Abstract
Background. Haemophilus ducreyi encounters several classes of antimicrobial peptides (APs) in vivo and utilizes the sensitive-to-antimicrobial-peptides (Sap) transporter as one mechanism of AP resistance. A mutant lacking the periplasmic solute–binding component, SapA, was somewhat more sensitive to the cathelicidin LL-37 than the parent strain and was partially attenuated for virulence. The partial attenuation led us to question whether the transporter is fully abrogated in the sapA mutant.
Methods. We generated a nonpolar sapBC mutant, which lacks both inner membrane permeases of the Sap transporter, and tested the mutant for virulence in human volunteers. In vitro, we compared LL-37 resistance phenotypes of the sapBC and sapA mutants.
Results. Unlike the sapA mutant, the sapBC mutant was fully attenuated for virulence in human volunteers. In vitro, the sapBC mutant exhibited significantly greater sensitivity than the sapA mutant to killing by LL-37. Similar to the sapA mutant, the sapBC mutant did not affect H. ducreyi's resistance to human defensins.
Conclusions. Compared with the sapA mutant, the sapBC mutant exhibited greater attenuation in vivo, which directly correlated with increased sensitivity to LL-37 in vitro. These results strongly suggest that the SapBC channel retains activity when SapA is removed.
Haemophilus ducreyi is the causative agent of chancroid, a sexually transmitted genital ulcerative disease. Throughout infection, H. ducreyi primarily remains extracellular in the dermis and epidermis, where the organism encounters attack by the host's innate immune system [1, 2]. Survival in this environment requires that H. ducreyi express mechanisms to overcome innate immune pressures such as phagocytosis, complement-mediated killing, and release of antimicrobial peptides (APs) [3]. Accordingly, H. ducreyi secretes the antiphagocytic proteins LspA1 and LspA2 and expresses outer membrane proteins DsrA, DltA, and MOMP for serum resistance [4–7]. APs are small, cationic components of the innate immune system that are thought to work in gram-negative bacteria by disrupting the integrity of the inner membrane [8]. During human infection, H. ducreyi is surrounded by AP-secreting cells, including resident keratinocytes and infiltrating macrophages and neutrophils, which collectively secrete 3 classes of APs, including α- and β-defensins and the cathelicidin LL-37 [1, 2]. We have recently shown that H. ducreyi utilizes 2 mechanisms of AP resistance, including the sensitive-to-antimicrobial-peptides (Sap) uptake transporter, which contributes to LL-37 resistance, and the multiple-transferable-resistance (MTR) efflux transporter, which contributes to resistance against both LL-37 and β-defensins [9, 10].
First characterized in Salmonella enterica serovar Typhimurium and later identified in Erwinia chrysanthemi and nontypeable H. influenzae (NTHi), the Sap transporter is important for host-pathogen–specific AP resistance and virulence [11–14]. Structurally, the Sap transporter is a member of the oligopeptide (Opp)–dipeptide (Dpp) family of peptide and metal ion–uptake ABC transporters, and consists of the periplasmic solute–binding protein, SapA, the inner membrane permease proteins SapB and SapC (which form a heterodimeric channel), and the cytoplasmic ATP binding cassette (ABC) proteins SapD and SapF. The current model of Sap-mediated AP resistance involves SapA binding APs in the periplasm and shuttling them to the SapBC channel for uptake to the cytoplasm, using energy provided by SapDF [12, 15, 16]. The cytoplasmic AP is then degraded; this process prevents direct interactions between the AP and the inner membrane [15].
Previously, we showed that SapA contributes to LL-37 resistance and virulence of H. ducreyi [9]. However, unlike the Sap transporters in other pathogens, deletion of sapA in H. ducreyi led to only an approximately 25%–50% reduction in LL-37 resistance and a 50% reduction in pustule formation in the human model of H. ducreyi infection. Although the NTHi Sap transporter interacts with human α- and β-defensins, we were unable to detect any effect of the Sap transporter on defensin resistance [9, 15, 17]. These partial in vitro and in vivo phenotypes led us to question whether loss of the periplasmic SapA component renders the H. ducreyi Sap transporter fully inactive. Reasoning that the inner membrane permease components SapB and SapC are essential for transporter function, we deleted the sapBC genes to define the full contribution of the Sap transporter to the virulence of H. ducreyi in humans. We also directly compared the contributions of transporter components SapA and SapBC to AP resistance of H. ducreyi.
METHODS
Bacterial Strains and Growth Conditions
Strains and plasmids are listed in Table 1. H. ducreyi strains were grown at 33°C with 5% CO2 on chocolate agar plates supplemented with 1% IsoVitaleX and the appropriate antibiotic to maintain plasmids or antibiotic cassettes when indicated, including spectinomycin (200 μg/mL), kanamycin (20 μg/mL), or streptomycin (100 μg/mL). For in vitro studies, broth cultures of H. ducreyi strains were grown in Columbia broth supplemented with 5% heat-inactivated fetal bovine serum (HyClone, Logan, UT), 1% IsoVitaleX, hemin (50 μg/mL; Aldrich Chemical Co, Milwaukee, WI), and, where appropriate, streptomycin (12.5 µg/mL). For in vivo studies, H. ducreyi strains were grown in a proteose-peptone broth–based medium with supplements, as described [18]. Escherichia coli strains, TOP10 unless otherwise stated, were grown in broth at 37°C in Luria-Bertani (LB) broth supplemented with appropriate antibiotics, including spectinomycin (50 μg/mL), ampicillin (50 μg/mL), kanamycin (50 μg/mL), or streptomycin (100 μg/mL), except for strain DY380, which was grown in L-Broth at 32°C or 42°C as indicated [10, 19, 20]. Plate-grown E. coli strains were cultured on LB agar with half the concentration of appropriate antibiotics.
Table 1.
Bacterial Strains and Plasmids Used in This Study
| Strain or Plasmid | Genotype or Description | Source |
|---|---|---|
| E. coli strains | ||
| TOP10 | F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araΔ139 Δ(ara-leu)7697 galU galK rpsL (StrR) endA1 nupG | Invitrogen |
| DY380 | F- mcrA Δ(mrr-hsdRMS-mcrBC) Φ80dlacZ M15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara, leu) 7649 galU galK rspL nupG (λcI857 (cro-bioA) <> tet) | [19] |
| H. ducreyi strains | ||
| 35000HP | Class I clinical isolate; human-passaged variant of strain 35000, Winnipeg, Canada, 1975 |
[23] |
| 35000HP(pLSSK) | 35000HP with vector pLSSK, StrR | [10] |
| 35000HPsapA(pLSSK) | sapA insertion-deletion mutation of 35000HP with vector pLSSK, KanR, StrR | [9] |
| 35000HPsapBC | sapBC unmarked deletion mutant of 35000HP | This study |
| 35000HPsapBC(pLSSK) | sapBC unmarked deletion mutant of 35000HP with vector pLSSK, StrR | This study |
| 35000HPsapBC(psapBC) | sapBC unmarked deletion mutant of 35000HP with plasmid pMEB244, StrR | This study |
| Plasmids | ||
| pGEM-T-Easy | TA cloning vector, AmpR | Promega |
| pLSSK | H. ducreyi shuttle vector, StrR | [21, 31] |
| pRSM2072 | Suicide vector, AmpR | [32] |
| pMEB165 | sapBC + flank in pGEM-T-Easy; AmpR | This study |
| pMEB180 |
sapBC replaced with SpecR cassette in pGEM-T-Easy; AmpR, SpecR |
This study |
| pMEB185 | sapBC replaced with SpecR cassette in pRSM2072; AmpR, SpecR | This study |
| pMEB244 | sapBC in pLSSK; SpecR | This study |
Abbreviations: AmpR, resistance to ampicillin; KanR, resistance to kanamycin; SpecR, resistance to spectinomycin; StrR, resistance to streptomycin.
Construction and Complementation of Nonpolar, Unmarked sapBC Deletion Mutation in H. ducreyi
We constructed a nonpolar, unmarked deletion of the sapBC genes, using the previously described “recombineering” technique [10, 20]. This technique deletes the open reading frame (ORF) of the desired gene(s), leaving only a 35-codon ORF containing a single flippase recognition target (FRT) flanked by the start codon of the 5′ deleted gene and last 7 codons of the 3′ deleted gene. Primers for the mutant construction are listed in Table 2. Briefly, the sapBC genes and flanking sequences were cloned into vector pGEM-T-Easy to generate pMEB165, which was used to transform DY380, a λ-red recombinase–expressing strain of E. coli. Next, a polymerase chain reaction (PCR) product containing the FRT-SpecR-FRT cassette flanked by 50 bases of homology to sapBC was introduced into the above strain for recombination, generating pMEB180. The locus disrupted by the FRT-SpecR-FRT cassette was subcloned into the suicide vector pRSM2072 (generating pMEB185) for delivery and recombination in H. ducreyi. The FRT-SpecR-FRT cassette was subsequently removed with the flippase enzyme, as described [10]. PCR was used to verify the recombination events, and the final desired construct was confirmed by sequencing in the DNA Sequencing Core Facility at the Indiana University School of Medicine.
Table 2.
Primers Used in This Study
| Primer | Construct or Use | Sequence |
|---|---|---|
| SapAFor2 | pMEB165 | TGGATTTCGACCGCTTATTCCGC |
| SapDRev2 | pMEB165 | GCCTTAATGTAGCAACCTTCCACG |
| H1P1sapB | pMEB180 | GGGCAAGTTCGTTTATCTGAATTAACTTTACATCAAGAGTAACAA TCATGATTCCGGGGATCCGTCGACC |
| H2P2sapC | pMEB180 | TCTAATAGAGCCATATTTTATTCCATTTATTAATAACGATGTTTTTC TAGTGTAGGCTGGAGCTGCTTCG |
| Sapfor6 | pMEB244/colony phenotyping | AATGCGCATATTGAGCCATTCGGG |
| Saprev8 | pMEB244/colony phenotyping | TACTCGCCCTGTTGGCGTATCAAT |
| SapDfor2 | qRT-PCR | GCATTGAAATTGATACGCCAACAGGGCGAG |
| SapDrev | qRT-PCR | TCCGCGGTCACAATCCACTCATCTTTCATC |
| HD1643for1 | qRT-PCR | TGAAGGGCTTGTTGCGGTGATTTC |
| HD1643rev1 | qRT-PCR | ACGATCACGACCTTGTTTAGCGGA |
| P5 | dnaE colony phenotyping | AACGTTACCTTCAGCAAGCGGTTC |
| P6 | dnaE colony phenotyping | GCCGTTTGGGATCGTCGAGTGTATA |
A mutant with the correct deletion of sapBC from 35000HP was named 35000HPsapBC; sequencing results showed 3 silent single base changes in the ORF of the upstream gene sapA. Growth curves indicated no significant changes in growth rates of 35000HPsapBC mutant compared with the parent strain (data not shown). Quantitative reverse transcriptase PCR, performed exactly as described previously, showed that expression of the downstream gene sapD was unaffected in 35000HPsapBC (data not shown) [9, 10].
The mutant 35000HPsapBC was transcomplemented by expressing the intact sapBC genes from the H. ducreyi shuttle vector pLSSK in the sapBC mutant [9, 10, 21] (see Table 2 for primers used in constructing the complementing plasmid, pMEB244). The resulting strain was designated 35000HPsapBC(psapBC).
Virulence Study of the sapBC Mutant in Human Volunteers
Human inoculation studies with H. ducreyi were carried out under the guidelines of the US Department of Health and Human Services, the US Food and Drug Administration, and the Institutional Review Board of Indiana University–Purdue University at Indianapolis. Each volunteer gave informed consent for participation in the study and underwent human immunodeficiency virus serology testing. Eight healthy adult volunteers (6 males and 2 females; 4 white, 4 black; age range, 22 to 49 years; mean age ± SD, 39 ± 9 years) were enrolled in the study. Of the 8, 3 volunteers withdrew from the study prior to inoculation; 5 volunteers were inoculated in the upper arm with 35000HP and 35000HPsapBC and monitored to the clinical endpoint, exactly as described in previous parent–mutant comparison trials [22–24]. Throughout the trial, we targeted a parent dose of 90 colony-forming units (CFUs). Intended doses for the mutant were 45–180 CFUs (first iteration) and 180–720 CFUs (second iteration). Because of the propensity of the organism to clump, exact dosing is difficult to obtain; actual doses used are reported in Table 3.
Table 3.
Effects of sapBC on Human Inoculation With H. ducreyia
| Volunteer (gender) | No. of Days of Observation | Strain | Dose (CFUs) | No. of Initial Papules | No. of Pustules | Final Outcome of Sites |
||
|---|---|---|---|---|---|---|---|---|
| Papule | Pustule | Resolved | ||||||
| 387 (female) | 6 | P | 66 | 3 | 1 | 1 | 2 | |
| M | 67–267 | 0 | 0 | 3 | ||||
| 388 (male) | 6 | P | 66 | 3 | 3 | 3 | ||
| M | 67–267 | 3 | 1 | 1b | 2 | |||
| 390 (male) | 6 | P | 66 | 3 | 3 | 3 | ||
| M | 67–267 | 3 | 0 | 3 | ||||
| 391 (male) | 5 | P | 17 | 3 | 0 | 3 | ||
| M | 144–577 | 3 | 0 | 3 | ||||
| 394 (male) | 6 | P | 17 | 2 | 1 | 1 | 1 | |
| M | 144–577 | 2 | 0 | 2 | ||||
Abbreviations: CFUs, colony-forming units; M, mutant strain 35000HPsapBC; P, parent strain 35000HP.
a Volunteers 387, 388, and 390 were inoculated in the first iteration. Volunteers 391 and 394 were inoculated in the second iteration.
b One pustule formed at the site inoculated with 267 CFUs.
There are gender and other host effects on outcome in the model [24]; each volunteer serves as their own control for these effects. To account for correlation among inoculated sites within a subject, papule and pustule formation rates for mutant and parent inoculated sites were compared by using a logistic regression model with generalized estimating equations, as described previously [25]. Papule sizes formed after inoculation with parent or mutant strains were compared using a mixed model with subject as a random effect to account for within-person correlations. The sole pustule that formed at a mutant-inoculated site was biopsied, along with a parent-inoculated pustule from the same volunteer. Both biopsies were sectioned, semiquantitatively cultured, and stained with hematoxylin–eosin and anti-CD3 antibodies for comparison, as described previously [26]. Bacteria isolated from the inocula, surface cultures, and biopsies were tested to confirm parent or mutant phenotype with primers specific for dnaE and sapBC (Table 2) [20].
Bactericidal Antimicrobial Peptide Assay
Assays were performed as previously described [10]. Briefly, a midlogarithmic phase culture was washed and diluted to approximately 2000 CFUs per well of 96-well polypropylene plates in 10 mM sodium phosphate buffer pH 7.4 supplemented with 1% Columbia broth. The desired peptide was added at the indicated doses and incubated for 1 hour. Each well was plated in triplicate, and percent survival for each well was calculated based on control wells that received bacteria but no peptide. All assays were performed in duplicate and repeated 3–6 times. We used Student t test with Sidak adjustment for multiple comparisons to determine statistical significance.
RESULTS
A sapBC Transporter Mutant Is Fully Attenuated for Virulence in Humans
To define the effects of the SapBC channel on virulence in H. ducreyi, we directly compared the virulence of strain 35000HP with the isogenic sapBC mutant in the human challenge model of H. ducreyi infection (Table 3). In the first iteration, 3 volunteers were inoculated with 35000HP at 3 sites in 1 arm with an estimated delivered dose of 66 CFUs and at 3 sites on the other arm with 67, 134, and 267 CFUs of 35000HPsapBC. For the parent-challenged sites, papules formed at all 9 sites and pustules formed at 7 of 9 sites, while papules formed at 6 of 9 mutant-inoculated sites and pustules formed at 1 of 9 sites (Table 3). In the second iteration, 2 volunteers were inoculated with 35000HP with an estimated delivered does of 17 CFUs at 3 sites in 1 arm and at 3 sites on the other arm with 144, 289, and 577 CFUs of 35000HPsapBC. For the parent-challenged sites, papules formed at 5 of 6 sites and pustules formed at 1 of 6 sites, while for the mutant-challenged sites, papules formed at 5 of 6 sites and pustules formed at 0 of 6 sites.
Overall, the papule formation rates were 93.3% for parent-inoculated sites and 73.3% for mutant-inoculated sites (P = .26). The mean sizes ±SD of papules at 24 hours were 9.7 ± 6.0 mm2 for parent and 3.6 ± 2.8 mm2 for mutant (P = .0005). The pustule formation rate was 53.3% for parent and 6.7% for mutant (P = .002). Given that the sapBC mutant generally did not form pustules at doses even 10-fold that of the parent, the sapBC mutant was judged to be fully attenuated in the model [3]. Thus, loss of the SapBC channel has a more profound effect than loss of the periplasmic SapA on virulence in humans.
One subject (388) developed pustules at 1 mutant-inoculated and 3 parent-inoculated sites; the mutant-inoculated pustule and 1 parent-inoculated pustule were biopsied and compared for bacterial load and histology. The mutant biopsy yielded 2.0 × 103 CFUs of H. ducreyi per gram of tissue and the parent biopsy yielded 2.6 × 103 CFUs of H. ducreyi per gram of tissue. Both samples contained a dermal infiltrate of perivascular CD3+ cells and were indistinguishable (data not shown).
Colonies recovered from the inocula, surface cultures, and biopsies were tested by colony hybridization to confirm that the sites had been inoculated correctly. Parent colonies hybridize to probes for dnaE and sapBC, while sapBC colonies hybridize to the dnaE probe only. At least 1 positive surface culture for H. ducreyi was obtained during the follow-up visits from 5 of the 15 parent-inoculated and none of the 15 mutant-inoculated sites. All colonies recovered from the surface cultures of parent sites (n = 96), all colonies recovered from biopsies of parent (n = 68) and mutant (n = 25) sites, and all colonies tested from the parent (n = 72) and mutant (n = 72) inocula had the expected phenotypes and showed no evidence of cross-contamination between sites.
SapB and SapC Permeases Contribute to LL-37 Resistance Without SapA
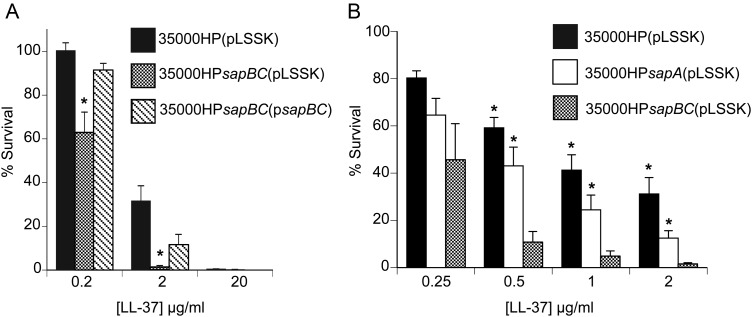
Results of the human challenge studies with the sapBC mutant showed that the Sap transporter plays a greater role in virulence of H. ducreyi than was observed by inactivating only sapA, which yielded a roughly 50% reduction in pustule formation [9]. These data suggested that the SapBC channel may retain some activity when SapA is removed. To test this hypothesis, we examined the contribution of the SapBC permeases to LL-37 resistance. Compared with the parental strain, 35000HPsapBC(pLSSK) was significantly more sensitive to LL-37; wild-type resistance was restored by transcomplementation in 35000HPsapBC(psapBC) (Figure 1A). These data confirmed that the SapBC channel is involved in LL-37 resistance.
Figure 1.
Relative contributions of SapA and the SapBC permeases to LL-37 resistance. A, Survival of 35000HP(pLSSK), 35000HPsapBC (pLSSK), and 35000HPsapBC (psapBC) exposed to the indicated concentrations of LL-37. The data represent the mean and standard error of 5 independent assays. Asterisks indicate statistical significance from the parent strain (P < .05). B, Survival of 35000HP (pLSSK), 35000HPsapA (pLSSK), and 35000HPsapBC (pLSSK) exposed to the indicated concentrations of LL-37. The data represent the mean and standard error of 8 independent assays. Asterisks indicate statistical significance from the sapBC mutant (P < .05).
To directly compare the contributions of SapA and the SapBC permeases to LL-37 resistance, we assayed the sapA and sapBC mutants side by side, along with the parent strain. When challenged with LL-37, the sapBC mutant was significantly more sensitive than the sapA mutant (Figure 1B). These results showed that loss of SapA alone did not fully abrogate Sap transporter function; rather, the SapBC channel retained partial activity against LL-37 without the periplasmic solute binding component SapA.
The H. ducreyi Sap Transporter Does Not Confer Resistance to α- or β-Defensins
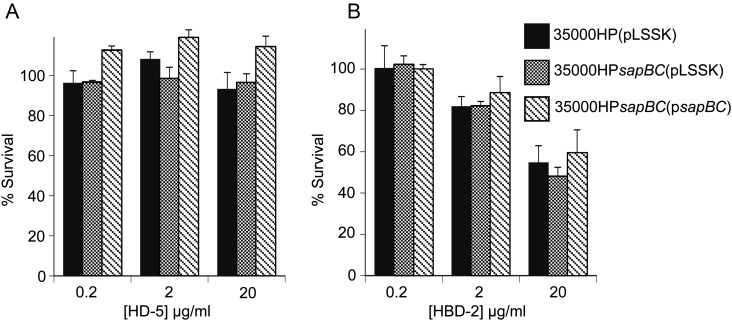
We previously reported that, unlike the Sap transporter of NTHi, SapA in H. ducreyi was not required for either α- or β-defensin resistance [9]. However, the partial activity retained in the sapA mutant could mask Sap-mediated effects on defensin resistance. To determine whether the Sap transporter confers resistance to other classes of APs, we assayed 35000HPsapBC for susceptibility to human α- and β-defensins. When compared to the parent strain, 35000HPsapBC(pLSSK) showed similar resistance levels for both α-defensins HD-5 (Figure 2A) and HNP-2 (data not shown) and the β-defensin HBD-2 (Figure 2B). These results confirm that, in H. ducreyi, the Sap transporter does not contribute significantly to defensin resistance.
Figure 2.
The Sap transporter does not confer resistance to α- or β-defensins. Survival of 35000HP(pLSSK), 35000HPsapBC (pLSSK), and 35000HPsapBC (psapBC) exposed to the indicated concentrations of HD-5 (A) and HBD-2 (B). The data represent the mean and standard error of 3 independent assays.
DISCUSSION
In this study, we defined the relative contributions of the periplasmic peptide binding protein and the structural channel components of the Sap transporter to LL-37 resistance and virulence. The sapBC mutant is fully attenuated for virulence in the human challenge model of infection (Table 3); in contrast, the sapA mutant, in which the sapBCDF genes are transcribed at wild-type levels, is only partially attenuated for virulence [9]. Similarly, the sapBC mutant is significantly more sensitive than the sapA mutant to the bactericidal effects of LL-37 (Figure 1B). Thus, the differential contributions of SapA and SapBC to virulence in vivo correlate with their relative contributions to LL-37 resistance in vitro. These results confirm the importance of the Sap transporter as a virulence factor in human disease and strongly suggest that the SapBCDF transport machinery retains activity in the absence of its cognate periplasmic peptide binding protein SapA.
To define the extent of Sap-mediated AP resistance in H. ducreyi, we sought to generate a mutation that would fully abrogate transport across the Sap channel. In other bacteria, the ABC protein SapD provides energy not only for the Sap transporter but also for the Trk low-affinity potassium uptake transporter [16, 27, 28]. The H. ducreyi genome contains homologs of a Trk transporter but no other known potassium transporters, indicating that the Trk transporter may be required for potassium uptake in H. ducreyi, as was shown for NTHi [16]. Importantly, no other sap genes are involved in Trk activity [27]. Thus, to generate a Sap-deficient mutant but avoid potentially confounding effects from loss of SapD, we specifically deleted the permease-encoding sapBC genes and confirmed that sapD expression was wild-type in the sapBC mutant. We then characterized the role of the SapBC permeases in human virulence (Table 3) and in AP resistance (Figures 1 and 2).
In the human challenge studies, the sapBC mutant was highly attenuated for development of pustules. Further, although the papule formation rate of the sapBC mutant did not achieve significance, the papules formed at the mutant-inoculated sites were significantly smaller than at parent-inoculated sites. These data suggest that the sapBC mutant was inefficient at establishing infection as well as being attenuated for disease progression. In the human model, the outcome of infected sites inoculated with the parent within a subject is not independent; more subjects form pustules or resolve infection at all inoculated sites than have mixed outcomes, suggesting a strong host effect on outcome [25]. Inoculation with a high dose (267 CFUs) of the sapBC mutant led to the development of 1 pustule in a volunteer who formed pustules at all parent-inoculated sites (Table 3). Interestingly, a similar pattern was observed in another participant who formed pustules with escalated doses of the otherwise fully attenuated pal mutant [29]. We speculate that these volunteers formed pustules with the mutants because they are hyperprone to pustule formation.
In the second iteration of the trial, the parent strain inoculum was below our target dose (Table 3). However, the inoculum still yielded an 83% infection rate. More importantly, the mutant dose, escalated in the second iteration, well exceeded 90 CFUs. Based on an analysis of 150 participants inoculated at 3 sites, a dose of 92 CFUs produced at least 1 pustule in 80% of volunteers overall and 91% of male volunteers. Although the low parent inoculum may have affected pustule formation of parent-inoculated sites in 2 volunteers, the mutant-inoculated sites received sufficient doses to determine effects on virulence.
To determine the contributions of the periplasmic and channel components of the Sap transporter to LL-37 resistance, we compared the LL-37 susceptibilities of the isogenic sapA and sapBC mutants alongside parental 35000HP. Because we find batch-to-batch variation in the potency of LL-37 for H. ducreyi, we assayed these mutants side by side. The sapBC mutant was significantly more sensitive than the sapA mutant to LL-37 (Figure 1B). These findings correlate well with our results of the human challenge trial; the data also indicate that the SapBCDF machinery can provide partial resistance by transporting LL-37, albeit less efficiently, without SapA. Although Parra-Lopez et al described a nonpolar sapA mutant in S. Typhimurium qualitatively as less sensitive than polar sapC and sapD mutants to protamine [12], our current studies are the first to directly compare the contributions of the periplasmic solute–binding protein SapA and the SapBC permeases to AP resistance. Our results suggest that the SapBC permease components can transport substrates independent of the periplasmic SapA component. The mechanism of SapA-independent Sap transporter activity is unknown; however, other ABC transporters have been shown to interact with more than 1 periplasmic solute–binding component [30]. Such cross-talk between the SapBCDF transport machinery and noncognate periplasmic peptide–binding proteins of related ABC transporters could account for the residual activity observed in the sapA mutant.
In other pathogens, Sap transporters confer resistance to multiple APs encountered during infection; in human pathogens, Sap transporter substrates include LL-37 and defensins [11–14]. Curiously, although H. ducreyi is surrounded during infection by cells that secrete multiple human APs, including several α- and β-defensins in addition to LL-37, the H. ducreyi sapA mutant showed no increased sensitivity to defensins [9]. Because partial activity of the Sap channel in 35000HPsapA could mask Sap-mediated defensin resistance, we examined 35000HPsapBC for susceptibility to representative α- and β-defensins. The sapBC mutant showed wild-type resistance to all defensins tested (Figure 2), suggesting that the H. ducreyi Sap transporter may have a much more narrow specificity for AP substrates than other Sap family members.
The Sap transporter is encoded by many significant pathogens in the γ-proteobacteria class of gram-negative bacteria, including members of the Enterobacteriaceae, Pasteurellaceae, Pseudomonadaceae, and Vibrionaceae. Its widespread conservation and demonstrated importance for virulence make the Sap transporter a viable target for antimicrobial agents that could be effective against drug-resistant gram-negative pathogens [9, 11–14]. Relevant to developing therapeutics, our data raise important questions for understanding how the Sap transporter functions. First, the ability of the SapBCDF machinery to transport LL-37 without SapA (Figure 1B) strongly suggests that the Sap transporter utilizes additional periplasmic solute–binding proteins. Thus, the repertoire of proteins involved in Sap-mediated AP resistance may extend beyond the recognized SapABCDF components. Thus, for a therapeutic agent to be effective against the Sap transporter, it must block SapBC permease activity, rather than disrupt SapA-substrate binding. Additionally, the narrow substrate range observed for the H. ducreyi Sap transporter (Figure 2) raises questions about the basis of substrate specificity for these pumps. In related ABC uptake transporters, specificity is thought to be determined by the substrate binding affinity of the periplasmic solute binding protein. Consistent with this model, the periplasmic SapA component of H. influenzae Sap transporter directly binds all known substrates of that transporter [17]. However, the ability of the H. ducreyi Sap transporter to function without SapA suggests that the basis of substrate specificity involves other proteins in addition to SapA.
In summary, this study demonstrated that the SapBC channel is more critical for virulence than SapA, which correlates with a greater contribution of the SapBC channel than of SapA to LL-37 resistance. Our results further suggest that the Sap transporter remains partially active without its cognate periplasmic component. Studies are underway to define the mechanism of SapA-independent Sap transporter activity and to define the basis of the transporter's substrate specificity.
Notes
Acknowledgements. We thank the volunteers who participated in the study, and Sheila Ellinger, who recruited them.
Financial support. This work was supported by the National Institutes of Health (R21 AI075008 to M. E. B., R21 AI096056 to M. E. B., U19 AI31494 to S. M. S., T32 AI007637 and T32 AI060519 fellowships to S. D. R., and UL RR052761 to Indiana Clinical and Translational Sciences Institute [Indiana Clinical Research Center]); Indiana University School of Medicine (Biomedical Research grant to M. E. B.); and Indiana University–Purdue University-Indianapolis (Research Support Funds grant to M. E. B).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bauer ME, Goheen MP, Townsend CA, Spinola SM. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect Immun. 2001;69:2549–57. doi: 10.1128/IAI.69.4.2549-2557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer ME, Townsend CA, Ronald AR, Spinola SM. Localization of Haemophilus ducreyi in naturally acquired chancroidal ulcers. Microb Infect. 2006;8:2465–8. doi: 10.1016/j.micinf.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Janowicz DM, Li W, Bauer ME. Host–pathogen interplay of Haemophilus ducreyi. Curr Opin Infect Dis. 2010;23:64–9. doi: 10.1097/QCO.0b013e328334c0cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vakevainen M, Greenberg S, Hansen EJ. Inhibition of phagocytosis by Haemophilus ducreyi requires expression of the LspA1 and LspA2 proteins. Infect Immun. 2003;71:5994–6003. doi: 10.1128/IAI.71.10.5994-6003.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elkins C, Morrow KJ, Olsen B. Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect Immun. 2000;68:1608–19. doi: 10.1128/iai.68.3.1608-1619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leduc I, Richards P, Davis C, Schilling B, Elkins C. A novel lectin, DltA, is required for expression of a full serum resistance phenotype in Haemophilus ducreyi. Infect Immun. 2004;72:3418–28. doi: 10.1128/IAI.72.6.3418-3428.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiltke TJ, Bauer ME, Klesney-Tait J, Hansen EJ, Munson RS, Jr., Spinola SM. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb Pathog. 1999;26:93–102. doi: 10.1006/mpat.1998.0250. [DOI] [PubMed] [Google Scholar]
- 8.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mount KLB, Townsend CA, Rinker SD, et al. Haemophilus ducreyi SapA contributes to cathelicidin resistance and virulence in humans. Infect Immun. 2010;78:1176–84. doi: 10.1128/IAI.01014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinker SD, Trombley MP, Gu X, Fortney KR, Bauer ME. Deletion of mtrC in Haemophilus ducreyi increases sensitivity to human antimicrobial peptides and activates the CpxRA regulon. Infect Immun. 2011;79:2324–34. doi: 10.1128/IAI.01316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groisman EA, Parra-Lopez C, Salcedo M, Lipps CJ, Heffron F. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11939–43. doi: 10.1073/pnas.89.24.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parra-Lopez C, Baer MT, Groisman EA. Molecular genetic analysis of a locus required for resistance to antimicrobial peptides in Salmonella typhimurium. EMBO J. 1993;12:4053–62. doi: 10.1002/j.1460-2075.1993.tb06089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Solanilla E, García-Olmedo F, Rodríguez-Palenzuela P. Inactivation of the sapA to sapF locus of Erwinia chrysanthemi reveals common features in plant and animal bacterial pathogenesis. Plant Cell. 1998;10:917–24. [PMC free article] [PubMed] [Google Scholar]
- 14.Mason KM, Munson RS, Jr, Bakaletz LO. A mutation in the sap operon attenuates survival of nontypeable Haemophilus influenzae in a chinchilla model of otitis media. Infect Immun. 2005;73:599–608. doi: 10.1128/IAI.73.1.599-608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shelton CL, Raffel FK, Beatty WL, Johnson SM, Mason KM. Sap transporter mediated import and subsequent degradation of antimicrobial peptides in Haemophilus. PLoS Pathog. 2011;7:e1002360. doi: 10.1371/journal.ppat.1002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mason KM, Bruggeman ME, Munson RS, Jr, Bakaletz LO. The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol Microbiol. 2006;62:1357–72. doi: 10.1111/j.1365-2958.2006.05460.x. [DOI] [PubMed] [Google Scholar]
- 17.Mason KM, Raffel FK, Ray WC, Bakaletz LO. Heme utilization by nontypeable Haemophilus influenzae is essential and dependent on Sap transporter function. J Bacteriol. 2011;193:2527–35. doi: 10.1128/JB.01313-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janowicz DM, Fortney KR, Katz BP, et al. Expression of the LspA1 and LspA2 proteins by Haemophilus ducreyi is required for virulence in human volunteers. Infect Immun. 2004;72:4528–33. doi: 10.1128/IAI.72.8.4528-4533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tracy E, Ye F, Baker BD, Munson RS., Jr Construction of non-polar mutants in Haemophilus influenzae using FLP recombinase technology. BMC Molec Biol. 2008;9:101–10. doi: 10.1186/1471-2199-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spinola SM, Fortney KR, Baker B, et al. Activation of the CpxRA system by deletion of cpxA impairs the ability of Haemophilus ducreyi to infect humans. Infect Immun. 2010;78:3898–904. doi: 10.1128/IAI.00432-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood GE, Dutro SM, Totten PA. Target cell range of Haemophilus ducreyi hemolysin and its involvement in invasion of human epithelial cells. Infect Immun. 1999;67:3740–9. doi: 10.1128/iai.67.8.3740-3749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Tawfiq JA, Harezlak J, Katz BP, Spinola SM. Cumulative experience with Haemophilus ducreyi in the human model of experimental infection. Sex Transm Dis. 2000;27:111–4. doi: 10.1097/00007435-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Al-Tawfiq JA, Thornton AC, Katz BP, et al. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J Infect Dis. 1998;178:1684–7. doi: 10.1086/314483. [DOI] [PubMed] [Google Scholar]
- 24.Janowicz DM, Ofner S, Katz BP, Spinola SM. Experimental infection of human volunteers with Haemophilus ducreyi: fifteen years of clinical data and experience. J Infect Dis. 2009;199:1671–9. doi: 10.1086/598966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spinola SM, Bong CTH, Faber AL, et al. Differences in host susceptibility to disease progression in the human challenge model of Haemophilus ducreyi infection. Infect Immun. 2003;71:6658–63. doi: 10.1128/IAI.71.11.6658-6663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinola SM, Wild LM, Apicella MA, Gaspari AA, Campagnari AA. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–50. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 27.Harms C, Domoto Y, Celik C, et al. Identification of the ABC protein SapD as the subunit that confers ATP dependence to the K+-uptake systems TrkH and TrkG from Escherichia coli K-12. Microbiology. 2001;147:2991–3003. doi: 10.1099/00221287-147-11-2991. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Yamamuro N, Stumpe S, Unemoto T, Bakker EP. Cloning of the trkAH gene cluster and characterization of the Trk K+-uptake system of Vibrio alginolyticus. Microbiology. 1998;144:2281–9. doi: 10.1099/00221287-144-8-2281. [DOI] [PubMed] [Google Scholar]
- 29.Fortney KR, Young RS, Bauer ME, et al. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect Immun. 2000;68:6441–8. doi: 10.1128/iai.68.11.6441-6448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Letoffe S, Delepelaire P, Wandersman C. The housekeeping dipeptide permease is the Escherichia coli heme transporter and functions with two optional peptide binding proteins. Proc Natl Acad Sci USA. 2006;103:12891–6. doi: 10.1073/pnas.0605440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dutro SM, Wood GE, Totten PA. Prevalence of, antibody response to, and immunity induced by Haemophilus ducreyi hemolysin. Infect Immun. 1999;67:3317–28. doi: 10.1128/iai.67.7.3317-3328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bozue JA, Tarantino L, Munson RS., Jr Facile construction of mutations in Haemophilus ducreyi using lacz as a counter-selectable marker. FEMS Microbiol Lett. 1998;164:269–73. doi: 10.1111/j.1574-6968.1998.tb13097.x. [DOI] [PubMed] [Google Scholar]