Abstract
By 2030, nearly half of Americans will have nonalcoholic fatty liver disease. In part, this epidemic is fueled by the increasing consumption of caloric sweeteners coupled with an innate capacity to convert sugar into fat via hepatic de novo lipogenesis. In addition to serving as substrates, monosaccharides also increase the expression of key enzymes involved in de novo lipogenesis via the carbohydrate response element-binding protein (ChREBP). To determine whether ChREBP is a potential therapeutic target, we decreased hepatic expression of ChREBP with a specific antisense oligonucleotide (ASO) in male Sprague-Dawley rats fed either a high-fructose or high-fat diet. ChREBP ASO treatment decreased plasma triglyceride concentrations compared with control ASO treatment in both diet groups. The reduction was more pronounced in the fructose-fed group and attributed to decreased hepatic expression of ACC2, FAS, SCD1, and MTTP and a decrease in the rate of hepatic triglyceride secretion. This was associated with an increase in insulin-stimulated peripheral glucose uptake, as assessed by the hyperinsulinemic-euglycemic clamp. In contrast, ChREBP ASO did not alter hepatic lipid content or hepatic insulin sensitivity. Interestingly, fructose-fed rats treated with ChREBP ASO had increased plasma uric acid, alanine transaminase, and aspartate aminotransferase concentrations. This was associated with decreased expression of fructose aldolase and fructokinase, reminiscent of inherited disorders of fructose metabolism. In summary, these studies suggest that targeting ChREBP may prevent fructose-induced hypertriglyceridemia but without the improvements in hepatic steatosis and hepatic insulin responsiveness.
Over the last 40 yr, dietary fructose consumption has increased dramatically in the United States and worldwide and is implicated in the increasing prevalence of nonalcoholic fatty liver disease (NAFLD), hyperlipidemia, and type 2 diabetes mellitus (1, 2). Fructose is both a potent substrate for and stimulator of de novo lipogenesis (DNL) in both humans and rodents (3, 4). Excess dietary fructose contributes to the development of NAFLD and hepatic insulin resistance (5, 6) via diacylglycerol-mediated activation of novel protein kinase Cs and impairment of insulin signaling (7–9). The ability of fructose to increase DNL is linked to the induction of the expression and activity of SREBP-1c (sterol regulatory element-binding protein 1c), a master regulator of hepatic lipogenesis (3). This induction is regulated by peroxisome proliferator-activated receptor gamma coactivator 1-β and X-box binding protein 1 (6, 10). However, in SREBP-1c-knockout mice, there is still a dramatic up-regulation of lipogenic genes during carbohydrate refeeding, suggesting the presence of SREBP-1c-independent pathways for lipogenesis (11). One of these pathways is regulated by the carbohydrate response element-binding protein (ChREBP, also known as MLX-interacting protein-like). ChREBP, a glucose-regulated transcription factor, increases the expression of lipogenic and glycolytic genes when glucose is abundant (12, 13). In one proposed model, glucose availability increases concentrations of xylulose-5-phosphate (X-5-P), a metabolite in the hexose monophosphate shunt pathway (14). X-5-P activates protein phosphatase 2A, which in turn dephosphorylates ChREBP, increasing nuclear translocation of ChREBP (12). But recent studies have challenged this paradigm. Fructose feeding has been shown to increase the activation of ChREBP without an increase in X-5-P, implying that ChREBP translocation can occur from mechanisms independent of X-5-P (15). Others have shown that protein phosphatase 2A is also not required for ChREBP activation (16). Additionally, the recent discovery of novel splice forms that have alternate first exons (ChREBP-α and -β) has led to a proposed model in which glucose initially increases expression of ChREBP-α. This isoform then promotes transcription of ChREBP-β, which appears to be the main regulator of lipogenesis (17).
The ability of ChREBP to regulate DNL links this pathway to the development of insulin resistance. In ob/ob mice, a liver-specific adenovirus-mediated RNA interference of ChREBP reduced hepatic steatosis and improved insulin sensitivity, as assessed during a glucose tolerance test (18). Additionally, ChREBP in the adipose tissue has been implicated in regulating adipose tissue DNL and systemic insulin sensitivity (17). But neither of these models specifically tested the ability of ChREBP in mediating DNL and the development of insulin resistance in response to fructose feeding.
We hypothesized that decreasing hepatic and adipose ChREBP expression would prevent fructose-induced lipid accumulation in liver and hepatic insulin resistance. To test this hypothesis, we used a ChREBP-specific antisense oligonucleotide (ASO) to specifically knock down ChREBP expression in liver and fat in chronic fructose-fed rats as well as high-fat-fed rats and assessed whole-body insulin sensitivity using the hyperinsulinemic-euglycemic clamp. Additionally, we assessed lipid dynamics by measuring hepatic triglyceride and diacylglycerol content and plasma triglyceride concentrations along with very-low-density lipoprotein (VLDL)-triglyceride secretion rates.
Materials and Methods
Animals
All procedures were approved by the Institutional Animal Care and Use Committee of Yale University School of Medicine. Approximately 150-g Male Sprague Dawley rats were received from Charles River Laboratories (Wilmington, MA). After acclimatization, the rats were assigned to a specific diet group and started receiving semiweekly 75-mg/kg ip ASO injections for 4 wk. Body weight and food consumption were monitored daily. The food consumed consisted of regular rodent chow (60% carbohydrate, 10% fat, 30% protein calories), a high-fat diet (26% carbohydrate, 59% fat, 15% protein calories, Dyets 112245) in which the major constitute is safflower oil, or a high-fructose diet (67% carbohydrate, 13% fat, 20% protein calories, TD89247) in which the major constituent is fructose. For the pair-fed fructose-feeding studies, control-ASO food intake was weighed out daily to exactly match the food intake of the ChREBP-ASO-treated rats.
ASO identification
To identify ASOs against ChREBP (also known as MLX-interacting protein-like), rapid-throughput screens were performed in vitro as described previously (19). In brief, 80 ASOs were designed to the ChREBP mRNA sequence, and initial screens identified several potent and specific ASOs, all of which targeted a binding site within the coding region of the ChREBP mRNAs. After extensive dose-response characterization, the most potent ASO from the screen was chosen, ISIS-233325, with the following sequence: 5′-CAGGGCTCTAAGCCATGCAC-3′. The control ASO, ISIS-141923, has the sequence 5′-CCTTCCCTGAAGGTTCCTCC-3′ and does not have perfect complementarity to any known gene in public databases. Both ASOs were dissolved in sterile saline for IP injection.
Hyperinsulinemic-euglycemic clamp studies
Catheters were inserted into the right internal jugular vein, extending to the right atrium, and left carotid artery, extending into the aortic arch. After the catheter placement, the rats were given 1 wk to recover to their initial body weight. Rats that did not recover 90% of their presurgical body weight were excluded from the study. At 1800 h, rats were fasted. The following morning at 0600 h, rats were infused with 99% [6,6-2H]glucose for 2 h (1.1 mg/kg prime, 0.1 mg/kg) to assess basal glucose turnover. After the basal period, the hyperinsulinemic-euglycemic clamp was conducted for 140 min with an infusion of insulin (400 mU/kg primed over 5 min followed by 4 mU/kg·min constant infusion) and a variable infusion of 20% dextrose spiked with approximately 2.5% [6,6-2H]glucose. During the steady state, each rat was given a 40-μCi bolus injection of [14C]deoxyglucose via the jugular catheter to determine tissue-specific glucose uptake. The calculations for glucose turnover were performed as previously described, and white adipose tissue (WAT) uptake reflects the epididymal fat pad and the muscle glucose uptake was the glucose update in both extensor digitorum longus and soleus muscles (20).
Tyloxapol challenge
After an overnight fast, rats were dosed with 500 mg/g tyloxapol (Sigma-Aldrich, St. Louis, MO) iv. Plasma samples were taken at baseline and subsequently every 30 min for the duration of the 150 min experiment to measure plasma triglyceride concentration to measure the rate of hepatic triglyceride secretion.
Biochemical analysis and calculations
Plasma insulin, glucagon, leptin, and adiponectin concentrations were determined using RIA kits (Linco, St. Charles, MO). Plasma glucose values were determined by using a glucose oxidase method (Beckman Glucose Analyzer II; Beckman Coulter, Brea, CA). The enrichment of plasma glucose during the hyperinsulinemic-euglycemic clamp was determined by using 30 μl of sample added directly to 150 μl of 100% methanol. After centrifugation, the supernatant was dried overnight and derivatized with 1:1 acetic anhydride/pyridine to produce the pentacetate derivative of glucose. The atom percentage enrichment of [6,6-2H]glucose was determined as previously described (21). The adipose and muscle [14C]2-deoxyglucose uptake was determined as previously described (8).
Tissue lipid and plasma measurement
The purification and measurements of diacylglycerols, triacylglycerols, and long-chain fatty acyl-coenzymes A (CoA) from liver were performed as previously described (22, 23).
Liver histology
Liver sections for histology were obtained after overnight fasting. The tissue was fixed in 10% formalin, and after processing and paraffin embedding, sections were stained with hematoxylin and eosin. Histology was read by a pathologist with expertise in diagnostic liver pathology (K.M.) who was blinded to the treatment groups. Briefly, steatosis, lobular inflammation, portal inflammation, microgranulomas, lipogranulomas, ballooning, and fibrosis were scored as described by Kleiner et al. (24). NAFLD activity score is expressed as the unweighted sum of steatotosis, lobular inflammation, and ballooning degeneration.
Lipogenesis
The incorporation of deuterium into plasma VLDL during administration of deuterium-labeled water was used to determine the fractional synthetic rate of fatty acids as described (25, 26). Rats were given a bolus of 10 ml/kg of 100% D2O (Sigma-Aldrich) and subsequently placed on 4% D2O in the drinking water for 5 d before the end of the ASO study. Deuterium enrichment in palmitate-derived triglyceride in liver and plasma measured by gas chromatography-mass spectrometry (5971A Mass Selective Detector; Hewlett-Packard, Palo Alto, CA) as described (26), and fractional rates of DNL were calculated as described (27).
Total RNA preparation, real-time quantitative RT-PCR analysis, and immunoblotting analysis
PCR was performed as previously described (20). For Western blot analysis, 20 mg powdered tissue in 200 μl homogenization buffer Laemmli sample buffer was homogenized. After centrifugation for 15 min at 10,000 × g, 40 μg crude protein was then separated on a 4–12% gradient polyacrylamide gel in a MOPS buffer system (Invitrogen, Carlsbad, CA). Subsequently, membranes were transferred to nitrocellulose membranes, and membranes were incubated in blocking buffer (5% milk) for 1 h and immunoblotted with anti-fatty acid synthase (FAS) (Cell Signaling Technology, Danvers, MA) or anti-ChREBP antibody (Abcam, Cambridge, MA) overnight, and subsequently with the appropriate secondary antibody.
Statistical analysis
All values are expressed as the average ± sem. The significance between the mean values for each study was evaluated by two-tailed unpaired Student's t test. Histological scoring was compared by Fisher's exact test in GraphPad Prism.
Results
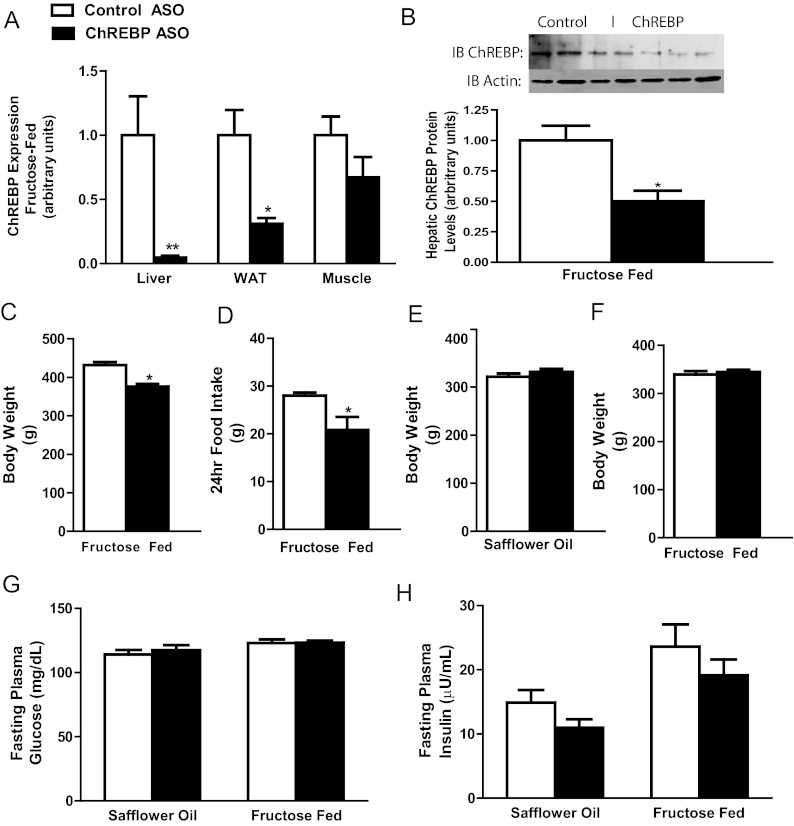
After 4 wk of semiweekly injections, ChREBP ASO reduced ChREBP mRNA levels 95% in liver and 69% in epididymal WAT but not muscle (Fig. 1A) in comparison with pair-fed rats on the fructose diet. The reduction in ChREBP mRNA levels corresponded with a 50% reduction in hepatic ChREBP protein in these rats on the fructose diet (Fig. 1B). ChREBP ASO produced similar reductions in ChREBP expression in high-fat-fed rats (data not shown). Pair feeding was necessary for fructose-fed rats because ad libitum-fed ChREBP-ASO-treated rats on a fructose diet were 13% lighter compared with control-ASO-treated rats (Fig. 1C) due to a 26% reduction in overnight food intake (Fig. 1D). In contrast, body weights were identical to the high-fat-fed control-ASO-treated rats (Fig. 1E). Body weight is a significant variable in the assessment of the metabolic changes after ChREBP knockdown. Thus, all additional studies were performed in pair-fed fructose-fed rats, eliminating the differences in body weight that in turn could affect metabolic measurements (Fig. 1F). Fasting plasma insulin and glucose concentrations were unaltered with ChREBP ASO on both diets administered (Fig. 1, G and H). Epididymal WAT (eWAT) weight was decreased with ChREBP ASO, and the decrease in eWAT weight (control ASO, 4.0 g, vs. ChREBP ASO, 2.5 g; P < 0.01) corresponded with substantial decreases in plasma leptin concentrations (Table 1).
Fig. 1.
ChREBP ASO reduces hepatic and adipose tissue but not muscle ChREBP levels. A and B, ChREBP ASO decreased the expression in liver and eWAT but not muscle (A), and the decreased expression in liver corresponded with decreases in ChREBP protein (B) in pair-fed rats with matched body weights (C); C and D, ChREBP ASO reduced body weight (C) and 24-h food intake (D) in rats fed a fructose diet ad libitum; F and G, rats fed safflower oil had no changes in body weight (E), or in rats that were pair-fed fructose diet (F), and ChREBP ASO did not affect either plasma glucose (G) or insulin (H) concentrations. *, P < 0.05; **, P < 0.005.
Table 1.
Metabolic profiles in control ASO and ChREBP ASO
| High-fat diet |
High-fructose diet |
|||
|---|---|---|---|---|
| Control ASO | ChREBP ASO | Control ASO | ChREBP ASO | |
| Fatty acids (mEq/liter) | 0.77 ± 0.05 | 0.58 ± 0.02a | 0.86 ± 0.05 | 0.64 ± 0.03a |
| Plasma leptin (ng/ml) | 1.28 ± 0.22 | 0.66 ± 0.13a | 2.45 ± 0.23 | 1.58 ± 0.20a |
| Plasma adiponectin (μg/ml) | 1.8 ± 0.2 | 2.0 ± 0.2 | 2.9 ± 0.5 | 2.7 ± 0.4 |
| Total plasma cholesterol (mg/dl) | 56.0 ± 2.6 | 53.9 ± 5.5 | 63.7 ± 6.2 | 60.5 ± 5.9 |
| HDL plasma cholesterol (mg/dl) | 21.7 ± 2.4 | 21.6 ± 0.9 | 25.9 ± 3.7 | 27.4 ± 2.7 |
| Plasma β-hydroxybutyrate (mmol/liter) | 2.11 ± 0.17 | 1.81 ± 0.13 | 1.62 ± 0.14 | 1.14 ± 0.08a |
| Hepatic glycogen (mg/g liver) | ND | ND | 2.58 ± 0.90 | 4.37 ± 1.74 |
Data are presented as mean ± sem. HDL, High-density lipoprotein; ND, not determined.
P < 0.05 between ChREBP ASO vs. control ASO on the specified diet.
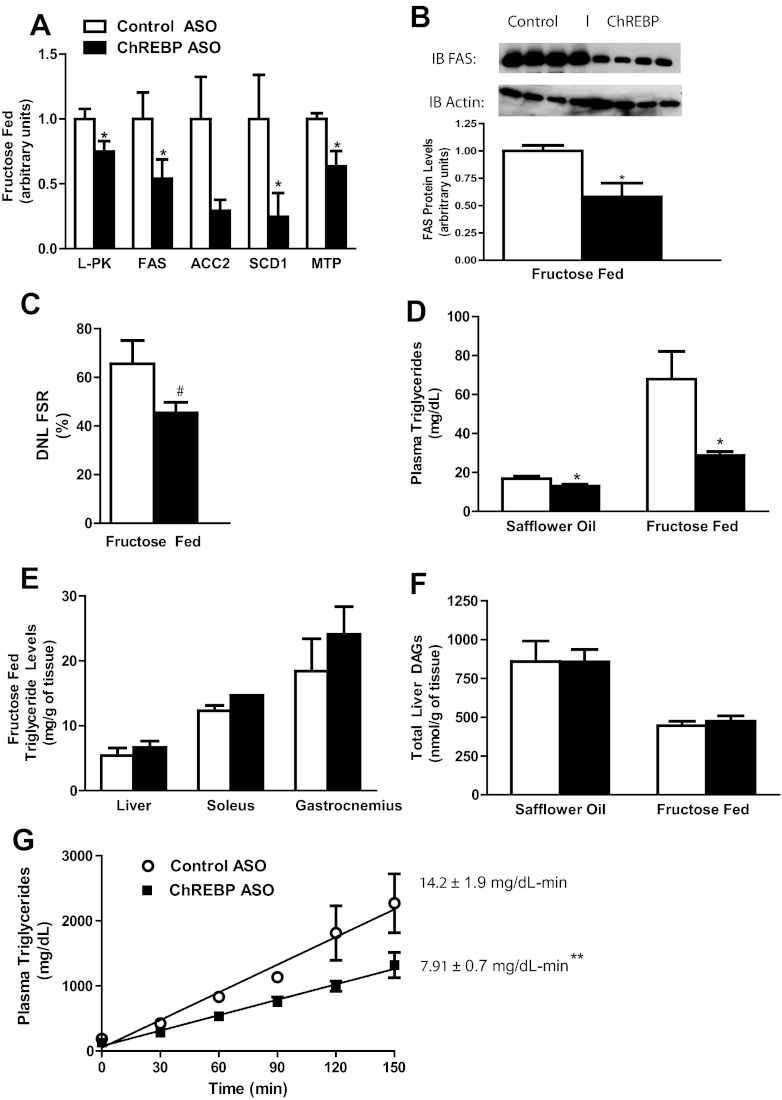
ChREBP is a key transcription factor that links glucose metabolism to expression of glucose-sensitive genes, such as liver-pyruvate kinase (L-PK) and FAS. In fructose-fed rats, ChREBP ASO reduced L-PK mRNA expression by 25%. Additionally, ChREBP ASO reduced mRNA expression of key lipogenic genes such as FAS, acetyl CoA-carboxylase 2 (ACC2), and stearoyl-CoA desaturase 1 (SCD1) (Fig. 2A). The reductions in FAS mRNA corresponded with reduced FAS protein levels (Fig. 2B). The reduced expression of these key lipogenic enzymes was associated with a trend toward reduced DNL (∼31% reduction) in vivo, as assessed by deuterium incorporation into palmitate triacylglycerol (P = 0.1, Fig. 2C). Although plasma triglyceride concentration was reduced in both high-fat-fed rats and fructose-fed rats treated with ChREBP ASO, the relative and absolute reductions were more marked in the fructose-fed rats (Fig. 2D). Despite the decreased expression of lipogenic genes and plasma triglycerides concentration, hepatic and muscle (soleus and gastrocnemius) triglyceride content was unchanged (Fig. 2E). Diacylglycerol content was also unchanged with ChREBP ASO in both the fructose-fed rats and high-fat-fed rats (Fig. 2F). The discordance between hepatic lipid content and plasma triglyceride concentration may be due to a decrease in hepatic lipid export. Expression of microsomal triglyceride transfer protein (MTTP) was decreased by 37% after ChREBP ASO treatment (Fig. 2A), and ChREBP ASO reduced the rate of plasma triglyceride secretion by 44% during a tyloxapol challenge (Fig. 2G).
Fig. 2.
ChREBP ASO decreases lipogenic gene expression and protein but only reduces plasma triglycerides. A, Key lipogenic genes FAS and MTP were decreased with ChREBP ASO as assessed by quantitative RT-PCR; B, hepatic FAS protein levels were decreased with ChREBP ASO; C, the contribution of DNL as assessed with heavy water; D, the reductions in lipogenic genes resulted in significant reductions in plasma triglycerides on either safflower oil or fructose diet; E, however, there were no significant differences in triglyceride concentrations in liver, soleus, and gastrocnemius; F, additionally, hepatic diacylglycerols were unchanged on both safflower oil and fructose diets in ChREBP-ASO-treated-rats; G, lastly, ChREBP ASO reduced the plasma triglycerides during a tyloxapol challenge. #, P = 0.1; *, P < 0.05; **, P < 0.005.
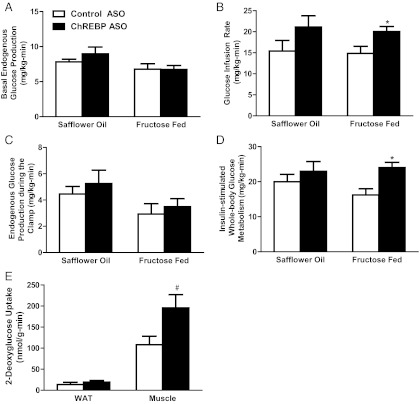
To assess changes in hepatic and peripheral insulin sensitivity after ChREBP ASO treatment, we performed hyperinsulinemic-euglycemic clamp studies. Basal rates of endogenous glucose production were similar in all groups tested (Fig. 3A). Fructose feeding decreased glucose infusion rates 38% compared with normal chow-fed rats (data not shown). In fructose-fed rats, ChREBP ASO treatment led to a 40% higher rate of glucose infusion to maintain euglycemia (Fig. 3B). There was no difference in endogenous glucose production under hyperinsulinemic-euglycemic conditions (Fig. 4C). In contrast, the increased glucose requirements were attributed to a 48% increase in insulin-stimulated whole-body glucose uptake (Fig. 4D). We measured tissue 2′-deoxyglucose uptake to determine tissue-specific changes in insulin-mediated glucose uptake. Glucose uptake was increased in skeletal muscle (soleus and extensor digitorum longus) but not in eWAT (Fig. 3E). These effects were specific to fructose-fed rats; ChREBP ASO did not lead to significant differences in either glucose infusion rate or insulin-stimulated glucose uptake in the rats fed the high-fat diet.
Fig. 3.
ChREBP ASO modestly improves peripheral insulin sensitivity. Glucose metabolism was assessed using the hyperinsulinemic-euglycemic clamp. A, Basal endogenous glucose production was unchanged between control ASO and ChREBP ASO for each group; B, ChREBP ASO increased the glucose infusion rate in the fructose-fed rats but not the safflower-oil-treated rats; C and D, insulin-stimulated endogenous glucose production (C) and whole-body glucose disposal (D) during the hyperinsulinemic-euglycemic clamps; E, the increases in glucose disposal in the fructose-fed ChREBP ASO-treated rats can be attributed to increases in glucose disposal in the muscle. #, P = 0.053; *, P < 0.05.
Fig. 4.
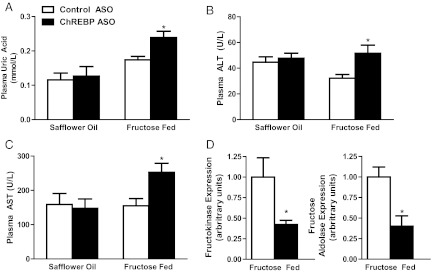
ChREBP ASO induces mild increases in ALT and AST only on the high-fructose diet. A–C, Plasma uric acid (A), ALT (B), and AST (C) were increased in ChREBP-ASO-treated rats on the fructose diet; D, expression of genes related to fructose entry into the glycolytic pathway were decreased with ChREBP ASO. *, P < 0.05.
Of note, fructose-fed rats treated with ChREBP ASO developed a mild transaminitis and hyperuricemia (Fig. 4, A–C). These changes were not evident in fat-fed ChBREP-ASO-treated rats and thus unlikely to be due to ChREBP ASO per se. Histological examination of livers from fructose-fed rats treated with either control ASO or ChREBP ASO showed similar findings of predominantly portal steatosis and mild inflammation (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). There was no significant difference in the histology between these two groups (Supplemental Table 1). Specifically, the increase in uric acid concentration is likely due to the reductions in fructokinase and fructose aldolase (Fig. 4D). Overall, these changes were reminiscent of the clinical changes present in patients with hereditary fructose intolerance.
Discussion
The observation that carbohydrate substrates could induce the expression of lipogenic enzymes led to the discovery of ChREBP as a key regulator of lipogenesis. Although ChREBP primarily regulates lipogenesis from glucose, its role in lipogenesis from fructose remains unclear. An intriguing possibility to explain the increased ChREBP activity seen with fructose feeding is a recent in vitro study demonstrating that glucose-6-phosphate directly activates ChREBP (28). With fructose feeding, the increase in hepatic fructose-1-phosphate activates glucokinase (29), which in turn could increase hepatic glucose-6-phosphate concentrations and activate ChREBP. Thus, ChREBP may be involved in lipogenesis from fructose (15) and, consequently, mediate changes in insulin action under high-fructose diets. Here, we sought to understand the role of ChREBP in regulating lipogenesis and insulin action in vivo.
Hepatic ChREBP expression was reduced using a specific ASO in adult male Sprague Dawley rats fed either a high-fat or high-fructose diet. ChREBP ASO had several key effects in fructose-fed animals. First, ChREBP ASO produced the expected decrease in hepatic lipogenic gene expression with the anticipated reduction in plasma triglyceride concentration and a trend for the reduction in the contribution of DNL to plasma triglycerides. Surprisingly, this was not associated with a reduction in hepatic lipid content in fructose-fed rats and, consequently, was not associated with improvements in hepatic insulin responsiveness. Although basal glucose turnover was similar between the groups, ChREBP ASO increased insulin-stimulated whole-body glucose disposal in fructose-fed rats. This was attributable to increased insulin-stimulated muscle glucose uptake, suggesting improved muscle insulin sensitivity. These improvements developed without alterations in muscle lipid content, suggesting that other mechanisms may be responsible that include substrate preference.
There was an interesting disassociation between plasma and hepatic lipid concentrations, with a decrease in plasma triglyceride concentration but not liver lipid content. This is attributed to decreased expression of MTTP. MTTP is critical for packaging and export of triglycerides and phospholipids from the liver endoplasmic reticulum into VLDL particles (30) and is transcriptionally regulated by ChREBP (31). Inhibition of MTTP, by either pharmacological inhibition or genetic manipulation, decreases plasma triglycerides concentration but leads to pronounced hepatic steatosis (32–34). In the present study, the reduction in MTTP expression was associated with a reduction in the rate of triglyceride secretion in the ChREBP-ASO-treated rats. Thus, although there was a reduction in hepatic lipogenic gene mRNA and protein expression, the concomitant decrease in lipid export may have prevented the reduction in hepatic lipid content. This also highlights the ability of ChREBP to broadly regulate hepatic lipid synthesis in response to the appropriate substrate, from the regulation of glycolysis and lipogenesis to the final export of lipids from the liver.
These results differ from previous studies in murine ChREBP-knockout models. ChREBP−/− mice fed a high-starch diet had decreases in liver triglyceride and plasma cholesterol but not plasma triglyceride (35). Crossing ChREBP−/− mice with ob/ob mice resulted in mice with reductions in both liver and plasma triglyceride concentrations. In these mice, although there was a marked reduction in fasting glucose, the alterations in insulin response were modest (36). Dentin et al. (18) used adenoviral-mediated liver-specific silencing of ChREBP in ob/ob mice and observed a decrease in hepatic lipids with associated improvements in glucose and insulin tolerance. However, ob/ob mice are hyperphagic and also have alterations in corticosterone, and thus those studies are not directly comparable to the present studies (37, 38). Recent studies demonstrate that glucose entry into adipocytes may alter glucose tolerance in a ChREBP-dependent manner; the enhanced glucose tolerance in adipose-specific GLUT4-overexpressing mice is blunted in the progeny produced when they are crossed with ChREBP−/− mice (17). In the rats treated with ChREBP ASO, there was a reduction in the eWAT mass and circulating free fatty acids, suggesting direct effects of ChREBP ASO on the adipocyte. The reductions in free fatty acids are consistent with the reductions in plasma ketones, suggesting decreased availability of lipids for oxidation. However, as described, we did not observe any alterations in insulin action in this model. These differences may reflect the presence of residual ChREBP activity with ASO-mediated knockdown.
ChREBP ASO decreased ad libitum food intake in fructose-fed, but not high-fat-fed, rats. This parallels studies reporting that ChREBP-knockout mouse die shortly after being placed on a fructose diet because of decreased expression of genes required for fructose metabolism (35). In contrast, ChREBP-ASO-treated rats survived on a high-fructose diet, possibly because of the residual ChREBP activity. However, we did observe a mild toxicity with transaminitis and hyperuricemia. These changes were not due specifically to the ChREBP ASO because there were no increases in alanine transaminase and aspartate aminotransferase in rats treated with ChREBP ASO when rats were fed a high-fat diet. Moreover, although there were modest increases in transaminase concentrations, there were no significant histological changes between the two groups. The transaminitis is likely due to the reduced expression of enzymes necessary for fructose metabolism and mimics the changes seen in hereditary fructose intolerance. Patients deficient in these enzymes have been known to have adverse reactions to fructose feeding such as increased uric acid levels that is mimicked with ChREBP knockdown (39). The adverse reaction to fructose should be taken into consideration when attempting to pharmacologically inhibit ChREBP because most Western diets include a high percentage of calories from fructose.
In summary, the data show that ChREBP broadly regulates DNL from fructose. Although knockdown of ChREBP high-fructose-fed rats can decrease plasma triglyceride concentration and modestly improve insulin-stimulated peripheral glucose disposal, it fails to alter the hepatic lipids and improve hepatic insulin sensitivity. In part, this may be due to a decrease in hepatic lipid export that effectively negates the reduction in hepatic DNL. Furthermore, knockdown of ChREBP induced a degree of fructose intolerance. Taken together, these data suggest that developing therapies targeting ChREBP will be difficult, especially in populations that continue to have a high consumption of dietary fructose.
Supplementary Material
Acknowledgments
We thank Christopher Carmean, Gary Cline, Romana Stark, Yanna Kosover, and Todd May for expert technical assistance and advice with the studies. We also thank Aida Groszmann for performing the hormone assays.
This work was supported by grants from the U.S. Public Health Service (R01 DK-40936 and P30 DK-45735 to G.I.S.) and a Distinguished Clinical Scientist Award from the American Diabetes Association. G.I.S. is an investigator of the Howard Hughes Medical Institute. V.T.S. is supported by VA Merit Review Award.
Disclosure Summary: S.F.M., V.P.M., and S.B. own stock and/or hold stock options in Isis Pharmaceuticals. D.E.M., V.P., J.J.H., C.V., K.M., S.Y., Y.N., M.K., M.P.G., J.F., G.W.C., G.I.S., and V.T.S. have nothing to disclose.
Footnotes
- ASO
- Antisense oligonucleotide
- ChREBP
- carbohydrate response element-binding protein
- CoA
- coenzyme A
- DNL
- de novo lipogenesis
- eWAT
- epididymal WAT
- FAS
- fatty acid synthase
- MTTP
- microsomal triglyceride transfer protein
- NAFLD
- nonalcoholic fatty liver disease
- SREBP-1c
- sterol regulatory element-binding protein 1c
- VLDL
- very-low-density lipoprotein
- WAT
- white adipose tissue
- X-5-P
- xylulose-5-phosphate.
References
- 1. Samuel VT. 2011. Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metab 22:60–65 [DOI] [PubMed] [Google Scholar]
- 2. Bray GA, Nielsen SJ, Popkin BM. 2004. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79:537–543 [DOI] [PubMed] [Google Scholar]
- 3. Nagai Y, Nishio Y, Nakamura T, Maegawa H, Kikkawa R, Kashiwagi A. 2002. Amelioration of high fructose-induced metabolic derangements by activation of PPARα. Am J Physiol Endocrinol Metab 282:E1180–E1190 [DOI] [PubMed] [Google Scholar]
- 4. Hudgins LC, Parker TS, Levine DM, Hellerstein MK. 2011. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. J Clin Endocrinol Metab 96:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. 2009. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119:1322–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D, Dong J, Zhang D, Jurczak MJ, Löffler MG, Cresswell J, Yu XX, Murray SF, Bhanot S, Monia BP, Bogan JS, Samuel V, Shulman GI. 2009. The role of peroxisome proliferator-activated receptor γ coactivator-1β in the pathogenesis of fructose-induced insulin resistance. Cell metabolism 9:252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erion DM, Shulman GI. 2010. Diacylglycerol-mediated insulin resistance. Nat Med 16:400–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. 2007. Inhibition of protein kinase Cϵ prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest 117:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, Still CD, Gerhard GS, Han X, Dziura J, Petersen KF, Samuel VT, Shulman GI. 2011. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA 108:16381–16385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee AH, Scapa EF, Cohen DE, Glimcher LH. 2008. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320:1492–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. 2002. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem 277:9520–9528 [DOI] [PubMed] [Google Scholar]
- 12. Uyeda K, Repa JJ. 2006. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab 4:107–110 [DOI] [PubMed] [Google Scholar]
- 13. Iizuka K, Horikawa Y. 2008. ChREBP: a glucose-activated transcription factor involved in the development of metabolic syndrome. Endocr J 55:617–624 [DOI] [PubMed] [Google Scholar]
- 14. Kabashima T, Kawaguchi T, Wadzinski BE, Uyeda K. 2003. Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver. Proc Natl Acad Sci USA 100:5107–5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koo HY, Miyashita M, Cho BH, Nakamura MT. 2009. Replacing dietary glucose with fructose increases ChREBP activity and SREBP-1 protein in rat liver nucleus. Biochem Biophys Res Commun 390:285–289 [DOI] [PubMed] [Google Scholar]
- 16. Dentin R, Tomas-Cobos L, Foufelle F, Leopold J, Girard J, Postic C, Ferré P. 2012. Glucose 6-phosphate, rather than xylulose 5-phosphate, is required for the activation of ChREBP in response to glucose in the liver. J Hepatol 56:199–209 [DOI] [PubMed] [Google Scholar]
- 17. Herman MA, Peroni OD, Villoria J, Schön MR, Abumrad NA, Blüher M, Klein S, Kahn BB. 2012. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 484:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dentin R, Benhamed F, Hainault I, Fauveau V, Foufelle F, Dyck JR, Girard J, Postic C. 2006. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes 55:2159–2170 [DOI] [PubMed] [Google Scholar]
- 19. Watts LM, Manchem VP, Leedom TA, Rivard AL, McKay RA, Bao D, Neroladakis T, Monia BP, Bodenmiller DM, Cao JX, Zhang HY, Cox AL, Jacobs SJ, Michael MD, Sloop KW, Bhanot S. 2005. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes 54:1846–1853 [DOI] [PubMed] [Google Scholar]
- 20. Erion DM, Yonemitsu S, Nie Y, Nagai Y, Gillum MP, Hsiao JJ, Iwasaki T, Stark R, Weismann D, Yu XX, Murray SF, Bhanot S, Monia BP, Horvath TL, Gao Q, Samuel VT, Shulman GI. 2009. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc Natl Acad Sci USA 106:11288–11293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Erion DM, Ignatova ID, Yonemitsu S, Nagai Y, Chatterjee P, Weismann D, Hsiao JJ, Zhang D, Iwasaki T, Stark R, Flannery C, Kahn M, Carmean CM, Yu XX, Murray SF, Bhanot S, Monia BP, Cline GW, Samuel VT, Shulman GI. 2009. Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein. Cell Metab 10:499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917 [DOI] [PubMed] [Google Scholar]
- 23. Neschen S, Moore I, Regittnig W, Yu CL, Wang Y, Pypaert M, Petersen KF, Shulman GI. 2002. Contrasting effects of fish oil and safflower oil on hepatic peroxisomal and tissue lipid content. Am J Physiol Endocrinol Metab 282:E395–E401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. 2005. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321 [DOI] [PubMed] [Google Scholar]
- 25. Previs SF, Hazey JW, Diraison F, Beylot M, David F, Brunengraber H. 1996. Assay of the deuterium enrichment of water via acetylene. J Mass Spectrom 31:639–642 [DOI] [PubMed] [Google Scholar]
- 26. Diraison F, Pachiaudi C, Beylot M. 1997. Measuring lipogenesis and cholesterol synthesis in humans with deuterated water: use of simple gas chromatographic/mass spectrometric techniques. J Mass Spectrom 32:81–86 [DOI] [PubMed] [Google Scholar]
- 27. Diraison F, Pachiaudi C, Beylot M. 1996. In vivo measurement of plasma cholesterol and fatty acid synthesis with deuterated water: determination of the average number of deuterium atoms incorporated. Metabolism 45:817–821 [DOI] [PubMed] [Google Scholar]
- 28. Li MV, Chen W, Harmancey RN, Nuotio-Antar AM, Imamura M, Saha P, Taegtmeyer H, Chan L. 2011. Glucose-6-phosphate mediates activation of the carbohydrate responsive binding protein (ChREBP). Biochem Biophys Res Commun 395:395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Schaftingen E, Vandercammen A. 1989. Stimulation of glucose phosphorylation by fructose in isolated rat hepatocytes. Eur J Biochem 179:173–177 [DOI] [PubMed] [Google Scholar]
- 30. Hussain MM, Rava P, Pan X, Dai K, Dougan SK, Iqbal J, Lazare F, Khatun I. 2008. Microsomal triglyceride transfer protein in plasma and cellular lipid metabolism. Curr Opin Lipidol 19:277–284 [DOI] [PubMed] [Google Scholar]
- 31. Ma L, Robinson LN, Towle HC. 2006. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem 281:28721–28730 [DOI] [PubMed] [Google Scholar]
- 32. Raabe M, Véniant MM, Sullivan MA, Zlot CH, Björkegren J, Nielsen LB, Wong JS, Hamilton RL, Young SG. 1999. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest 103:1287–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chandler CE, Wilder DE, Pettini JL, Savoy YE, Petras SF, Chang G, Vincent J, Harwood HJ., Jr 2003. CP-346086: an MTP inhibitor that lowers plasma cholesterol and triglycerides in experimental animals and in humans. J Lipid Res 44:1887–1901 [DOI] [PubMed] [Google Scholar]
- 34. Lettéron P, Sutton A, Mansouri A, Fromenty B, Pessayre D. 2003. Inhibition of microsomal triglyceride transfer protein: another mechanism for drug-induced steatosis in mice. Hepatology 38:133–140 [DOI] [PubMed] [Google Scholar]
- 35. Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. 2004. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci USA 101:7281–7286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iizuka K, Miller B, Uyeda K. 2006. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. Am J Physiol Endocrinol Metab 291:E358–E364 [DOI] [PubMed] [Google Scholar]
- 37. Samuel VT, Beddow SA, Iwasaki T, Zhang XM, Chu X, Still CD, Gerhard GS, Shulman GI. 2009. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proc Natl Acad Sci USA 106:12121–12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saito M, Bray GA. 1983. Diurnal rhythm for corticosterone in obese (ob/ob) diabetes (db/db) and gold-thioglucose-induced obesity in mice. Endocrinology 113:2181–2185 [DOI] [PubMed] [Google Scholar]
- 39. Oberhaensli RD, Rajagopalan B, Taylor DJ, Radda GK, Collins JE, Leonard JV, Schwarz H, Herschkowitz N. 1987. Study of hereditary fructose intolerance by use of 31P magnetic resonance spectroscopy. Lancet 2:931–934 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.