Abstract
We investigate whether the muscle response evoked by an electrically induced vestibular perturbation during standing is related to congruent sensory and motor signals. A robotic platform that simulated the mechanics of a standing person was used to manipulate the relationship between the action of the calf muscles and the movement of the body. Subjects braced on top of the platform with the ankles sway referenced to its motion were required to balance its simulated body-like load by modulating ankle plantar-flexor torque. Here, afferent signals of body motion were congruent with the motor command to the calf muscles to balance the body. Stochastic vestibular stimulation (±4 mA, 0–25 Hz) applied during this task evoked a biphasic response in both soleus muscles that was similar to the response observed during standing for all subjects. When the body was rotated through the same motion experienced during the balancing task, a small muscle response was observed in only the right soleus and in only half of the subjects. However, the timing and shape of this response did not resemble the vestibular-evoked response obtained during standing. When the balancing task was interspersed with periods of computer-controlled platform rotations that emulated the balancing motion so that subjects thought that they were constantly balancing the platform, coherence between the input vestibular stimulus and soleus electromyogram activity decreased significantly (P < 0.05) during the period when plantar-flexor activity did not affect the motion of the body. The decrease in coherence occurred at 175 ms after the transition to computer-controlled motion, which subjects did not detect until after 2247 ms (Confidence Interval 1801, 2693), and then only half of the time. Our results indicate that the response to an electrically induced vestibular perturbation is organised in the absence of conscious perception when sensory feedback is congruent with the underlying motor behaviour.
Key points
Electrical vestibular stimulation delivered at the mastoid processes evokes a reflex response in the appendicular muscles only when they are actively involved in keeping the unsupported head and body balanced.
We show that the vestibular-evoked muscle response was present during a task that simulated the control of standing where sensory feedback was congruent with the motor-generated expectation to balance the body, and absent when sensory feedback did not match.
The present results indicate that the task dependency of the vestibular-evoked muscle response relies on congruent sensory and motor signals, and that this is organised in the absence of a conscious perception of postural control.
These findings help us understand how our brain combines sensory and motor signals to provide an internal representation of standing balance that can be used to assess whether a perturbation poses a postural threat.
Introduction
The technique of galvanic (Coats, 1973; Nashner & Wolfson, 1974; Lund & Broberg, 1983; Britton et al. 1993; Fitzpatrick et al. 1994) and, more recently, stochastic (Fitzpatrick et al. 1996; Pavlik et al. 1999; Dakin et al. 2007, 2011) vestibular stimulation has been used extensively in humans to investigate vestibular function during standing. This normally entails a bipolar current stimulus applied bilaterally to the mastoid processes that bypasses the mechanosensitive transduction of the hair cells to modulate vestibular afferent firing rates (Goldberg et al. 1984). This type of vestibular stimulation is compelling, producing a pure vestibular signal of head motion without actual movement of the head or whole-body. When standing freely, the CNS interprets the spurious signal of head motion as a pure vestibular perturbation requiring a response to prevent the body from falling (Fitzpatrick & Day, 2004). Consequently, vestibular stimulation results in a muscle response, which can be measured with electromyography (EMG), and a stereotyped whole-body postural response that occurs regardless of whether the stimulus is delivered randomly, at a predictable time interval with an auditory cue, or when self-administered by the subject (Guerraz & Day, 2005).
In spite of the irresistible effect of electrical vestibular stimulation on postural activity, the appearance of a muscle response to this stimulus has been shown to be task dependent. Previous studies reveal that the CNS only responds to the electrically induced vestibular perturbation when it is engaged in a task that balances the unsupported head and body. The muscle response is present in the dependent legs during standing (Nashner & Wolfson, 1974; Fitzpatrick et al. 1994), in the trunk while seated on a stool (Ali et al. 2003), and even in the arms, which are not typically used for postural control, when they are used to stabilise a person standing on top of a balance board (Britton et al. 1993). Conversely, when the CNS is engaged in a non-postural task, for instance, co-contracting the muscles of the lower leg while seated (Fitzpatrick et al. 1994), electrical vestibular stimulation does not evoke a reflex response in those muscles. This task dependence is best highlighted by Fitzpatrick et al.'s (1994) equivalent body experiment. In their set-up, the body was supported upright by a rigid beam and held to an earth-fixed reference with only ankle motion available to balance an inverted pendulum. They show that even though the lower leg muscles were engaged in a task that required similar balance control as when keeping the body upright during normal standing, electrical vestibular stimulation did not evoke a reflex muscle response. Fitzpatrick et al. reasoned that holding the body to an earth-fixed reference minimised vestibular-directed changes in muscle activity and, therefore, concluded that an electrically induced vestibular perturbation only results in a reflex response when vestibular information is relevant to postural control.
In the context of standing, the CNS must be aware of the current state of the sensorimotor system, for example, that the leg muscles are engaged in a task that balances the head and body, in order to determine whether a vestibular perturbation poses a threat to postural stability. Presumably, the integration of visual, vestibular or somatosensory signals that detect the gravitational orientation and motion of the head and body together with the motor control of the leg muscles accommodates this sensibility. In the present study, we determine whether simulating the sensory and motor aspects of standing in a balancing task, whilst the body is supported, results in the CNS associating this motor behaviour with the postural control of standing. The simulation of standing balance was performed with the subject's body braced in a standing posture on top of a motion platform programmed with the mechanics of an inverted pendulum (Fig. 1A). The platform's motion is controlled by modulating ankle torque to balance the virtual pendulum (body) so that the splinted body is rotated through space in a motion similar to that experienced during normal postural sway, rather than being held to an earth-fixed reference (Fitzpatrick et al. 1994). We hypothesised that, even though the body is supported, when subjects modulate ankle torque to balance their virtual body, sensory signals of whole-body motion will match an internal model of the expected sensory consequences from the motor command to balance the body and the CNS will associate the balance task with the postural control of standing. Therefore, we expected that an electrically induced vestibular perturbation during our balancing task will evoke a reflex muscle response. This also led us to determine whether the CNS's interpretation of congruent sensory and motor signals of body motion during a balance task as postural activity could be generalised to a non-postural intrinsic hand muscle when it is used to balance the body seated on top of the inverted pendulum-like platform.
Figure 1. Experimental set-up.
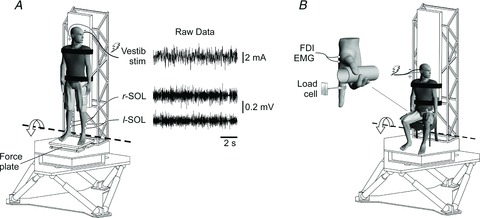
A, the subject was securely strapped to a rigid backboard on top of a motion platform that could be controlled by modulating the ankle torque exerted on the force plate. The platform rotated about an axis that passed through the subject's ankles (broken line). Seatbelts placed across the shoulders and around the waist prevented the subject from falling forward without supporting the load of the body acting through the feet. Raw data of the vestibular stimulus and electromyography (EMG) activity of the right (r-SOL) and left (l-SOL) soleus muscles are shown during a trial where the subject balanced the platform programmed with the mechanics of an inverted pendulum. During standing trials, the platform was stationary, seatbelts were removed and the rigid backboard was retracted. B, the subject was seated and balanced the inverted pendulum-like platform by modulating the torque generated by abducting the extended index finger against an immovable load cell. The subject stabilised the right hand by grasping a steel cylinder that was mounted to the backboard. A vestibular stimulus was delivered as the subject balanced the platform, and EMG activity was recorded for the first dorsal interosseus (FDI).
Our study also assesses whether, in addition to congruent sensory and motor signals of body motion, the reflex muscle response to an electrical vestibular stimulus requires a conscious perception of the postural control of standing. In all previous studies, subjects were aware of whether they were balancing their own body, i.e. during normal standing, or in the case of Fitzpatrick et al.'s (1994) equivalent body experiment, that the body was still and the leg muscles balanced an external load. We hypothesised that due to the irresistible effect of electrical vestibular stimulation on postural activity, the dependence of the vestibular-evoked muscle response on congruent sensory and motor signals for body motion is independent from a conscious perception of postural control. Here, we introduce a pseudo-balance condition that consisted of dynamic changes in the control of the platform's motion. Unbeknownst to the subjects, the control of the platform would switch from human-controlled balancing of the virtual pendulum to a computer-controlled motion that simulated balancing but was not influenced by the applied ankle torque.
Methods
Twelve healthy adults (10 males, mean age 27.6 years; SD 4.4 years) with no history of neurological disorder participated as subjects. The experiments were conducted in accordance with the standards set by the Declaration of Helsinki, and the University of British Columbia's Clinical Research Ethics Board approved all experimental procedures. All subjects provided written informed consent before participating.
Experimental set-up
A motion platform (6DOF2000E; MOOG, East Aurora, NY, USA) was programmed with the mechanics of an inverted pendulum to simulate the load of the body during standing. A real-time system (PXI-8196; National Instruments, Austin, TX, USA), operating at 60 Hz, controlled the motion of the robotic platform in response to a change in ankle torque exerted on a force plate (OR6-7-1000; AMTI, Watertown, MA, USA) fixed to the surface of the platform (Fig. 1A). The system delay between the position command and the actual position of the platform was ∼41.5 ms. A detailed description of the set-up and design of the motion platform was presented by Luu et al. (2011). Briefly, the inverted pendulum was modelled on the physical dimensions of each subject's body. The pendulum's mass was distributed into three adjoining segments that corresponded to the grouped masses of the subject's head, torso and upper limbs, the pelvis and thighs, and the shanks. A distributed-mass model was used instead of the conventional concentrated-mass model (Fitzpatrick et al. 1992, 1994; Winter et al. 1998; Loram et al. 2001) as, for this robotic system, the inertia about the ankles of the distributed mass provided a more accurate representation of the load stiffness of the human body at the sway frequencies experienced during standing (Luu et al. 2011). The height of the pendulum's centre of mass from the pivot point of the ankles was matched to the centre of mass measured for each subject's body. The virtual angle of the pendulum was calibrated so that the subject was required to exert the same amount of ankle torque as during standing in order to keep the motion platform horizontal and the supported body upright in space. This corresponded to a mean pendulum angle of 2.9 deg (SD 0.5 deg) from vertical.
The motion platform rotated in the sagittal plane about an axis that passed through the ankle joints. Angular limits of 6 deg anterior and 3 deg posterior from vertical were imposed on the platform's motion to ensure that the subject could generate the required stabilising torque to balance the platform. As the platform rotates forward, the subject must increase ankle plantar-flexor torque in order to stabilise the platform, and hence the body, in the same way that ankle torque must increase to prevent the body from toppling during forward sway. With the ankle joints sway referenced to the platform's motion, the normal contribution to the total ankle torque from changes in passive ankle torque (Moseley et al. 2001) and ankle damping (Loram & Lakie, 2002) during ankle rotation were simulated for each subject according to Luu et al. (2011).
The motion platform was also configured for control by a non-postural hand muscle. Seated on top of the platform with arms by the side and the torso supported by the rigid backboard (Fig. 1B), subjects balanced the inverted pendulum-like platform by modulating the abduction torque generated at the first metacarpophalangeal joint of the right hand. The torque generated by contracting the first dorsal interosseus (FDI) muscle isometrically was measured with a load cell (Model 31; Honeywell, Columbus, OH, USA) placed at the proximal interphalangeal joint of the extended right index finger. The output of the load cell was adjusted for each subject so that contracting the FDI at 10% of the maximal voluntary abduction torque kept the motion platform horizontal. Like ankle-controlled motion, an increase in abduction torque was required to stabilise the platform as it rotated forward.
Vestibular stimulation
An isolated current unit (Model 2200 Analog Stimulus Isolator; AM Systems, Sequim, WA, USA) was used to deliver a bipolar stochastic vestibular stimulus through carbon rubber electrodes (9 cm2) coated with conductive gel (Spectra 360; Parker Laboratories, Fairfield, NJ, USA) and adhered over the mastoid processes. The stochastic signal was delivered as a continuous analog signal, with a bandwidth of 0–25 Hz and peak amplitude of ±4 mA (average root mean square (RMS) 0.78 mA; SD 0.02 mA). The vestibular stimulus was 3 min in duration.
Data recordings
Data were acquired with a real-time data acquisition board (PXI-6289; National Instruments) at 2000 Hz. EMG activities of the right FDI and both soleus muscles were amplified (×1000) and recorded at a bandwidth of 30–1000 Hz (P511 AC Amplifier; Grass Technologies, West Warwick, RI, USA) using Ag–AgCl surface electrodes (Blue Sensor M; Ambu A/S, Ballerup, Denmark).
Protocol
All trials were performed with the eyes open and head turned 90 deg to the left. A laser pointer attached above the right ear and oriented to the subject's line of sight kept Reid's plane, which passes bilaterally through the inferior orbital margin and the external acoustic meatus, tilted ∼18 deg up from horizontal. Arranging the head in this position relative to the ankle joints maximised the muscle and postural responses to electrical vestibular stimulation for anterior–posterior rotations about the ankles (Cathers et al. 2005; Day & Fitzpatrick, 2005), in line with the action of the calf muscles. Stochastic vestibular stimulation was applied for 3 min in all trials. The vestibular stimulus was used to probe vestibular-motor activity during the experiments explained in the sections below. These experiments were completed over several days. A total of 12 subjects was recruited: 10 subjects participated in the experiments described in the congruent sensory and motor signals section; and eight subjects participated in the experiments in the pseudo-balance section.
Congruent sensory and motor signals
A series of trials was conducted to assess whether the muscle response to electrical vestibular stimulation depended on congruent sensory and motor signals. The first trial for all subjects required them to stand on a flat surface with bare feet approximately 10 cm apart and the body unsupported. The vestibular-evoked muscle response obtained during this standing trial served as a reference for the remaining trials. Subjects then participated in three separate trials in which the body was braced in an upright position on top of the motion platform (Fig. 1A). In these braced trials, afferent signals of body motion were either congruent with or decoupled from the motor command to the calf muscles.
For congruent sensory and motor signals, subjects modulated ankle torque to balance the body braced on top of the inverted pendulum-like platform. They were instructed to keep the body balanced and upright in space, and informed that relaxing the calf muscles would cause the platform to fall forward. For decoupled sensory and motor signals, subjects contracted the calf muscles at a constant intensity while the platform's motion was controlled by a computer programmed to rotate the body to follow the same trajectory recorded in the previous balancing trial. The target contraction level was matched to the mean RMS amplitude of EMG activity (time constant: 200 ms) recorded earlier for the right soleus muscle as each subject stood unsupported for 1 min without vestibular stimulation. Feedback for muscle contraction intensity was provided verbally, and the subject was reminded throughout the trial to maintain a similar level of activity in both calf muscles. Here, the subject was informed that a computer controlled the motion of the platform and that ankle plantar-flexor torque would not influence its motion. A control trial required subjects to contract the calf muscles to reach the same target intensity while the braced body and platform remained stationary.
A further study was conducted where subjects were seated on top of the platform (Fig. 1B) when balancing the inverted pendulum-like platform with the FDI. The best of three maximum voluntary contractions of FDI, performed with verbal encouragement by the experimenter, was used to calibrate the motion platform for control by the right index finger. Each subject was instructed to balance the platform by modulating the activity in the FDI, and that relaxing the FDI would cause the platform to fall forward.
Pseudo-balance
The pseudo-balance condition was designed to assess whether the task dependency of the vestibular-evoked muscle response was related to the perception of balancing the body. This involved a set of two experiments that were conducted on separate days. Both contained a series of trials where the control of the robotic platform transitioned from the subject balancing the inverted pendulum-like platform with the feet to following a computer-controlled fixed trajectory for 4 s at a time before human control was reinstated. The withdrawal and then reinstatement of human-controlled balancing of the platform occurred several times within a trial.
To maintain the illusion that the subject was still balancing the platform during the computer-controlled period, the trajectory recorded during an earlier 3 min balancing trial served as a template from which a 4 s portion was selected to encode the fixed trajectory. Prior to each transition, the template trajectory was analysed to find a suitable start point that was within 0.034 rad of the robotic platform's current position and within 0.00085 rad s−1 of its current velocity. Once a suitable start point was selected, the robotic system would only transition into the computer-controlled mode when the current position and velocity of the platform was within 0.017 rad and 0.00085 rad s−1, respectively, of the selected portion of the template trajectory. This ensured that the robotic system remained in the human-controlled balance mode until a smooth transition could occur. Note that a new portion of the template trajectory was analysed for each transition so that no computer-controlled trajectory was repeated. The minimum time between transitions was set at 8 s, and ranged up to 65 s. At the end of each period of computer-controlled motion, the balance mechanics of the inverted pendulum were programmed to correspond with the final position and velocity of the fixed trajectory to facilitate a smooth transition back to human control.
Of the five subjects who participated in the first experiment, only one subject was aware of the pseudo-balance protocol. The remaining subjects were only told that they had to balance the inverted pendulum-like platform for 3 min at a time. No feedback was provided as to when each transition occurred, and subjects were advised that relaxing the calf muscles would cause the platform to fall forward. Additionally, subjects were unaware that the robotic system was capable of performing an online transition from human- to computer-controlled motion. A total of 100 transitions was obtained for each subject in 11–13 trials of 3 min duration.
In the second experiment, all subjects were informed about the protocol. Five participants were recruited; only two of whom had participated in the first pseudo-balance experiment. Subjects were instructed to indicate by pressing and holding down a hand-held button switch the moment they felt that the control of the platform's motion had been withdrawn. Similarly, subjects were instructed to release the button once they felt that they had regained control of the platform. To ensure that subjects were balancing the robotic platform and not actively seeking to detect the change in control, they were not permitted to rotate the platform back and forth in an oscillating motion, nor were they allowed to simply relax the calf muscles and let the platform fall forward. The experimenter carefully monitored the use of these strategies in each trial. Thirty transitions were completed for each subject. The duration of the computer-controlled motion and the number of transitions that occurred within each trial were not conveyed to the subjects.
Measurement and analysis
Coherence and cumulant density functions were constructed for each trial from the stochastic vestibular stimulus and rectified EMG signals using the method described by Rosenberg et al. (1989), with the stochastic vestibular signal as the reference (input) signal. Frequency-specific coherence was calculated using a window of 2048 data points (∼1 s) to give a resolution of ∼0.98 Hz. Cumulant density functions were derived by transforming the cross-spectra between the stochastic vestibular signal and the EMG signal into the time domain, and then normalising by the product of the vector norms of the two signals (Dakin et al. 2010). The cumulant density function, therefore, is equivalent to the cross-correlation between the vestibular stimulus and the EMG signal, and has been shown to provide similar temporal and spatial characteristics to the trigger-averaged muscle responses obtained with square-wave galvanic vestibular stimulation (Dakin et al. 2007). In this study, the correlation between the controlled input signal (vestibular stimulus) and the measured physiological signal (EMG) will be referred to as an evoked muscle response that is dimensionless. Coherence and cumulant density functions for each subject were deemed to be significant when values exceeded the 95% confidence limits described by Halliday et al. (1995).
Time-varying changes in coherence between the stochastic vestibular stimulus and rectified EMG signals were determined for the pseudo-balance trials at the transition points from the human-controlled balance mode of the robotic platform to the computer-controlled mode. A 16 s segment of data that included data points 6 s prior to the pseudo-balance mode and 6 s after was extracted from each occurrence for processing. Time–frequency coherence was calculated based on the continuous Mortlet wavelet transform described by Zhan et al. (2006) and modified by Blouin et al. (2011), and averaged over 100 repeat occurrences for each subject. Time–frequency plots are presented with the first and last 2 s of data removed due to the distortion by edge effects when applying wavelet analysis. For two correlated processes, this technique is able to detect inserted time intervals that have no correlation with a temporal resolution of between 20 and 30 ms for the peak frequencies obtained in the coherence estimates in this study. The total coherence across frequencies at each time point was used to compare the overall strength of the relationship between the vestibular stimulus and soleus EMG activity during the period of human-controlled balance versus pseudo-balance. Total coherence was calculated as the mean of the coherence values from 0 to 25 Hz.
Data were averaged across subjects to provide grouped means with a 95% confidence interval (CI). Significant differences between the vestibular-evoked muscle responses for standing and the human-controlled inverted pendulum trial that simulated the load of the body during standing were determined statistically with Student's paired t test, with significance set at P < 0.05. In the pseudo-balance trials, significant differences between the total coherence during the periods of human-controlled balance and computer-controlled fixed trajectory motions were also determined with Student's paired t test.
Results
Congruent sensory and motor signals
Data for a representative subject are shown in Fig. 2. Coherence between the input vestibular stimulus and soleus EMG was significant at frequencies below 20 Hz in both of the subject's legs for the reference standing trial (Fig. 2A). This corresponded with the biphasic muscle response to vestibular stimulation in the cumulant density function, which showed the characteristic short- and medium-latency peaks exceeding the 95% confidence limits. The timing of the muscle response was similar for both legs, with the positive short-latency peak occurring at 61 ms and the negative medium-latency peak occurring at 96 ms for the right soleus muscle (Fig. 2, vertical lines). Vestibular stimulation during the control trial, where the subject contracted the plantar flexors while the body was held still in space, did not evoke a detectable muscle response in either leg (Fig. 2B). When the subject's body was braced, vestibular stimulation evoked a muscle response that was similar to the reference standing trial only when the subject balanced the virtual pendulum to keep his own body upright in space (Fig. 2C). When the computer controlled the motion of the subject's body, vestibular stimulation did not evoke a muscle response that was comparable to the reference standing trial (Fig. 2D).
Figure 2. Vestibular-evoked muscle responses for a single subject.
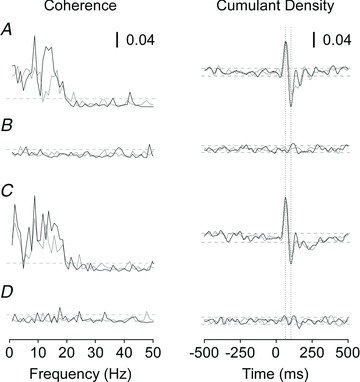
Coherence was computed between the vestibular stimulus and left (grey) or right (black) soleus EMG activity. Cumulant density functions represent the evoked muscle response to vestibular stimulation. Data are shown during the reference standing trial (A), the control trial where the subject was braced to a stationary platform (B), and for the trials in which the sensory signals of body motion were either congruent (C) with or decoupled (D) from the motor command to the leg muscles. Horizontal lines represent the 95% CIs, and vertical lines indicate the short- and medium-latency peaks for the right soleus during the reference standing trial.
A similar trend was observed across subjects for each condition (Fig. 3). For the reference standing trial (Fig. 3A), mean latencies for the positive peak in the evoked muscle responses were 63 ms (CI 60, 67) in the right and 67 ms (CI 63, 71) in the left soleus. Mean latencies for the negative peak were 105 ms (CI 97, 114) in the right and 114 ms (CI 105, 123) in the left leg. A similar biphasic muscle response was evoked by vestibular stimulation during the braced trial in which subjects balanced the inverted pendulum-like platform to keep the body upright in space (Fig. 3C). The mean latencies for the braced balancing trial were 67 ms (CI 64, 69) and 120 ms (CI 110, 130) for the right, and 67 ms (CI 64, 70) and 117 ms (CI 108, 127) for the left soleus. When the computer controlled the platform to rotate the body in a motion that replayed the sway experienced in the previous balancing trial, no significant vestibular-evoked muscle response was detected in the left soleus for all subjects (Fig. 3D). In the right soleus, a small but significant negative peak was detected in five out of 10 subjects. This negative peak had a mean amplitude of –0.010 (CI –0.015, –0.0057) and mean latency of 90 ms (CI 84, 95), which did not correspond to the short- or medium-latency peaks in the muscle response obtained during the reference standing trial for these subjects.
Figure 3. Averaged muscle responses to electrical vestibular stimulation.
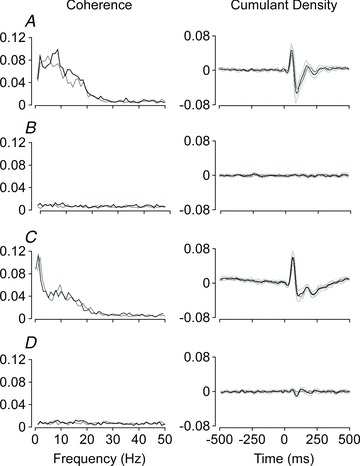
Group mean (n= 10) data are shown for the reference standing trial (A), the control trial where the platform was stationary (B), the balancing trial where sensory signals of body motion were congruent with the motor command to balance the body (C), and when sensory signals of body motion were decoupled from the motor command (D). Coherence between the vestibular stimulus and soleus EMG activity is shown for the left (grey) and right (black) legs. Thin lines represent the 95% confidence limits about the group means (thick lines) for the cumulant density functions.
The amplitudes of the vestibular-evoked muscle response in the reference standing trial were 0.048 (CI 0.034, 0.061) in the right and 0.041 (CI 0.027, 0.055) in the left soleus for the short-latency peak, and –0.055 (CI –0.072, –0.038) in the right and –0.044 (CI –0.057, –0.031) in the left soleus for the medium-latency peak. In the braced balancing trial, the amplitude of the short-latency peak in the right soleus was 0.061 (CI 0.046, 0.075), and the amplitude of the medium-latency peak in the right soleus was –0.032 (CI –0.045, –0.018). When the amplitudes of the vestibular-evoked muscle response in the braced balancing trial were compared with the reference standing trial, there were no significant (P > 0.05) differences between corresponding peaks for the left or right soleus muscles.
Subjects were able to balance the inverted pendulum-like platform with the index finger while seated. A significant biphasic muscle response to vestibular stimulation was observed in the FDI muscle in nine out of 10 subjects (Fig. 4). A positive short-latency peak at 78 ms (CI 70, 87), with a mean amplitude of 0.021 (CI 0.014, 0.029), was followed by a prolonged negative peak at 261 ms (CI 215, 306), with a mean amplitude of –0.020 (CI –0.029, –0.012).
Figure 4. Human-controlled balancing with a non-postural hand muscle.
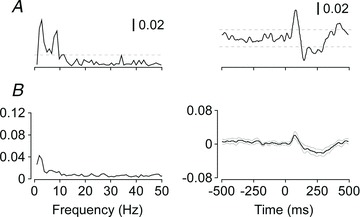
Data are shown for a single subject (A) in the top panel with group means (n= 10) below (B). Subjects were seated on top of an inverted pendulum-like platform that they balanced by modulating the abduction torque generated by the right index finger. Coherence between the vestibular stimulus and EMG activity of the right FDI is shown on the left, and the vestibular-evoked muscle response in FDI is shown on the right. Horizontal lines in the single subject data and thin lines in the group mean data represent the 95% confidence limits.
Pseudo-balance
In the first set of trials where subjects were not briefed about the pseudo-balance protocol, they rarely detected that the applied ankle torque no longer controlled the motion of the platform. On the occasions when subjects did notice a disturbance, it was perceived as if it ‘felt like the response of the system was delayed’ rather than not being in control of the platform. Indeed, mean rectified EMG activity over 100 transitions for individual and group mean data did not change when human control of the robotic system was withdrawn (Fig. 5).
Figure 5. Mean rectified EMG data during the pseudo-balance transitions.
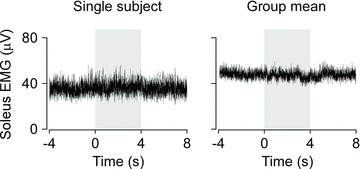
Single-subject data are presented for the right soleus muscle as mean rectified electromyogram (EMG) from 100 transitions. Data are shown as the subject balanced the platform prior to the withdrawal of human control at time zero, during the period of computer-controlled motion (shaded area), and after the reinstatement of human control at 4 s. The group mean (n= 5) data are shown for the same muscle.
Transitioning from human-controlled balancing of the robotic platform to a computer-controlled motion affected the coherence between the input vestibular stimulus and soleus EMG activity. Data from a single subject (Fig. 6A) show that prior to this transition there was significant coherence between these two signals mainly at frequencies below 10 Hz. Coherence then decreased in both legs during the period of computer-controlled motion before recovering to pre-transition levels when control of the robotic platform's motion reverted back to human control. This behaviour was reflected in the data for all subjects (Fig. 6B). The mean total coherence from 0 to 25 Hz across the 4 s pre-transition period was 0.026 (CI 0.008, 0.044) in the left soleus and 0.027 (CI 0.008, 0.046) in the right soleus. Total coherence declined rapidly toward a new baseline when plantar-flexor torque no longer controlled the motion of the platform, and remained attenuated throughout this period at a mean of 0.016 (CI 0.007, 0.025) in the left and 0.015 (CI 0.006, 0.024) in the right leg. This represented a significant (P < 0.05) decrease in the mean total coherence of 40.1% (CI 33.4, 46.9) in the left and 42.4% (CI 32.7, 52.1) in the right soleus.
Figure 6. Pseudo-balance.
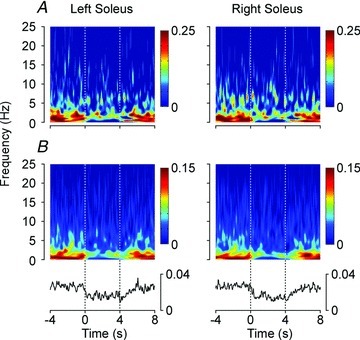
Time-varying changes in coherence between the vestibular stimulus and soleus EMG activity during 100 transitions from human- to computer-controlled motion of the robotic platform. Data are shown for a single subject (A) and the group mean (B) for all five subjects. The 4 s period prior to time zero shows frequency-specific coherence as subjects were braced on top of the platform and balanced its body-like load with their feet. Time zero represents the transition point from human-controlled balancing of the platform to a computer-controlled rotation of the platform along a predetermined path, which lasted for 4 s (between the vertical lines), before subjects regained control of the platform. The mean of the coherence from 0 to 25 Hz at each time point is shown across the bottom panel. Non-significant data points have been removed so that zero coherence represents the values below the threshold of the 99% confidence limit; 0.046 for the single subject and 0.0093 for the group mean data.
The change in coherence when transitioning from human-controlled to computer-controlled motion was estimated from the total coherence at each time point. Total coherence decreased to more than 3 SDs below the pre-transition mean after 150 ms for the left and 200 ms for the right soleus (175 ms for both legs combined). Once the control of the robotic platform's motion reverted back to human control, total coherence returned towards the pre-transition mean, recovering to within 3 SDs of this mean after 850 ms for the left and 900 ms for the right soleus (875 ms for both legs combined).
In the second experiment, when subjects were asked to indicate each time they detected that the control of the robotic platform had been withdrawn, the rate of detection varied between nine and 22 out of 30 transitions, and the detection times ranged between 1503 and 2880 ms. For all subjects, the mean detection rate was 52% (CI 38, 66), which was achieved with 1.8 (CI 0.1, 3.5) false positives, and the mean detection time was 2247 ms (CI 1801, 2693). When human control of the platform was reinstated, the average time required for subjects to perceive that they had regained control was 1644 ms (CI 931, 2357). Interestingly, 23% (CI 7, 40) of the time subjects incorrectly perceived that the control of the platform had been reinstated before the end of the computer-controlled period.
Discussion
Our study has shown that the typical biphasic muscle response to electrical vestibular stimulation depends on congruent sensory and motor signals, thus supporting Fitzpatrick et al.'s (1994) view that relevant vestibular information is required to mediate the electrically induced vestibular-motor pathway. Our results further refine this concept, showing that the efficacy of the vestibular-evoked motor pathway is not dependent on a conscious perception of balancing the body. The pseudo-balance condition clearly shows a significant decrease in coherence between the vestibular stimulus and soleus EMG activity when, unbeknownst to the subject, human control of the inverted pendulum-like platform was withdrawn. This suppression occurred rapidly, and during the period when subjects perceived that they were still performing the balancing task.
Task dependency of the vestibular-evoked muscle response
The task-dependent nature of the vestibular-evoked muscle response seems to be related to the CNS's ability to associate the motor behaviour with the control of posture. This is evident by the differential effect of vestibular stimulation in the active muscle for the human- versus computer-controlled trials. With the body supported, simulating the sensory signals normally associated with motor control of standing balance resulted in a reflex muscle response to the electrically induced vestibular perturbation that was comparable to the reference standing trial. The motor command to balance the platform's virtual load induced a body sway that had similar load stiffness properties (i.e. ankle torque vs. angle) as the expected movement of the body if the same motor command had been issued when the subject was standing freely (Luu et al. 2011). Accordingly, the sensory signals of body motion were congruent with the underlying motor behaviour and would resemble the expected reafferent signals that arise from the control of standing balance. Applying this interpretation to the condition in which the movement of the body was decoupled from the actions of the calf muscles explains the lack of a vestibular-evoked muscle response. The mismatch between the actual sensory signals of body motion and an internal model of the expected sensory consequences generated by the motor command to maintain a constant-intensity contraction did not produce a motor behaviour that was expected to be associated with postural control. As such, vestibular stimulation evoked a small muscle response that appeared only in the right leg, and in only half of the subjects, which could not be classified according to the characteristic short- or medium-latency responses obtained in the reference standing trial.
The dependence of the vestibular-evoked motor pathway on congruent sensory and motor signals for body motion during our braced balancing task was not strictly limited to the context of standing balance. Vestibular stimulation evoked a reflex response in seated subjects when the FDI was the only muscle available to balance the body. This confirms and expands Britton et al.'s (1993) finding of a vestibular-evoked response in a non-postural muscle when it was used to mechanically stabilise a standing person. Our result shows that a muscle does not need to directly support the load of the body in order to be engaged in postural control, the CNS only requires that the force output of the muscle contributes to balancing the body in space.
Rapid, unconscious suppression of vestibular-motor pathways
Dynamic changes in the control of the platform's motion, and therefore the motion of the body, produced rapid changes in coherence between the vestibular stimulus and soleus EMG activity. Transitioning from human-controlled balancing of the platform to a computer-controlled balance simulation resulted in a significant decrease in vestibular-muscular coherence after 175 ms. This suggests that the CNS is able to almost immediately recognise that the motor command to balance the body is decoupled from the actual movement of the body so that the sensorimotor system is no longer involved in postural control. The reduction in vestibular-motor coupling occurred much faster than can be attributed to the subjects consciously detecting a loss of control of the platform. This suggests that the neural processes that create the association between congruent sensory and motor signals of body motion during the braced balancing task with postural control most likely operate separately from a conscious perception of balancing the body. When accounting for the time it takes to send a motor command to indicate with the thumb that a transition had occurred, less than 30 ms for an intrinsic hand muscle (Day et al. 1989), the attenuation in coherence occurred an order of magnitude faster (175 vs. 2217 ms) than the mean detection time for the transition from human- to computer-controlled platform motion.
Subjects generally performed poorly at detecting the subtle transitions from human- to computer-controlled motion. They were only able to detect half the number of transitions presented and, in approximately a quarter of these detected transitions, subjects incorrectly perceived that human control of the platform had been reinstated before the end of the computer-controlled period. The timing of the attenuation in coherence and failure to consciously detect the discrepancy between the motor command and related sensory feedback is in line with reported perceptual thresholds for associating somatosensory stimuli from a robot arm (Blakemore et al. 1999) or visual stimuli relating to hand movements (Shimada et al. 2010) as generated by our own actions, which involve delays of less than 200 ms between the motor command and sensory feedback.
While the transition from human- to computer-controlled platform motion was accompanied with a rapid suppression of vestibular-muscular coherence, reinstatement of human-controlled balancing of the platform resulted in a relatively slower (875 vs. 175 ms) recovery of coherence to pre-transition levels. This indicates that the CNS takes longer to re-associate the balancing task with postural activity than identifying a discrepancy between the expected sensory consequences from the motor command to balance the body and the actual sensory feedback. One possible explanation for the longer time frame for reacquiring postural control during the braced balancing task is related to the congruency between the platform's control signal and its motion. When switching from human- to computer-controlled motion, the platform instantaneously transitions to the predetermined trajectory recorded from a previous balancing trial so that the platform's motion is immediately congruent with the computer's control signal but incongruent with the subject's motor command to balance the platform's virtual body. That is, the link between the subject's motor output and the expected sensory consequence is abruptly broken. When the subject regains control of the platform, he must acquire the properties of the platform (an unstable inverted pendulum) in order to balance it, which inevitably takes more time. For normal standing, the calf muscles make an average of 2.6 adjustments per second (Loram et al. 2005), with each adjustment providing information from the sensory and motor systems to the CNS. Based on the time for recovery of vestibular-muscular coherence in this study, the CNS requires between two and three adjustments in muscle activity in order to make this re-association.
Integration of congruent sensory and motor signals during a whole-body balancing task
Several models (Sperry, 1950; von Holst & Mittelstaedt, 1973; Wolpert et al. 1995) have been proposed that may explain how the CNS associates the information obtained from the sensory and motor systems during the braced balancing task with the motor behaviour normally generated during standing. These generally involve comparing an efference copy of the motor command, which can be transformed into an internal representation of the expected sensory consequence of that motor command, with the actual sensory feedback to characterise otherwise ambiguous signals. These models have generally been applied to discrete voluntary tasks such as moving a limb towards a target. However, these models may still apply to a postural task such as standing (van der Kooij et al. 1999; Kuo, 2005; Gawthrop et al. 2009), where the engagement of vestibular-motor pathways occurs independently from a conscious perception of balancing the body, if we consider that the sensory information generated during the balancing task is constantly being compared with the expected sensory consequences of the motor command within the neural networks that underlie the control of balance.
A comparison of our braced balancing task with Fitzpatrick et al.'s (1994) equivalent-body experiment suggests that the vestibular system plays a crucial role in the association of a motor task with the control of posture. While both studies simulate congruent sensory and motor signals during a balancing task, their study limited this to the ankle joint as the body was held still relative to an earth-fixed reference. This arrangement did not produce a vestibular-evoked muscle response, whereas we show the presence of a vestibular-evoked reflex response even though the body was supported. The key difference with our study is that with the body braced on top of the motion platform, our balancing task simulated congruent sensory and motor signals of the whole body during standing, in which vestibular information was available for balance control. Indeed, the vestibular system is ideally suited to process sensory and motor signals during the balancing task as vestibular neurons receive convergent inputs from several sensory channels and they project to lower-limb motoneurons (Carpenter, 1988; Highstein & Holstein, 2006). Roy & Cullen (2001, 2004) have provided empirical evidence in primates for differential processing of vestibular afferents within the vestibular nuclei based on whether these sensory signals are congruent with the expected sensory consequences to a motor command for head motion. We propose that similar mechanisms may be responsible for mediating the task dependency of vestibular-evoked muscle responses in the legs during standing balance. Cullen et al. (2011) suggested that while vestibular afferents are differentially processed at the vestibular nuclei, this process may require integration of motor signals and other sensory signals within the cerebellum, a structure that shares reciprocal connections with vestibular neurons (Carleton & Carpenter, 1983; Walberg & Dietrichs, 1988), and has been implicated in the regulation of postural activity.
A limitation of the current experimental approach relates to the non-physiological nature of the electrical vestibular stimulus. Normal movement of the head and body through space would stimulate vestibular afferents in conjunction with other sensory modalities, such as visual and somatosensory inputs. Stochastic vestibular stimulation, however, modulates vestibular afferent activity independently from these other sensory modalities, which the CNS may not equate with physiological vestibular activation. Secondly, although our data show that the engagement of vestibular-motor pathways relies on congruent sensory and motor signals, the present experiments do not address how this hypothesis could explain the tuning of vestibular-evoked reflexes based on the task at hand. For example, the increased gain of vestibular-evoked reflexes when subjects stand on a compliant surface (Fitzpatrick et al. 1994; Welgampola & Colebatch, 2001) may also be sensitive to the reliance placed upon the vestibular system to maintain standing balance (Fitzpatrick et al. 1994), or the availability and reliability of sensory signals for body motion.
Conclusions
The present study has demonstrated that the task-dependent nature of the vestibular-evoked muscle response is related to congruent sensory and motor signals during standing. This can be simulated in a non-postural task by having subjects balance a body-like load so that the activation of sensory signals related to body motion is matched to the expected sensory consequences had the motor command been issued during standing. Vestibular-evoked responses in the active muscle were always present when subjects actively controlled the motion of their own body and not when a contraction of similar intensity was performed that did not affect body motion. The task dependence of this vestibular-motor pathway is not influenced by a conscious perception of the task, but rather related to the CNS's association of the motor behaviour with the postural activity present during the control of standing.
Acknowledgments
This work was supported in part by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Institutes of Health Research, the Canadian Chiropractic Research Foundation, and the Michael Smith Foundation for Health Research. The work of B.L.L. was supported by a NSERC Discovery Accelerator Supplements Program, and the work of T.P.H. was supported in part by a University of British Columbia Affiliated Fellowship and a NSERC Alexander Graham Bell Canada Graduate Scholarship.
Glossary
- CI
confidence interval
- EMG
electromyography
- FDI
first dorsal interosseous
- RMS
root mean square
Author contributions
B.L.L. and J.-S.B. contributed to the conception and design of the studies. B.L.L. conducted all of the experiments, analysed the data and prepared the initial draft. All authors contributed to the interpretation of data, critically revising the manuscript, and approving the final version to be published.
References
- Ali AS, Rowen KA, Iles JF. Vestibular actions on back and lower limb muscles during postural tasks in man. J Physiol. 2003;546:615–624. doi: 10.1113/jphysiol.2002.030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Frith CD, Wolpert DM. Spatio-temporal prediction modulates the perception of self-produced stimuli. J Cogn Neurosci. 1999;11:551–559. doi: 10.1162/089892999563607. [DOI] [PubMed] [Google Scholar]
- Blouin JS, Dakin CJ, van den Doel K, Chua R, McFadyen BJ, Inglis JT. Extracting phase-dependent human vestibular reflexes during locomotion using both time and frequency correlation approaches. J Appl Physiol. 2011;111:1484–1490. doi: 10.1152/japplphysiol.00621.2011. [DOI] [PubMed] [Google Scholar]
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Carleton SC, Carpenter MB. Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res. 1983;278:29–51. doi: 10.1016/0006-8993(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Carpenter MB. Vestibular nuclei: afferent and efferent projections. Prog Brain Res. 1988;76:5–15. doi: 10.1016/s0079-6123(08)64487-8. [DOI] [PubMed] [Google Scholar]
- Cathers I, Day BL, Fitzpatrick RC. Otolith and canal reflexes in human standing. J Physiol. 2005;563:229–234. doi: 10.1113/jphysiol.2004.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats AC. Effects of varying stimulus parameters on the galvanic body-sway response. Ann Otol Rhinol Laryngol. 1973;82:96–102. doi: 10.1177/000348947308200119. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Brooks JX, Jamali M, Carriot J, Massot C. Internal models of self-motion: computations that suppress vestibular reafference in early vestibular processing. Exp Brain Res. 2011;210:377–388. doi: 10.1007/s00221-011-2555-9. [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Inglis JT, Blouin JS. Short and medium latency muscle responses evoked by electrical vestibular stimulation are a composite of all stimulus frequencies. Exp Brain Res. 2011;209:345–354. doi: 10.1007/s00221-011-2549-7. [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Luu BL, van den Doel K, Inglis JT, Blouin JS. Frequency-specific modulation of vestibular-evoked sway responses in humans. J Neurophysiol. 2010;103:1048–1056. doi: 10.1152/jn.00881.2009. [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Son GM, Inglis JT, Blouin JS. Frequency response of human vestibular reflexes characterized by stochastic stimuli. J Physiol. 2007;583:1117–1127. doi: 10.1113/jphysiol.2007.133264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Fitzpatrick RC. Virtual head rotation reveals a process of route reconstruction from human vestibular signals. J Physiol. 2005;567:591–597. doi: 10.1113/jphysiol.2005.092544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol. 1994;478:363–372. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances. J Neurophysiol. 1996;76:3994–4008. doi: 10.1152/jn.1996.76.6.3994. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. 2004;96:2301–2316. doi: 10.1152/japplphysiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Taylor JL, McCloskey DI. Ankle stiffness of standing humans in response to imperceptible perturbation: reflex and task-dependent components. J Physiol. 1992;454:533–547. doi: 10.1113/jphysiol.1992.sp019278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawthrop P, Loram I, Lakie M. Predictive feedback in human simulated pendulum balancing. Biol Cybern. 2009;101:131–146. doi: 10.1007/s00422-009-0325-6. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Guerraz M, Day BL. Expectation and the vestibular control of balance. J Cogn Neurosci. 2005;17:463–469. doi: 10.1162/0898929053279540. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data–theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Holstein GR. The anatomy of the vestibular nuclei. Prog Brain Res. 2006;151:157–203. doi: 10.1016/S0079-6123(05)51006-9. [DOI] [PubMed] [Google Scholar]
- Kuo AD. An optimal state estimation model of sensory integration in human postural balance. J Neural Eng. 2005;2:S235–249. doi: 10.1088/1741-2560/2/3/S07. [DOI] [PubMed] [Google Scholar]
- Loram ID, Kelly SM, Lakie M. Human balancing of an inverted pendulum: is sway size controlled by ankle impedance. J Physiol. 2001;532:879–891. doi: 10.1111/j.1469-7793.2001.0879e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Lakie M. Direct measurement of human ankle stiffness during quiet standing: the intrinsic mechanical stiffness is insufficient for stability. J Physiol. 2002;545:1041–1053. doi: 10.1113/jphysiol.2002.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Human postural sway results from frequent, ballistic bias impulses by soleus and gastrocnemius. J Physiol. 2005;564:295–311. doi: 10.1113/jphysiol.2004.076307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Broberg C. Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand. 1983;117:307–309. doi: 10.1111/j.1748-1716.1983.tb07212.x. [DOI] [PubMed] [Google Scholar]
- Luu BL, Huryn TP, Van der Loos HF, Croft EA, Blouin JS. Validation of a robotic balance system for investigations in the control of human standing balance. IEEE Trans Neural Syst Rehabil Eng. 2011;19:382–390. doi: 10.1109/TNSRE.2011.2140332. [DOI] [PubMed] [Google Scholar]
- Moseley AM, Crosbie J, Adams R. Normative data for passive ankle plantarflexion-dorsiflexion flexibility. Clin Biomech. 2001;16:514–521. doi: 10.1016/s0268-0033(01)00030-4. [DOI] [PubMed] [Google Scholar]
- Nashner LM, Wolfson P. Influence of head position and proprioceptive cues on short latency postural reflexes evoked by galvanic stimulation of the human labyrinth. Brain Res. 1974;67:255–268. doi: 10.1016/0006-8993(74)90276-5. [DOI] [PubMed] [Google Scholar]
- Pavlik AE, Inglis JT, Lauk M, Oddsson L, Collins JJ. The effects of stochastic galvanic vestibular stimulation on human postural sway. Exp Brain Res. 1999;124:273–280. doi: 10.1007/s002210050623. [DOI] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol. 1989;53:1–31. doi: 10.1016/0079-6107(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Selective processing of vestibular reafference during self-generated head motion. J Neurosci. 2001;21:2131–2142. doi: 10.1523/JNEUROSCI.21-06-02131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci. 2004;24:2102–2111. doi: 10.1523/JNEUROSCI.3988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Qi Y, Hiraki K. Detection of visual feedback delay in active and passive self-body movements. Exp Brain Res. 2010;201:359–364. doi: 10.1007/s00221-009-2028-6. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- van der Kooij H, Jacobs R, Koopman B, Grootenboer H. A multisensory integration model of human stance control. Biol Cybern. 1999;80:299–308. doi: 10.1007/s004220050527. [DOI] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt H. The Behavioral Physiology of Animals and Man: The Collected Papers of Erich von Holst, Trans. Martin R. Methuen, London: 1973. The reafference principle; pp. 139–173. [Google Scholar]
- Walberg F, Dietrichs E. The interconnection between the vestibular nuclei and the nodulus: a study of reciprocity. Brain Res. 1988;449:47–53. doi: 10.1016/0006-8993(88)91022-0. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Colebatch JG. Vestibulospinal reflexes: quantitative effects of sensory feedback and postural task. Exp Brain Res. 2001;139:345–353. doi: 10.1007/s002210100754. [DOI] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J Neurophysiol. 1998;80:1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Halliday D, Jiang P, Liu X, Feng J. Detecting time-dependent coherence between non-stationary electrophysiological signals–a combined statistical and time-frequency approach. J Neurosci Methods. 2006;156:322–332. doi: 10.1016/j.jneumeth.2006.02.013. [DOI] [PubMed] [Google Scholar]


