Abstract
Nematodes use an extensive chemical language based on glycosides of the dideoxysugar ascarylose for developmental regulation (dauer formation), male sex attraction, aggregation, and dispersal. However, no examples of a female- or hermaphrodite-specific sex attractant have been identified to date. In this study, we investigated the pheromone system of the gonochoristic sour paste nematode Panagrellus redivivus, which produces sex-specific attractants of the opposite sex. Activity-guided fractionation of the P. redivivus exometabolome revealed that males are strongly attracted to ascr#1 (also known as daumone), an ascaroside previously identified from Caenorhabditis elegans hermaphrodites. Female P. redivivus are repelled by high concentrations of ascr#1 but are specifically attracted to a previously unknown ascaroside that we named dhas#18, a dihydroxy derivative of the known ascr#18 and an ascaroside that features extensive functionalization of the lipid-derived side chain. Targeted profiling of the P. redivivus exometabolome revealed several additional ascarosides that did not induce strong chemotaxis. We show that P. redivivus females, but not males, produce the male-attracting ascr#1, whereas males, but not females, produce the female-attracting dhas#18. These results show that ascaroside biosynthesis in P. redivivus is highly sex-specific. Furthermore, the extensive side chain functionalization in dhas#18, which is reminiscent of polyketide-derived natural products, indicates unanticipated biosynthetic capabilities in nematodes.
Keywords: chemical ecology, chemical signaling, peroxisomal β-oxidation, metabolomics
Chemical communication plays an important role in the biology of many free-living and parasitic nematodes (1). Recent studies have shown that a family of small-molecule signals, the ascarosides, control sexual attraction (2–5), aggregation (6, 7), olfactory plasticity (8), dauer formation (9–12), and dispersal (13) in the model organism Caenorhabditis elegans. Ascarosides (Fig. 1) are glycolipids derived from the dideoxysugar ascarylose, which in some cases, bear additional moieties, such as para-aminobenzoic acid and indole carboxy- or p-hydroxybenzoyl groups. The diversity of behavioral effects modulated by ascarosides in C. elegans is paralleled by complexity of ascaroside structures. Targeted metabolomics-based analysis of wild-type C. elegans exudates (i.e., the exometabolome) resulted in the detection of over 50 ascaroside components, many of which have no known function (14). Using LC-MS analysis, we have previously shown that ascaroside signaling is not restricted to C. elegans and is widely conserved among nematodes (15). Many free-living and parasitic nematode species were shown to release ascarosides. However, the species-specific functions of the identified ascarosides remain largely unknown. Moreover, this targeted analysis focused exclusively on ascarosides previously identified from C. elegans, and it seems likely that other nematode species produce additional ascarosides not present in C. elegans.
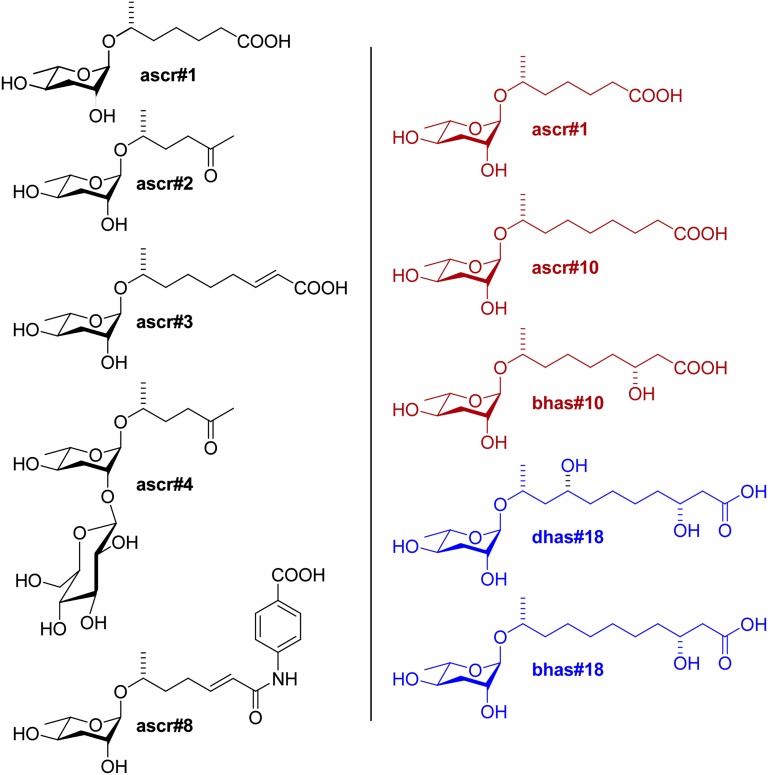
Fig. 1.
(Left) Select ascaroside-based signaling molecules that regulate development and behavior in C. elegans (black) (14). Ascr#1, ascr#2, ascr#3, and ascr#8 are dauer pheromone components (4, 11, 12); ascr#2, ascr#3, ascr#4, and ascr#8 synergize in male attraction (3, 4), and ascr#3 contributes to hermaphrodite repulsion (6, 7). (Right) Ascarosides discovered in P. redivivus from this study that are produced specifically by females (red) and males (blue).
In this study, we used activity-guided fractionation to isolate both male- and female-specific sex pheromones in the free-living sour-paste nematode Panagrellus redivivus. P. redivivus shares the same ecological niche as C. elegans but belongs to a different clade based on small subunit ribosomal DNA sequence comparisons (16). Ascaroside biosynthesis patterns correlate with phylogeny in some cases and lifestyle or ecological niche in other cases (15). In particular, species from Clade 9 (which include C. elegans) produce ascarosides with longer side chains containing 12–15 carbons, whereas species from Clade 10 (which include P. redivivus) lack such ascarosides (15). Because these studies did not take an activity-guided approach, it is unclear whether these ascarosides mediate species-specific mate-finding behavior.
In contrast to hermaphroditic C. elegans, which under laboratory conditions, produces more than 99.5% hermaphrodites and very few males, gonochoristic P. redivivus produces both females and males in roughly equal numbers. A previous study reported that P. redivivus virgin females attract and are attracted by males, but neither sex attracts itself (17). This finding is in contrast to C. elegans, in which males are attracted to hermaphrodites, but hermaphrodites show no apparent attraction to males (2). Here, we report that, in P. redivivus, both males and females use ascarosides as sex pheromones. We show that only females produce the male-attracting ascaroside, whereas only males produce the female-attracting ascaroside. Specifically, males are attracted to ascr#1, which is produced by females and not males, and females are attracted to dhas#18, a previously unreported ascaroside that is only produced by males and not females. Targeted metabolomic analyses of the male and female P. redivivus exometabolomes revealed sex-specific production of four additional ascarosides, which may represent biosynthetic intermediates or serve other signaling functions. The chemical structure of the female-attracting ascaroside shows previously unexpected biosynthetic capabilities in nematodes, and together with the structures of the other identified ascarosides, it indicates that sex pheromone biosynthesis in P. redivivus depends, in part, on sex-specific regulation of peroxisomal β-oxidation.
Results
Isolation of P. redivivus Sex-Specific Attractants.
We initially based our study on a report by Duggal (17) that showed the existence of both male- and female-specific pheromones in P. redivivus. We isolated 25 virgin female and 25 male young adult P. redivivus and incubated them in 25 μL M9 buffer for 6 h. Exudates were then tested in a two-spot bioassay, previously described in the work by Choe et al. (15), which indicated that males were attracted to female exudates, females were attracted to male exudates, and neither was attracted to exudates of their own sex (Fig. S1).
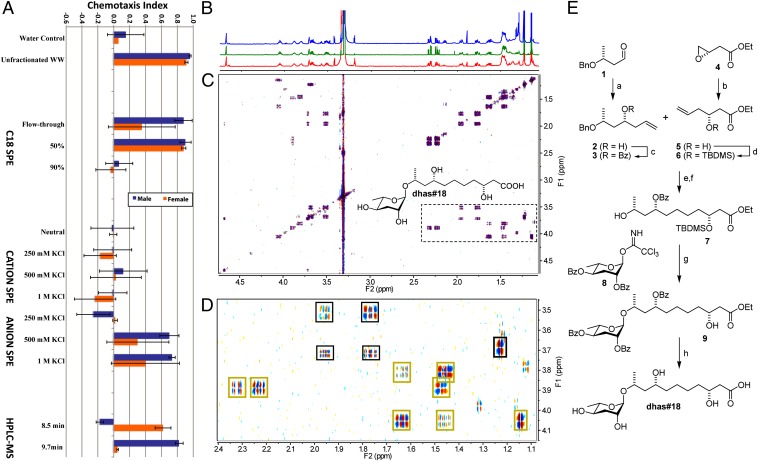
To identify the compounds responsible for P. redivivus sex-specific attraction, we used activity-guided fractionation (Fig. S2) of a preparation called worm water (WW), in which we transfer ∼4,000,000 worms at a specific developmental stage from a semisynchronized liquid bacterial coculture to double distilled water and collect the worm exudates for a prescribed period (3). This amount of starting material was necessary to isolate and identify the active components described below. Detailed methods used to generate the large number of worms are provided in SI Text. P. redivivus WW from mixed sex worms was tested for activity and found to attract both males and females as expected. We then fractionated the WW, according to protocols previously developed for the C. elegans mating pheromone (3). The WW was initially fractionated by lipophilicity using a C18 solid-phase extraction column (Fig. S2). Males and females were both attracted to the flow-through and 50% methanol (MeOH) fractions, but they did not respond to the 90% fraction. Based on previous studies with C. elegans, the flow-through fraction contains many common metabolites, such as amino acids and sugars (18, 19); some of these metabolites are attractive to nematodes (20), whereas the 50% MeOH fraction is enriched in ascarosides (3, 5, 21). We, therefore, focused our attention on the 50% MeOH fraction, which was further fractionated using ion exchange solid-phase extraction. The desalted 500 mM and 1 M KCl fractions from the anion exchange column had the greatest activity for both male and female attraction, and therefore, we analyzed the combined fractions by high-performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC-ESI-MS) in positive ion mode using a C18 column to separate the components. We observed two major total ion chromatogram peaks that eluted at 8.5 and 9.7 min with m/z values of 382 [M+NH4]+ and 294 [M+NH4]+, respectively. We assayed all of the HPLC fractions by making several different combinations, and we found that the 8.5 min peak selectively attracted females and the 9.7 min peak selectively attracted males (Fig. 2A); however, none of the other combined fractions showed any significant activity. Using samples isolated by preparative HPLC, we collected 1D and 2D NMR datasets as well as LC-MS/MS data on both compounds.
Fig. 2.
Identification of ascr#1 and dhas#18 as sex pheromones in P. redivivus. (A) Activity-guided fractionation of P. redivivus WW. (B) 1H NMR spectra of isolated natural dhas#18 (red), synthetic dhas#18 (green), and a mixture of natural and synthetic samples (blue). (C) dqf-COSY spectrum of isolated dhas#18. (D) Section of the dqf-COSY spectrum in C showing cross-peaks diagnostic of the ascarylose ring (black boxes) and the highly functionalized side chain (yellow boxes). (E) Synthesis of dhas#18. a, 1. TiCl4, 2. allylSn(PPh3); b, vinylmagnesium bromide, Me2S-CuBr, tetrahydrofuran (THF); c, BzCl, pyridine; d, t-butyldimethylsilyl chloride (TBDMSCl), imdidazole, dichloromethane (DCM); e, Grubbs II, 1,4-benzochinone, DCM; f, H2, Pd/C, EtOH; g, trimethylsilyl triflate (TMSOTf), DCM; h, aqueous LiOH, THF, dioxane.
Identification of the Male-Attracting Signal.
Using a combination of HPLC-MS and NMR techniques, the male-specific attractant was readily identified as 6-(3′,5′-dihydroxy-6’-methyltetrahydropyran-2’-yloxy)-heptanoic acid, more commonly known as ascr#1, daumone, or C7 (the first ascaroside that was originally identified in C. elegans) (9). To verify the identity of ascr#1 as the male attractant in P. redivivus and quantify the amount produced by worms under laboratory conditions, we spiked the natural sample with synthetic ascr#1 and collected LC-MS and NMR data that were indistinguishable from the data of natural ascr#1 (Fig. S3).
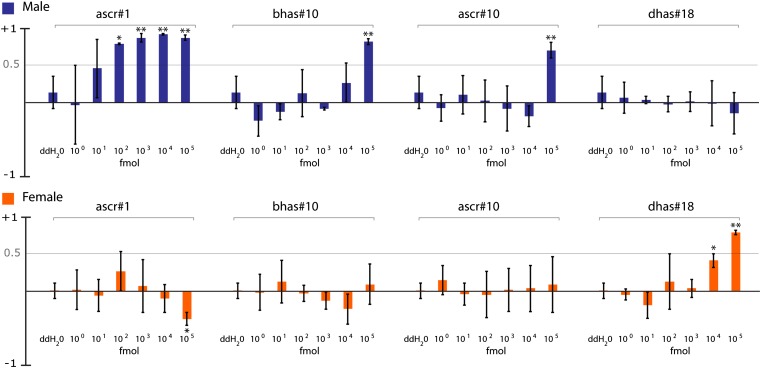
Using the two-spot assay, we tested several concentrations of synthetic ascr#1, similar to the concentrations derived from worms, on P. redivivus males and females (Fig. 3). Consistent with the activity-guided fractionation results, we found that ascr#1 caused strong male attraction, with measurable responses to amounts as little as 10 fmol ascr#1 (Fig. 3). Unlike C. elegans males, which show a bell-shaped concentration-dependent response (3, 5) to different mixtures of ascr#2, ascr#3, ascr#4 (3), and ascr#8 (4) (Fig. 1), P. redivivus males responded robustly to ascr#1 as a single component, and the response did not decrease with increasing concentrations of attractant. Considering that ascr#1 has only very weak dauer-forming activity in C. elegans (9) and no apparent mating attraction activity (3), these results indicate that a given ascaroside might serve different functions in different nematodes. We also tested ascr#1 on P. redivivus females and found that high concentrations repelled females. This finding is similar, for example, to the repulsion of C. elegans hermaphrodites to hermaphrodite-derived ascr#2 and ascr#3 (3).
Fig. 3.
Dose–response for male and female attraction to major P. redivivus ascarosides. P. redivivus males and females were separately scored for their response to areas conditioned with different amounts of synthesized ascarosides (100–105 fmol). Their occupancy in the area containing the ascaroside was compared with occupancy in the control region (containing the same concentration of ethanol in water as the ascaroside dilution for each trial) for the duration of 20 min. Experiments in which males or females spent significantly more time in the control region have a negative index score, indicating avoidance of the ascaroside. Experiments in which males or female spent significantly more time in the ascaroside region have a positive index score, indicating attraction to the ascaroside. Error bars are SD. Statistical significance for each value was calculated compared with response to ddH2O when present in both scoring regions, which was shown first in each set. (One-factor ANOVA followed by Dunnett’s posttest; *P < 0.05, **P < 0.01.)
Identification of the Female-Attracting Signal.
The female-attracting P. redivivus pheromone seemed to have a mass of 364 Da. High-resolution MS yielded a mass of 387.2022 for the sodium adduct (positive-ion ESI), which indicated a molecular formula of C17H32O8 (calculated mass of the sodium adduct 387.1989). Both the NMR spectra and MS fragmentation patterns suggested an ascaroside-based structure (Fig. S4). Analysis of the double quantum filtered-correlated spectroscopy (dqf-COSY) NMR spectrum further suggested a β-hydroxyacid fragment and a (ω-1)-linkage between the side chain and the ascarylose sugar (Fig. 2 B–D). The remaining alkyl bridge connecting these two fragments, thus, had the formula C6H12O and included a secondary hydroxygroup as judged from the NMR data. Careful analysis of the high-resolution dqf-COSY spectrum revealed a (ω-3)-position of this hydroxyl group and thus, led to the proposal of 10-(3′,5′-dihydroxy-6’-methyltetrahydro-pyran-2’-yloxy)-3,8-dihydroxy-undecanoic acid. We named this compound dhas#18, because it constitutes the 3,8-dihydroxyderivative of the known ascr#18 (14) (Fig. S4).
Confirmation of these structural assignments as well as elucidation of the absolute configuration of dhas#18 required developing a chemical synthesis for this compound. Considering the absolute stereochemistry of the 3,8,10-trihydroxyundecanoic acid-derived side chain, we assumed a 10R-configuration, because all l-ascarylose–linked ascarosides identified so far have been found to share the same configuration at the (ω-1)-position. Furthermore, we assumed an 8R-configuration based on careful examination of H-H coupling constants derived from the dqfCOSY spectrum (Table S1), whereas the 3R-configuration was anticipated based on the fact that enoyl-CoA hydratase (MAOC-1) from C. elegans, which introduces the 3-hydroxy group during peroxisomal β-oxidation, exhibits highest homology to 3R-selective enoyl-CoA hydratase (14).
We consequently synthesized ethyl (10R)-benzyloxy-(8R)-benzoyloxy-(3R)-tert-butyldimethylsilyloxy-undec-5E-enoate by Grubbs' ruthenium-catalyzed olefin cross-metathesis of 3 and 6, which were obtained from the chiral synthons 1 and 4 as previously described (22, 23) (Fig. 2E). Hydrogenation of this compound afforded the selectively deprotected side chain (7) that was coupled to di-O-benzoyl-protected ascarylose to give, after deprotection and hydrolysis, dhas#18 (Fig. 2E and Fig. S5).
Synthetic dhas#18 was shown to be indistinguishable from natural material isolated from P. redivivus by coinjection of natural and synthetic samples in HPLC-MS analyses (Fig. S4 A and B). In addition, we analyzed a mixture of natural and synthetic dhas#18 by NMR spectroscopy, which revealed one single set of peaks, indicating that natural and synthetic materials have the same relative configuration (Fig. 2 B and C and Fig. S4D). Finally, we verified the absolute stereochemistry of natural dhas#18 by preparation of the O-methyl ester and subsequent conversion to the tetra-(R)-Mosher ester, which was compared by 1H NMR with O-methyl tetra-(R)- and (S)-Mosher esters of synthetic dhas#18. Next, we tested dhas#18 on both female and male P. redivivus responses using our two-spot assay. Males were not attracted to a wide range of dhas#18 concentrations, whereas females were attracted to as little as 100 nmol dhas#18 (Fig. 3).
Targeted Analysis Identifies Additional Ascarosides.
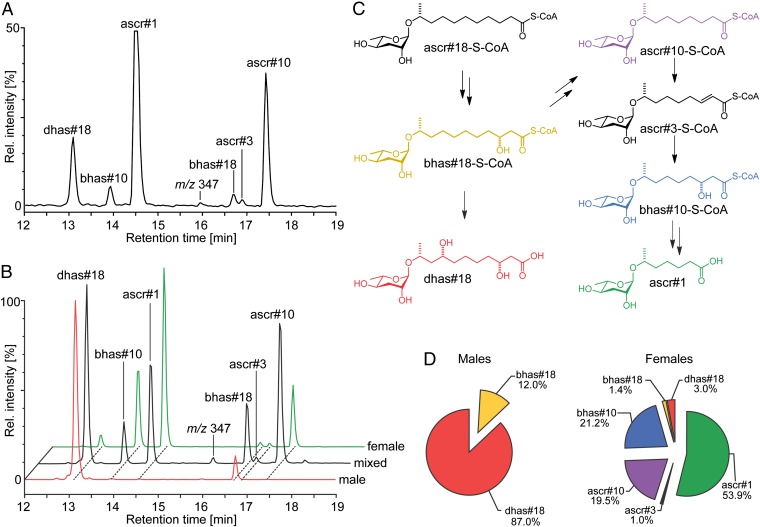
Having established ascaroside structures for both the male and female attractant, we used a targeted HPLC-MS/MS-based approach to screen P. redivivus exudates for the presence of additional ascarosides. We have previously shown that ascarosides can be selectively detected by screening for precursor ions of m/z = 73 in negative ion HPLC-ESI-MS/MS (14). Analysis of P. redivivus WW using the same protocol revealed the presence of ascr#1 (15) and dhas#18 identified in the activity-guided fractionation along with a variety of additional components, such as ascr#10, ascr#3, bhas#18, and bhas#10 (Fig. 4), which we isolated from P. redivivus liquid culture supernatant and verified their structures by NMR. Trace quantities of ascr#9 (C5 side chain), ascr#12 (C6 side chain), ascr#14 (C8 side chain), and ascr#18 (C11 side chain) were also detected. Because bhas#10 and ascr#10 composed a significant proportion of the female ascaroside blend (Fig. 4D), we tested these synthesized ascarosides for male or female attraction. We found that high concentrations (105 fmol) of both bhas#10 and ascr#10 attracted males but not females (Fig. 3). The ascarosides bhas#10 and ascr#10 may comprise components of the male sex attractant; however, our results show that they are required at much higher amounts than ascr#1.
Fig. 4.
Sex-specific expression of ascarosides in P. redivivus. (A) HPLC-MS/MS screen for precursor ions of m/z = 73 selectively detects ascarosides. (B) SIR for molecular ions of ascarosides detected by MS/MS in P. redivivus samples reveals sex-specific expression of ascarosides. Shown chromatograms are derived from mixed populations (black), females (green), and males (red). (C) Proposed biosynthetic pathway for ascaroside biosynthesis in P. redivivus. (D) Distribution of major ascaroside in male and female WW samples.
Sex-Specific Ascaroside Expression Suggests Sex-Specific Regulation of Peroxisomal β-Oxidation.
Because our activity-guided fractionation as well as our MS/MS analysis relied on exudates of mixed sex populations, it was initially unclear whether there was sex-specific production of ascr#1, dhas#18, both, or neither. To determine whether male and female attractants are both produced by the opposite sex and hence, represent bona fide sex pheromones, we compared P. redivivus mixed populations with male-only and female-only samples using HPLC with MS in the selective ion recording (SIR) mode (Fig. 4B). SIR scanning for ascaroside masses previously detected by MS/MS revealed that expression of ascarosides in P. redivivus is highly sex-specific (Fig. 4 B and D). Most importantly, we found that both male and female attractants are almost exclusively produced by the opposite sex. Ascarosides exhibiting longer side chains (C11), such as the female-attracting dihydroxy ascaroside dhas#18 and its putative monohydroxylated precursor bhas#18, were almost exclusively detected in the exudates of male worms. The very small amount of these components detected in female samples likely originates from incomplete separation of different sexes. Furthermore, ascarosides with shorter side chains (C7–C9), such as the male-attracting ascr#1, were exclusively detected in female exudates.
We have previously shown that ascaroside biosynthesis in C. elegans proceeds through peroxisomal β-oxidation of long-chain precursors (24), which involves four biosynthetic steps catalyzed by acyl-CoA oxidase, enoyl-CoA hydratase, (3R)-hydroxyacyl-CoA dehydrogenase, and 3-ketoacyl-CoA thiolase (14). The structures of the major ascarosides in P. redivivus showing odd-numbered side chains with 7, 9, or 11 carbons suggest that a homologous β-oxidation pathway is operating in P. redivivus (Fig. 4C). Consequently, the sex-specific distribution of ascarosides in male and female P. redivivus (Fig. 4D) suggests that sex-specific ascaroside biogenesis depends in part on differential regulation of peroxisomal β-oxidation. In a possible model, a CoA thioester of ascr#18 (ascr#18 was detected in trace quantities) is β-hydroxylated during peroxisomal β-oxidation to afford the S-CoA derivative of bhas#18, which represents the key branching point for sex-specific ascaroside biosynthesis in P. redivivus. In females, up to two additional cycles of chain-shortening by peroxisomal β-oxidation afford the potent male-attracting ascr#1 (7 carbon side chain) along with ascr#10, ascr#3, and bhas#10 as side products (9 carbon side chain). However, in males, chain-shortening of bhas#18 seems to be stalled, possibly because of sex-specific differences in (3R)-hydroxyacyl-CoA dehydrogenase activity. Instead, bhas#18 or its S-CoA derivative is selectively hydroxylated at the unusual (ω-3)-position, presumably by male-specific cytochrome P450 activity, to afford the dihydroxylated dhas#18. In conclusion, our results suggest that chemical communication through ascaroside-based sex pheromones in P. redivivus depends on sex-specific regulation of a primary metabolic pathway and that these sex-dependent differences in peroxisomal β-oxidation affect β-hydroxyacyl-CoA oxidase and (ω-3)-hydroxylase activity acting on bhas#18 or its S-CoA derivative as a common precursor to all ascarosides identified in males and females.
Discussion
Mate finding is critical for the reproduction of obligate gonochoristic species such as P. redivivus. Nematodes have adapted several different reproductive modes, including gonochorism (male–female mating), self-fertilizing hermaphroditism, heterogeny (alternating gonochorism and hermaphrodism), and parthenogenesis (lack of sperm in reproduction). Surveys of rhabditids show that most of known species are gonochoristic and that gonochorism is the ancestral form of reproduction (25). Hermaphrodism is thought to have evolved independently 10 times within rhabditids (25), suggesting that, in nematodes, the molecular underpinnings of sex and reproduction are flexible. A mate-finding cue is likely more important in gonochoristic species than hermaphrodites.
We have previously shown that hermaphroditic C. elegans produces a diversity of ascarosides (7, 14) and that mixtures of ascr#2, ascr#3, ascr#4, and ascr#8 can synergistically act as male-specific attractants at very low concentrations (3–5). However, hermaphrodite-specific attraction to males has not been reported in C. elegans.
In this study, we have elucidated the sex-specific pheromones for a gonochoristic nematode, including the identification of a female-specific nematode attractant. Using activity-guided fractionation, we have identified two ascarosides, ascr#1 and dhas#18, as highly potent male- and female-specific attractants in the gonochoristic P. redivivus, respectively. Ascr#1 is exclusively produced by females, specifically attracts males, and repels females. Ascr#1 was originally isolated as the dauer-inducing pheromone of C. elegans, but subsequent studies showed that ascr#1 was, at best, a minor constituent of the dauer pheromone and that two other ascarosides, ascr#2 and ascr#3, were ∼100× more potent than ascr#1 in dauer formation (12). We have recently detected ascr#1 in various rhabditids, but its function in these species remains unknown (15). Our current finding that ascr#1 is a potent male attractant and a female repellent in P. redivivus, thus, shows that a given ascaroside can serve different functions in different nematode species. These results would suggest the possibility that ascarosides could also function as allelochemicals between different nematode species occupying overlapping habitats. However, knowledge on nematode ecology and interspecific nematode interactions is still very limited, and therefore, testing of this hypothesis is difficult.
The previously unreported ascaroside dhas#18, a dihydroxyderivative of the known ascr#18, is exclusively produced by males and specifically attracts females. In addition to a 3R-hydroxy group, which most likely originates from peroxisomal β-oxidation, dhas#18 also carries an additional R-configured hydroxy function at the (ω-3)-position of the side chain, which shows previously unexpected biosynthetic capabilities in nematodes. Because ascarosides seem to be widespread among nematodes from widely diverged clades, species-specific modifications of the ascaroside core structure may be used to ensure that essential intraspecific communication processes (e.g., mating) remain species-specific.
Using targeted metabolomics, we have identified sex-specific production of four additional ascarosides: ascr#10, ascr#3, bhas#18, and bhas#10. They may represent biosynthetic intermediates or shunt metabolites, but they may also serve other unidentified signaling functions. Lastly, the sex-specific production of ascarosides indicates that sex pheromone biosynthesis in P. redivivus depends, in part, on sex-specific regulation of a conserved primary metabolic pathway, peroxisomal β-oxidation.
Methods
OP50 Escherichia coli was grown on a standard 5-cm agar plate (made with standard Nematode Growth Medium). The 16-mm bacterial lawn was grown overnight at 20 °C before being used in trials. Two 4-mm spots (0.6 μL) were placed on opposite sides of the bacterial lawn (using a transparent template to guide spot placement), and several minutes were allowed to elapse for the liquid to settle in before placing nematodes down on the assay. Recording began immediately on worm placement; 0.6 μL control were placed on one side of the lawn, and 0.6 μL experimental cue were placed on the other side of the lawn; the location of the cue was changed throughout trials between left/right and top/bottom to avoid bias. Nematodes were isolated by sex at the L4 stage the day before being used in trials as developed adults. Worms were evenly divided and placed at two points equidistant from the foci of the scoring region. Trials were recorded for 20 min, and frames were collected for analysis at one frame per 1 s. Results were averaged from at least three different trials. We used the Automated Software described in the work by Choe et al. (15) to compare worm occupancy in each scoring region over time and then adapted the Chemotaxis Index described in the work by Bargmann et al. (26) to score preference or avoidance to each ascaroside. One-factor ANOVA followed by Dunnett’s posttest was used; ascarosides were grouped according to dosage per sex (*P < 0.05, **P < 0.01).
Activity-guided purification of ascr#1 and dhas#18 was conducted as previously described for the purification of mating cues in C. elegans (3). Details on large-scale cultures for P. redivivus and NMR and LC-MS spectra are given in SI Text. The syntheses of dhas#18 and Mosher esters to establish the absolute configuration are provided in SI Text.
Targeted and sex-specific identifications of ascr#1, ascr#10, bhas#10, bhas#18, and dhas#18 were done according to previously described methods (14).
Supplementary Material
Acknowledgments
We thank James R. Rocca for expert assistance in NMR data collection and interpretation. NMR data were collected in the University of Florida Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) Facility, which is supported in part by the National Science Foundation-funded National High Magnetic Field Laboratory. Support for this study was from National Institutes of Health Grant R01GM085285.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218302109/-/DCSupplemental.
References
- 1.Huettel RN. Chemical communicators in nematodes. J Nematol. 1986;18(1):3–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Simon JM, Sternberg PW. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99(3):1598–1603. doi: 10.1073/pnas.032225799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srinivasan J, et al. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454(7208):1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pungaliya C, et al. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2009;106(19):7708–7713. doi: 10.1073/pnas.0811918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan F, et al. Ascaroside expression in Caenorhabditis elegans is strongly dependent on diet and developmental stage. PLoS One. 2011;6(3):e17804. doi: 10.1371/journal.pone.0017804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macosko EZ, et al. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458(7242):1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasan J, et al. A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 2012;10(1):e1001237. doi: 10.1371/journal.pbio.1001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada K, et al. Olfactory plasticity is regulated by pheromonal signaling in Caenorhabditis elegans. Science. 2010;329(5999):1647–1650. doi: 10.1126/science.1192020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong PY, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433(7025):541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 10.Butcher RA, Ragains JR, Clardy J. An indole-containing dauer pheromone component with unusual dauer inhibitory activity at higher concentrations. Org Lett. 2009;11(14):3100–3103. doi: 10.1021/ol901011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butcher RA, Ragains JR, Kim E, Clardy J. A potent dauer pheromone component in Caenorhabditis elegans that acts synergistically with other components. Proc Natl Acad Sci USA. 2008;105(38):14288–14292. doi: 10.1073/pnas.0806676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3(7):420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan F, et al. Interspecific nematode signals regulate dispersal behavior. PLoS One. 2012;7(6):e38735. doi: 10.1371/journal.pone.0038735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Reuss SH, et al. Comparative metabolomics reveals biogenesis of ascarosides, a modular library of small-molecule signals in C. elegans. J Am Chem Soc. 2012;134(3):1817–1824. doi: 10.1021/ja210202y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choe A, et al. Ascaroside signaling is widely conserved among nematodes. Curr Biol. 2012;22(9):772–780. doi: 10.1016/j.cub.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Megen H, et al. A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology. 2009;11:927–950. [Google Scholar]
- 17.Duggal CL. Sex attraction in the free-living nematode Panagrellus redivivus. Nematologica. 1978;24:213–221. [Google Scholar]
- 18.Robinette SL, et al. Hierarchical alignment and full resolution pattern recognition of 2D NMR spectra: Application to nematode chemical ecology. Anal Chem. 2011;83(5):1649–1657. doi: 10.1021/ac102724x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan F, et al. Bacterial attraction and quorum sensing inhibition in Caenorhabditis elegans exudates. J Chem Ecol. 2009;35(8):878–892. doi: 10.1007/s10886-009-9670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward S. Chemotaxis by the nematode Caenorhabditis elegans: Identification of attractants and analysis of the response by use of mutants. Proc Natl Acad Sci USA. 1973;70(3):817–821. doi: 10.1073/pnas.70.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edison AS. Caenorhabditis elegans pheromones regulate multiple complex behaviors. Curr Opin Neurobiol. 2009;19(4):378–388. doi: 10.1016/j.conb.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keck GE, Murry JA. Total synthesis of (-)-colletol. J Org Chem. 1991;56(23):6606–6611. [Google Scholar]
- 23.Evans PA, Andrews WJ. A sequential two-component etherification/oxa-conjugate addition reaction: Asymmetric synthesis of (+)-leucascandrolide A macrolactone. Angew Chem Int Ed Engl. 2008;47(29):5426–5429. doi: 10.1002/anie.200801357. [DOI] [PubMed] [Google Scholar]
- 24.Butcher RA, et al. Biosynthesis of the Caenorhabditis elegans dauer pheromone. Proc Natl Acad Sci USA. 2009;106(6):1875–1879. doi: 10.1073/pnas.0810338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiontke K, Fitch DH. The phylogenetic relationships of Caenorhabditis and other rhabditids. WormBook. 2005;11:1–11. doi: 10.1895/wormbook.1.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74(3):515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.