Abstract
Overexpression of CD24, a glycosyl phosphatidylinositol-linked sialoglycoprotein, is associated with poor outcome in urothelial carcinoma and contributes to experimental tumor growth and metastasis. However, the requirement for CD24 (Cd24a in mice) in tumorigenesis and spontaneous metastasis from the orthotopic site remains uncharacterized. Using N-butyl-N-(4-hydroxybutyl) nitrosamine induction of invasive and metastatic bladder cancer, we show that Cd24a-deficient male mice developed fewer bladder tumors than C57BL/6 control male mice. Evaluating only mice with evidence of primary tumors, we observed that Cd24a-deficient male mice also had fewer metastases than wild-type counterparts. In parallel observations, stratification of patients based on CD24 immunohistochemical expression in their tumors revealed that high levels of CD24 are associated with poor prognosis in males. In female patients and mice the above observations were not present. Given the significant role of CD24 in males, we sought to assess the relationship between androgen and CD24 regulation. We discovered that androgen receptor knockdown in UM-UC-3 and TCCSUP human urothelial carcinoma cell lines resulted in suppression of CD24 expression and cell proliferation. Androgen treatment also led to increased CD24 promoter activity, dependent on the presence of androgen receptor. In vivo, androgen deprivation resulted in reduced growth and CD24 expression of UM-UC-3 xenografts, and the latter was rescued by exogenous CD24 overexpression. These findings demonstrate an important role for CD24 in urothelial tumorigenesis and metastasis in male mice and indicate that CD24 is androgen regulated, providing the foundation for urothelial bladder cancer therapy with antiandrogens.
Keywords: carcinogenesis, genetically engineered mice
Bladder cancer represents a significant public health burden (1) and is the most costly cancer to treat in the Medicare population (2). Bladder cancer presents as both superficial (noninvasive) and invasive tumors (3); the invasive phenotype heralds metastasis in nearly half of diagnosed patients. An emerging molecule of interest in bladder cancer is CD24 (4, 5). CD24 is a glycosylated, mucin-like protein (6) which is rarely expressed in normal tissue (7). However, CD24 is detected in a wide range of carcinomas and has prognostic significance (8). CD24 is integral to tumor cell proliferation and anchorage-independent growth (4, 9), both hallmarks of cancer development (10). Additionally, CD24 is linked to tumor cell invasion (11) and metastatic progression (5, 12). Notably, Cd24a-deficient mice have no increased incidence of spontaneous cancer (13–15).
We sought to evaluate the contribution of CD24 in bladder cancer incidence and progression within a defined genetic model by exposing Cd24a-deficient mice to a carcinogen. Because carcinogens, such as those found in cigarette smoke (16), are the primary causative agents for bladder cancer, using a carcinogenesis-based animal model approximates the spontaneous tumor formation and progression of this disease. To initiate this carcinogenesis, we fed mice N-butyl-N-(4-hydroxybutyl) nitrosamine (hereafter, OH-BBN), a carcinogen known to induce bladder cancer selectively (17, 18). Molecular characterization of OH-BBN–induced tumors reveals that their genetic profile resembles that of invasive human bladder cancer (19, 20). Notably, this model system also accurately recapitulates the increased incidence of bladder cancer found in males (21). This predilection to males persists despite considering differential exposure to environmental and occupational risk factors such as cigarette smoking and aromatic amines (16, 22), the primary risk factors for bladder cancer in humans (23). Interestingly, a recent meta-analysis suggests a role for additional factors in the etiology of human bladder cancer (24), such as hormonal effects, that could explain the male preponderance of bladder cancer (3:1 ratio) (1, 16) observed in human epidemiology studies (21, 25).
Accordingly, the results from our study suggest that CD24 plays a significant role in bladder tumorigenesis and metastasis, especially in males. These data also implicate CD24 as an important androgen-regulated effector in male human bladder cancer and thus provide a rationale for novel therapeutic approaches targeting the androgen receptor (AR) in male bladder cancer.
Results
Bladder Tumor Incidence Is Reduced in Cd24a-Deficient Mice.
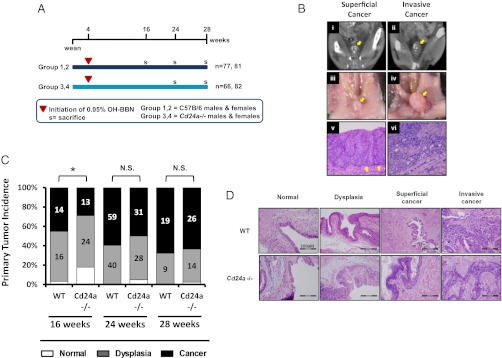
To study the effect of CD24 deficiency on the incidence of bladder tumors, we exposed paired cohorts of Cd24a-deficient and WT C57BL/6 control mice to water containing OH-BBN, ad libitum (Fig. 1A). Results from serial micro-computed tomography (mCT) imaging with subsequent necropsy and pathologic evaluation suggested that 16-, 24-, and 28-wk assessment points following OH-BBN initiation were optimal for stratifying tumor progression (Fig. 1B). The incidence of cancer was lower in Cd24a-deficient mice (29%) than in WT mice (45%) at 16 wk (Fig. 1C) and remained lower in Cd24a-deficient mice at 24 and 28 wk. Bladder tissues from Cd24a-deficient and WT mice, when matched for tumor grade, displayed no discernible histopathological differences (Fig. 1D).
Fig. 1.
Germline Cd24a loss is associated with reduced incidence of OH-BBN–induced bladder cancer. (A) Schematic of the experimental design for assessing bladder tumor incidence. WT and Cd24a-deficient (Cd24a−/−) mice were started on OH-BBN ∼4 wk after weaning. Successive mCT imaging of pilot study animals suggested the use of assessment points at 16, 24, and 28 wk for best assessment of tumor development, so cohorts were analyzed for the presence of bladder tumors at those time points. (B) Corresponding mCT scans (Top), necropsy images (Middle), and histological preparations (Bottom) collected at 24 wk. Images i, iii, and v are of a superficial (i.e., noninvasive) bladder tumor; ii, iv, and vi are of invasive tumor. Arrows indicate the bladder wall in i–iv and the basement membrane in v. L, lumen of bladder. (C) Comparison of tumor incidence over time in populations of WT and Cd24a-deficient mice using binary and logistic regression models revealed that the incidence of malignancy was significantly higher in WT mice than in Cd24a-deficient mice. *P < 0.05. N.S., not significant. (D) Representative H&E-stained bladder tissues displayed no differences in histology between grade-matched WT and Cd24a−/− tissues.
Cd24a mRNA and Protein Expression Are Increased in OH-BBN–Induced Bladder Cancer.
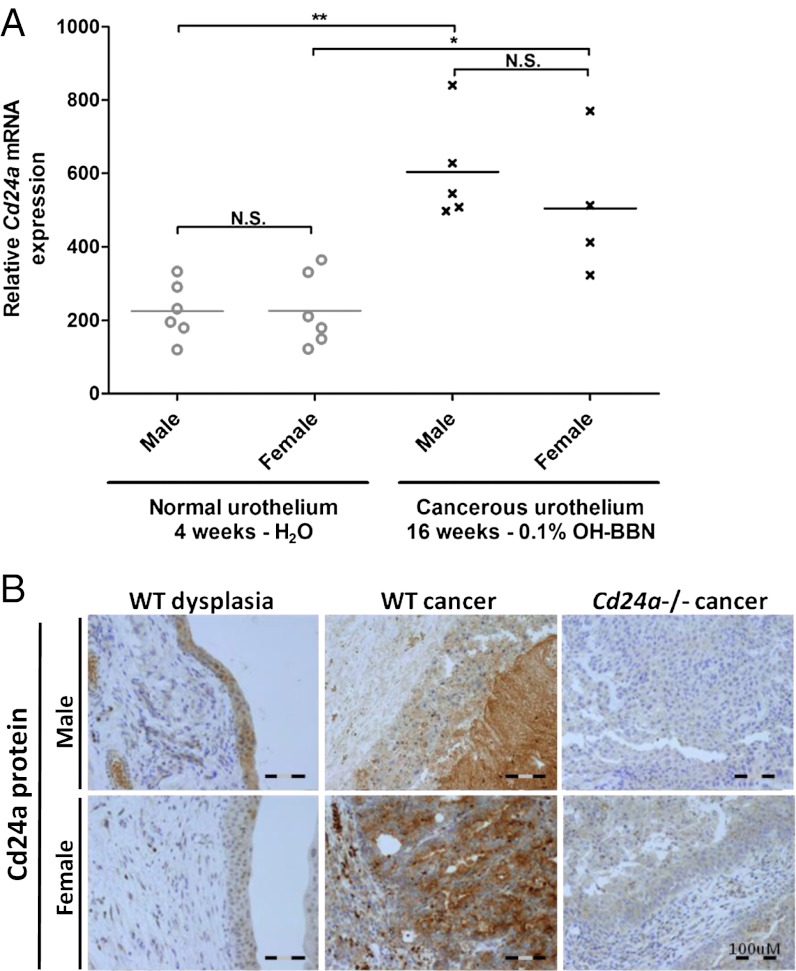
Because loss of Cd24a appears to protect bladder urothelium from OH-BBN–induced tumorigenesis, we sought to determine if Cd24a expression increased in OH-BBN–induced cancers compared with normal bladder tissue. Cd24a mRNA levels in urothelium samples from mice exposed to OH-BBN in drinking water were analyzed using quantitative RT-PCR (qRT-PCR). We found that Cd24a mRNA levels were significantly elevated in cancerous urothelium from both male and female mice exposed to OH-BBN as compared with normal urothelium (P < 0.01 and P < 0.05, respectively by Mann–Whitney test) (Fig. 2A). This finding was consistent with other studies (26) that have associated CD24 overexpression with malignant transformation.
Fig. 2.
OH-BBN–induced bladder tumors have high levels of Cd24a mRNA and protein expression. (A) C57BL/6 WT mice (n = 21) were provided with normal drinking water until age 4 wk; then 12 mice were killed, and the remaining nine mice were exposed to drinking water containing OH-BBN ad libitum for an additional12 wk. Bladders from each cohort were processed for histological and qRT-PCR analysis. Cd24a mRNA analysis revealed that cancerous urothelium from the cohort exposed to OH-BBN had significantly more Cd24a than comparative control normal urothelium, regardless of the sex of the mice. **P < 0.01, *P < 0.05; Mann–Whitney test; N.S., not significant. (B) The expression of Cd24a protein increases as a function of tumor progression. After exposure to OH-BBN, bladders from WT mice with cancer were found to express higher levels of Cd24a protein than dysplastic counterparts (n = 17). Cancerous urothelial samples from Cd24a-deficient mice were stained similarly to control for antibody specificity (n = 4).
In parallel with our assessment of Cd24a mRNA levels, we used immunohistochemical analysis to evaluate whether Cd24a protein levels were increased as a function of histopathologic findings. Compared with dysplastic tissue from WT mice, WT cancerous urothelium demonstrated significantly higher levels of Cd24a staining (Fig. 2B). Urothelium from male and female mice did not express a quantifiable difference in Cd24a. Cancerous urothelium from Cd24a-deficient mice showed no appreciable staining. Urothelial tissue obtained from WT mice without histologically apparent tumors also showed no evidence of Cd24a staining. Thus, Cd24a mRNA and protein levels increased as urothelial cells were transformed into a cancerous pathology, consistent with CD24 being a driver for tumorigenesis.
Cd24a Deficiency in Male Mice Delays Bladder Malignancy.
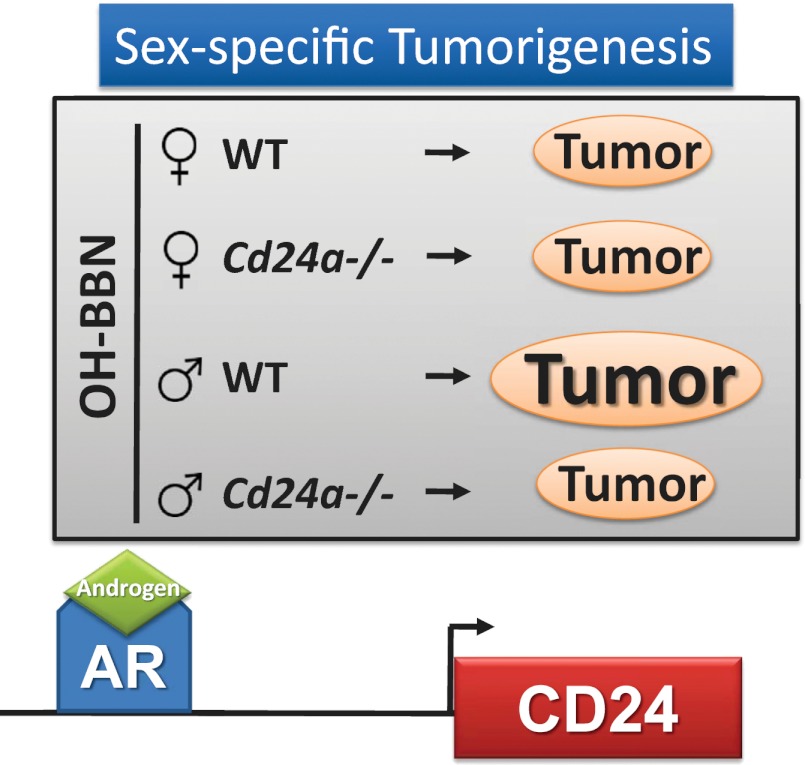
Human bladder cancer rates are higher in males (16, 22). Similarly, OH-BBN–induced bladder tumor rates are higher in male WT mice than in female WT mice (21, 25). Consistent with these observations, our studies found that after 16-wk exposure to OH-BBN males had a greater proportion of malignant tumors (56%) and fewer normal results (0%) than females (33% and 7%, respectively) (Table 1). Based on ordinal logistic regression models, these differences were statistically significant (P = 0.050). Similar results were found at 24 wk, although the differences were not significant (P = 0.059). Expanding these studies to look at the effect of Cd24a deficiency on these rates revealed that the cancer incidence was significantly lower in Cd24a-deficient male mice than in WT mice at all time points (Table 1). Thus, loss of CD24 is protective against OH-BBN–induced bladder cancer in male mice but not in female mice.
Table 1.
Histological analysis of bladders from C57B/6 control (WT) and Cd24a-deficient mice exposed to OH-BBN
| WT male, n (%) | Cd24a−/− male, n (%) | WT female, n (%) | Cd24a−/− female, n (%) | |
| 16 wk | ||||
| Normal | 0 (0) | 5 (21) | 1 (7) | 3 (14) |
| Dysplasia | 7 (44) | 11 (46) | 9 (60) | 13 (62) |
| Cancer | 9 (56*†) | 8 (33†) | 5 (33*) | 5 (24) |
| 24 wk | ||||
| Normal | 0 (0) | 1 (3) | 0 (0) | 2 (6) |
| Dysplasia | 14 (30) | 16 (54) | 26 (49) | 12 (38) |
| Cancer | 32 (70†) | 13 (43†) | 27 (51‡) | 18 (56‡) |
| 28 wk | ||||
| Normal | 0 (0) | 0 (0) | 0 (0) | 1 (3) |
| Dysplasia | 3 (20) | 4 (33) | 6 (46) | 10 (35) |
| Cancer | 12 (80†) | 8 (67†) | 7 (54‡) | 18 (62‡) |
| Total | 77 | 66 | 81 | 82 |
*Increased proportion of WT males with advanced disease compared with WT females at 16 wk (P = 0.05; χ2 for trend).
†Increased proportion of WT males with advanced disease compared with Cd24a−/− males (P < 0.05; χ2 for trend).
‡No statistically significant difference between WT females with advanced disease and Cd24a−/− females.
Cd24a Deficiency Reduces Metastasis in Male Mice Harboring Primary Tumors.
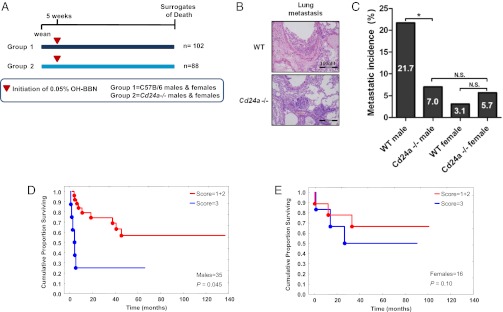
Previous studies have shown that CD24 expression is higher in nodal metastases than in matched primary tumors (4, 5). To determine whether Cd24a deficiency would limit metastatic incidence and burden, new cohorts of Cd24a-deficient and WT mice subjected to OH-BBN carcinogenesis were monitored until they reached previously established experimental surrogates of death (Fig. 3A). To ensure that our evaluation of metastatic incidence was independent of tumor occurrence, only mice with evidence of primary bladder cancer by histopathologic analysis were included in our assessment of metastatic frequency. Histological analysis of lung metastasis samples revealed no apparent differences in tumor architecture between metastases obtained from WT and from Cd24a-deficient mice of either sex (Fig. 3B). In analyzing metastatic incidence by sex, we found that the incidence of metastasis was higher in male WT mice than in female WT mice (Fig. 3C), similar to previous reports (21). In accordance with data on tumor incidence, we also observed that loss of Cd24a resulted in dramatic protection against metastatic incidence. The incidence of metastasis was significantly lower in Cd24a-deficient male mice (7%) than in WT (21.7%) male mice (P = 0.039, one-tailed Wald test). Cd24a-deficient female mice did not show a reduction in metastatic incidence relative to WT female mice. There was no statistical difference in metastatic incidence between male and female Cd24a-deficient mice (Fig. 3C).
Fig. 3.
Loss of Cd24a decreases metastasis in male mice. (A) Schematic of the experimental design for assessing metastatic incidence. WT (n = 102) and Cd24a-deficient (n = 88) mice were provided OH-BBN until they reached previously established surrogates of death (cachexia >20% of control animal weight, lethargy/behavioral changes, or overt respiratory/general distress). (B) Lung metastatic tissues from Cd24a-deficient and WT mice were stained by H&E and showed similar histological architecture. (C) Of animals with documented primary tumors, WT males had higher rates of metastasis (21.7%) than WT females (3.1%). Additionally, Cd24a-deficient males had decreased metastatic incidence (7.0%) relative to their WT counterparts. *P = 0.039; one-tailed Wald test. Comparisons of WT and Cd24a-deficient females showed no statistically significant difference (N.S.) in metastasis. (D) Evaluation of a previously reported tissue microarray with immunohistochemical staining for CD24 (4). Stratification of disease-free survival data from human bladder cancer patients as a function of patient sex and CD24 expression level. The red line indicates a score of 1 or 2; the blue line indicates a score of 3. [Scoring was described previously (4)]. Kaplan–Meier analysis suggests that CD24 is an independent prognostic factor for disease-free survival in males (P < 0.05).
Tumor CD24 Expression Is Prognostic of Outcomes in Bladder Cancer Patients.
Based on the reduction of OH-BBN–induced bladder metastases in Cd24a-deficient mice, we reevaluated a previously reported tissue immunohistochemical analysis of CD24 in human bladder cancer samples (4) to determine if CD24 expression has sex-specific prognostic significance in bladder cancer patients. Male patients with tumors expressing higher levels of CD24 had shorter disease-free survival times than males whose tumors expressed lower levels of CD24 (Fig. 3D), even in a small (n = 35) patient cohort (P = 0.045). This correlation with high CD24 expression was not as apparent in female patients (P = 0.10) (Fig. 3E). Our limited human cohort study corroborates results from the murine metastasis model and supports the hypothesis that CD24 is an important contributor to the development of bladder metastasis in males and, to a lesser extent, in females. Thus, loss of CD24/Cd24a appears to protect against both tumorigenesis and metastasis in male mice and in humans.
Androgen-Induced Growth Is Dependent on CD24 Expression in UM-UC-3 Male Bladder Tumor Cells.
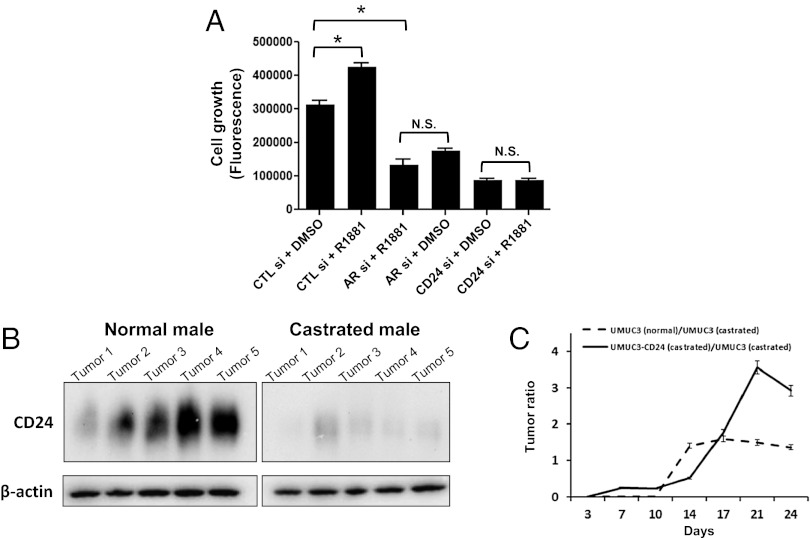
Interestingly, males appear to be more protected than females by loss of CD24/Cd24a even though the sexes exhibit similar CD24/Cd24a mRNA and protein levels (Fig. 2B). One explanation for this sex difference is that CD24/Cd24a may function in a signaling network that is less important in females than in males. Androgen regulation is the most broadly studied of such pathways. Therefore we investigated androgen-mediated cell proliferation in the context of CD24 depletion to assess the importance of CD24/Cd24a in androgen-induced cellular growth. UM-UC-3 cells were treated with CD24 siRNA and analyzed for differential growth with alamarBlue following treatment with R1881. Consistent with previous studies (25, 27, 28), R1881 induced the growth of control cells (Fig. 4A). In contrast, neither CD24-silenced cells nor AR-silenced cells exhibited significant changes in proliferation after R1881 treatment. These findings suggest that CD24 has a significant role in androgen-mediated growth in bladder tumors.
Fig. 4.
Tumor growth promoted by androgen signaling is dependent on CD24 in vitro and in vivo. (A) Relative growth of UM-UC-3 cells as assessed by alamarBlue 72 h after indicated treatments and siRNA transfections. Treatment with R1881 increases growth compared with DMSO. Transfection of cells with either AR or CD24 siRNA inhibits R1881-induced growth. *P < 0.05. (B) Xenograft in vivo tumorigenesis model. Normal and castrated male nude mice (n = 10) were injected s.c. with 5 × 105 UM-UC-3 cells. After 4 wk, tumors were resected and evaluated for CD24 protein expression with human CD24-specific antibodies. Tumors isolated from normal male mice expressed higher levels of CD24 than tumors resected from castrated male mice. (C) Normal and castrated male nude mice (n = 10) were injected s.c. with 5 × 105 UM-UC-3 cells that had been vector transfected or CD24 transfected as described previously (5). Graph represents tumor size over time. Data show that castration can reduce growth of UM-UC-3 tumors and that stable exogenous expression of CD24 in UM-UC-3 cells can rescue this growth reduction. Error bars indicate SEM.
To assess whether androgen signaling mediates CD24 expression in vivo, we used a previously developed xenograft model of bladder cancer (5). Normal and castrated male mice were injected s.c. with 5 × 105 UM-UC-3 cells, which express moderate levels of CD24 (5). Tumors resected from control male mice after 4 wk showed distinctly higher expression of CD24 than tumors resected from castrated male mice (Fig. 4B). Thus, in vivo, in the absence of androgen (i.e., with castration), CD24 expression is reduced dramatically. Additional analysis of these mice revealed that tumors generated in normal male mice were larger than tumors grown in castrated male mice (Fig. 4C, dotted line). To investigate further the in vivo functional impact of CD24 as an effector of androgen-mediated tumor growth, we compared xenografts from parental UM-UC-3 cells and from UM-UC-3 cells that stably overexpress CD24 (5). In this setting, overexpression of CD24 could restore tumor growth characteristics that previously had been reduced by castration (Fig. 4C, solid line). Thus, CD24 appears to have a pronounced functional role as a downstream effector in androgen-mediated growth signaling in bladder cancer.
CD24 mRNA and Protein Expression Are Androgen Dependent in Male Bladder Cancer Cells.
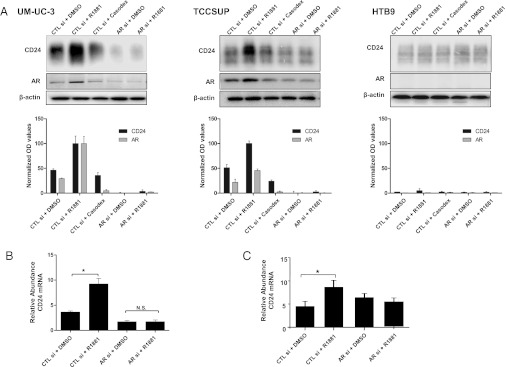
Previous studies using a similar OH-BBN carcinogenesis model suggest that both AR and androgen signaling function in the development of bladder cancer, both in vitro and in vivo (25). Given that Cd24a deficiency resulted in fewer malignant bladder tumors in male mice and that AR depletion or blockade reduced tumor growth in a manner dependent on CD24 expression, we questioned whether CD24 may function as part of an androgen-signaling cascade. To address this question, three bladder cancer cell lines were analyzed based on their AR expression status: AR-expressing UM-UC-3 and TCCSUP cells and HTB9 (5637) cells that do not express AR (25). Activation of AR signaling using R1881 (synthetic androgen) treatment in UM-UC-3 and TCCSUP cells led to a 2.5- and twofold induction of AR protein, respectively (Fig. 5A). Strikingly, R1881 treatment also led to a 2.2- and 3.1-fold induction in CD24 protein expression. Treatment of the cell lines with Casodex or siRNA to silence AR expression reduced both AR and CD24 protein expression in UM-UC-3 and TCCSUP cells. Furthermore, R1881 treatment failed to induce CD24 expression in AR-silenced UM-UC-3 and TCCSUP cells. These observations strongly suggest that androgen signaling controls CD24 expression. The expression of CD24 in AR-null HTB9 cells was not affected by R1881-, Casodex-, or siRNA-mediated AR silencing (Fig. 5A).
Fig. 5.
CD24 mRNA and protein expression is regulated by androgen. (A) UM-UC-3, TCCSUP, and HTB9 cells were transfected with control (CTL si) or AR siRNA (AR si) for 48 h before being treated with DMSO, R1881 (1 nM), or Casodex (30 µM). After 24 h, lysates were collected and analyzed for CD24 and AR protein expression. OD values obtained with Alpha Innotech software (normalized for protein loading using β-actin staining) are plotted below the corresponding immunoblots. Representative blots from three separate experiments are shown. (B and C) Total RNA extracted from UM-UC-3 (B) and TCCSUP (C) cells in A was analyzed for CD24 expression by qRT-PCR. Treatment with R1881 increases CD24 expression, an effect that is abrogated when cells are transfected first with AR siRNA. *P < 0.05.
To assess whether these androgen-induced changes in CD24 protein levels also occurred at the mRNA level, qRT-PCR was performed on total RNA from the UM-UC-3 cells used above for immunoblotting. Here, we observed that R1881 significantly stimulates CD24 mRNA expression in UM-UC-3 (Fig. 5B) and TCCSUP cells (Fig. 5C), whereas siRNA silencing of AR prevents this increase (P < 0.05). Together these results suggest that androgen and AR function in controlling expression of CD24 at the point of transcription.
AR Activation Stimulates CD24 Promoter Activity in Human Bladder Cancer Cells.
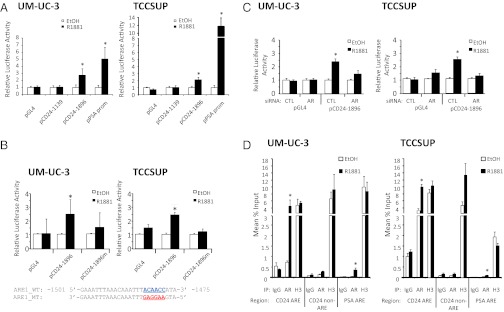
To test the hypothesis that androgen controls CD24 transcription we generated luciferase reporter constructs containing roughly 1 kbp and 2 kbp of the CD24 promoter upstream from the start codon. UM-UC-3 cells were transiently transfected with each of these reporter constructs and incubated with or without R1881 for 24 h. Although none of the reporter constructs exhibited an increase in basal activity relative to control (pGL4), R1881 treatment induced a 2.8-fold increase in the activity of a 1,896-bp CD24 promoter (Fig. 6A). R1881 also induced an increase in the activity of a prostate-specific antigen (PSA) promoter construct, as expected (29). The ability of R1881 treatment to induce CD24 promoter activity also was observed in TSSCUP cells (Fig. 6A).
Fig. 6.
Androgen signaling promotes activation of the CD24 promoter. (A) Two CD24 promoters of different length fused to a luciferase reporter were tested for sensitivity to androgen treatment. The plasmids were transiently transfected into UM-UC-3 or TCCSUP cells and subsequently were incubated with or without R1881 for 24 h. The PSA promoter reporter was used as a positive control, and the GL4 empty vector was used as a negative control for response to R1881. *P < 0.05. (B) The ARE in the 1,896-bp promoter was mutated (pCD24-1896m); this mutation blocks R1881-induced activity of the CD24 promoter. The precise base pairs of the ARE in the CD24 promoter are shown. (C) The 1,896-bp reporter no longer responds to R1881 when cells are treated with AR siRNA. (D) ChIP with AR antibody confirms that AR associates with the CD24 promoter at the aforementioned ARE. Positive controls included histone H3 antibody and PSA ARE-based primers. Mouse IgG and primers targeting a non-ARE element in the CD24 promoter were used as negative controls.
Interestingly, the shorter CD24 promoter construct (1,139 bp) did not exhibit any change in activity following R1881 treatment, suggesting that an androgen-response element (ARE) lies between 1,139 and 1,896 bp upstream of the CD24 start codon. Analysis of this region of the CD24 promoter by Genomatix MatInspector software suggested the presence of an ARE in this region. We subsequently mutated six base pairs within this element and tested the ability of R1881 to promote activity. We found that R1881 induction was abolished in the mutated reporter in both UM-UC-3 and TCCSUP cell lines (Fig. 6B), suggesting that androgen-activated AR directly activates CD24 transcription. As a further test of this hypothesis, we investigated the ability of R1881 to increase CD24 promoter activity in the absence of AR. Treatment of both UM-UC-3 and TCCSUP with AR siRNA abrogates the R1881-dependent increase in CD24 promoter activity (Fig. 6C). Together these in vitro studies suggest a model in which androgen activation of AR results in an AR- and ARE-dependent increase in CD24 promoter activity.
To test this model using the endogenous AR and CD24 promoter, we used ChIP on cells treated with or without R1881. Nonspecific mouse IgG and histone H3 antibodies served as negative and positive controls, respectively. We found that AR was associated with the aforementioned ARE within the CD24 promoter but only in an R1881-dependent manner (Fig. 6D). Similarly, AR was associated with the known ARE in the promoter of PSA. However, AR was not associated with a region of the CD24 promoter that did not contain a computationally predicted ARE. Taken together, these findings demonstrate that there is an ARE about 1.5 kbp upstream from the CD24 transcription start site and that androgen-activated AR forms a complex with this DNA element to promote an increase in CD24 transcription.
Discussion
CD24 is a cell-surface protein that functions as a mediator of proliferation, invasion, and motility in multiple types of cancer cell lines from multiple tissues (4, 11, 12, 30, 31). Previous efforts to investigate the role of CD24 in the primary tumor setting have been limited to using exogenous overexpression and suppression in xenograft models. In this study, the role of CD24 in bladder tumor formation and metastasis was analyzed using Cd24a-deficient mice to model better the natural history of bladder cancer in a more physiologic setting. Mice were exposed to OH-BBN, a validated and commonly used model of bladder cancer induction (17, 21). Treatment of mice with OH-BBN resulted in progressive neoplastic development similar to that seen in prior reports (19, 21, 32). We observed fewer malignant lesions in Cd24a-deficient mice than in WT mice, demonstrating that Cd24a deficiency inhibits tumor development in an OH-BBN model of bladder cancer. This finding is consistent with a role of CD24 as a driver of bladder cancer and is supported by previous in vivo data showing that CD24-specific antibodies offer therapeutic benefit against bladder tumor xenograft formation (5). Interestingly, further analysis of the OH-BBN data by sex revealed that only males showed significant decreases in tumor incidence with loss of Cd24a.
Investigating the effect of Cd24a deficiency on metastatic incidence revealed that loss of Cd24a in male mice resulted in a nearly threefold decrease in metastatic incidence relative to WT male mice. This finding was independent of the observed reduction of tumor incidence, because only mice with evidence of bladder tumors were included in this metastasis analysis. This important finding demonstrates that CD24 plays a role in both cancer formation and metastasis. A similar trend was seen in female mice when metastatic incidence and loss of Cd24a were analyzed, but the results were not statistically significant.
Assessment of human tumor samples revealed a similar relationship between CD24 expression and metastatic recurrence after radical primary therapy: High CD24 expression in tumors from males was associated with a reduction in disease-free survival. Because metastasis is rarely curable and metastasis of bladder tumors is strongly correlated with death in patients with bladder cancer, this observation is consistent with the mouse metastasis data. A similar trend was observed in female patients. Taken together, these findings show that CD24/Cd24a expression levels in males correlate to a high degree with metastatic recurrence and that this protein is important in tumor formation. CD24/CD24a expression levels do not correlate with tumor formation rates in female mice but may play a role in metastasis in female mice and humans. The ability of CD24 to mediate metastasis is consistent with prior data on this molecule (5, 11, 12, 33).
The finding that CD24/CD24a affects tumor formation and metastasis differentially in males and females is surprising, particularly given the similar expression levels of CD24a mRNA and protein in male and female normal and malignant murine urothelium. In males, loss of CD24a in mice or a lower level of CD24 in human tumor samples correlates with protection against both tumor formation and metastatic recurrence. Although the data suggest that female mice appear to gain protection from metastasis development with lower CD24a levels, this protective effect was not observed during primary tumor formation. This observation suggests that the role of CD24a in tumor formation is less important in females and may be muted by a more dominant, female-specific pathway or that the downstream effectors of CD24 found in males are not expressed or functional in females.
That hormones may play a role in regulating sex-specific tumorigenesis in bladder cancer was shown by Miyamoto et al. (25), who used the OH-BBN model to demonstrate that both androgen signaling-mediated pathways and AR are important in promoting tumorigenesis. Moreover, they reported that androgen signaling may regulate the expression of molecules involved in angiogenesis and metastasis. Given our observations that Cd24a-deficient male mice are preferentially protected from OH-BBN–induced tumorigenesis and metastasis, we decided to investigate whether CD24 is responsive to androgen regulation. Because multiple groups have demonstrated AR expression in normal urothelium and AR signals in bladder cancer cells from both males and females (34–36), our model system provides an excellent platform for investigating the ability of androgen and AR to regulate CD24 expression. We discovered that androgen signaling increases CD24 promoter activity, yielding higher CD24 mRNA and protein expression. Furthermore, diminished tumor xenograft growth as a result of depleted androgen was offset by exogenous overexpression of CD24 in vivo. Because CD24 depletion reduces cellular proliferation to the same extent as AR knockdown, our results suggest that the ability of androgen and AR to promote tumorigenesis in males is dependent on CD24 expression. The studies presented here also demonstrate that other, non-androgen/AR mechanisms up-regulate CD24, because female mice exhibit similar levels of CD24 in normal and cancerous urothelium even though androgen is much lower in females. Ongoing investigative efforts in our laboratory are focused on analyzing differences in gene expression between the sexes, with a particular focus on genes that correlate strongly with hormone expression. These efforts are aimed at identifying sex-specific CD24 effectors to understand better the role of CD24 in bladder tumor formation in males. Currently, the effectors that allow CD24 to execute its cancerous agenda have not been identified. The observations made here reveal an avenue to discover these effectors.
Finally, because therapeutic targeting of CD24 has proven beneficial in multiple xenograft models of human bladder (5), colon, and pancreatic (37) cancer, our work provides a rationale for the use of androgen deprivation as a therapeutic modality to reduce CD24 expression in males with CD24-dependent cancers. Furthermore, because oral antiandrogens (such as Casodex) have been shown to be safe for prolonged use (38), clinical investigation of their use in patients with bladder cancer, either in conjunction with therapy for metastatic disease or to prevent the development of metastatic disease in high-risk patients (39), appears warranted.
Materials and Methods
Experimental Animals, Cell Lines, and PCR.
Mice deficient for Cd24a (Cd24a−/−), the murine homolog of CD24, were previously developed in a C57BL/6 background using embryonic stem cells as described (14, 15) and were a gift of Yang Liu (University of Michigan Comprehensive Cancer Center, Ann Arbor, MI). WTC57BL/6 mice of equivalent age and sex were used as controls. All animals were monitored under protocols approved by the National Institutes of Health and the University of Virginia Animal Care and Use Committee (IACUC). We used a set of three primers: neo-rev primer (5′-GCCATGATGGATACTTTCTCG-3′), an upstream flanking primer (5′-ACCGCGAGAGTTTGTGCAGTC-3′), and a CD24 exon primer (5′-GGTTGCTGCTTCTGGCACTG-3′) for confirmatory genotyping PCR of the WT and Cd24a-deficient colonies. The PCR protocol using Platinum Taq DNA Polymerase and High Fidelity PCR buffer (Invitrogen) was 30 cycles of 94 °C for 45 s, 61 °C for 45 s, and 72 °C for 1 min. Resultant products were 945 bp for the Cd24a-deficient allele and 416 bp for the WT allele, as visualized by electrophoresis on 1.1% agarose gel (Fig. S1). After OH-BBN treatment, mRNA was isolated from sample tissues and evaluated by qRT-PCR, according to previously described methods using the primers CD24cds_F (5′-CTGCTGGCACTGCTCCTAC) and CD24cds_R (5′-GGTGGTGGCATTAGTTGGAT) (5).
UM-UC-3 and TCCSUP human urothelial carcinoma cell lines were cultured as described (5), and 5637 (HTB9) cells (40) were cultured using RPMI with 10% FBS (Gibco) supplementation. Select cells were transfected with siRNA targeting luciferase (negative control), AR, and CD24. AR siRNA was designed against the following sequence in the 3′-untranslated region: 5′-GATGTCTTCTGCCTGTTAT-3′ (41). CD24 siRNA was directed against the following sequence, as previously described: 5′-CAACTAATGCCACCACCAA-3′ (4). Cells subsequently were treated with DMSO, 0.5 nM R1881 (Sigma-Aldrich), or 30 uM Casodex (AstraZeneca). Immunoblotting analysis was performed with antibodies detecting human CD24 (SWA11; a gift of Peter Altevogt, German Cancer Research Center Heidelberg, Germany), AR (Cell Signaling Technologies), and β-actin (Sigma) using previously described techniques, including optical density normalization with Alpha Innotech software based on relative β-actin expression (4). Cell proliferation was assessed using alamarBlue (Invitrogen) 24 h after androgen modulation treatment with R1881 or Casodex. UM-UC-3 cells obtained from ATCC were cultured and injected s.c. into the flank regions of castrated or normal NCrnu/nu mice according to previously described protocols (5).
Carcinogenesis Treatment Protocol.
Male and female 7-wk-old Cd24a-deficient mice and WT C57BL/6 control mice were supplied ad libitum with tap water containing 0.1% OH-BBN (TCI America); bottles were refreshed twice a week. Mice were inspected weekly and observed for signs of distress associated with bladder lesions, including hematuria and firm bladders. Cohorts of treated Cd24a-deficient and WT mice were killed at 16-, 24-, and 28-wk time points. To evaluate disease-specific survival, animals were killed when they exhibited IACUC-accepted humane surrogates of death (cachexia >20% of control animal weight, lethargy/behavioral changes, or overt respiratory/general distress). We harvested bladder, periaortic lymph nodes, liver, lung, and kidney tissues. A portion of each tissue was preserved in phosphate-buffered 10% formalin and paraffin embedding for eventual sectioning and staining with H&E. The remaining tissues were snap frozen in liquid nitrogen and stored at −80 °C for RNA and protein analyses.
CD24 Promoter, Reporter Assays, and Computational Binding-Site Predictions.
Two previously published CD24 promoter sequences (42, 43) showed considerable mismatch between each other and a partial promoter from GenBank. Therefore we cloned and sequenced the CD24 promoter from several distinct human cell lines to identify a common sequence for our studies as described (44). The sequences can be found in GenBank with the following accession numbers: UM-UC-3 CD24 promoter, JN565036; J82 CD24 promoter, JN565037; LNCaP CD24 promoter, JN565040; LuL-2 CD24 promoter, JN565041; and EJ CD24 promoter, JN565042.
In this study we used the promoter from UM-UC-3 cells called “CD24-1896,” representing −1936 to −30 of CD24 5′ UTR from UM-UC-3 cells. Use of Genomatix MatInspector software (Genomatix Software GmbH) on CD24-1896 suggested that an ARE is located at −1501 to −1475. Mutation of this site was executed according to instructions from the site-directed mutagenesis protocol (Stratagene). Primers were AREmS: 5′-CTA-ACG-AGA-GTG-TTC-TGC-AAA-ACT-GAA-ATT-TAA-ACA-AAT-TTG-AGT-CCA-TAC-TAG-GTT-TGG-TCT-CTG-3′ and AREmAS: 5′- CAG-AGA-CCA-AAC-CTA-GTA-TGG-ACT-CAA-ATT-TGT-TTA-AAT-TTC-AGT-TTT-GCA-GAA-CAC-TCT-CGT-TAG-3′. The PSA promoter construct has been described previously (45). DNA transfections of UM-UC-3 or TCCSUP cells were carried out using Lipofectamine (Invitrogen) and 1 µg of plasmid. For specific experiments, siRNA (20 pmol) against AR or luciferase was cotransfected with DNA. After 24 h cell medium was replaced with medium containing charcoal-stripped serum. Cells then were treated with R1881 (10 nM) or vehicle (ethanol). After 24 h cells were lysed and assayed for luciferase activity according to the manufacturer’s instructions (Promega). Luciferase values were normalized to cell number generated from Cyquant analysis (Life Technologies).
ChIP.
UM-UC-3 and TCCSUP cells were grown to 70% confluence in two 15-cm dishes per treatment. Medium was removed, and fresh medium containing charcoal-stripped serum and R1881 or EtOH was added for 2 h. Then, according to instructions in the SimpleChIP Enzymatic Chromatin IP Kit (Cell Signaling), paraformaldehyde was added directly to cell medium (1% final concentration) and quenched with 1 M glycine at room temperature, and cells (4 × 107) were collected. The chromatin was sheared using Micrococcal Nuclease and sonicated for three 10-s pulses at 60% output and 50% duty cycle. Overnight ChIP was set up using 10 µg of chromatin and antibody (histone H3, AR, or control IgG). After purification of the immunoprecipitated DNA, three-step qRT-PCR was used to quantify the DNA levels using an iQ5 thermocycler and iQ SYBR Green Supermix (Bio-Rad). Primers used were CD24-ARE S: 5′-TGA-ACC-AAA-AAT-ACT-AAC-GAG-AGT-G-3′; CD24-ARE AS: 5′-AGC-AAA-CAG-CAG-AGA-CCA-AA-3′; CD24-upstream S: 5′-TCA-AGT-GTG-AAA-ATG-TTC-TC-3′; CD24-upstream AS: 5′-CGA-AGT-GTT-CCT-GTT-TAA-AT-3′; PSA-ARE S: 5′-CTG-CCT-TTG-TCC-CCT-AGA-T-3′; and PSA-ARE AS: 5′-AAC-CTT-CAT-TCC-CCA-GGA-CT-3′ (46). The input cycle threshold (Ct) values were adjusted to 100% efficiency by subtracting 5.64 from each Ct value because the starting input fraction was 2% of the total immunoprecipitation. All Ct values subsequently were adjusted to percent of input using the equation 100*2(adjusted input Ct − sample Ct).
Imaging and Histological Analysis of Murine and Human Tissues.
Serial mCT scans were acquired 10 min after the administration of 0.3 mmol/kg Gd- diethylene triamine pentaacetic acid (DTPA) (Magnevist). The images were acquired using 512 slices of a 512 × 512 pixel 2D matrix and a reconstructed pixel element with a spatial resolution of 0.17 mm3. Murine tissues were examined as a series of 20-μm sections and were reviewed by a pathologist (H.F.F.) blinded to genotype origins of the sample mouse tissues. Histological tissue architectures were classified as normal, dysplasia, and cancer (encompassing carcinoma in situ). Samples of murine tissues were analyzed with immunohistochemical analysis using rat anti-mouse Cd24a antibody (M169 clone; BD Pharmingen). CD24 immunohistochemistry on human tissues, the specific tissue microarray used, and stratification techniques were reported previously (4).
Statistical Analysis.
Binary and proportional odds ordinal logistic regression models (47) were used to estimate the effects of CD24a deficiency vs. WT, sex, and time (e.g., 16 wk vs. 24 wk) on the incidence of normal, dysplasia, and cancer cells in bladder tissue samples. The binary and ordinal models included main effects for CD24a deficiency vs. WT, sex, and time and all two-way interactions. The score test was used to check the proportional odds assumption, resulting in a P value of 0.28, indicating that the data displayed no significant deviation from the proportional odds assumption. Logistic regression models also were used to assess metastatic incidence by sex and Cd24a deficiency. Specific comparisons were made with one-sided Wald tests because of our hypothesis that Cd24a deficiency would limit metastases. The log-rank test was used to correlate patient disease-free survival with CD24 expression levels. For the luciferase assay and ChIP data, a mixed-model ANOVA with a random effect for date of replicate experiment was used, and the Levene’s test was used to check for homogeneity of variances among groups. Preplanned contrasts were used to test for significant differences between EtOH- and R1881-treated groups. Statistical analysis was carried out with Prism (GraphPad Software); for the binary and ordinal models, SAS 9.2 (SAS Institute, Cary, NC) was used.
Supplementary Material
Acknowledgments
We thank Drs. Christopher Moskaluk and Pat Pramoonjago for their technical support. This study was supported by National Institutes of Health (NIH) Grant CA075115 (to D.T.) and NIH Cancer Research Training in Molecular Biology Award 5T32CA009109 (to J.B.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper can be found in GenBank database [JN565036 (UM-UC-3 CD24 promoter); JN565037 (J82 CD24 promoter); JN565040 (LNCaP CD24 promoter); JN565041 (LuL-2 CD24 promoter); and JN565042 (EJ CD24 promoter)].
See Author Summary on page 20788 (volume 109, number 51).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113960109/-/DCSupplemental.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Botteman MF, Pashos CL, Hauser RS, Laskin BL, Redaelli A. Quality of life aspects of bladder cancer: A review of the literature. Qual Life Res. 2003;12:675–688. doi: 10.1023/a:1025144617752. [DOI] [PubMed] [Google Scholar]
- 3.Wu X-R. Urothelial tumorigenesis: A tale of divergent pathways. Nat Rev Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 4.Smith SC, et al. The metastasis-associated gene CD24 is regulated by Ral GTPase and is a mediator of cell proliferation and survival in human cancer. Cancer Res. 2006;66:1917–1922. doi: 10.1158/0008-5472.CAN-05-3855. [DOI] [PubMed] [Google Scholar]
- 5.Overdevest JB, et al. CD24 offers a therapeutic target for control of bladder cancer metastasis based on a requirement for lung colonization. Cancer Res. 2011;71:3802–3811. doi: 10.1158/0008-5472.CAN-11-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kay R, Rosten PM, Humphries RK. CD24, a signal transducer modulating B cell activation responses, is a very short peptide with a glycosyl phosphatidylinositol membrane anchor. J Immunol. 1991;147:1412–1416. [PubMed] [Google Scholar]
- 7.Kristiansen G, Sammar M, Altevogt P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol. 2004;35:255–262. doi: 10.1023/b:hijo.0000032357.16261.c5. [DOI] [PubMed] [Google Scholar]
- 8.Lee J-H, Kim S-H, Lee E-S, Kim Y-S. CD24 overexpression in cancer development and progression: A meta-analysis. Oncol Rep. 2009;22:1149–1156. doi: 10.3892/or_00000548. [DOI] [PubMed] [Google Scholar]
- 9.Sagiv E, Arber N. The novel oncogene CD24 and its arising role in the carcinogenesis of the GI tract: From research to therapy. Expert Rev Gastroenterol Hepatol. 2008;2:125–133. doi: 10.1586/17474124.2.1.125. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 11.Mierke CT, Bretz N, Altevogt P. Contractile forces contribute to increased glycosylphosphatidylinositol-anchored receptor CD24-facilitated cancer cell invasion. J Biol Chem. 2011;286:34858–34871. doi: 10.1074/jbc.M111.245183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann P, et al. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005;65:10783–10793. doi: 10.1158/0008-5472.CAN-05-0619. [DOI] [PubMed] [Google Scholar]
- 13.Li O, Zheng P, Liu Y. CD24 expression on T cells is required for optimal T cell proliferation in lymphopenic host. J Exp Med. 2004;200:1083–1089. doi: 10.1084/jem.20040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen PJ, et al. Altered erythrocytes and a leaky block in B-cell development in CD24/HSA-deficient mice. Blood. 1997;89:1058–1067. [PubMed] [Google Scholar]
- 15.Wenger RH, et al. B-cell maturation in chimaeric mice deficient for the heat stable antigen (HSA/mouse CD24) Transgenic Res. 1995;4:173–183. doi: 10.1007/BF01968782. [DOI] [PubMed] [Google Scholar]
- 16.Madeb R, Messing EM. Gender, racial and age differences in bladder cancer incidence and mortality. Urol Oncol. 2004;22:86–92. doi: 10.1016/S1078-1439(03)00139-X. [DOI] [PubMed] [Google Scholar]
- 17. Druckrey H, et al. (1964) Selektive Erzeugung Von Blasenkrebs an Ratten Durch Dibutyl- und N-Butyl-N-Butanol(4)-Nitrosamin [Selective induction of bladder cancer in rats by dibutyl- and N-butyl-N-butanol(4)-]. Z Krebsforsch 66:280–290 [in German] [PubMed]
- 18.Bertram JS, Craig AW. Induction of bladder tumours in mice with dibutylnitrosamine. Br J Cancer. 1970;24:352–359. doi: 10.1038/bjc.1970.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtani M, et al. Sequential changes of mouse bladder epithelium during induction of invasive carcinomas by N-butyl-N-(4-hydroxybutyl)nitrosamine. Cancer Res. 1986;46:2001–2004. [PubMed] [Google Scholar]
- 20.Williams PD, Lee JK, Theodorescu D. Molecular credentialing of rodent bladder carcinogenesis models. Neoplasia. 2008;10:838–846. doi: 10.1593/neo.08432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertram JS, Craig AW. Specific induction of bladder cancer in mice by butyl-(4-hydroxybutyl)-nitrosamine and the effects of hormonal modifications on the sex difference in response. Eur J Cancer. 1972;8:587–594. doi: 10.1016/0014-2964(72)90137-5. [DOI] [PubMed] [Google Scholar]
- 22.Hartge P, et al. Unexplained excess risk of bladder cancer in men. J Natl Cancer Inst. 1990;82:1636–1640. doi: 10.1093/jnci/82.20.1636. [DOI] [PubMed] [Google Scholar]
- 23.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306:737–745. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemelt M, Yamamoto H, Cheng KK, Zeegers MPA. The effect of smoking on the male excess of bladder cancer: A meta-analysis and geographical analyses. Int J Cancer. 2009;124:412–419. doi: 10.1002/ijc.23856. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto H, et al. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 26.Lim SC, Oh SH. The role of CD24 in various human epithelial neoplasias. Pathol Res Pract. 2005;201:479–486. doi: 10.1016/j.prp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Boorjian SA, et al. Expression and significance of androgen receptor coactivators in urothelial carcinoma of the bladder. Endocr Relat Cancer. 2009;16:123–137. doi: 10.1677/ERC-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kauffman EC, et al. (2011) Role of androgen receptor and associated lysine-demethylase coregulators, LSD1 and JMJD2A, in localized and advanced human bladder cancer. Mol Carcinog 50:931–944. [DOI] [PMC free article] [PubMed]
- 29.Cheng H, Snoek R, Ghaidi F, Cox ME, Rennie PS. Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res. 2006;66:10613–10620. doi: 10.1158/0008-5472.CAN-06-0028. [DOI] [PubMed] [Google Scholar]
- 30.Sagiv E, et al. CD24 is a new oncogene, early at the multistep process of colorectal cancer carcinogenesis. Gastroenterology. 2006;131:630–639. doi: 10.1053/j.gastro.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 31.Senner V, et al. CD24 promotes invasion of glioma cells in vivo. J Neuropathol Exp Neurol. 1999;58:795–802. doi: 10.1097/00005072-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Baffa R, et al. Fez1/Lzts1-deficient mice are more susceptible to N-butyl-N-(4-hydroxybutil) nitrosamine (BBN) carcinogenesis. Carcinogenesis. 2008;29:846–848. doi: 10.1093/carcin/bgn006. [DOI] [PubMed] [Google Scholar]
- 33.Friederichs J, et al. The CD24/P-selectin binding pathway initiates lung arrest of human A125 adenocarcinoma cells. Cancer Res. 2000;60:6714–6722. [PubMed] [Google Scholar]
- 34.Matsushita K, et al. Immunohistochemical biomarkers for bladder cancer prognosis. Int J Urol. 2011;18:616–629. doi: 10.1111/j.1442-2042.2011.02809.x. [DOI] [PubMed] [Google Scholar]
- 35.Mir C, et al. Loss of androgen receptor expression is not associated with pathological stage, grade, gender or outcome in bladder cancer: A large multi-institutional study. BJU Int. 2011;108:24–30. doi: 10.1111/j.1464-410X.2010.09834.x. [DOI] [PubMed] [Google Scholar]
- 36.Waliszewski P, Waliszewska MK, Hemstreet GP, 3rd, Hurst RE. Expression of sex steroid receptor genes and comodulation with retinoid signaling in normal human uroepithelial cells and bladder cancer cell lines. Urol Oncol. 1997;3:141–147. doi: 10.1016/s1078-1439(98)00011-8. [DOI] [PubMed] [Google Scholar]
- 37.Sagiv E, et al. Targeting CD24 for treatment of colorectal and pancreatic cancer by monoclonal antibodies or small interfering RNA. Cancer Res. 2008;68:2803–2812. doi: 10.1158/0008-5472.CAN-07-6463. [DOI] [PubMed] [Google Scholar]
- 38.Wirth M, et al. Bicalutamide (Casodex) 150 mg plus standard care in early non-metastatic prostate cancer: Results from Early Prostate Cancer Trial 24 at a median 7 years’ follow-up. Prostate Cancer Prostatic Dis. 2007;10:87–93. doi: 10.1038/sj.pcan.4500916. [DOI] [PubMed] [Google Scholar]
- 39.Steeg PS, Theodorescu D. Metastasis: A therapeutic target for cancer. Nat Clin Pract Oncol. 2008;5:206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welte K, et al. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci USA. 1985;82:1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gioeli D, et al. Stress kinase signaling regulates androgen receptor phosphorylation, transcription, and localization. Mol Endocrinol. 2006;20:503–515. doi: 10.1210/me.2005-0351. [DOI] [PubMed] [Google Scholar]
- 42.Shulewitz M, et al. Repressor roles for TCF-4 and Sfrp1 in Wnt signaling in breast cancer. Oncogene. 2006;25:4361–4369. doi: 10.1038/sj.onc.1209470. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, et al. A dinucleotide deletion in CD24 confers protection against autoimmune diseases. PLoS Genet. 2007;3:e49. doi: 10.1371/journal.pgen.0030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas S, et al. CD24 is an effector of HIF-1 driven primary tumor growth and metastasis. Cancer Res. 2012;72:5600–5612. doi: 10.1158/0008-5472.CAN-11-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bigler D, et al. Gene profiling and promoter reporter assays: Novel tools for comparing the biological effects of botanical extracts on human prostate cancer cells and understanding their mechanisms of action. Oncogene. 2003;22:1261–1272. doi: 10.1038/sj.onc.1206242. [DOI] [PubMed] [Google Scholar]
- 46.Dart DA, Brooke GN, Sita-Lumsden A, Waxman J, Bevan CL. Reducing prohibitin increases histone acetylation, and promotes androgen independence in prostate tumours by increasing androgen receptor activation by adrenal androgens. Oncogene. 2011 doi: 10.1038/onc.2011.591. 10.1038/onc.2011.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agresti A. Categorical Data Analysis. 2nd Ed. New York: Wiley; 2002. [Google Scholar]