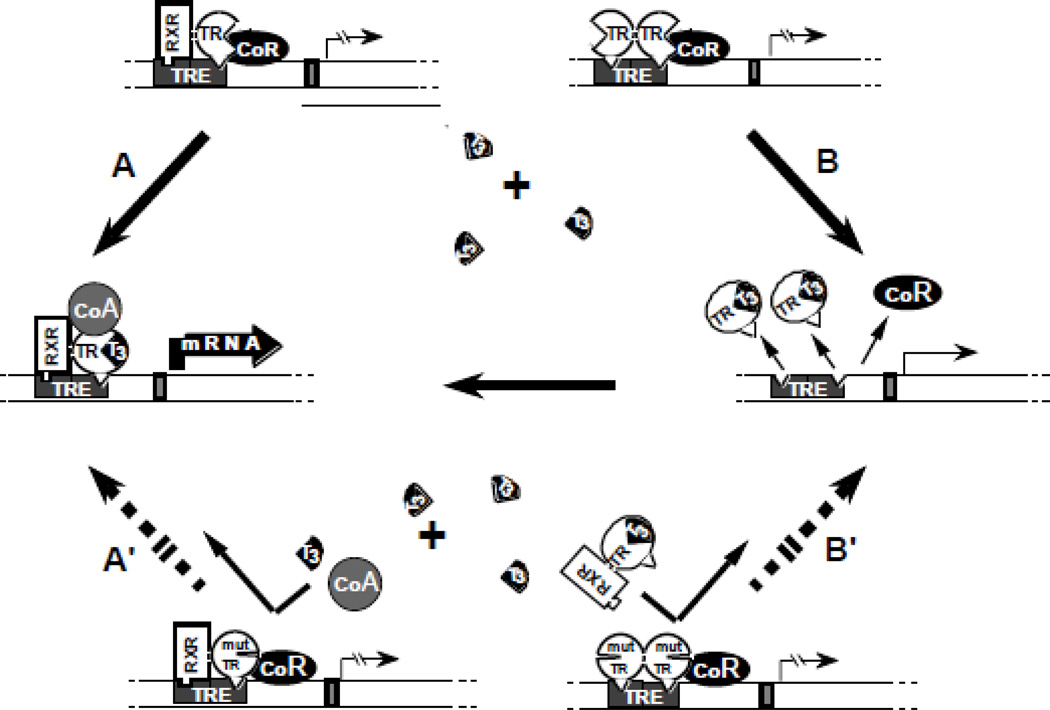
An optimal amount of intracellular TH, in its active form T3, is required for the expression of TH action. Rapid, non-genomic action, involving ion channels, oxidative phosphorylation and second messengers, is exerted at the level of the plasma membrane and cytoplasm [14]. The principal and best studied, the genomic effect of TH requires T3 translocation into the nucleus where it interacts with TRs to activate or repress transcription of target genes. Specific sequences (TH response elements or TREs), at or near the promoter region of these genes, allow binding of TRs to DNA. In the absence of T3, TRs interact with other molecules, most notably the coregulator retinoid X receptor, and corepressors. The latter produce a silencing effect on genes positively regulated by TH. T3 binding induces conformational changes in the TR molecule, triggering a chain of events that include, release of the corepressors, recruitment of coactivators and a large number of other proteins, some with histone acetylation activity. In genes positively controlled by TH, this results in the loosening of the nucleosome structure making the DNA more accessible to transcription factors that mediate binding of RNA polymerase II and general transcription initiation factors [19] (Fig. 2).
Figure 2.
Schematic representation of the DNE mechanism: In the absence of T3, occupancy of TRE by TR heterodimers (TR-RXR) or dimers (TR-TR) suppresses transactivation through association with a corepressor (CoR). (A) T3-activated transcription mediated by TR-RXR heterodimers involves the release of the CoR and association with coactivators (CoA) as well as (B) the removal of TR dimers from TRE releases their silencing effect and liberates TREs for the binding of active TR-RXR heterodimers. The DNE of a mutant TR (mut TR), that does not bind T3, can be explained by the inhibitory effect of mut TR-containing-dimers and heterodimers that occupy TRE. Thus, T3 is unable to activate the mut TR-RXR heterodimer (A') or release TREs from the inactive mut TR homodimers (B'). (Modified from Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev 1993;14:348–399.)
Cloning of the TR genes in 1986 provided an explanation for a syndrome recognized two decades earlier as refractoriness to TH. Most subjects with RTH were subsequently found to have mutations in the TRβ gene. While the clinical presentation is variable, the cardinal features are (a) high serum FT4 and usually also T3 concentration, (b) non-suppressed (normal or high) serum TSH, (c) absence of the usual symptoms and signs of thyrotoxicosis, and (d) commonly a goiter [17].
Variants: clinical and genetic
Before TRβ gene mutations were recognized, RTH was subdivided on clinical basis into generalized, isolated pituitary and peripheral tissues resistance [20]. Although based on symptoms and signs this sub classification appeared to have a clinical usefulness, it has no logical etiologic grounds since the former two are encountered in individuals with identical TRβ gene mutations and show no distinctive laboratory differences [21]. The latter clinical subtype of only peripheral tissue resistance was reported in a single patient [22] and represents the development of tolerance to the ingestion of excess TH.
NonTR-RTH
The term nonTR-RTH refers to a subgroup of individuals with the cardinal features of RTH but no mutations in the TRβ gene. It occurs in 15% of families with RTH. In several of these families mutations in both TRβ and TRα genes have been excluded by cosegregation analysis, thus ruling out mosaicism [23].
First reported in 1996 [24], nonTR-RTH it is clinically and biochemically undistinguishable from RTH with TRβ gene mutations. In families with more than one affected subject, the inheritance is autosomal dominant. It is 3-fold more frequent in women. The TRβ gene transcripts are of normal size and abundance. While the genetic defect remains unknown, cultured fibroblasts from such individuals are resistant to the in-vitro effect of TH. The finding of an aberrant interaction of nuclear extracts of fibroblasts from such an individual with the wild-type (WT) TRβ suggests that the defect involves an aberrant cofactor. However, cosegregation analysis, and direct screening for mutations by DNA sequencing of several families with nonTR-RTH excluded the involvement of coactivators (SRC-1/NcoA-1; and NcoA-3/SRC-3/AIB1/RAC-3), two corepressors (NCoR and SMRT) and two coregulators (RXRγ and TRIP1) as well as the cell-transporter LST-1 (OATP1B1) [23].
RTH due to a TRα gene mutation
The long sought mutation in the TRα gene was identified this year by exomic sequencing of DNA obtained from a 6 year-old girl with chronic constipation noted upon weaning at 7 months of age, with growth and developmental delay [10]. The nonsense mutation identified produces a truncated TRα1 (E403X) that lacks the C-terminal α-helix. Findings suggestive of TH deficiency involved organs expressing predominantly TRα, including bones, gastrointestinal tract, heart, striated muscle and central nervous system. Indeed, X-rays showed patent cranial sutures with wormian bones, delayed dentition, femoral epiphyseal dysgenesis and retarded bone age. In addition, diminished colonic motility with megacolon, slow heart rate, reduced muscle strength were suggestive of hypothyroidism, as was her placid affect, slow monotonous speech and cognitive impairment. Recently, another family with TRα gene mutation was reported [11]. In the first three years of life the proposita had macroglossia, omphalocele, congenital hip dislocation, delayed bone age, delayed closure of skull sutures, delayed tooth eruption, delayed motor development, and macrocephaly. In both reports, thyroid function tests were distinct from those found in classical RTH with or without TRβ gene mutations. They had some similarity to those in mice with TRα gene mutations, low serum T4, high T3, and very low rT3. In addition they are somewhat reminiscent of MCT8 defects (see the THCMTD section below), presumably due to alterations in iodothyronine metabolism.
Clinical features and course of the disorder
Characteristic of the RTH syndrome is the sparseness of specific clinical manifestations. When present, they are variable from one patient to another [17, 21]. Common features that bring subjects to medical attention are goiter, hyperactive behavior, learning disabilities, developmental delay and sinus tachycardia. The finding of elevated serum free T4 (FT4) concentration in association with nonsuppressed TSH usually trigger further studies that lead to the diagnosis.
With the exception of some rare subjects, particularly those homozygous for TRβ mutations [25, 26], the majority maintain a near normal metabolic state at the expense of high TH levels. The degree of compensation for the hyposensitivity to TH is variable among individuals as well as among different tissues, and results in the coexistence of clinical and laboratory evidence for TH deficiency and excess. Thus, delayed growth and bone maturation, learning disabilities, which are compatible with hypothyroidism, can be present along with hyperactivity and tachycardia, suggestive of thyrotoxicosis. Typical symptoms of hypothyroidism are common in individuals who received treatment to normalize their serum TH levels.
Goiter is the most common finding, reported in 66–95% of cases. Enlargement is usually diffuse with gross asymmetry. Nodularity often occurs in recurrent goiters after surgery. Sinus tachycardia is very common and together with goiter could lead to the erroneous diagnosis of autoimmune thyrotoxicosis.
About one-half of subjects with RTH have learning disability but mental retardation (IQ <60) was found only in 3% of cases [17]. Attention deficit hyperactivity disorder is also present in half of the children with RTH [27]. However, RTH in children with attention deficit disorder is extremely rare [28, 29]. Recurrent ear infections are very common in childhood but hearing impairment of variable degree has been reported in 10 to 20% of cases [30]. Various somatic malformations have been reported, mostly coincidental.
The course of the condition is also variable. Most individual achieve normal stature and development, and lead a normal life at the expense of high TH levels and a slight thyroid gland enlargement. In others, low stature and intellectual impairment persist. Symptoms of hyperactivity tend to improve with age. Goiter usually recurs after surgery. As a consequence, some of the RTH subjects have been submitted to repeat thyroidectomies or treatments with radioiodide.
Genetics
Inheritance and incidence
Neonatal screening programs based on blood TSH determination rarely identify RTH. However, a limited survey of newborn for high blood T4 found 1 case per 40,000 life births [31, 32]. Known cases surpass 3,000 with wide geographic distribution and reported in Whites, Blacks, Asians and Amerindians. In the majority of families, the syndrome is transmitted in an autosomal dominant fashion and occurs with equal frequency in both sexes. In one family with complete deletion of the TRβ gene, inheritance was recessive [33].
TR genes and mutations
The TRβ and TRα are members of the nuclear receptor family. They have structural and sequence similarities and are encoded by genes located on chromosomes 17 and 3, respectively. Alternative splicing and promoter usage produce 2 major TRα (α1, α2) and 2 major TRβ (β1, β2) isoforms [14]. Most important are the three isoforms that bind TH (TRα1, TRβ1 and TRβ2). The absence of the ligand-binding domain (LBD) precludes TH binding to TRα2, but does not prevent binding to TREs through its DNA-binding domain (DBD). Although it does not function as a proper TR, [34] it appears to have a weak antagonistic effect [35]. The significance of the other TR isoforms in humans remains unknown [36]. The level of expression and relative product distribution of the two TR genes vary among tissues and at different stages of development. TRβ and TRα are to a certain degree interchangeable [36, 37]. However, the compensatory effects observed in the absence of one of the receptors are not complete and some TH effects are TR isoform specific.
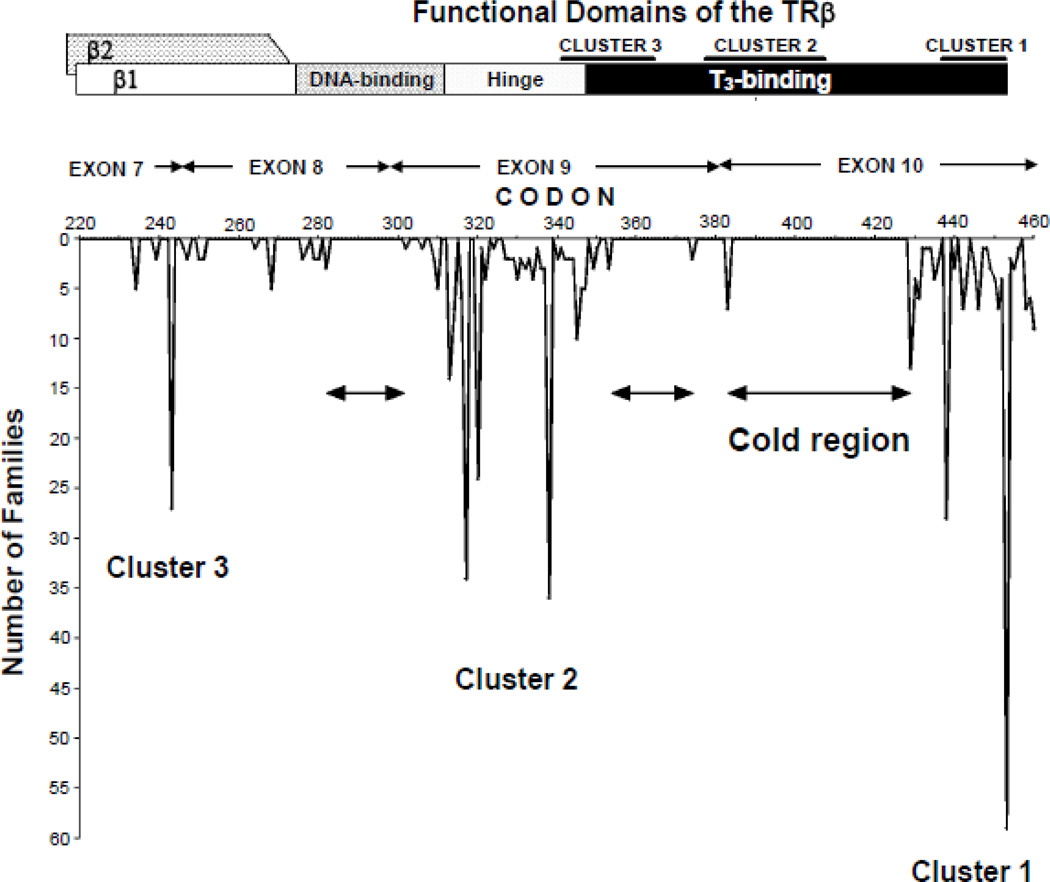
TRβ gene mutations are located in the functionally relevant domain of the LBD and its adjacent hinge region. They are mostly contained within three clusters rich in CpG "hot spots” [38–40] (Fig. 3). The mutant (mut) TRβ molecules have either reduced affinity for T3 [39, 41], or abnormal interaction with one of the cofactors involved in TH action [40, 42].
Figure 3.
Location of natural mutations in the TRβ molecule associated with RTH.
TOP PORTION: Schematic representation of the TRβ and its functional domains for interaction with TREs (DNA-binding) and with hormone (T3-binding). Their relationship to the three clusters of natural mutations is also indicated. TRβ2 has 15 more residues than TRβ1 at the amino-terminus.
BOTTOM PORTION: The location of the 170 different mutations detected and their frequencies in the total of 459 unrelated families (published and our unpublished data). Amino acids are numbered consecutively starting at the amino terminus of the TRβ1 molecule according to the consensus statement of the First International Workshop on RTH [134]. "Cold regions" are areas devoid of mutations associated with RTH.
Mutations have been now identified in 459 families, 432 of which have single nucleotide substitutions, resulting in one amino acid replacement and in 11 truncated molecules. Twenty other families have nucleotide deletions, insertions or duplications, some producing frameshifts that create nonsense proteins. From the 171 different mutations identified, some are shared by more than one family. Proof that they developed independently has been obtained [38]. TRβ R338W, caused by a C to T replacement in a CpG has been identified in 33 unrelated families. Mutations have also produced different amino acids in the same codon. Seven such different substitutions were identified in codon 453 (P453T,S,A,N,Y,H,L).
In contrast to the androgen receptor gene [43], belonging to the same receptor family, not a single TRβ gene mutation has been reported in non-coding regions or reported to produce abnormal splice variants. Sequencing of cDNA is important in subjects with clinically proven RTH in whom no mutations could be found in genomic DNA. Somatic TRβ gene mutations have been identified in two TSH-producing pituitary adenomas [44, 45].
Laboratory findings
A high serum FT4 concentration and nonsuppressed TSH are required to raise the suspicion of RTH. Usually they are accompanied by high serum T3 and rT3 levels. Serum T4 and T3 can be just above to several fold the upper limit of normal, but, contrary to autoimmune thyrotoxicosis, can maintain a near normal T3:T4 ratio [17]. Serum thyroglobulin concentration tends also to be high as the result of TSH induced thyroid gland over activity. The response of TSH to TSH-releasing hormone is normal or exaggerated, in proportion to the baseline TSH level. The relatively high TSH bioactivity explains why goiters develop despite normal levels of immunoreactive TSH [46]. Thyroidal radioiodide uptake is high and not dischargeable by perchlorate. The presence of thyroperoxidase and thyroglobulin antibodies does not exclude the diagnosis of RTH. As a matter of fact, their cooccurrence is slightly more frequent [47].
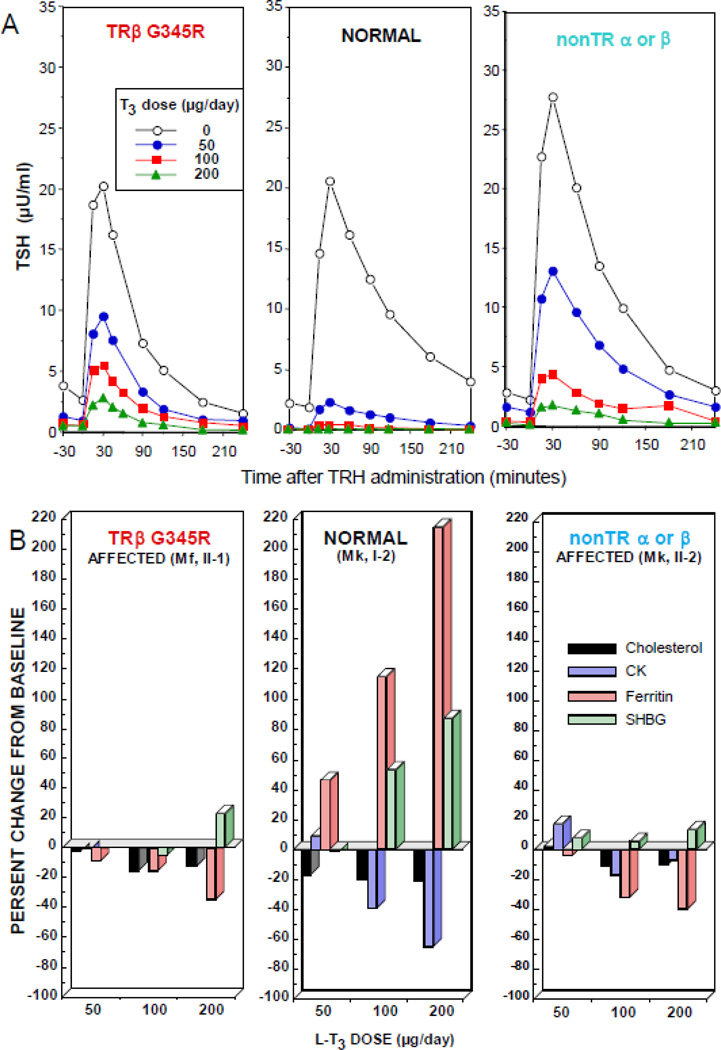
Tests assessing TH action on peripheral tissue are usually normal at baseline, but are not sensitive to small hormonal deficiencies or excess. A standardized protocol, using short-term administration of L-T3 to determine the sensitivity of central and peripheral tissues to TH is available [17]. Three incremental doses given to adults, each for three consecutive days, are a replacement dose of 50 µg/day and two supraphysiologic doses of 100 and 200 µg/day, administered in split daily doses. Attenuated stimulation of sex hormone binding globulin and ferritin and reduced suppression of TSH, cholesterol and creatine kinase are compatible with RTH (Fig. 4).
Figure 4.
Responses to the administration of L-T3 in subjects with RTH, with and without mutations in the TRβ gene and in a normal individual. The hormone was given in three incremental doses, each for 3 days. Results are shown at baseline and after each dose of L-T3 in patients with RTH in the presence (left) or absence (right) of a TRβ gene mutation, and the unaffected mother of the patient with nonTR-RTH (center). (A) TSH responses to TRH stimulation. (B) Responses of peripheral tissues. Note the stimulation of ferritin and sex hormone binding globulin (SHBG) and the suppression of cholesterol and creatine kinase (CK) in the normal subject. Responses in affected subjects, with or without a TRβ gene mutation, were blunted or paradoxical. (Modified from Refetoff S, Dumitrescu AM. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab. 2007 Jun;21(2):277–305.)
Evaluation for other endocrine abnormalities has yielded negative results. Earlier, in-vivo tests, measuring hormonal turnover, and in-vitro tests, using the patients skin fibroblasts in culture, have provided valuable diagnostic information which, with the advent of genetic diagnosis, have lost their utility [17].
These laboratory results were not found in a subject with a TRα gene mutation (see Variants, above)
Mechanisms of the Disorder
The more common forms of TH hypersecretion are TSH independent and thus, as in primary hypothyroidism, there is a reciprocal relation between the serum TH and TSH concentrations. The absence of such correlation in RTH, has introduced the term "inappropriate secretion of TSH". This is unfortunate, as the increased secretion of TH as consequence of the reduced feedback action of TH, is compensatory and fully appropriate for the defect.
Individuals with one WT TRβ allele due to deletion of the other allele are normal, while those expressing a mut TRβ and a WT allele, have RTH. The former is not due to a compensatory over expression of the WT allele [48], and the reduced amount of TRβ does not produce haploinsufficiency. In the latter situation, the presence of a defective mut TRβ interferes with the function of the WT TRβ, a phenomenon known as dominant negative effect (DNE). This explains the dominant form of RTH inheritance when caused by mut TRβs and recessive in subjects with TRβ gene deletion [33, 49].
Expression of a DNE requires that the mut TRs with defective T3-binding preserve a DNA-binding domain and the ability to dimerize with a homologous or heterologous partner (Fig. 2). Mut TRs interfere with WT TR function by occupying TREs on target genes and by engaging WT TRβ in homodimerization. This explains why no mutations have been identified in CpGs located in regions of the TRβ molecule involved in homodimerization [50]. Dominant negative effect can also manifest through altered association of a mut TRβ with a cofactor, including increased affinity to or decreased release of a corepressor [42, 51], or reduced association with a coactivator [40]. In contrast, mut TRβs with complete inability to bind T3 can paradoxically produce minimal dysfunction if association to corepressor is also reduced [41]. Less obvious was the reason for the reverse situation in which mut TRβs manifesting strong DNE have minimal impairment or even, intact T3-binding. The two mutations R243Q and R243W, located in the hinge domain and with normal T3-binding in solution, become resistant to T3 when bound to TRE, thus maintaining repressive dimers [52, 53]. On some occasions, mut TRβs may show greater impairment of transactivation on genes negatively rather than positively regulated by T3 [41, 54]. Recent work suggests that the mut TRβ R429Q affects predominantly the TRβ2 mediated action [55] while mut TRβ R338W, through its association with a single nucleotide polymorphism in the enhancer region, produces over expression of the mut TRβ allele [56]. These two mechanisms give credence to the existence of predominantly pituitary RTH.
Different mechanisms are responsible for the variable phenotype of RTH. Homozygotes devoid of WT TRβ are deaf and color blind even though the magnitude of thyroid function tests abnormalities are comparable to heterozygotes with mut TRβs. This is due to the total absence of TRβ mediated action required for cochlear and photoreceptor development (see Mouse Models below). Conversely, the severe signs of hypothyroidism in bone and brain of homozygotes with mut TRβs can be explained by the DNE of the double dose of the mut TRβs that interfere with TRα function as well [26].
Differences in the magnitude of hormonal resistance in different tissue are due to the absolute and relative levels of TRβ and TRα expression. For example, the hypothalamus and pituitary which are dependent on TRβ, manifest relative hormone deprivation, while the heart which depends on TRα, exhibits signs of hormone excess in the form of tachycardia [17]. Thus, it is not surprising that the phenotype of the patient with TRα gene mutation was completely distinct [10]
Although differences in functional impairment and, rarely, level of expression of the mut TRβs can explain some of the clinical differences, the molecular basis for the heterogeneity of the RTH phenotype in individuals with the same mutation remains unknown. This phenotypic variation can occur across families [38] and within the same family [57]. Finding differences in the relative expression level of the mutant relative to the normal TRβ allele has not been consistent [48, 58]. Genetic variability of factors other than TRβ may modulate the phenotype of RTH.
Mouse Models
Gene targeting has allowed the generation of mice with TRβ deletion (knockout, KO) and with various mutations (knockin, KI), which replicate defects observed in man. These animals have been invaluable in understanding the physiology of TR and the pathophysiology of RTH [59].
TRβ gene manipulations
TRβKO mice exhibit all the features of humans with TRβ gene deletion. Heterozygotes are normal and homozygotes have, in addition to the characteristic thyroid test abnormalities, sensorineural deafness and monochromatic vision, indicating that the deaf-mutism and color blindness in humans can be fully explained by the complete absence of TRβ. These mice allowed in-depth investigations establishing that TRβ2 deficiency was responsible for the color blindness [60] but not for the hearing loss [61]. TRβKO mice have increased heart rate that normalizes with reduction on the TH level [62, 63]. These findings, together with the lower heart rate in TRα1KO mice [59], indicates that TH affects heart rate through TRα1, and explains the tachycardia observed in some patients with RTH.
TRβKI mice, targeted to replicate known human TRβ gene mutations (frame-shift PV and T337D) [64, 65], are true models of the dominantly inherited form or RTH. Heterozygous KI mice manifest many of the abnormalities observed in man. In addition, homozygotes develop metastatic thyroid cancer [64].
TRα gene manipulation
TRα gene deletions, total or only α1, do not produce important alterations in TH or TSH concentrations [35]. Several mutations naturally occurring in the human TRβ gene (PV, R438C and P453H) were targeted in homologous regions of the TRα1 gene of the mouse [66–68]. The resulting phenotypes were variable but did not exhibit RTH as that found in TRβ gene mutations. In the heterozygous state, the first two KIs mentioned show severe postnatal development and growth retardation, while the latter has increased body fat and insulin resistance. Decreased heart rate and cold-induced thermogenesis, as well as reduced fertility, were also observed. Serum thyroid tests were variable with the type of mutations and with age, but T4 had the tendency to be low and T3 high. Some of the abnormalities are reminiscent to those observed in the heterozygous human with TRα1 gene mutation. All three types of TRα1KIs did not survive in the homozygous state, recapitulating the noxious effect of unliganded TRα1.
Combined TR and related gene deletions
Deletion of both α and β TRs is compatible with life [36, 37]. This contrasts with the complete TH deficiency in the athyreotic Pax8KO mouse that dies prior to weaning, unless rescued by TH. The survival in the absence of TR is not due to the presence of an unidentified TR but to the absence of the noxious effect of unliganded TRs. Indeed, removal of all TRα isoforms rescues the Pax8KO mice from death [69, 70]. Deletion of the TRα1 gene also prevents the development of cerebellar abnormalities during TH deprivation [71]. The aporeceptor does not seem to be required for the upregulation of TSH.
NCoA-1 (SRC1) KO mice have resistance to TH in addition to sex-hormones [72]. Mice deficient in retinoid-X receptor γ, the dimerization partner of TR, are also mildly resistant to TH [73].
Thyroid hormone cell membrane transporter defect (THCMTD)
Cell membrane TH transporters
The previously accepted paradigm of passive TH diffusion into cells [74] has been abandoned with the identification of several classes of molecules that transport TH across cell membranes [75]. These proteins belong to different families of solute carriers. Among them, the X-linked monocarboxylate transporter 8 (MCT8) has been shown to be a potent and specific TH transporter [76]. The important role of MCT8 was convincingly demonstrated by the identification in two different laboratories of the first inherited THCMTD caused by mutations in the MCT8 (SLC16A2) gene [6, 7]. All affected subjects tested to date have 1) a complex and severe neurodevelopmental defect and 2) a combination of pathognomonic test abnormalities of high serum T3, low rT3, low normal to reduced T4 with TSH levels normal or slightly elevated.
Clinical features and course of the disorder
Male subjects later found to have MCT8 gene mutations, are referred for medical investigation during infancy or early childhood because of neurodevelopmental abnormalities. Newborns have normal Apgar scores and in most cases there is history of normal gestation, though polyhydramnios and reduced fetal movements have been reported [77, 78] [Dumitrescu, A.M. and Refetoff, S, unpublished data].
Truncal hypotonia and feeding problems are the most common early signs of the defect, appearing in the first 6 months of life. Characteristically, the neurological manifestations progress from flaccidity to limb rigidity, which, with advancing age, leads to spastic quadriplegia. Most subjects are unable to walk, stand or sit independently and do not develop speech. Only members of three families [78, 79] were able to walk with ataxic gait or with support and had a limited, dysarthric speech. A possible explanation for milder neurological phenotype in these patients is a residual 15–37% TH-transport activity of their mutant MCT8 molecules [80].
Dystonia and purposeless movements are common and characteristic paroxysms of kinesigenic dyskinesias have been reported in several patients [81, 82]. In addition, true seizures occur in one quarter of the patients. Difficulty falling asleep and frequent awakenings, can represent an important clinical issue for caregivers [82]. Reflexes are usually brisk and clonus is often present.
With advancing age, weight gain lags and microcephaly becomes apparent, while linear growth proceeds normally [83]. Muscle mass is diminished and there is generalized muscle weakness with typical poor head control, originally described as “limber neck” [84]. A prominent and common feature in MCT8 deficient patients is the failure to thrive, which may require in some cases a gastric feeding tube. Possible reasons for low weight and muscle wasting are difficulty swallowing on neurological basis, and increased metabolism due to the thyrotoxic state of peripheral tissues caused by the high serum T3 levels [82, 85–87].
Facial features, attributed to the prenatal and infantile hypotonia, include ptosis, open mouth, and a tented upper lip. Ears are long, thick and cup-shaped while there is a decrease in facial creases. Pectus excavatum and scoliosis are common.
Cognitive impairment is severe but MCT8 deficient patients tend to be non-aggressive. Death during childhood or teens is common, usually caused by recurrent infections and/or aspiration pneumonia. However, survival beyond age 70 years has been observed in a few instances of more mild neurologic involvement [78].
Female carriers have none of the psychomotor abnormalities described above. Nevertheless, intellectual delay and frank mental retardation was reported in six carrier females [6, 78, 86, 88]. Although an unfavorable nonrandom X-inactivation could alter the phenotype in these females [78], cognitive impairment can be due to a variety of unrelated causes [89].
Genetics
Inheritance and incidence
MCT8 deficiency is a X-linked defect. Mutations have 100% penetrance in males who manifest both neuropsychomotor and characteristic thyroid tests abnormalities Carrier females may show only mild thyroid test abnormalities [6, 89, 90]. A single female manifesting the typical features of MCT8-specific THCMTD had a de novo translocation disrupting the MCT8 gene and unfavorable nonrandom X-inactivation [88]. The defect has been reported in individuals of all races and diverse ethnic origins. In a study of 448 subjects with X-linked metal retardation, MCT8 gene mutations responsible for the phenotype were found in three [88]. Although no affected male has reproduced, MCT8 gene mutations are maintained in the population due to the fact that carrier females are asymptomatic and fertile.
MCT8 gene and mutations
The MCT8 gene was cloned in the process of characterizing the Xq13.2 region containing the X-inactivation center [91]. The deduced products are proteins of 613 and 539 amino acids (translated from two in-frame start sites) containing 12 transmembrane domains (TMD) with intracellular amino- and carboxyl- ends [92]. The upstream translation start site is absent in most species. Thus, the additional N-terminal sequence present in humans has unknown functional importance.
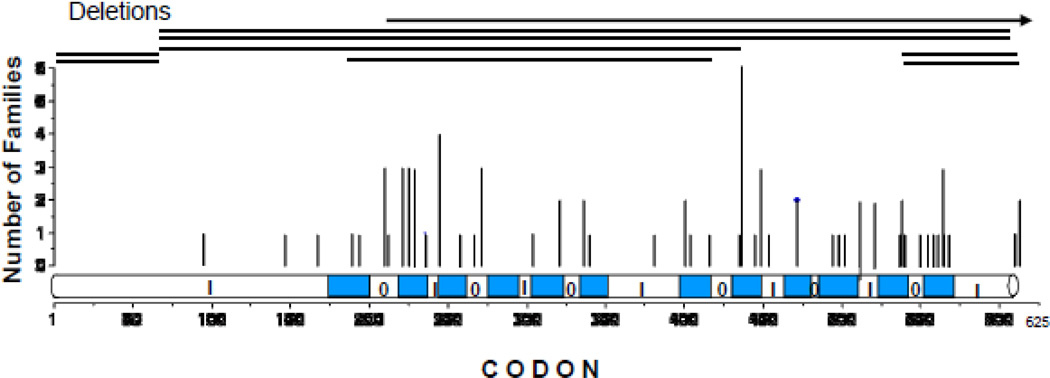
Mutations are distributed throughout the coding region of the gene with apparent increased distribution in the TMDs (Fig. 5). As mutations are relatively underrepresented in the extracellular and intracellular loops, it is likely that missense mutations in these domains result in a milder phenotype, escaping detection. In fact, sequences in these regions are less conserved across species compared to the TMD regions [93].
Figure 5.
Location of mutations in the MCT8 molecule associated with THCMTD Shown by vertical lines are 58 known mutant MCT8 proteins and their frequency in 80 families (published and our unpublished data). Horizontal lines indicate the mutations with deletions of large regions. Numbering is consecutive, starting at the amino terminus of the 613 amino acid human molecule. The 12 TMDs are indicated in blue. Loops predicted to be outside the cell are indicated by an O and those inside the cell, by an I.
MCT8 gene mutations range from single nucleotide substitutions to large deletions involving one or more exons. Twelve different mutations occurred each in at least two or more unrelated families. The majority of single nucleotide substitutions are located in mutational hotspots such as CpG dinucleotides, or C and A repeats. Of cases who’s mothers were genotyped, de novo mutations were identified in 25%.
Laboratory findings
Biochemical
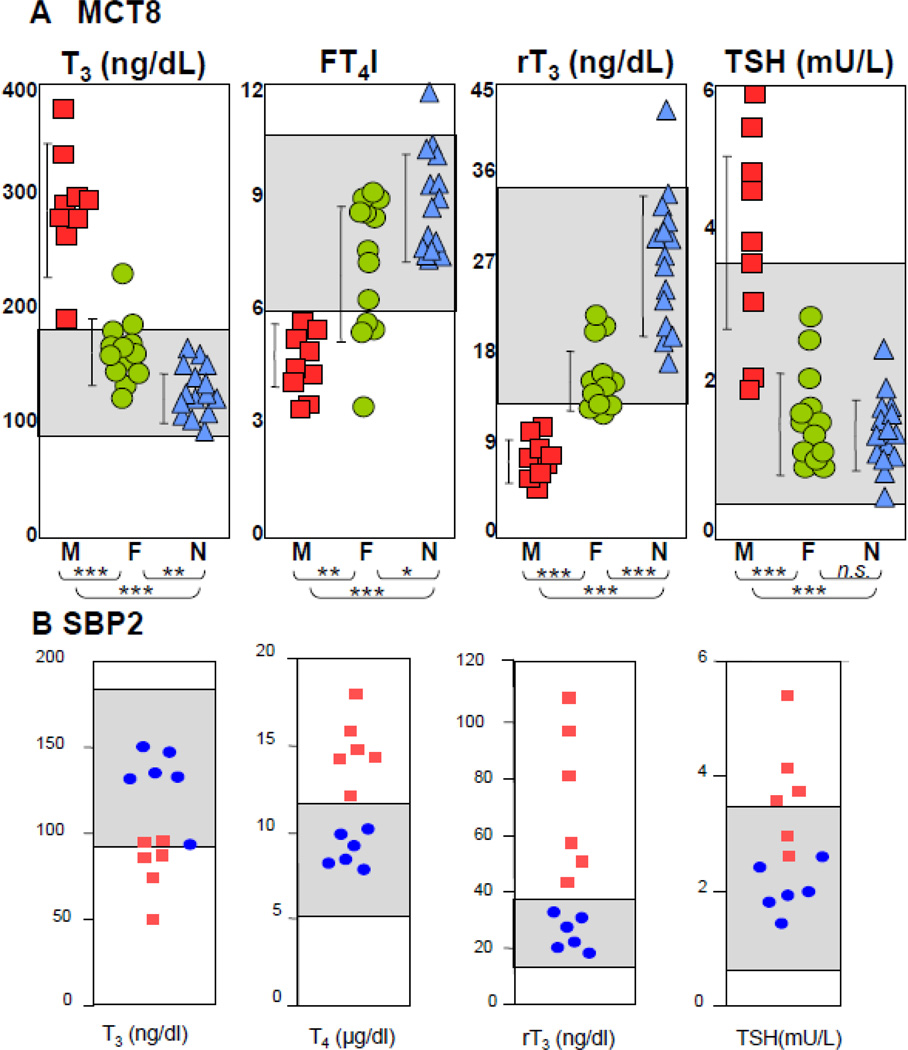
Pathognomonic are the high serum total and free T3 and low rT3 concentrations. T4 is reduced in most cases and TSH levels can be slightly elevated but rarely above 6 mU/L (Fig. 6A).
Figure 6.
(A) Thyroid function tests in several families with MCT8 deficiency studied in the authors’ laboratory. Grey regions indicate the normal range for the respective test. Hemizygous males (M) are represented as red squares, heterozygous carrier females (F), as green circles and unaffected members of the families, as blue triangles (N). With the exception of TSH, mean values of iodothyronines in carrier females are significantly different than those in affected males and normal relatives. (B) Thyroid function tests in subjects from 4 families with SBP2 deficiency studied in the authors’ laboratory. Grey regions indicate the normal range for the respective test. Affected individuals are represented as red squares and unaffected members of the families, as blue circles. (Modified from Refetoff S, Dumitrescu AM. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab. 2007 Jun;21(2):277–305.)
TSH is usually normal at neonatal screening. Neonatal T4 levels available in 8 cases revealed low values in six and normal in two [78, 82] [Dumitrescu, A.M. and Refetoff, S, unpublished data], but information on T3 and rT3 concentration in the first days of life is not available. The typical thyroid tests abnormalities of MCT8 deficiency become apparent within one month.
Heterozygous female carriers have all three serum iodothyronine concentrations intermediate between affected males and unaffected family members [6, 78, 86]. Serum TSH concentrations are, however, normal (Fig. 6A).
Urinary organic acids, serum amino acids and fatty acids, CSF neurotransmitters, and glucose are usually normal. Other test results were abnormal only in some patients, such as, elevated serum SHBG, transaminases, ammonia, lactate and pyruvate; mildly elevated medium chain products in plasma acylcarnitine profile, elevated hydroxybutyric acid in urine [77, 86] [Dumitrescu, A.M. and Refetoff, S, unpublished data] and reduced serum cholesterol. While the relation of some test abnormalities with MCT8 deficiency is unclear, others, such as reduced cholesterol, and increased SHBG and lactate, can be ascribed to the effect of the high serum T3 levels on peripheral tissues.
Other endocrine tests, including pituitary function were normal when tested in a several individuals. However, administration of incremental doses of L-T3, using the protocol described for the study of patients with RTH, showed reduced pituitary sensitivity to the hormone [Dumitrescu, A.M. and Refetoff, S, unpublished data]. This is probably caused by the reduced suppressive effect of T3 on the hypothalamic-pituitary axis, as well as the diminished incremental effect of the hormone on peripheral tissues already exposed to high levels of T3.
Altered activity of muscle mitochondrial complexes II and IV was identified in two cases [94] [Dumitrescu, A.M. and Refetoff, S, unpublished data]. Whether this is due to the abnormal TH status of the muscle or to a yet unidentified effect of MCT8 on the mitochondria, it remains unknown.
Imaging
Reports on bone age have been inconsistent as they were found to be delayed in four cases and slightly advanced in one [86, 95, 96] [Dumitrescu, A.M. and Refetoff, S, unpublished data].
Mild to severe delayed myelination or dysmyelination [97, 98] [Dumitrescu, A.M. and Refetoff, S, unpublished data] is a common finding when brain magnetic resonance imaging (MRI) is performed in early life. Decreased myelination is less apparent by 4 years of age. This distinguishes MCT8 deficiency from other leukodystrophies with persistent myelination defect, one such example being the Pelizaeus-Merzbacher syndrome [97]. Other MRI abnormalities reported in single cases might be non-specific. They include subtle cortical and subcortical atrophy [85], mild cerebellar atrophy [86], putaminal lesions [99] and a small corpus callosum [Dumitrescu, A.M. and Refetoff, S, unpublished data]. Increased choline and myoinositol levels and decreased N-acetyl aspartate detected by MR-spectroscopy were associated with the degree of dysmyelination found on MRI [100].
Mechanisms of the disorder
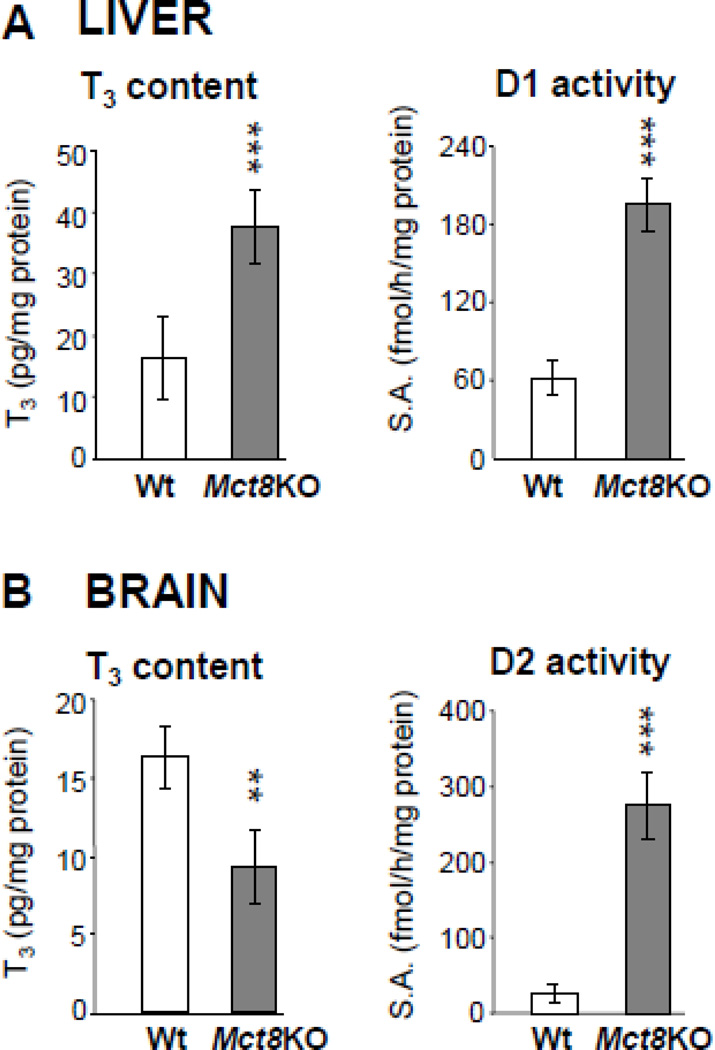
Mct8-deficient (Mct8KO) mice [18, 101] replicate the characteristic thyroid tests abnormalities found in humans and, in this respect, have been invaluable in understanding the mechanisms responsible for the thyroid phenotype [102]. The variable availability of the circulating hormone to tissues, depending on the redundant presence of TH cell membrane transporters was demonstrated by measurement of tissue T3. Tissues such as the liver, that express other transporters [12], have high T3 concentrations reflecting the high levels in serum, despite the absence of Mct8. The effect of TH excess was demonstrated by an increase in the D1 enzymatic activity (Fig. 7A), decrease in serum cholesterol and increase in serum alkaline phosphatase concentrations. In contrast, tissues with limited redundancy in cell membrane TH transporters, such as the brain [12], have decreased T3 content in Mct8KO mice, which together with the increase in D2, indicate local TH deprivation (Fig. 7B). The high D2 in the context of TH deficiency [13] has only partial compensatory effect. The coexistent tissue specific T3 abundance and deficiency in the Mct8KO mouse explain, in part, the mechanisms underlying the manifestation of TH excess and deficiency in humans with MCT8 defects.
Figure 7.
Tissue T3 content Mct8KO and Wt mice and its corresponding effect. A. T3 content and D1 enzymatic activity in liver. B. T3 content and D2 enzymatic activity in brain. Data from Mct8KO mice are represented as grey bars and those from Wt littermates are in open bars. ** p-value <0.01, *** p-value <0.001. S.A., specific activity. (Modified from Refetoff S, Dumitrescu AM. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab. 2007 Jun;21(2):277–305.)
Mct8 also has a role in TH efflux and excretion in kidney and secretion from the thyroid gland, respectively [103, 104]. The content of T4 and T3 in kidney is increased, producing an increase in D1 activity. In the thyroid, Mct8 is localized at the basolateral membrane of thyrocytes. Thyroidal T4 and T3 content is increased in Mct8KO mice, while the rate of their secretion and appearance in serum is reduced [104].
The increased D1 and D2 activities, stimulated by opposite states of intracellular TH availability, have an additive consumptive effect on T4 levels and together increase T3 Generation. The important role of D1 in maintaining a high serum T3 level has became evident by the normalization of serum T3 and rT3 in Mct8KO mice also made deficient in D1 [105]. The low serum T4 in Mct8 deficiency is not only the result of attrition trough deiodination but also caused by the reduced secretion from the thyroid gland and possibly increased renal loss.
Serum TSH is usually modestly increased in MCT8 deficient subjects, a finding compatible with the decrease in serum T4 concentration but not with the elevated serum T3 level. However, as MCT8 is expressed in the hypothalamus and pituitary, its inactivation likely interferes with the negative feedback of TH at both sites [106]. Hypothalamic TRH expression is markedly increased in Mct8KO mice, and high T3 doses are needed to suppress it, indicating T3 resistance at the hypothalamic level.
Mct8KO mice are useful for testing thyromimetic compounds with the potential of bypassing the Mct8 defect in tissues. The same dose of the TH analogue, diiodothyropropionic acid (DITPA), was found to be equally effective in the Mct8KO and WT animal to replace the TH requirements in animals rendered hypothyroid [107]. In contrast, 2.5 and 8-fold higher doses of L-T4 and L-T3, respectively, were necessary to produce a central effect in the Mct8KO compared to that in WT animal. These high doses of TH produced “hyperthyroidism” in peripheral tissues of the Mct8KO mice.
The absence of a clear neurological phenotype in Mct8KO mice limits their use in the understanding the mechanisms of the neurological manifestations in patients with MCT8 deficiency. If combined with deficiencies of other TH transporters in brain, Mct8 has the potential of producing an obvious neurological phenotype. This is being currently explored in several laboratories.
Mutant MCT8 molecules identified in humans and tested by transfection in heterologous systems, or in fibroblasts derived from affected individuals, show absent or greatly reduced ability to transport iodothyronines, primarily T3 [80]. However, the magnitude of serum T3 elevation does not correlate with the degree of T3 transport defect probably due to the important contribution of the concomitant perturbation in iodothyronine metabolism on the production of T3, as demonstrated in the Mct8KO mice. Similarly, there is no correlation between the magnitude of serum T3 elevation or rT3 reduction in affected males compared to corresponding values in their carrier mothers [Dumitrescu, A.M. and Refetoff, S, unpublished data]. Nevertheless, an imperfect correlation does exist between the degree of the MCT8 defect and clinical consequences. Less severely affected individuals, capable of some locomotion, have mutations with partial preservation of T3 transport function. In contrast, early death is more common in patients with mutations that completely disrupt the MCT8 molecule. However, it should be kept in mind that genetic factors, variability in tissue expression of MCT8, and other iodothyronine cell membrane transporters could be responsible for the lack of a stronger phenotype/genotype correlation. The possibility that MCT8 is involved in the transport of other ligands, or has functions other than TH transport, cannot be excluded.
Thyroid hormone metabolism defect (THMD)
Intracellular metabolism of TH
The proper intracellular hormone supply, dependent on cell types and the timing in development, is fine-tuned by intracellular TH metabolism, regulated by three selenoprotein iodothyronine deiodinases (Ds). The rare amino acid, selenocysteine (Sec), located in the center of these molecules, is required for their enzymatic activity. Their expression varies according to cell type and in response to alterations in the intracellular environment, regulated at the level of transcription, translation and metabolism [13]. D2 activity can change very rapidly being inactivated by T4 through ubiquitination, a reversible process that can regenerate active D2 enzyme through de-ubiquitination.
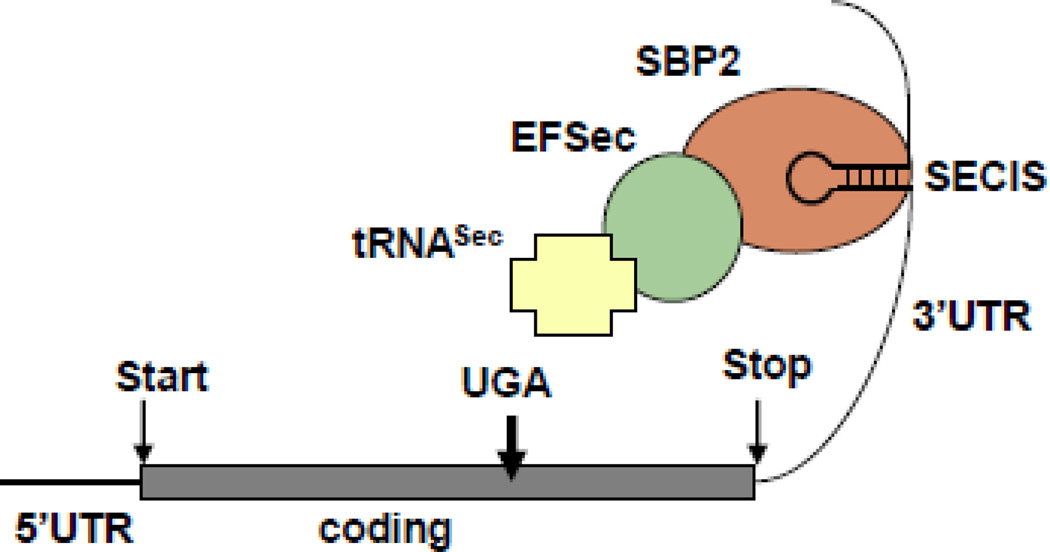
As other selenoproteins, deiodinases are synthesized through a unique mode of translation. Sec is encoded by UGA, a codon that in most circumstances serves as a signal to stop synthesis. The recoding of a specific UGA is determined by the presence of a selenocysteine insertion sequence (SECIS) in the 3’-untranslated region of the selenoprotein messenger RNA. A SECIS-binding protein 2 (in short SBP2) recognizes the SECIS and recruits multiple factors and the specific transfer RNA for the addition of Sec to the nascent protein chain (Fig. 8) [108].
Figure 8.
Schematic representation of the components involved in Sec incorporation that are central to the synthesis of selenoproteins. Elements present in the mRNA of selenoproteins are an in frame UGA codon and Sec incorporation sequence (SECIS) element, a stem loop structure located in the 3’UTR (untranslated region). SBP2 binds SECIS and recruits the Sec-specific elongation factor (EFSec) and Sec-specific tRNA (tRNASec) thus resulting in the recoding of the UGA codon and Sec incorporation.
Alterations of TH metabolism in man are typically acquired. The most frequent produces the “low T3 syndrome” of non-thyroidal illness [109]. The first inherited thyroid hormone metabolism defect was identified in 2005 [8]. It was caused by recessive mutations in SBP2 gene affecting selenoprotein synthesis, among which are the selenoenzymes deiodinases. Since this report a total of eight families were found to have mutations in the SBP2 gene. Affected individuals have delayed growth and characteristic thyroid tests abnormalities: high serum T4, low T3, high rT3 and normal or slightly elevated serum TSH. In addition they also have decreased serum selenium (Se) and selenoprotein levels or activities. The overall clinical phenotype is complex. The prominent findings in the 10 individuals so far identified are described in the following section.
Clinical features and course of the disorder
The probands of the first three families were brought to clinical attention because of growth delay [8, 110]. All were boys ranging in age from 6 to 14.5 years. Two young siblings, one male and one female, had the biochemical but not clinical abnormalities. The proband of a fourth family was a 12 years old girl with delayed bone maturation, congenital myopathy, impaired mental and motor coordination development, and bilateral sensorineural hearing loss [111]. In a fifth family, a male child, presented at age 2 years with failure to thrive as infant, followed by global developmental delay and short stature. Other features were eosinophilic colitis, fasting nonketotic hypoglycemia with low insulin levels, muscle weakness and mild bilateral high-frequency hearing loss [112]. A more recent report is that of 10 years old Japanese boy who at 3 months had short stature and failure to thrive. Through childhood he had delayed motor and intellectual development carrying the diagnosis of mild mental retardation and pervasive development disorder. The patient had rotary vertigo, recurrent exudative otitis media and bilateral mild conductive hearing loss [113]. We identified another patient (unpublished), an 11 years old Turkish girl born to non-consanguineous parents with mental and motor retardation, poor growth and abnormal thyroid function tests, typical of SBP2 deficiency.
Only one adult with SBP2 deficiency has been identified. He presented at age 35 years with primary infertility, skin photosensitivity, fatigue, muscle weakness, severe digital vasospasm, impaired hearing, and rotatory vertigo [112]. He had a history of delayed motor and speech development. Hearing problems persisted despite myringotomies for secretory otitis media at 6 years of age. He had difficulty walking and running in adolescence, with genu valgus and external rotation of the hip. At 13 years of age, marked sun photosensitivity was noted. Pubertal development was normal but, at the age of 15 years, he developed unilateral testicular torsion requiring orchiectomy and fixation of the remaining testis.
All affected subjects were found to have the characteristic serum thyroid test abnormalities but none had enlarged thyroid glands confirmed by ultrasound examinations. As most of the patients are young, the long-term evolution of this defect is unknown. While growth retardation is a common feature it is unknown whether adult stature would be impaired. Some of the patients have a complex phenotype involving multiple tissues and organs. It is conceivable that oxidative damage causing neoplasia, neurodegeneration, premature ageing may manifest with time.
Genetics
Incidence and inheritance
The incidence of THMD caused by SBP2 deficiency is unknown. Although a total of 8 families have been identified over the period of 6 years [8, 110–113] [Dumitrescu, A.M. and Refetoff, S, unpublished data], failure of detection is likely due to the relatively mild clinical symptoms in some subjects. The inheritance is autosomal recessive and males and females are equally affected. The ethnic origins of the reported patients are Bedouin from Saudi Arabia, African, Irish, Brazilian, English, Turkish and Japanese.
SBP2 gene and mutations
The human SBP2 gene, located on chromosome 9, encodes a protein of 854 amino acids widely expressed in tissues [114]. The C-terminal domain of the molecule is required for SECIS binding, ribosome binding and Sec incorporation [115]. Recent in vitro studies have shown a nuclear localization signal in the N-terminal part and a nuclear export signal in the C-terminal part, allowing SBP2 to shuttle between the nucleus and the cytoplasm [116]. The fourteen different mutations so far identified are shown in Table 1.
Table 1.
SBP2 gene mutations
| SBP2 gene | Protein | Comments on putative defect |
No of affected |
Defect | Ref |
|---|---|---|---|---|---|
| c.1619 G>A | R540Q | hypomorphic allele | 3 | homozygous | [8] |
| c.1312 A>T | K438X | missing C terminus | 1 | compund heterozygous | [8] |
| IVS8ds+29 G>A | fs | abnormal splicing | |||
| c.382 C>T | R128X | smaller isoforms* | 1 | homozygous | [110] |
| c.358 C>T | R120X | smaller isoforms* | 1 | compund heterozygous | [111] |
| c.2308 C>T | R770X | disrupted C-terminus | |||
| c.668delT | F223 fs 255X | truncation and smaller isorforms* | 1 | compund heterozygous | [112] |
| intron 6 –155 delC | fs | abnormal splicing, missing C-terminus | |||
| c.2071 T>C | C691R | increased proteasomal degradation | 1 | compund heterozygous | [112] |
| intronic SNP | fs | transcripts lacking exons 2–4, or 3–4 | |||
| c.1529_1541dup CCAGCGCCCCACT | M515 fs 563X | missing C terminus | 1 | compund heterozygous | [113] |
| c.235 C>T | Q79X | smaller isoforms* | |||
| c.2344 C>T | Q782X | missing C terminus | 1 | compund heterozygous | ** |
| c.2045–2048 delAACA | K682 fs 683X | missing C terminus |
generated from downstream ATGs;
Dumitrescu, A.M. and Refetoff, S, unpublished data
fs – frame shift
It is likely that defects in thyroid hormone metabolism caused by mutations in other genes will have different phenotypes. So far mutations in the deiodinase genes or in other proteins involved in selenoprotein synthesis have not been reported in humans.
Laboratory findings
Biochemical
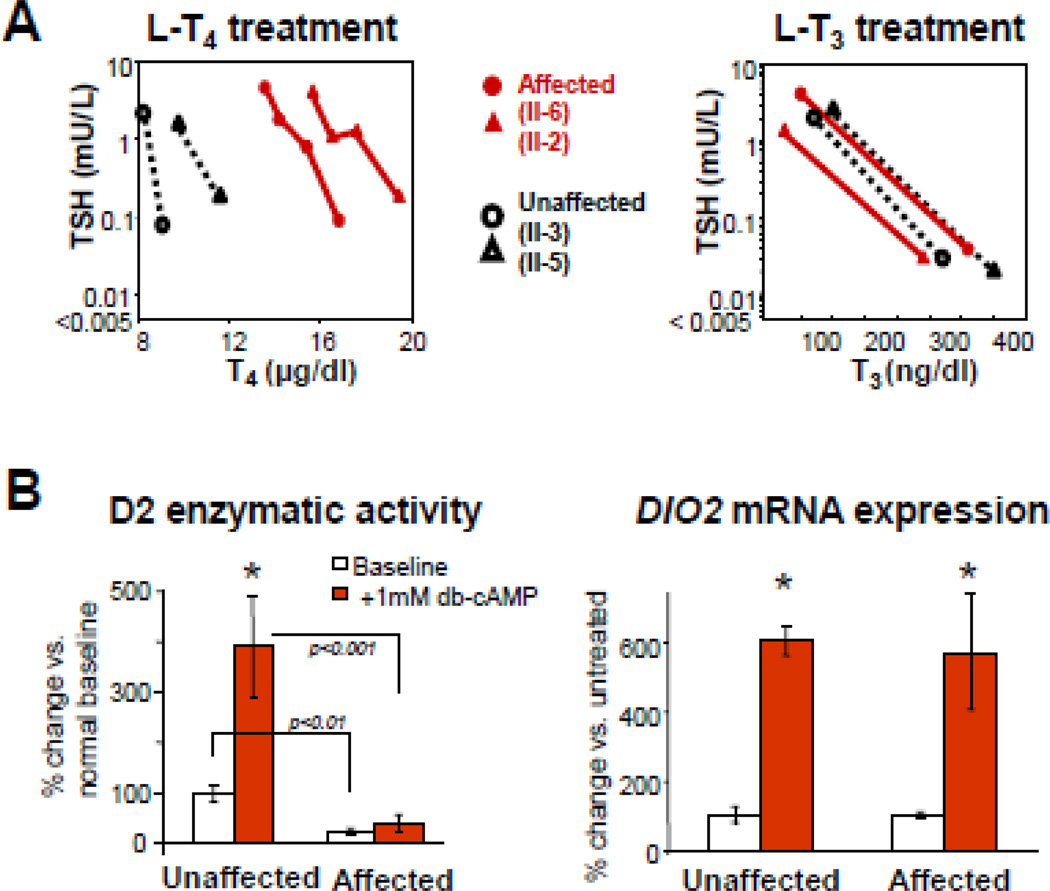
The characteristic thyroid tests abnormalities in subjects with SBP2 gene mutations are high total and free T4, low T3, high rT3 and slightly elevated serum TSH [117] (Fig. 6B). In vivo studies showed that affected children required higher amounts of T4, but not T3, to reduce their serum TSH levels (Fig. 9A).
Figure 9.
In-vivo and in-vitro studies in subjects with SBP2 deficiency. (A) In-vivo studies: Serum TSH and corresponding serum T4 and T3 levels, before and during the oral administration of incremental doses of L-T4 and L-T3. Note the higher concentrations of T4 required to reduce serum TSH in the affected subjects; (B) In-vitro studies of deiodinase 2 in cultured skin fbroblasts: Baseline and stimulated D2 activity is significantly lower in affected individuals. There is significant increase of DIO2 mRNA with dibutyryl cyclic adenosine monophosphate [(db)-cAMP), in both unaffected and affected (*p <0.001) while there are no significant differences in baseline (db)-cAMP stimulated DIO2 mRNA in affected versus the unaffected. (Modified from Refetoff S, Dumitrescu AM. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab. 2007 Jun;21(2):277–305.)
Cultured skin fibroblasts from affected individuals had reduced basal and cAMP-stimulated D2 enzymatic activity. However, basal and cAMP-stimulated D2 mRNA levels were not different than those in fibroblasts from normal individuals (See Fig. 9B).
Being epistatic to selenoprotein synthesis, SBP2 deficiency is expected to affect multiple selenoproteins. In fact, the concentrations of serum selenium, selenoprotein P are reduced, and skin fibroblasts have decreased D2 and glutathione peroxidase (Gpx) activities [117].
Detailed studies of three recent cases with severe SBP2 deficiency demonstrated multiple selenoproteins deficiencies [111, 112]: lack of testis-enriched selenoproteins producing a failure of the latter stages of spermatogenesis resulting in azoospermia; selenoprotein N (SEPN) like myopathy resulting in axial muscular dystrophy; cutaneous deficiencies of antioxidant selenoenzymes causing increased cellular reactive oxygen species (ROS); and reduced selenoproteins in peripheral blood cells resulting in immune deficits [112]. Deficiencies of other selenoproteins of unknown function, such as SELH, SELT, SELW, SELI, were found and their consequences are as yet unknown [112].
Imaging
T1-weighted MRI in three patients revealed connective tissue and fatty infiltration in the adductor muscle at the mid-thigh level [111–113]. Delayed bone age seems to be characteristic in this defect.
Mechanisms of the disorder
Investigations have established that the SBP2 gene mutations fully explain the observed abnormalities, as SBP2 is crucial for the synthesis of selenoproteins. Complete lack of SBP2 function is predicted to be lethal, as its immunodepletion eliminates Sec incorporation. The survival of reported patients with SBP2 deficiency is attributed to the preservation of partial SBP2 activity and to the hierarchy in selenoproteins synthesis.
The thyroid tests abnormalities found in all subjects with SBP2 deficiency are consistent with a defect in TH metabolism caused by deficiency in deiodinases. The mutant R540Q SBP2 behaves as a hypomorphic allele as shown in studies using the corresponding R531Q mutation of the rat Sbp2 [118]. The mutant molecule showed no binding to some but not all SECIS elements, resulting in selective loss in the expression of a subset of selenoproteins. In another family the compound heterozygous child expressed ~24% of a normal SBP2 transcripts. In the case of the homozygous R128X mutation, smaller SBP2 isoforms translated from downstream ATGs provided functional molecules containing the C-terminus domains.
Because the human selenoproteome comprises at least 25 selenoproteins [119, 120] it is not surprising that the phenotype of SBP2 deficiency is complex and has additional manifestations than the thyroid tests abnormalities. The more severe phenotype, recently reported in three families, is due to a more extensive impairment in SBP2 function [117]. In the patient with two nonsense mutations the R770X mutation truncates the C-terminal functional domain and likely abolishes SBP2 function, while the R120X allele likely generates smaller functionally active SBP2 isoforms [111]. However, the overall amount would be lesser than that of the homozygous R128X patient [110], thus explaining the more severe phenotype. Low expression of functional SBP2 is also at the basis of the phenotype of the two patients from the UK. Increased proteasomal degradation was demonstrated for the C691R mutation and Western blotting of skin fibroblasts from both probands showed lack of full length SBP2 protein expression [112]
There is no mouse model of a SBP2 defect or other components of the Sec incorporation machinery except for tRNASec [121]. A partial synthesis defect results in uneven deficiency in the different types of selenoproteins, reflecting the hierarchy in selenoprotein expression known to occur under conditions of selenium deprivation.
Homologous recombination has created mice deficient in each of the three deiodinases [122–124]. Dio1KO mice have elevated levels of T4 and rT3 while the concentrations of T3 and TSH are unimpaired. Dio2KO mice have significantly elevated serum T4 and normal T3 levels but, contrary to Dio1KO mice, TSH concentration is elevated. Dio2KO mice show also some growth retardation and defective auditory function [125]. Total deficiency in D3 is associated with partial embryonic and neonatal lethality. Surviving mice show severe growth retardation, impaired reproduction and central hypothyroidism [124]. Mice with combined D1 and D2 deficiency have high serum T4, and rT3, reminiscent of the phenotype in SBP2 deficient patients. However, different from the patients, their T3 is normal while TSH is markedly elevated. The putative, partial and uneven involvement of all three deiodinases in the thyroid phenotype of SBP2 defect, might explain the noted difference in the thyroid tests abnormalities.
Differential diagnosis
Common to the syndromes of reduced sensitivity to thyroid hormone is the non-suppressed TSH despite an increase in TH levels. Indeed, the combination of non-suppressed (normal or slightly elevated) serum TSH with increased concentrations of T4, T3 or both, is characteristic of the three syndromes of reduced sensitivity to TH (Table 2). This applies to a lesser degree to the single patient with TRα gene mutation. The ratio of T3 to rT3 is characteristically high in MCT8 deficiency while it is normal or low in RTH and low in other causes of abnormal T3 and rT3 levels, such as binding defects, iodine deficiency and non-thyroidal illness.
Table 2.
Comparison of TFT Abnormalities in Syndromes of RSTH
| Syndrome | FT4 | FT3 | rT3 | TSH | Common clinical manifestations |
Gene defect |
|---|---|---|---|---|---|---|
| RTH β* | ↑↑ | ↑ N | ↑↑ | N, sl ↑ | Attention deficit disorder, learning disability, tachycardia, goiter | TRβ |
| RTH α# | ↓ | N, sl ↑ | ↓ | N | Bradycardia, constipation, mental retardation, short lower limbs, delayed bone age | TRα |
| THCMTD** | ↓ | ↑↑ | ↓ | N, sl ↑ | Severe psychomotor impairment (no talk, no walk, poor head control) | MCT8 |
| THMD | ↑↑ | ↓ | ↑↑ | N, sl ↑ | Growth delay | SBP2 |
Includes nonTR-RTH
Males
based on three cases.
RSTH = reduced sensitivity to thyroid hormone
THCMTD = thyroid hormone cell membrane transport defect
THMD = thyroid hormone metabolism defect
sl, slight; ↑, increased; ↓, decreased; N, normal.
Exclusion of serum TH transport protein abnormalities by direct assessment or measurement of the free TH levels (preferably by equilibrium dialysis) is recommended before proceeding with further testing. The laboratory test abnormalities must be confirmed by repeated testing. Laboratory errors, transient changes in thyroid function tests and the consequence of a variety of non-thyroidal illnesses and drugs should be excluded. Other rare causes of increased TH concentration, such as the presence of iodothyronine antibodies should be considered when thyroglobulin and/or thyroperoxidase antibodies are positive. It should be noted that in MCT8 and TRα gene mutations serum T4 concentration is usually low, rather than high.
Although the clinical presentation of THCMTD involving other cell-membrane transporters than MCT8 is unknown, the latter always presents in males and is accompanied by psychomotor abnormalities, including truncal hypotonia, limb spasticity, poor head control, dyskinetic movements and absent or garbled speech. However, presence of the characteristic thyroid function test abnormalities is mandatory. Sequencing of the MCT8 gene in subjects with similar psychomotor manifestations but no characteristic thyroid tests abnormalities have yielded negative results.
Because the clinical presentations of RTH and defects of SBP2, are variable and non-specific, the differential diagnosis includes all possible causes of hyperthyroxinemia accompanied by non-suppressed TSH (Table 3). A typical feature of RTH and SBP2-linked THMD is high free T4 concentration. It should be noted that in mutant albumins with high affinity for T4, free T4 can be falsely elevated when measured by a direct method. A distinguishing feature of SBP2 defects is the low serum T3 and the absence of goiter. In RTH the possibility of a TSH producing pituitary adenoma should be considered, particularly when first presenting in an adult and when the parents have normal thyroid function tests or are not available for testing. Measurement of the α-subunit of pituitary glycoproteins should precede imaging studies.
Table 3.
Conditions Associated with Hyperthyroxinemia
| Thyroid Function Tests | ||||||
|---|---|---|---|---|---|---|
| Defect | T4 | T3 | rT3 | TSH | FT4 direct | FT4 dialysis |
| ↑TBG | ↑ | ↑ | ↑ | N | N | N |
| ↑TTR* | ↑ | N | ↑ | N | N | N |
| FDH | ↑ | ↑ or N | ↑ | N | ↑ | N |
| RTH β** | ↑ | ↑ or N | ↑ | sl↑ or N | ↑ | ↑ |
| SBP2 | ↑ | ↓ | ↑ | sl↑ or N | ↑ | ↑ |
| Acute NTI | ↑ | ↓↓ | ↑ | N | ↑ or N | N |
Refers to TTR with increased affinity for T4 and rT3
Includes nonTR-RTH
TBG, T4 binding globulin; TTR, transthyretin;
FDH, familial dysalbuminemic hyperthyroxinemia;
SBP2, selenocysteine insertion binding sequence protein;
NTI, nonthyroidal illness;
sl, slight; ↑, increased; ↓, decreased; N, normal.
Most rewarding and cost effective is to obtain thyroid function tests in first-degree relatives. In RTH, which usually is dominantly inherited, testing both parents may suffice. Identification of affected siblings and children can help in sorting out symptoms and signs that are unrelated to the condition under investigation. Mothers of males suspected of having MCT8 gene defect, should be also tested as they could present a mild thyroid phenotype.
Genetic testing can be sufficient to provide the diagnosis under the following circumstances: identification of a mutation in the candidate gene is pathognomonic if it results in a functionally defective protein or failure to synthesize the protein. Linkage analysis, if informative, can exclude the involvement of a specific gene. However, absence of a mutation does not rule out the suspected defect, particularly when dealing with mosaicism, nonTR-RTH or TH metabolism defect caused by a mutation in a gene other than SBP2. In such instances a biochemical diagnosis should be secured by measuring the responses to incremental doses of L-T4 and/or L-T3, as described in the respective section of Laboratory Tests.
Routine neonatal testing for hypothyroidism by measurement of blood TSH is inadequate of identifying affected subjects. However, measurement of blood T4 will detect RTH [31, 32], especially the more severe cases. Low T4 concentrations are commonly found at birth in MCT8 defects. Unfortunately low T4 values are not uncommon, and are more often associated with low levels of T4-binding protein and prematurity.
Treatment
Because the syndromes of reduced sensitivity to TH manifest in the same individual, at the same time, either cell dependent TH deficiency, sufficiency or excess, manipulation of the TH level cannot fully and specifically correct the defects. Furthermore, treatment depends on the timing of the diagnosis, age of the subject and the effect of prior treatment. It should be kept in mind that routine neonatal screening based on the measurement of TSH will rarely lead to the identification of the defects, though measurement of blood T4 would identify neonates with TRβ or non-TR RTH. On the other hand, in all syndromes of reduced sensitivity to TH, a prenatal diagnosis can be made in mothers known to carry a specific mutation by genotyping DNA obtained by chorionic villus sampling or amniocentesis. When contemplating treatment, most important is not to intervene with the sole purpose of normalizing the TH levels.
RTH
The common occurrence of sinus tachycardia can be controlled with the β-adrenergic blocking agent, atenolol. In many cases, this treatment also improves the hyperactivity. However, more severe attention deficit disorder may require treatment aimed at this condition. Fortunately, in most subjects with RTH, the partial tissue resistance to TH is reasonably well compensated by an increase in the endogenous supply of TH. This is not the case in patients with limited thyroidal reserve due to prior ablative therapy. In these patients, the serum TSH level can be used as a guideline for hormone dosage. Sometimes the compensation is incomplete and requires the judicious administration of supraphysiological doses of the hormone. This is particularly common in homozygotes for TRβ gene mutations. In such cases, attention must be paid to growth, bone maturation and mental development in children. It is suggested that TH be given in incremental doses and that the basal metabolic rate, nitrogen balance and serum SHBG be monitored at each dose, and bone age and growth on a longer term. Development of a catabolic state is an indication of overtreatment.
Even more rarely, infants may present with failure to thrive accompanied by hypermetabolism and severe tachycardia not controlled by beta-blockers. This has been observed with frameshift mutations producing a TRβ with an extended nonsense carboxyl-terminal sequence [126]. In such instances, temporary reduction of the TH level with somatostatin, and if ineffective, the judicious administration of antithyroid drugs could be tried. Among the TH analogues used to alleviate symptoms of apparent TH excess [127], triiodothyroacetic acid (TRIAC) has had the widest use [128, 129]. The combination of greater affinity for TRIAC than T3 of some mut TRβs [130] and its short half-life, produce greater effect centrally than on peripheral tissues. This reduces TSH and TH secretion with apparent amelioration of hypermetabolism. The value of treatment with D-T4 is questionable.
Prenatal diagnosis and counseling is particularly important in families whose affected members show evidence of growth or mental retardation. In addition, women with RTH, that carry unaffected fetuses, have increased risk of abortion and to give birth to infants with low weight for gestational age [131]. This effect, caused by the excess of maternal TH reaching the normal fetus, may be averted by not allowing the maternal free T4 to rise more than 20% above the upper limit of normal [Dumitrescu, A.M. and Refetoff, S, unpublished data]. The wisdom of in-utero treatment is questionable.
The indications for treatment of RTH in infancy have not been established. This is often an issue when the diagnosis is known or made at birth or in early infancy. Any signs of hypothyroidism, other than serum TSH elevation, such as retarded bone development and failure to thrive deserve consideration for treatment with TH. Though experience is lacking, treatment with high doses of TH may be indicated in homozygotes for TRβ gene mutations that often manifest stigmata indicative of hormone deficiency. The outcome of affected older members of the family who did not receive treatment may serve as a guideline. Longer follow-up and psychological testing of infants who have been given treatment will determine the efficacy of early intervention.
Reducing goiter size, without causing side effects, can be achieved with supraphysiological doses of L-T3, given as a single dose every other day [132]. Such treatment is preferable as postoperative recurrence of goiter is the rule. The L-T3 dose must be adjusted until TSH and thyroglobulin are suppressed.
MCT8 defects
Treatment options for patients with MCT8 gene mutations are currently limited. Supportive measures include the use of braces to prevent mal-position contractures that could lead to orthopedic surgery. Aspiration should be prevented by diet adjustment. Dystonia might be improved with medications such as anticholinergics, L-DOPA, carbamazepine and lioresol. Drooling might be reduced with glycopyrolate or scopolamine. Seizures should be treated with standard anticonvulsants. When refractory, ketogenic diet has been successful as well as administration of supraphysiologic doses of L-T4. Experience with such treatments is, however, limited to only a few cases.
Early treatment with L-T4 doses used for generalized hypothyroidism was not beneficial, presumably because of the impaired uptake of the hormone in MCT8-dependent tissues. Treatment with supraphysiological doses of L-T4 to increase the availability of TH to the brain will aggravate the hypermetabolism caused by an excess of D1 generated T3 on peripheral tissues. Therefore, high L-T4 dose treatment has been used in combination with propylthiouracil (PTU), which is a specific inhibitor of D1. Benefit was limited to reduction of the hypermetabolism and weight loss, without improvement of the neuropsychomotor deficit [87] [Dumitrescu, A.M. and Refetoff, S, unpublished data]. It is unknown what effect such treatment will have if initiated at birth.
The use of thyromimetic drugs that do not require MCT8 to enter tissues are currently under investigation. One such TH analogue, DITPA, is effectively transported into mouse brain in the absence of Mct8 [107]. Preliminary results show normalization of the thyroid tests and possible improvement in the nutritional status but no objective change in the neuropsychiatric deficit [Dumitrescu, A.M. and Refetoff, S, unpublished data]. TH metabolites, such as TRIAC and its precursor TETRAC (tetraiodothyroacetic acid) are being tested. It is possible that for any TH mediated treatment to be effective on brain development, that it will have to be initiated at, or before birth.
SBP2
Although all subjects with SBP2 gene defects have deficiency in selenoprotein synthesis, in some cases the phenotype is mild, owing to the fact that the deficiency is not complete. The common clinical abnormality of growth retardation, due to reduced generation of T3, can be corrected by the administration of L-T3 [110]. Although administration of selenium, in the form of selenomethionine normalized the serum selenium concentration it did not increase the selenoprotein P levels nor restored the TH metabolism dysfunction [133].
More severe consequences caused by the defect require supportive treatment. The benefit of treatment with anti-oxidants has not been established.
Conclusions
Syndromes of reduced sensitivity to thyroid hormone are more common than formerly suspected. The coexistence of cell-specific TH deprivation and excess is characteristic of these syndromes.
RTH
Goiter, attention deficit hyperactivity disorder and tachycardia are the most common reasons that lead to the testing and ultimately, the diagnosis of RTH. Genetic analysis of subjects suspected of having RTH provides a short cut to diagnosis. Failure to identify a TRβ gene mutation in genomic DNA from circulating mononuclear cells of subjects presenting the RTH phenotype could be due to mosaicism in a de-novo mutation or to a yet unidentified etiology of the syndrome (nonTR RTH). Ablative treatment in RTH complicates the follow up and outcome, as adjustment of TH replacement is not easy.
THCMTD
The neurological manifestations of MCT8 deficiency cannot be explained by the thyroid serum tests abnormalities observed in childhood and adult life. The phenotype is different than that of global TH deficiency or excess. Psychomotor abnormalities without characteristic serum thyroid tests abnormalities are unlikely to be caused by a MCT8 defect. As carrier females are asymptomatic, the presence of a MCT8 defect is not suspected until the birth of the first affected male. Aspiration pneumonia is the most common cause of death in affected males. Treatment with physiological doses of L-T4 has not corrected the phenotype in several patients.
THMD
The phenotype of SBP2 deficiency is variable and depends on the severity of functional impairment of the mutant protein. Thyroid tests abnormalities are always present, and delayed growth and puberty are seen even in the more mild cases. Treatment with physiological doses of L-T3 may accelerate growth and maturation but does not correct other manifestations of the defect.
Highlights.
-
-
Defects of TH action manifest as tissue and cell specific TH deficiency and excess
-
-
The syndrome of resistance to TH has helped elucidate the mechanism of TH action
-
-
Neurological deficits of MCT8 defects are more severe than in congenital hypothyroidism
-
-
SBP2 defects reduce selenoprotein synthesis and alter intracellular TH metabolism
Acknowledgements
Supported in part by grants DK15070, DK205955, RR04999 and DK091016, from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alexandra M. Dumitrescu, Department of Medicine, The University of Chicago, Chicago, Illinois, USA.
Samuel Refetoff, Departments of Medicine, Paediatrics and Genetics, The University of Chicago, Chicago, Illinois, USA.
References
- 1.Refetoff S, DeWind LT, DeGroot LJ. Familial syndrome combining deaf-mutism, stippled epiphyses, goiter, and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab. 1967;27:279–294. doi: 10.1210/jcem-27-2-279. [DOI] [PubMed] [Google Scholar]
- 2.Refetoff S, DeGroot LJ, Benard B, DeWind LT. Studies of a sibship with apparent hereditary resistance to the intracellular action of thyroid hormone. Metabolism. 1972;21:723–756. doi: 10.1016/0026-0495(72)90121-7. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986;324:641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- 4.Sap J, Munoz A, Schmitt J, Stunnenberg H, Vennström B. Repression of transcription mediated at thyroid hormone response element by the v-erbA oncogene product. Nature. 1989;340:242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai A, Takeda K, Ain K, Ceccarelli P, Nakai A, Seino S, Bell GI, Refetoff S, DeGroot LJ. Generalized resistance to thyroid hormone associated with a mutation in the ligand-binding domain of the human thyroid hormone receptor b. Procedings of the National Academy of Sciences (USA) 1989;86:8977–8981. doi: 10.1073/pnas.86.22.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A Novel Syndrome Combining Thyroid and Neurological Abnormalities Is Associated with Mutations in a Monocarboxylate Transporter Gene. Am J Hum Genet. 2004;74:168–175. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet (The) 2004;364:1435–1437. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- 8.Dumitrescu AM, Liao X-H, Abdullah SYM, Lado-Abeal J, Abdul-Majed F, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet. 2005;37:1247–1252. doi: 10.1038/ng1654. [DOI] [PubMed] [Google Scholar]
- 9.Refetoff S. Resistance to thyroid hormone: one of several defects causing reduced sensitivity to thyroid hormone. Nat Clin Pract Endocrinol Metab. 2008;4:1. doi: 10.1038/ncpendmet0703. [DOI] [PubMed] [Google Scholar]
- 10.Bochukova E, Schoenmakers N, Agostini M, Schoenmakers E, Rajanayagam O, Keogh JM, Henning E, Reinemund J, Gevers E, Sarri M, Downes K, Offiah A, Albanese A, Halsall D, Schwabe JWR, Bain M, Lindley K, Muntoni F, Vargha Khadem F, Dattani M, Farooqi IS, Gurnell M, Chatterjee K. A Mutation in the Thyroid Hormone Receptor Alpha Gene. N Engl J Med. 2012 doi: 10.1056/NEJMoa1110296. [DOI] [PubMed] [Google Scholar]
- 11.van Mullem A, van Heerebeek R, Chrysis D, Visser E, Medici M, Andrikoula M, Tsatsoulis A, Peeters R, Visser TJ. Clinical phenotype and mutant TRalpha1. N Engl J Med. 2012;366:1451–1453. doi: 10.1056/NEJMc1113940. [DOI] [PubMed] [Google Scholar]
- 12.Friesema EC, Jansen J, Milici C, Visser TJ. Thyroid hormone transporters. Vitam Horm. 2005;70:137–167. doi: 10.1016/S0083-6729(05)70005-4. [DOI] [PubMed] [Google Scholar]
- 13.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocrine Reviews. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 14.Bassett JH, Harvey CB, Williams GR. Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol Cell Endocrinol. 2003;213:1–11. doi: 10.1016/j.mce.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Lazar MA. The mechanism of action of thyroid hormones. Annu Rev Physiol. 2000;62:439–466. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- 16.Yen PM, Ando S, Feng X, Liu Y, Maruvada P, Xia X. Thyroid hormone action at the cellular, genomic and target gene levels. Mol Cell Endocrinol. 2006;246:121–127. doi: 10.1016/j.mce.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14:348–399. doi: 10.1210/edrv-14-3-348. [DOI] [PubMed] [Google Scholar]
- 18.Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. Tissue specific thyroid hormone deprivation and excess in Mct8 deficient mice. Endocrinology. 2006:4036–4043. doi: 10.1210/en.2006-0390. [DOI] [PubMed] [Google Scholar]
- 19.Fondell JD, Guermah M, Malik S, Roeder RG. Thyroid hormone receptor-associated proteins and general positive cofactors mediate thyroid hormone receptor function in the absence of the TATA box-binding protein-associated factors of TFIID. Proc, Natl. Acad. Sci. USA. 1999;96:1959–1964. doi: 10.1073/pnas.96.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weintraub BD, Gershengorn MC, Kourides IA, Fein H. Inappropriate secretion of thyroid stimulating hormone. Annals of Internal Medicine. 1981;95:339–351. doi: 10.7326/0003-4819-95-3-339. [DOI] [PubMed] [Google Scholar]
- 21.Beck-Peccoz P, Chatterjee VKK. The variable clinical phenotype in thyroid hormone resistance syndrome. Thyroid. 1994;4:225–232. doi: 10.1089/thy.1994.4.225. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan MM, Swartz SL, Larsen PR. Partial peripheral resistance to thyroid hormone. American Journal of Medicine. 1981;70:1115–1121. doi: 10.1016/0002-9343(81)90885-8. [DOI] [PubMed] [Google Scholar]
- 23.Sadow P, Reutrakul S, Weiss RE, Refetoff S. Resistnace to thyroid hormone in the absence of mutations in the thyroid hormone receptor genes. Curr. Opin. Endocrinol. Diabetes. 2000;7:253–259. [Google Scholar]
- 24.Weiss RE, Hayashi Y, Nagaya T, Petty KJ, Murata Y, Tunka H, Seo H, Refetoff S. Dominant inheritance of resistance to thyroid hormone not linked to defects in the thyroid hormone receptors a or β genes may be due to a defective co-factor. Journal of Clinical Endocrinology and Metabolism (The) 1996;81:4196–4203. doi: 10.1210/jcem.81.12.8954015. [DOI] [PubMed] [Google Scholar]
- 25.Ono S, Schwartz ID, Mueller OT, Root AW, Usala SJ, Bercu BB. Homozygosity for a "dominant negative" thyroid hormone receptor gene responsible for generalized resistance to thyroid hormone. Journal of Clinical Endocrinology and Metabolism (The) 1991;73:990–994. doi: 10.1210/jcem-73-5-990. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara AM, Onigata K, Ercan O, Woodhead H, Weiss RE, Refetoff S. Homozygous Thyroid Hormone Receptor beta-Gene Mutations in Resistance to Thyroid Hormone: Three New Cases and Review of the Literature. J Clin Endocrinol Metab. 2012;97:1328–1336. doi: 10.1210/jc.2011-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hauser P, Zametkin AJ, Martinez P, Vitiello B, Matochik JA, Mixson AJ, Weintraub BD. Attention deficit-hyperactivity disorder in people with generalized resistance to thyroid hormone. New England Journal of Medicine (The) 1993;328:997–1001. doi: 10.1056/NEJM199304083281403. [DOI] [PubMed] [Google Scholar]
- 28.Weiss RE, Stein MA, Trommer B, Refetoff S. Attention-deficit hyperactivity disorder and thyroid function. J. Pediatr. 1993;123:539–545. doi: 10.1016/s0022-3476(05)80947-3. [DOI] [PubMed] [Google Scholar]
- 29.Elia J, Gulotta C, Rose SR, Martin G, Rapoport J. Thyroid function and attention-deficit hyperactivity disorder. Journal of the American Academy of Child Adolescent Psychiatry. 1994;33:169–172. doi: 10.1097/00004583-199402000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Brucker-Davis F, Skarulis MC, Grace MB, Benichou J, Hauser P, Wiggs E, Weintraub BD. Genetic and clinical features of 42 kindreds with resistance to thyroid hormone. The National Institutes of Health prospective study. Annals of Internal Medicine. 1995;123:573–583. doi: 10.7326/0003-4819-123-8-199510150-00002. [DOI] [PubMed] [Google Scholar]
- 31.LaFranchi SH, Snyder DB, Sesser DE, Skeels MR, Singh N, Brent GA, Nelson JC. Follow-up of newborns with elevated screening T4 concentrations. J. Pediatr. 2003;143:296–301. doi: 10.1067/S0022-3476(03)00184-7. [DOI] [PubMed] [Google Scholar]
- 32.Tajima T, Jo W, Fujikura K, Fukushi M, Fujieda K. Elevated free thyroxine levels detected by a neonatal screening system. Pediatr Res. 2009;66:312–316. doi: 10.1203/PDR.0b013e3181b1bcbd. [DOI] [PubMed] [Google Scholar]
- 33.Takeda K, Sakurai A, DeGroot LJ, Refetoff S. Recessive inheritance of thyroid hormone resistance caused by complete deletion of the protein-coding region of the thyroid hormone receptor-β gene. Journal of Clinical Endocrinology and Metabolism (The) 1992;74:49–55. doi: 10.1210/jcem.74.1.1727829. [DOI] [PubMed] [Google Scholar]
- 34.Mitsuhashi T, Tennyson GE, Nikodem VM. Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormones. Proc. Natl. Acad. Sci. USA. 1988;85:5804–5805. doi: 10.1073/pnas.85.16.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macchia PE, Takeuchi Y, Kawai T, Cua K, Gauthier K, Chassande O, Seo H, Hayashi Y, Samarut J, Murata Y, Weiss RE, Refetoff S. Increased sensitivity to thyroid hormone in mice with complete deficiency of thyroid hormone receptor alpha. Procedings of the National Academy of Sciences (USA) 2001;98:349–354. doi: 10.1073/pnas.011306998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gauthier K, Chassande O, Platerotti M, Roux J-P, Legrand C, Rousset B, Weiss R, Trouillas J, Samarut J. Different functions for the thyroid hormone receptors TRa and TRβ in the control of thyroid hormone production and post-natal development. EMBO Journal (European Molecular Biology Organization) 1999;18:623–631. doi: 10.1093/emboj/18.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Göthe S, Wang Z, Ng L, Kindblom JM, Campos barros A, Ohlsson C, Vennström B, Forrest D. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes & Dev. 1999;13:1329–1341. doi: 10.1101/gad.13.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss RE, Weinberg M, Refetoff S. Identical mutations in unrelated families with generalized resistance to thyroid hormone occur in cytosine-guanine-rich areas of the thyroid hormone receptor beta gene: Analysis of 15 families. J Clin Invest. 1993;91:2408–2415. doi: 10.1172/JCI116474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams M, Matthews C, Collingwood TN, Tone Y, Beck-Peccoz P, Chatterjee KK. Genetic analysis of 29 kindreds with generalized and pituitary resistance to thyroid hormone: identification of thirteen novel mutations in the thyroid hormone receptor β gene. Journal of Clinical Investigation (The) 1994;94:506–515. doi: 10.1172/JCI117362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collingwood TN, Wagner R, Matthews CH, Clifton-Bligh RJ, Mark G, Rajanayagam O, Agostini M, Fletterick RJ, Beck-Peccoz P, Reinhardt W, Binder G, Ranke MB, Hermus A, Hesch RD, Lazarus J, Paul N, Parfitt V, Ragatt P, de Zegher F, Chatterjee VKK. A role for helix 3 of the TRβ ligand-binding domain in coactivator recruitment identified by characterization of a third cluster of mutations in resistance to thyroid hormone. EMBO Journal (European Molecular Biology Organization) 1998;17:4760–4770. doi: 10.1093/emboj/17.16.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayashi Y, Weiss RE, Sarne DH, Yen PM, Sunthornthepvarakul T, Marcocci C, Chin WW, Refetoff S. Do clinical manifestations of resistance to thyroid hormone correlate with the functional alteration of the corresponding mutant thyroid hormone-β receptors? Journal of Clinical Endocrinology and Metabolism (The) 1995;80:3246–3256. doi: 10.1210/jcem.80.11.7593433. [DOI] [PubMed] [Google Scholar]
- 42.Yoh SM, Chatterjee VKK, Privalsky ML. Thyroid hormone resistance syndrome manifests as an aberrant interaction between mutant T3 receptor and transcriptional corepressor. Molecular Endocrinology. 1997;11:470–480. doi: 10.1210/mend.11.4.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottlieb B, Lehvaslaiho H, Beitel LK, Lumbroso R, Pinsky L, Trifiro M. The Androgen Receptor Gene Mutations Database. Nucleic. Acids. Res. 1998;26:234–238. doi: 10.1093/nar/26.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ando S, Sarlis NJ, Krishan J, Feng X, Refetoff S, Zhang MQ, Olfield EH, Yen PM. Aberrant alternative splicing of thyroid hormone receptor in a TSH-secreting pituitary tumor is a mechanism for hormone resistance. Molecular Endocrinology. 2001;15:1529–1538. doi: 10.1210/mend.15.9.0687. [DOI] [PubMed] [Google Scholar]
- 45.Ando S, Sarlis NJ, Oldfield EH, Yen PM. Somatic mutation of TRbeta can cause a defect in negative regulation of TSH in a TSH-secreting pituitary tumor. Journal of Clinical Endocrinology and Metabolism (The) 2001;86:5572–5576. doi: 10.1210/jcem.86.11.7984. [DOI] [PubMed] [Google Scholar]
- 46.Persani L, Asteria C, Tonacchera M, Vitti P, Chatterjee VKK, Beck-Peccoz P. Evidence for secretion of thyrotropin with enhanced bioactivity in syndromes of thyroid hormone resistance. Journal of Clinical Endocrinology and Metabolism (The) 1994;78:1034–1039. doi: 10.1210/jcem.78.5.8175956. [DOI] [PubMed] [Google Scholar]
- 47.Barkoff MS, Kocherginsky M, Anselmo J, Weiss RE, Refetoff S. Autoimmunity in Patients with Resistance to Thyroid Hormone. J Clin Endocrinol Metab. 2010;95:3189–3193. doi: 10.1210/jc.2009-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayashi Y, Janssen OE, Weiss RE, Murata Y, Seo H, Refetoff S. The relative expression of mutant and normal thyroid hormone receptor genes in patients with generalized resistance to thyroid hormone determined by estimation of their specific messenger ribonucleic acid products. Journal of Clinical Endocrinology and Metabolism (The) 1993;76:64–69. doi: 10.1210/jcem.76.1.8421105. [DOI] [PubMed] [Google Scholar]
- 49.Sakurai A, Miyamoto T, Refetoff S, DeGroot LJ. Dominant negative transcriptional regulation by a mutant thyroid hormone receptor β in a family with generalized resistance to thyroid hormone. Molecular Endocrinology. 1990;4:1988–1994. doi: 10.1210/mend-4-12-1988. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi Y, Sunthornthepvarakul T, Refetoff S. Mutations of CpG dinucleotides located in the triiodothyronine (T3)-binding domain of the thyroid hormone receptor (TR) β gene that appears to be devoid of natural mutations may not be detected because they are unlikely to produce the clinical phenotype of resistance to thyroid hormone. Journal of Clinical Investigation (The) 1994;94:607–615. doi: 10.1172/JCI117376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones I, Srinivas M, Ng L, Forrest D. The thyroid hormone receptor beta gene: structure and functions in the brain and sensory systems. Thyroid. 2003;13:1057–1068. doi: 10.1089/105072503770867228. [DOI] [PubMed] [Google Scholar]
- 52.Yagi H, Pohlenz J, Hayashi Y, Sakurai A, Refetoff S. Resistance to thyroid hormone caused by two mutant thyroid hormone receptor β, R243Q and R243W, with marked impairment of function that cannot be explained by altered in-vitro 3,5,3'-triiodothyronine binding affinity. J Clin Endocrinol Metab. 1997;82:1608–1614. doi: 10.1210/jcem.82.5.3945. [DOI] [PubMed] [Google Scholar]
- 53.Safer JD, Cohen RN, Hollenberg AN, Wondisford FE. Defective release of corepressor by hinge mutants of the thyroid hormone receptor found in patients with resistance to thyroid hormone. J Biol Chem. 1998;273:30175–30182. doi: 10.1074/jbc.273.46.30175. [DOI] [PubMed] [Google Scholar]
- 54.Clifton-Bligh RJ, de Zegher F, Wagner RL, Collingwood TN, François I, Van Helvoirt M, Fletterick RJ, Chatterjee VKK. A novel mutation (R383H) in resistance to thyroid hormone syndrome predominantly impairs corepressor release and negative transcriptional regulation. Molecular Endocrinology. 1998;12:609–621. doi: 10.1210/mend.12.5.0113. [DOI] [PubMed] [Google Scholar]
- 55.Lee S, Young BM, Wan W, Chan IH, Privalsky ML. A Mechanism for Pituitary-Resistance to Thyroid Hormone (PRTH) Syndrome: a Loss in Cooperative Coactivator Contacts by Thyroid Hormone Receptor (TR){beta}2. Mol Endocrinol. 2011;25:1111–1125. doi: 10.1210/me.2010-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Machado DS, Sabet A, Santiago LA, Sidhaye AR, Chiamolera MI, Ortiga-Carvalho TM, Wondisford FE. A thyroid hormone receptor mutation that dissociates thyroid hormone regulation of gene expression in vivo. Proc Natl Acad Sci U S A. 2009;106:9441–9446. doi: 10.1073/pnas.0903227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiss RE, Marcocci C, Bruno-Bossio G, Refetoff S. Multiple genetic factors in the heterogeneity of thyroid hormone resistance. Journal of Clinical Endocrinology and Metabolism (The) 1993;76:257–259. doi: 10.1210/jcem.76.1.8421095. [DOI] [PubMed] [Google Scholar]
- 58.Mixson AJ, Hauser P, Tennyson G, Renault JC, Bodenner DL, Weintraub BD. Differential expression of mutant and normal beta T3 receptor alleles in kindreds with generalized resistance to thyroid hormone. Journal of Clinical Investigation (The) 1993;91:2296–2300. doi: 10.1172/JCI116458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flamant F, Samarut J. Thyroid hormone receptors: lessons from knockout and knock-in mutant mice. Trends Endocrinol Metab. 2003;14:85–90. doi: 10.1016/s1043-2760(02)00043-7. [DOI] [PubMed] [Google Scholar]
- 60.Jones I, Ng L, Liu H, Forrest D. An intron control region differentially regulates expression of thyroid hormone receptor beta2 in the cochlea, pituitary, and cone photoreceptors. Mol Endocrinol. 2007;21:1108–1119. doi: 10.1210/me.2007-0037. [DOI] [PubMed] [Google Scholar]
- 61.Abel ED, Boers ME, Pazos-Moura C, Moura E, Kaulbach H, Zakaria M, Lowell B, Radovick S, Liberman MC, Wondisford F. Divergent roles for thyroid hormone receptor beta isoforms in the endocrine axis and auditory system. Journal of Clinical Investigation (The) 1999;104:291–300. doi: 10.1172/JCI6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiss RE, Murata Y, Cua K, Hayashi Y, Seo H, Refetoff S. Thyroid hormone action on liver, heart and energy expenditure in thyroid hormone receptor β deficient mice. (Erratum, 141:4767, 2000) Endocrinology. 1998;139:4945–4952. doi: 10.1210/endo.139.12.6412. [DOI] [PubMed] [Google Scholar]
- 63.Wikstrom L, Johansson C, Salto C, Barlow C, Campos Barros A, Baas F, Forrest D, Thoren P, Vennstrom B. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor alpha 1. EMBO J. 1998;17:455–461. doi: 10.1093/emboj/17.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki H, Willingham MC, Cheng SY. Mice with a mutation in the thyroid hormone receptor Beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002;12:963–969. doi: 10.1089/105072502320908295. [DOI] [PubMed] [Google Scholar]
- 65.Santiago LA, Santiago DA, Faustino LC, Cordeiro A, Lisboa PC, Wondisford FE, Pazos-Moura CC, Ortiga-Carvalho TM. The Delta337T mutation on the TRbeta causes alterations in growth, adiposity, and hepatic glucose homeostasis in mice. J Endocrinol. 2011;211:39–46. doi: 10.1530/JOE-11-0194. [DOI] [PubMed] [Google Scholar]
- 66.Kaneshige M, Suzuki H, Kaneshige K, Cheng J, Wimbrow H, Barlow C, Willingham MC, Cheng S-y. A targeted dominant negative mutation of the thyroid hormone alpha 1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc Natl Acad Sci (USA) 2001;98:15095–15100. doi: 10.1073/pnas.261565798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tinnikov A, Nordstrom K, Thoren P, Kindblom JM, Malin S, Rozell B, Adams M, Rajanayagam O, Pettersson S, Ohlsson C, Chatterjee K, Vennstrom B. Retardation of post-natal development caused by a negatively acting thyroid hormone receptor alpha1. Embo. J. 2002;21:5079–5087. doi: 10.1093/emboj/cdf523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu YY, Schultz JJ, Brent GA. A thyroid hormone receptor alpha gene mutation (P398H) Is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J. Biol. Chem. 2003;278:38913–38920. doi: 10.1074/jbc.M306120200. [DOI] [PubMed] [Google Scholar]
- 69.Flamant F, Poguet AL, Plateroti M, Chassande O, Gauthier K, Streichenberger N, Mansouri A, Samarut J. Congenital Hypothyroid Pax8(−/−) Mutant Mice Can Be Rescued by Inactivating the TRalpha Gene. Molecular Endocrinology. 2002;16:24–32. doi: 10.1210/mend.16.1.0766. [DOI] [PubMed] [Google Scholar]
- 70.Mittag J, Friedrichsen S, Heuer H, Polsfuss S, Visser TJ, Bauer K. Athyroid Pax8−/− mice cannot be rescued by the inactivation of thyroid hormone receptor alpha1. Endocrinology. 2005;146:3179–3184. doi: 10.1210/en.2005-0114. [DOI] [PubMed] [Google Scholar]
- 71.Morte B, Manzano J, Scanlan T, Vennstrom B, Bernal J. Deletion of the thyroid hormone receptor alpha 1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc Natl Acad Sci (USA) 2002;99:3985–3989. doi: 10.1073/pnas.062413299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weiss RE, Xu J, Ning G, Pohlenz J, O'Malley BW, Refetoff S. Mice deficient in the steroid receptor coactivator-1 (SRC-1) are resistant to thyroid hormone. EMBO Journal (European Molecular Biology Organization) 1999;18:1900–1904. doi: 10.1093/emboj/18.7.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brown NS, Smart A, Sharma V, Brinkmeier ML, Greenlee L, Camper SA, Jensen DR, Eckel RoH, Krezel W, Chambon P, Haugen BR. Thyroid hormone resistance and increased metabolic rate in the RXR-g-deficient mouse. Journal of Clinical Investigation (The) 2000;106:73–79. doi: 10.1172/JCI9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ekins R. The free hormone hypothesis and measurement of free hormones. Clinical Chemistry. 1992;38:1289–1293. [PubMed] [Google Scholar]
- 75.Hennemann G, Docter R, Friesema EC, de Jong M, Krenning EP, Visser TJ. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocrine Reviews. 2001;22:451–476. doi: 10.1210/edrv.22.4.0435. [DOI] [PubMed] [Google Scholar]
- 76.Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. Journal of Biological Chemistry. 2003;278:40128–40135. doi: 10.1074/jbc.M300909200. [DOI] [PubMed] [Google Scholar]
- 77.Holden KR, Zuniga OF, May MM, Su H, Molinero MR, Rogers RC, Schwartz CE. X-linked MCT8 gene mutations: characterization of the pediatric neurologic phenotype. J Child Neurol. 2005;20:852–857. doi: 10.1177/08830738050200101601. [DOI] [PubMed] [Google Scholar]
- 78.Schwartz CE, May MM, Carpenter NJ, Rogers RC, Martin J, Bialer MG, Ward J, Sanabria J, Marsa S, Lewis JA, Echeverri R, Lubs HA, Voeller K, Simensen RJ, Stevenson RE. Allan-Herndon-Dudley Syndrome and the Monocarboxylate Transporter 8 (MCT8) Gene. Am J Hum Genet. 2005;77:41–53. doi: 10.1086/431313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Visser WE, Jansen J, Friesema EC, Kester MH, Mancilla E, Lundgren J, van der Knaap MS, Lunsing RJ, Brouwer OF, Visser TJ. Novel pathogenic mechanism suggested by ex vivo analysis of MCT8 (SLC16A2) mutations. Hum Mutat. 2008;30:29–38. doi: 10.1002/humu.20808. [DOI] [PubMed] [Google Scholar]
- 80.Friesema EC, Visser WE, Visser TJ. Genetics and phenomics of thyroid hormone transport by MCT8. Mol Cell Endocrinol. 2010;322:107–113. doi: 10.1016/j.mce.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 81.Brockmann K, Dumitrescu AM, Best TT, Hanefeld F, Refetoff S. X-linked paroxysmal dyskinesia and severe global retardation caused by defective MCT8 gene. J Neurol. 2005;252:663–666. doi: 10.1007/s00415-005-0713-3. [DOI] [PubMed] [Google Scholar]
- 82.Boccone L, Mariotti S, Dessi V, Pruna D, Meloni A, Loudianos G. Allan-Herndon-Dudley syndrome (AHDS) caused by a novel SLC16A2 gene mutation showing severe neurologic features and unexpectedly low TRH-stimulated serum TSH. Eur J Med Genet. 2010;53:392–395. doi: 10.1016/j.ejmg.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 83.Visser WE, Friesema EC, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol. 2011;25:1–14. doi: 10.1210/me.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Allan W, Herndon CN, Dudley FC. Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microcephaly. Am J Ment Defic. 1944;48:325–334. [Google Scholar]
- 85.Biebermann H, Ambrugger P, Tarnow P, von Moers A, Schweizer U, Grueters A. Extended clinical phenotype, endocrine investigations and functional studies of a loss-of-function mutation A150V in the thyroid hormone specific transporter MCT8. Eur J Endocrinol. 2005;153:359–366. doi: 10.1530/eje.1.01980. [DOI] [PubMed] [Google Scholar]
- 86.Herzovich V, Vaiani E, Marino R, Dratler G, Lazzati JM, Tilitzky S, Ramirez P, Iorcansky S, Rivarola MA, Belgorosky A. Unexpected Peripheral Markers of Thyroid Function in a Patient with a Novel Mutation of the MCT8 Thyroid Hormone Transporter Gene. Horm Res. 2006;67:1–6. doi: 10.1159/000095805. [DOI] [PubMed] [Google Scholar]
- 87.Wemeau JL, Pigeyre M, Proust-Lemoine E, d'Herbomez M, Gottrand F, Jansen J, Visser TJ, Ladsous M. Beneficial effects of propylthiouracil plus L-thyroxine treatment in a patient with a mutation in MCT8. J Clin Endocrinol Metab. 2008;93:2084–2088. doi: 10.1210/jc.2007-2719. [DOI] [PubMed] [Google Scholar]
- 88.Frints SG, Lenzner S, Bauters M, Jensen LR, Van Esch H, des Portes V, Moog U, Macville MV, van Roozendaal K, Schrander-Stumpel CT, Tzschach A, Marynen P, Fryns JP, Hamel B, van Bokhoven H, Chelly J, Beldjord C, Turner G, Gecz J, Moraine C, Raynaud M, Ropers HH, Froyen G, Kuss AW. MCT8 mutation analysis and identification of the first female with Allan-Herndon-Dudley syndrome due to loss of MCT8 expression. Eur J Hum Genet. 2008;16:1029–1037. doi: 10.1038/ejhg.2008.66. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz CE, Stevenson RE. The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Pract Res Clin Endocrinol Metab. 2007;21:307–321. doi: 10.1016/j.beem.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Papadimitriou A, Dumitrescu AM, Papavasiliou A, Fretzayas A, Nicolaidou P, Refetoff S. A novel monocarboxylate transporter 8 gene mutation as a cause of severe neonatal hypotonia and developmental delay. Pediatrics. 2008;121:e199–e202. doi: 10.1542/peds.2007-1247. [DOI] [PubMed] [Google Scholar]
- 91.Lafreniere RG, Carrel L, Willard HF. A novel transmembrane transporter encoded by the XPCT gene in Xq13.2. Hum. Mol. Genet. 1994;3:1133–1139. doi: 10.1093/hmg/3.7.1133. [DOI] [PubMed] [Google Scholar]
- 92.Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 93.Kleinau G, Schweizer U, Kinne A, Kohrle J, Gruters A, Krude H, Biebermann H. Insights into molecular properties of the human monocarboxylate transporter 8 by combining functional with structural information. Thyroid Res. 2011;4(Suppl 1):S4. doi: 10.1186/1756-6614-4-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Crushell E, Reardon W. Elevated TSH levels in a mentally retarded boy. Eur J Pediatr. 2009;169:573–575. doi: 10.1007/s00431-009-1075-0. [DOI] [PubMed] [Google Scholar]
- 95.Fuchs O, Pfarr N, Pohlenz J, Schmidt H. Elevated serum triiodothyronine and intellectual and motor disability with paroxysmal dyskinesia caused by a monocarboxylate transporter 8 gene mutation. Dev Med Child Neurol. 2009;51:240–244. doi: 10.1111/j.1469-8749.2008.03125.x. [DOI] [PubMed] [Google Scholar]
- 96.Namba N, Etani Y, Kitaoka T, Nakamoto Y, Nakacho M, Bessho K, Miyoshi Y, Mushiake S, Mohri I, Arai H, Taniike M, Ozono K. Clinical phenotype and endocrinological investigations in a patient with a mutation in the MCT8 thyroid hormone transporter. Eur J Pediatr. 2007;167:785–791. doi: 10.1007/s00431-007-0589-6. [DOI] [PubMed] [Google Scholar]
- 97.Vaurs-Barriere C, Deville M, Sarret C, Giraud G, Des Portes V, Prats-Vinas JM, De Michele G, Dan B, Brady AF, Boespflug-Tanguy O, Touraine R. Pelizaeus-Merzbacher-Like disease presentation of MCT8 mutated male subjects. Ann Neurol. 2009;65:114–118. doi: 10.1002/ana.21579. [DOI] [PubMed] [Google Scholar]
- 98.Gika AD, Siddiqui A, Hulse AJ, Edwards S, Fallon P, McEntagart ME, Jan W, Josifova D, Lerman-Sagie T, Drummond J, Thompson E, Refetoff S, Bönnemann CG, Jungbluth H. White matter abnormalities and dystonic motor disorder associated with mutations in the SLC16A2 gene. Dev Med & Child Neurol. 2010;52:475–482. doi: 10.1111/j.1469-8749.2009.03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kakinuma H, Itoh M, Takahashi H. A novel mutation in the monocarboxylate transporter 8 gene in a boy with putamen lesions and low free T4 levels in cerebrospinal fluid. J Pediatr. 2005;147:552–554. doi: 10.1016/j.jpeds.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 100.Sijens PE, Rodiger LA, Meiners LC, Lunsing RJ. 1H magnetic resonance spectroscopy in monocarboxylate transporter 8 gene deficiency. J Clin Endocrinol Metab. 2008;93:1854–1859. doi: 10.1210/jc.2007-2441. [DOI] [PubMed] [Google Scholar]
- 101.Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, Raivich G, Bauer K, Heuer H. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest. 2007;117:627–635. doi: 10.1172/JCI28253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bernal J. Role of Monocarboxylate Anion Transporter 8 (MCT8) in Thyroid Hormone Transport: Answers from Mice. Endocrinology. 2006;147:4034–4035. doi: 10.1210/en.2006-0695. [DOI] [PubMed] [Google Scholar]
- 103.Trajkovic-Arsic M, Muller J, Darras VM, Groba C, Lee S, Weih D, Bauer K, Visser TJ, Heuer H. Impact of Monocarboxylate Transporter-8 Deficiency on the Hypothalamus-Pituitary-Thyroid Axis in Mice. Endocrinology. 2010;151:5053–5062. doi: 10.1210/en.2010-0593. [DOI] [PubMed] [Google Scholar]
- 104.Di Cosmo C, Liao XH, Dumitrescu AM, Philp NJ, Weiss RE, Refetoff S. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J Clin Invest. 2010;120:3377–3388. doi: 10.1172/JCI42113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liao XH, Di Cosmo C, Dumitrescu AM, Hernandez A, Van Sande J, St Germain DL, Weiss RE, Galton VA, Refetoff S. Distinct Roles of Deiodinases on the Phenotype of Mct8 Defect: A Comparison of Eight Different Mouse Genotypes. Endocrinology. 2011;152:1180–1191. doi: 10.1210/en.2010-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fliers E, Unmehopa UA, Alkemade A. Functional neuroanatomy of thyroid hormone feedback in the human hypothalamus and pituitary gland. Mol Cell Endocrinol. 2006;251:1–8. doi: 10.1016/j.mce.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 107.Di Cosmo C, Liao XH, Dumitrescu AM, Weiss RE, Refetoff S. A thyroid hormone analogue with reduced dependence on the monocarboxylate transporter 8 (MCT8) for tissue transport. Endocrinology. 2009;150:4450–4458. doi: 10.1210/en.2009-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Driscoll DM, Copeland PR. Mechanism and regulation of selenoprotein synthesis. Annu Rev Nutr. 2003;23:17–40. doi: 10.1146/annurev.nutr.23.011702.073318. [DOI] [PubMed] [Google Scholar]
- 109.Koenig RJ. Regulation of type 1 iodothyronine deiodinase in health and disease. Thyroid. 2005;15:835–840. doi: 10.1089/thy.2005.15.835. [DOI] [PubMed] [Google Scholar]
- 110.Di Cosmo C, McLellan N, Liao XH, Khanna KK, Weiss RE, Papp L, Refetoff S. Clinical and molecular characterization of a novel selenocysteine insertion sequence-binding protein 2 (SBP2) gene mutation (R128X) J Clin Endocrinol Metab. 2009;94:4003–4009. doi: 10.1210/jc.2009-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Azevedo MF, Barra GB, Naves LA, Ribeiro Velasco LF, Godoy Garcia Castro P, de Castro LC, Amato AA, Miniard A, Driscoll D, Schomburg L, de Assis Rocha Neves F. Selenoprotein-related disease in a young girl caused by nonsense mutations in the SBP2 gene. J Clin Endocrinol Metab. 2010;95:4066–4071. doi: 10.1210/jc.2009-2611. [DOI] [PubMed] [Google Scholar]
- 112.Schoenmakers E, Agostini M, Mitchell C, Schoenmakers N, Papp L, Rajanayagam O, Padidela R, Ceron-Gutierrez L, Doffinger R, Prevosto C, Luan J, Montano S, Lu J, Castanet M, Clemons N, Groeneveld M, Castets P, Karbaschi M, Aitken S, Dixon A, Williams J, Campi I, Blount M, Burton H, Muntoni F, O'Donovan D, Dean A, Warren A, Brierley C, Baguley D, Guicheney P, Fitzgerald R, Coles A, Gaston H, Todd P, Holmgren A, Khanna KK, Cooke M, Semple R, Halsall D, Wareham N, Schwabe J, Grasso L, Beck-Peccoz P, Ogunko A, Dattani M, Gurnell M, Chatterjee K. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest. 2010;120:4220–4235. doi: 10.1172/JCI43653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hamajima T, Mushimoto Y, Kobayashi H, Saito Y, Onigata K. Novel compound heterozygous mutations in the SBP2 gene: characteristic clinical manifestations and the implications of GH and triiodothyronine in longitudinal bone growth and maturation. Eur J Endocrinol. 2012;166:757–764. doi: 10.1530/EJE-11-0812. [DOI] [PubMed] [Google Scholar]
- 114.Lescure A, Allmang C, Yamada K, Carbon P, Krol A. cDNA cloning, expression pattern and RNA binding analysis of human selenocysteine insertion sequence (SECIS) binding protein 2. Gene. 2002;291:279–285. doi: 10.1016/s0378-1119(02)00629-7. [DOI] [PubMed] [Google Scholar]
- 115.Copeland PR. Regulation of gene expression by stop codon recoding: selenocysteine. Gene. 2003;312:17–25. doi: 10.1016/s0378-1119(03)00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Papp LV, Lu J, Striebel F, Kennedy D, Holmgren A, Khanna KK. The redox state of SECIS binding protein 2 controls its localization and selenocysteine incorporation function. Mol Cell Biol. 2006;26:4895–4910. doi: 10.1128/MCB.02284-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dumitrescu A, Di Cosmo C, Liao XH, Weiss R, Refetoff S. The syndrome of inherited partial SBP2 deficiency in humans. Antioxid Redox Signal. 2010;12:905–920. doi: 10.1089/ars.2009.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bubenik JL, Driscoll DM. Altered RNA-binding activity underlies abnormal thyroid hormone metabolism linked to a mutation in Sec insertion sequence binding protein 2. J Biol Chem. 2007;282:34653–34662. doi: 10.1074/jbc.M707059200. [DOI] [PubMed] [Google Scholar]
- 119.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 120.Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- 121.Bosl MR, Takaku K, Oshima M, Nishimura S, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp) Proc Natl Acad Sci (USA) 1997;94:5531–5534. doi: 10.1073/pnas.94.11.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schneider MJ, Fiering SN, Thai B, Wu SY, St Germain E, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 1 selenodeiodinase gene (dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147:580–589. doi: 10.1210/en.2005-0739. [DOI] [PubMed] [Google Scholar]
- 123.Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted Disruption of the Type 2 Selenodeiodinase Gene (DIO2) Results in a Phenotype of Pituitary Resistance to T(4) Molecular Endocrinology. 2001;15:2137–2148. doi: 10.1210/mend.15.12.0740. [DOI] [PubMed] [Google Scholar]
- 124.Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116:476–484. doi: 10.1172/JCI26240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP, Kelley MW, Germain DL, Galton VA, Forrest D. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci U S A. 2004;101:3474–3479. doi: 10.1073/pnas.0307402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu SY, Cohen RN, Simsek E, Senses DA, Yar NE, Grasberger H, Noel J, Refetoff S, Weiss RE. A novel thyroid Hormone receptor-beta mutation that fails to bind nuclear receptor corepressor in a patient as an apparent cause of severe, predominantly pituitary resistance to thyroid hormone. J Clin Endocrinol Metab. 2006;91:1887–1895. doi: 10.1210/jc.2005-2428. [DOI] [PubMed] [Google Scholar]
- 127.Iglesias P, Diez JJ. Therapeutic possibilities in patients with selective pituitary resistance to thyroid hormones. Med Clin (Barc) 2008;130:345–350. doi: 10.1157/13117351. [DOI] [PubMed] [Google Scholar]
- 128.Beck-Peccoz P, Piscitelli G, Cattaneo MG, Faglia G. Successful treatment of hyperthyroidism due to nonneoplastic pituitary TSH hypersecretion with 3,5,3'-triiodothyroacetic acid (TRIAC) Journal of Endocrinological Investigation. 1983;6:217–223. doi: 10.1007/BF03350611. [DOI] [PubMed] [Google Scholar]
- 129.Radetti G, Persani L, Molinaro G, Mannavola D, Cortelazzi D, Chatterjee VKK, Beck-Peccoz P. Clinical and hormonal outcome after two years of triiodothyroacetic acid treatment in a child with thyroid hormone resistance. Thyroid. 1997;7:775–778. doi: 10.1089/thy.1997.7.775. [DOI] [PubMed] [Google Scholar]
- 130.Takeda T, Suzuki S, Liu R-T, DeGroot LJ. Triiodothyroacetic acid has unique potential for therapy of resistance to thyroid hormone. Journal of Clinical Endocrinology and Metabolism (The) 1995;80:2033–2040. doi: 10.1210/jcem.80.7.7608251. [DOI] [PubMed] [Google Scholar]
- 131.Anselmo J, Cao D, Karrison T, Weiss RE, Refetoff S. Fetal loss associated with excess thyroid hormone exposure. Journal of the American Medical Association. 2004;292:691–695. doi: 10.1001/jama.292.6.691. [DOI] [PubMed] [Google Scholar]
- 132.Anselmo J, Refetoff S. Regression of a large goiter in a patient with resistance to thyroid hormone by every other day treatment with triiodothyronine. Thyroid. 2004;14:71–74. doi: 10.1089/105072504322783876. [DOI] [PubMed] [Google Scholar]
- 133.Schomburg L, Dumitrescu AM, Liao XH, Bin-Abbas B, Hoeflich J, Kohrle J, Refetoff S. Selenium supplementation fails to correct the selenoprotein synthesis defect in subjects with SBP2 gene mutations. Thyroid. 2009;19:277–281. doi: 10.1089/thy.2008.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beck-Peccoz P, Chatterjee VKK, Chin WW, DeGroot LJ, Jameson JL, Nakamura H, Refetoff S, Usala SJ, Weintraub BD. Nomenclature of thyroid hormone receptor β-gene mutations in resistance to thyroid hormone: Consensus statement from the first workshop on thyroid hormone resistance, July 10–11, 1993, Cambridge, United Kingdom. Journal of Clinical Endocrinology and Metabolism (The) 1993;78:990–993. doi: 10.1210/jcem.78.4.8157732. [DOI] [PubMed] [Google Scholar]