Abstract
Following antigenic stimulation, CD8+ T cells undergo clonal expansion and differentiation into cytotoxic T lymphocytes (CTLs) that can mount a strong defense against intracellular pathogens and tumors. SWAP-70-like adapter of T cells (SLAT), also known as Def6, is a novel guanine nucleotide exchange factor for the Cdc42 GTPase that plays a role in CD4+ T cell activation and T-helper cell differentiation by controlling Ca2+/NFAT signaling, but its requirement in CD8+ T cell response has not been explored. Using a range of transgenic and knockout in vivo systems, we show that SLAT is required for efficient expansion of CD8+ T cells during the primary response, but is not necessary for CTL differentiation. The reduced clonal expansion observed in the absence of SLAT resulted from a CD8+ T cell-intrinsic proliferation defect and a reduced IL-2-dependent cell survival. On a molecular level, we show that Def6 deficiency resulted in defective TCR/CD28-induced NFAT translocation to the nucleus in CD8+ T cells. Constitutively active Cdc42 or NFAT1 mutants fully restored the impaired expansion of Def6−/− CD8+ T cells. Taken together, these data ascribe a new and pivotal role to SLAT-mediated NFAT activation in CD8+ T cells, providing new insight into the signaling pathways involved in CD8+ T cell proliferation.
INTRODUCTION
A functional CD8+ T cell response is critical for host defense against intracellular pathogens and malignancies. Upon recognition of cognate antigen (Ag), naïve CD8+ T cells undergo a vigorous antigen-specific clonal expansion and differentiation into effector CD8+ cytotoxic T lymphocytes (CTL), producing cytokines, mainly interferon (IFN)-γ and tumor necrosis factor (TNF)-α and cytotoxic effector molecules, such as perforin and granzyme B, to mediate direct killing of target cells. Once an infection has been successfully cleared, the CTLs undergo a rapid contraction phase characterized by extensive cell death of the majority (>90%), leaving behind a stable pool of long-lived memory cells that provide long-term immunity against subsequent infections via rapid reactivation (1). It has been shown that extracellular stimuli such as Ag, costimulatory molecules (2, 3) and cytokines (4, 5) instruct naive CD8+ T cells for clonal expansion and memory formation. The integration of these instruction signals triggers downstream intracellular signaling pathways leading to specific transcriptional programs that govern the fate of CD8+ T cells (i.e., activation, proliferation, survival, differentiation). This research area remains intensively studied and is crucial for the design of novel vaccines against malignancies and pathogen infections.
SLAT (SWAP-70-like Adaptor of T cells, also known as IBP or Def6 (hereafter named SLAT), which is encoded by the Def6 gene, has been recently identified as a novel TCR-regulated guanine nucleotide factor (GEF) for Cdc42 (and to a lesser extent Rac1) (6–8). SLAT is abundantly expressed in central and peripheral lymphoid tissues, with high levels found in thymocytes and peripheral T cells (7, 9, 10). Our previous examination of Def6-deficient (Def6−/−) mice revealed SLAT to be a critical selective regulator of the TCR-coupled Ca2+/NFAT signaling pathway (10), controlling positively CD4+ T-helper cell activation and differentiation, as evidenced by its critical role in the development of T cell-dependent inflammatory diseases such as asthma (10) or experimental autoimmune encephalomyelitis (11). Moreover, the Ca2+/NFAT regulatory activity of SLAT depends on actin polymerization and functional Cdc42 activity (6). While the function of NFAT in CD4+ T cell activation and differentiation is well established, its role in CD8+ T cells is less clearly defined. Although NFAT transcriptional activity seems to be limited in Ag-stimulated CD8+ T cells by comparison with CD4+ T cells (12), NFAT1 has been shown to translocate to the nucleus of CD8+ T cells upon TCR stimulation and to regulate IFN-γ gene expression (13). In addition, NFAT1 and its Ca2+/calmodulin-dependent phosphatase, calcineurin, have been implicated in peripheral CD8 tolerance in vivo (14). Finally, silencing of cytokine production by CD8+ T cells during chronic viral infections, such as murine LCMV and human HIV, has been causally linked to impaired NFAT nuclear translocation (15).
In this study, we examined the involvement of SLAT in CD8+ T cell activation and differentiation in vivo. We found that SLAT was required for the expansion of Ag-specific CD8+ T cells during the primary response by intrinsically promoting their proliferation and by regulating the rate of CD8+ T cell survival in an IL-2-dependent manner. In contrast, SLAT was not critical for CD8+ T cell effector functions, such as cytokine production, degranulation and cytolytic activity. Furthermore, constitutively active Cdc42 or NFAT1 mutants restored the proliferation of Def6−/− CD8+ T cells in vivo. These data highlight a new role of SLAT and its proximal (Cdc42) and distal (NFAT) effectors, in CD8+ T cell biology. Thus, SLAT may represent a novel target for manipulating CD8+ T cell expansion in vaccination and other immunotherapies.
MATERIALS AND METHODS
Mice
Mice were maintained under specific pathogen-free conditions in accordance with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International. The studies described in this paper conform to the principles outlined by the Animal Welfare Act and the National Institutes of Health guidelines for the care and use of animals in biomedical research. C57BL/6J (B6; CD45.2+), B6.SJL (CD45.1+) and Rag1−/− mice were purchased from The Jackson Laboratory. B6 (CD45.1/2+) mice were generated by crossing B6 (CD45.2+) with B6.SJL (CD45.1+) mice. Def6−/− mice on a B6 background (10), Ovalbumin (OVA)-specific OT-I TCR-transgenic (Tg) CD45.1+ and Act-mOVA/Kb−/− mice on a B6 background (16) have been previously described. Def6−/− OT-I TCR-Tg CD45.2+ mice were generated by crossing Def6−/− mice with OT-I TCR-Tg CD45.2+ mice, and their T cells were used as a source of Vβ5Vα2 CD8+ T cells specific for amino acid residues 257–264 of OVA (OVA257–264; SIINFEKL peptide). Six- to 12 week-old mice were used in all experiments.
Immunizations and adoptive transfers
Groups of mice were primed either with 5 × 106 Act-mOVA/Kb−/− splenocytes i.v. or with 3000 cfu Ova-expressing Listeria monocytogenes (Lm-OVA.) i.v. The mice were rechallenged 7 days later with 1×107 ActA-deficient (ActA−) Lm-OVA i.v.. For adoptive cotransfer, Def6−/− CD45.2) and WT (CD45.1) OT-I TCR-Tg mice were bled and the number of OT-I cells was determined by counting and FACS staining for Vα2+Vβ5+ cells. Fifty each Def6−/− and WT OT-I CD8+ T cells per mouse were injected i.v. into naïve recipient mice one day prior to immunization.
Tetramer staining
Cells were stained for 10 min at room temperature with phycoerythrin-conjugated OVA257–264-H-2-Kb tetramer (BD Pharmingen), followed by staining with anti-CD8 (PE-TR), −CD62L (Alexa-Fluor 750), −CD44 (Alexa-Fluor 700), −CD127 (APC) and −KLRG-1 (Pe-Cy7) Abs. The antibodies were purchased from BD Pharmingen, eBiosciences or Biolegend. Samples were acquired and analyzed as described above.
CD107a staining
Splenocytes (1–2 × 106) from immunized mice were plated in 96-well round-bottom plates, in 200 µl of culture medium plus OVA257–264 peptide (1 µg/ml) in the presence of GolgiPlug, GolgiStop (BD Biosciences) and CD107a antibody for 5 h at 37°C. Cells were stained with anti-CD8 (PE-TR), CD44 (Alexa-Fluor 700), CD45.1 (Pacific Blue) and CD45.2 (Percp-Cy5.5) followed by fixation with Cytofix-Cytoperm (BD Biosciences) for 20 min at 4°C. Fixed cells were subjected to intracellular staining in Perm/Wash buffer (BD Biosciences) for 30 min at 4°C with anti-CD107a. Abs were purchased from BD Pharmingen, eBiosciences or Biolegend. Samples were acquired and analyzed as described.
In vitro CFSE, Annexin V and active caspase-3 analysis by flow cytometry
Cell division was analyzed by prelabeling purified CD8+ T cells with 1 µM CFSE (Molecular Probes; Invitrogen) and stimulating them (0.5 × 106/ml) with plate-coated anti-CD3 and soluble anti-CD28 mAbs (5 and 2.5 µg/ml, respectively) for 72 hours. CFSE dilution was measured by flow cytometry. For parallel analysis of apoptosis, cells were incubated with annexin V (APC) for 15 min in annexin-binding buffer (10 mM Hepes, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4), washed, fixed and permeabilized, and stained with a PE-conjugated antibody against active caspase-3 (BD Pharmingen).
Ex vivo restimulation and intracellular cytokine staining (ICS)
Cytokine production was assessed as previously described (17). Briefly, splenocytes (1–2 × 106) from immunized mice were plated in 96-well round-bottom plates in the case of Act-mOVA/Kb−/− priming or in 96-well flat-bottom plates in the case of Lm-OVA priming, in 200 µl of culture medium (IMDM; Invitrogen) supplemented with 8% FCS (Omega Scientific), 1% L-glutamine (Invitrogen), 100 µg/ml streptomycin, 100 U/ml penicillin, and 50 µM 2-ME (Sigma-Aldrich) plus OVA257–264 peptide (1 µg/ml) in the presence of GolgiPlug (BD Biosciences) for 5 h at 37°C. Cells were stained with antibodies (Abs) against CD8 (PE-TR), CD62L (Alexa-Fluor 750), CD44 (Alexa-Fluor 700), CD45.1 (Pacific Blue) and CD45.2 (Percp-Cy5.5) followed by fixation with Cytofix-Cytoperm (BD Biosciences) for 20 min at 4°C. Fixed cells were subjected to intracellular cytokine staining in Perm/Wash buffer (BD Biosciences) for 30 min at 4°C, using anti-TNF (PE-cy7), -IFN-γ (APC) or -IL-2 (PE) Abs. Abs were purchased from BD Pharmingen, eBiosciences, or Biolegend. The samples were acquired on a LSRII flow cytometer (Becton Dickinson) and data were analyzed with FlowJo software.
Ex vivo cytotoxicity assay
Def6−/− or WT OT-I mice were immunized i.v. with 5 × 106 Act-mOVA splenocytes. Activated (CD44high Vβ5+) OT-I CD8+ splenocytes were sorted 7 days later. Different numbers of effector cells were seeded in quadruplate in 96-well, round-bottom plates in the presence of 1 × 105 Ova-expressing EL4 (mOVA-EL4) target cells, which were previously labeled for 6 h with [3H]thymidine (2.5 µCi/ml). Basal 3H retention was determined by adding medium instead of effector cells. After overnight culture, cells were collected on glass-fiber filters, and the 3H label retained in live target cells was measured in a β counter. The percentage of specific killing was calculated by the following formula: ((spontaneous cpm – experimental cpm)/ spontaneaous cpm) × 100.
In vivo proliferation assay
Def6−/− and WT OT-I splenocytes were isolated by negative selection (MACS, Miltenyi). WT CD45.1+CD90.2+ B6.SJL mice were injected (day -2) with CFSE-labeled 5 × 105 Def6−/− or WT OT-I cells (both expressing CD45.2) one day prior to being injected with 1–2 × 106 splenocytes from P14 LCMV TCR-Tg CD45.2+ CD90.1+ mice, which served as an internal control. On day 0, mice were immunized with 5 × 106 Act-mOVA/Kb−/− splenocytes i.v. and three days later, spleens were harvested. Samples were acquired on a LSRII flow cytometer (Becton Dickinson) and data were analyzed with FlowJo software.
In vivo cytotoxic assay
Cytotoxicity assays were performed in immunized intact or in adoptively transfererd mice. For intact mouse immunization, mice were primed with Act-mOVA/Kb−/− splenocytes as described above. In the adoptive transfer system, B6 (CD45.2+) mice were injected i.v. with 5 × 103 naïve WT or Def6−/− (CD45.1+) OT-I CD8+ T cells. One day later (day 0), the mice were immunized i.v. with Act-mOVA/Kb−/− splenocytes. Six days after immunization of intact mice or 5 days after immunization of adoptive transfer recipients, the mice received i.v. 5 × 106 B6.SJL (CD45.1+) splenocytes, which were stained with 0.2 µM carboxyfluorescein diacetate succinimidyl ester (CFSEhigh) and loaded with the specific peptide (OVA257–264) as a source of antigen-specific target cells. As a control, the mice also simultaneously received a similar number of splenocytes labeled with 0.02 µM CFSE (CFSElow) and loaded with an irrelevant peptide (Ski9 peptide, E1B192–200). Spleen cell suspensions were prepared 15 h later, and samples were acquired and analyzed as described above. Percent specific killing was calculated by the following formula: 100-[(% CFSEhigh / % CFSElow in primed condition)/(% CFSEhigh / CFSElow in naïve condition)].
Generation of retrogenic mice
The empty RV-IRES-GFP retroviral vector and a retroviral vector expressing a constitutively active Cdc42 (Cdc42CA) mutant (pMX GFP Cdc42Q61L) have been described (18). The retroviral vector expressing a constitutively active form of NFAT1 containing two additional point mutations, which abolish NFAT homodimerization but not NFAT:AP-1 heterodimerization and, hence, leading to productive T cell activation (RV-CA-NFAT1-DCC’-QQEE or “NFATCAΔdimer”) has been kindly provided by Dr. F. Macian (18). Platinum-E packaging cells (19) (0.5 × 106 cells) in 2 ml DMEM plus 10% FBS were plated in a 6-well plate. After overnight incubation, the cells were transfected with 3 µg retroviral plasmid DNA with TransIT-LT1 transfection reagent. After 24 h, the medium was replaced with complete DMEM containing 10% FBS. Cultures were maintained for 24 h, and the retroviral supernatant was harvested, filtered through 0.45-µm filters, and used for infection.
BM cells were harvested from the femurs of Def6−/− OT-I TCR-Tg CD45.2+ or OT-I TCR-Tg CD45.1+ mice. Lin-negative cells were isolated by negative selection using a lineage cell depletion kit (MACS) and cultured in complete DMEM containing 20% FCS supplemented with IL-3 (10 ng/ml), IL-6 (20 ng/ml) and stem cell factor (SCF; 50 ng/ml). After 24 h, the cells were resuspended in 0.5 ml of complete DMEM and were added to 0.5 ml of retroviral supernatant, supplemented with polybrene (5 µg/ml final concentration) and recombinant cytokines (IL-3, IL-6 and SCF), and centrifuged at 2,000 rpm for 1 h at room temperature. Cells were then incubated for 6 h at 32°C, and cultured overnight at 37°C, followed by two additional retroviral infections at daily intervals. Transduced (GFP+) progenitor cells were sorted and injected (>2 × 105 cells) i.v. into sublethally irradiated (450 rad) recipient Rag1−/− B6 mice. Mice were analyzed 8 weeks later for engraftment by analyzing their peripheral blood lymphocytes for OVA257–264-H-2-Kb tetramer+ CD8+ T cells.
Subcellular fractionation and immunoblotting
Purified CD8+ T cells (1 × 107) were washed with ice-cold PBS, resuspended in 100 µl of buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 1 mM DTT, and proteases inhibitors) for 15 min on ice. Nonidet P-40 was then added to a final concentration of 0.5%, samples were quickly vortexed for 10 s, and centrifuged for 2 min (14,000 × g at 4°C). The supernatant was collected as the cytosolic fraction. Nuclear pellets were washed twice with buffer A lacking Nonidet P-40, resuspended in 40 µl of buffer B (20 mM HEPES, pH 7.9, 400 mM NaCl, 1 mM EDTA, 1 mM DTT, and proteases inhibitors), vortexed for 10 s, and rocked for 30 min at 4°C. Samples were centrifuged for 10 min at 14,000 ×g, and the supernatant was collected as the nuclear fraction. Samples were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with Abs against HA, lamin B, NFAT1 (4G6-G5) (both from Santa Cruz Biotechnology), β-actin (Biolegend), or rat α-tubulin (YL1/2; Serotec). Signals were detected using the ECL system (Amersham Biosciences).
Reporter assays
Simian virus 40 large T antigen-transfected human leukemic Jurkat T cells (Jurkat-TAg) were transfected with NFAT-luciferase plasmids plus a β-galactosidase (β-Gal) reporter plasmid as described (20). Transfected cells were cultured overnight, lysed, and luciferase or β-Gal activities were determined as described (20). The results are expressed in arbitrary luciferase units normalized to β-Gal activity in the same cells.
Statistical analysis
Data were analyzed using PRISM software (GraphPad, San Diego, CA). Differences between groups were examined for statistical significance using an unpaired two-tailed Student’s t test. Unless otherwise indicated, data represent the mean ± SEM, with p<0.05 considered statistically significant.
RESULTS
SLAT is critical for Ag-specific CD8+ T cell primary expansion
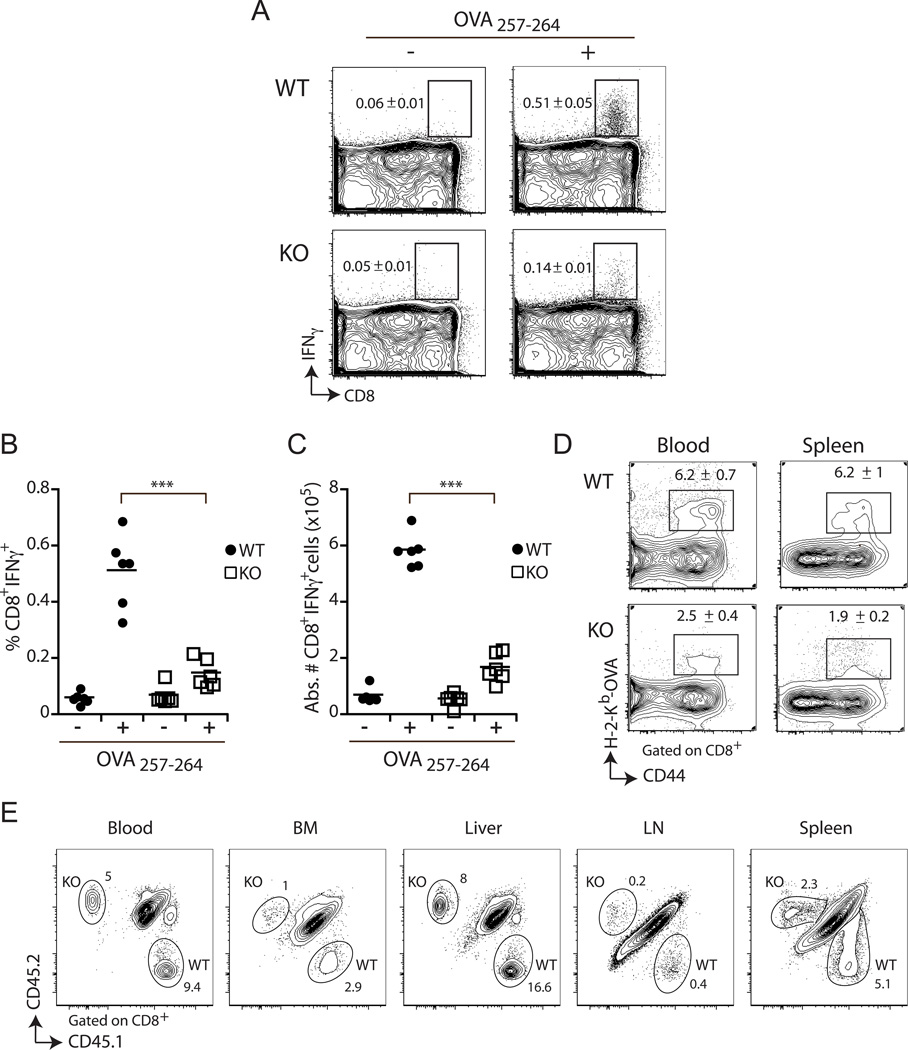
Although SLAT is required for CD4+ T cell activation and differentiation (6, 10, 11, 21), its role in CD8+ T cells has not been addressed. To directly assess the in vivo requirement of SLAT in CD8+ T cell responses, we immunized WT or Def6−/− mice with a non-replicating, non-inflammatory, cell based-immunogen, i.e., splenocytes from Act-mOVA mice, which express OVA under control of the actin promoter and have a homozygous deletion of the gene encoding H-2Kb (Act-mOVA/Kb−/−). These splenocytes cannot directly present the OVA antigen, leading to its cross-presentation by host APCs and, consequently, to cross-priming of functional H-2Kb-restricted OVA257–264-specific CD8+ T cells. Seven days after priming, the magnitude of the CD8+ T cell response was measured by using H-2Kb-OVA tetramers to track OVA257–264-specific CD8+ T cell response and by analyzing intracellular IFN-γ expression following restimulation of the Ag-specific CD8+ T cells with the relevant OVA peptide. We observed a 2–3-fold decrease in the proportion as well as the absolute number of OVA257–264-specific IFN-γ+ CD8+ T cells at the peak (day 7) of the primary response in Def6−/− mice when compared to WT mice (Fig. 1A–D). Furthermore, the reduced expansion of Def6−/− OVA-specific CD8+ T cells was not due to differential homing or trafficking of the cells as a similar degree of decrease was observed in all organs tested, namely, the bone marrow, liver, LN, blood, and spleen (Fig. 1D and E). Taken together, these results suggest that SLAT is required for optimal expansion of Ag-specific CD8+ T cells following in vivo priming.
Figure 1. Def6 deficiency results in defective CD8+ T cell primary expansion.
A–D, Mice were challenged and Ag-specific CD8+ T cell response in the spleen and blood was assayed 7 days later by IFN-γ ICS after 5h of ex vivo restimulation in the presence (+) or absence (−) of the OVA257–264 peptide (A–C) and OVA257–264/Kb tetramer staining (D). Representative FACS plots (A), frequency (B) and absolute number (C) of IFN-γ-producing wild-type (WT) and Def6−/− (KO) CD8+ T cells present in the spleen (n=6 per group) is shown. Numbers shown in the FACS plots are the percentage ± SEM for each group of mice. Dots and squares represent individual mice, and means ± SEM are indicated. ***P<0.0005 (two-tailed unpaired t-test). D, Representative FACS plots of WT and KO Ag-specific CD8+ T cells present in the blood and spleen. Numbers shown in the FACS plots are the percentage ± SEM for each group of mice. E, Five hundred naïve WT (CD45.1+) and Def6−/− (KO; CD45.2+) OT-I CD8+ T cells (expressing an OVA-specific TCR) were adoptively co-transferred into CD45.1/2+ B6 recipient mice that were immunized one day later with 5 × 106 Act-mOVA/Kb−/− splenocytes followed by flow cytometry analysis at day 7. The frequency of WT and KO OT-I CD8+ T cells among the lymphocytes from the blood, bone marrow (BM), liver, lymph node (LN) and spleen is indicated. Data are representative of five independent experiments.
SLAT is dispensable for CD8+ T cell differentiation into effector CTLs
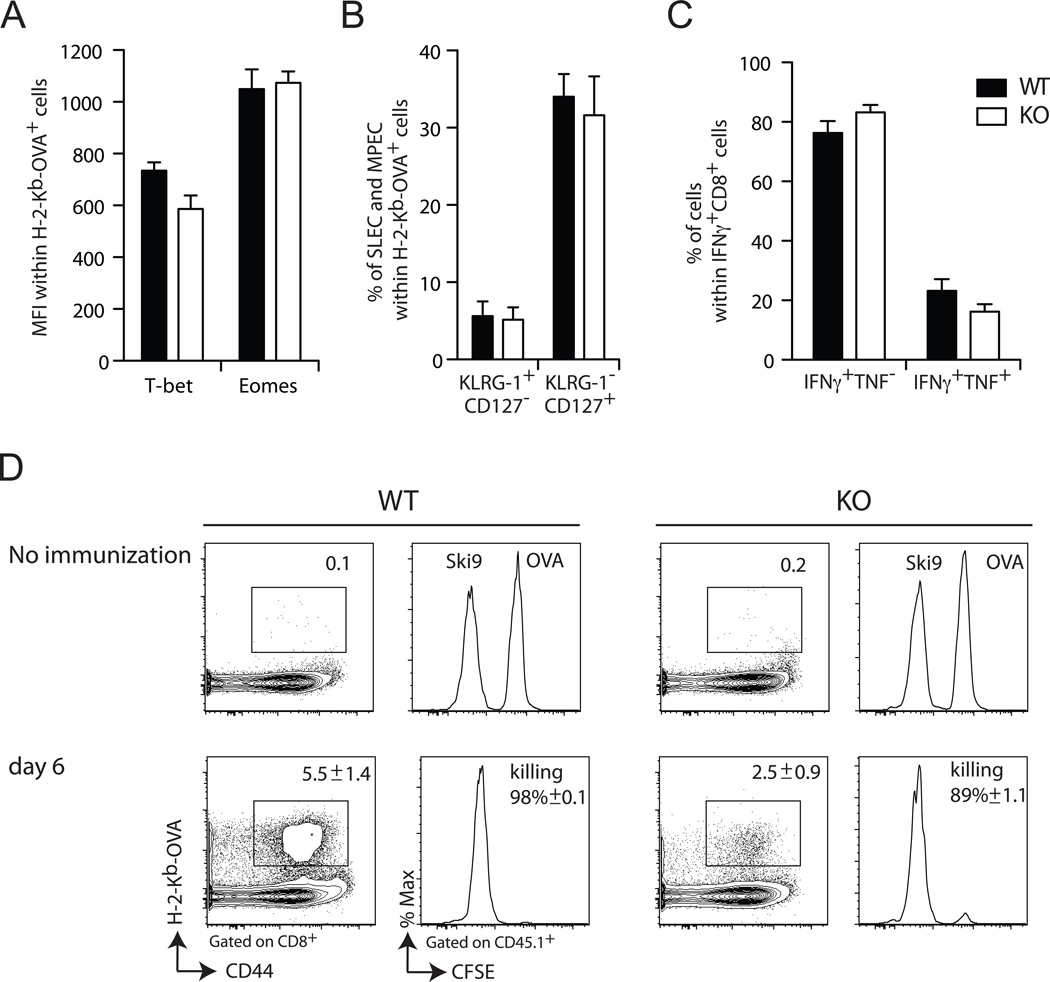
Next, we assessed the influence of Def6 deletion on the activation and effector function of CD8+ T cells by analyzing several phenotypic and functional parameters characteristic of effector CD8+ T cells. Expression of the key regulatory transcription factors, T-bet and Eomesodermin, shown to control CTL effector programming (22, 23), was similar in WT and Def6−/− OVA-specific CD8+ T cells following Ag priming (Fig. 2A). Moreover, analysis of surface expression patterns of KLRG-1 and CD127, which phenotypically discriminate terminally differentiated short-lived effector cells (SLEC; KLRG-1high CD127low) from long-lived memory precursor effector cells (MPEC; KLRG-1low CD127high), showed no difference between responding WT and Def6−/− CD8+ T cells (Fig. 2B). Furthermore, Def6−/− CD8+ T cells were proficient at effector cytokine production since similar frequencies of IFN-γ- or IFN-γ/TNF-α-producing CD8+ T cells were found among the responding CD8+ T cells from Act-mOVA-immunized WT and Def6−/− mice (Fig. 2C). Finally, despite a defective Ag-induced primary expansion of CD8+ T cells in Def6−/− mice, the remaining CD8+ T cells displayed intact in vivo cytolytic activity (Fig. 2D). Altogether, these data suggest that Def6 deficiency, while impairing the expansion of CD8+ T cells, did not alter either CD8+ T cell differentiation into effector CTLs, or the effector function of differentiated CTLs.
Figure 2. SLAT is not required for CD8+ T cell differentiation into primary effector CTLs.
Mice were primed with 5 × 106 Act-mOVA/Kb−/− splenocytes. On day 7, OVA257–264/Kb-specific CD8+ T cells were stained with H-2Kb-OVA tetramer and IFN-γ and TNF-α -producing CD8+ T cells were assessed by ICS following restimulation with OVA peptide for 5 h. A, Expression of T-Bet and Eomes within the OVA257–264/Kb-specific CD8+ T cell population. B, Expression of KLRG1 and CD127 on OVA/Kb-specific CD8+ T cells. KLRG-1high CD127low CD8+ T cells are referred to as short-lived effector cells (SLEC) whereas KLRG-1low CD127high CD8+ T cells are referred to as memory precursor effector cells (MPEC). C, Frequency of IFN-γ and TNF-α-producing CD8+ T cells within the IFN-γ+ CD8+ T cells. Each graph represents the mean of 6 mice/group and is representative of at least two independent experiments. D, In vivo cytolytic activity of Ag-specific CD8+ T cells from WT vs. KO mice 6 days after priming with Act-mOVA/Kb−/− splenocytes. Data are presented as the frequency of tetramer+ cells (numbers above outlined areas in first and third panels from left) and killing of target cells pulsed with OVA257–264 peptide and loaded with high concentration of the cytosolic dye CFSE (OVA) vs. control target cells pulsed with an irrelevant peptide and loaded with a low concentration of CFSE (Ski9) (second and fourth panels from left). Cytotoxicity was determined 16 h after adoptive transfer of target cells. Numbers in plots indicate percentage ± SEM of specific killing of CFSEhigh, OVA-loaded cells). Data are representative of five independent experiments.
Intrinsic effects of SLAT deficiency on CD8+ T cell expansion
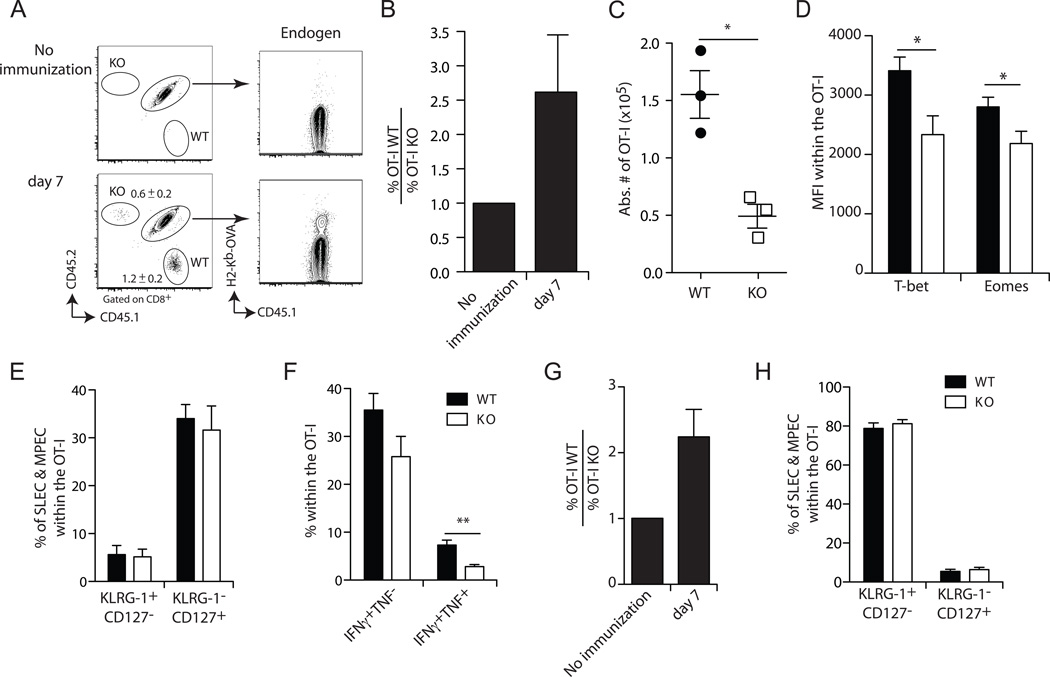
The defect in Def6−/− CD8+ T cell priming could reflect defective activation of CD4+ T helper cells or impaired function of antigen-presenting dendritic cells (DCs), which also express SLAT. Therefore, we wanted to determine whether the observed defect is CD8+ T cell-intrinsic. To this end, we crossed Def6−/− mice onto an OT-I background, in which the majority of CD8+ T cells express a Vα2Vβ5 TCR recognizing the OVA257–264 peptide presented in the context of H-2Kb (24). We adoptively co-transferred equal numbers of naïve (CD62Lhigh CD44low) WT CD45.1+ and Def6−/− CD45.2+ OT-I CD8+ T cells into WT CD45.1/2+ recipient mice. Of note, we transferred a very small number of cells (50 each) in order to most closely mimic the endogenous CD8+ T cell response without suppressing it (17, 25) (as shown in Fig. 3A, right column). The next day, we immunized the recipient mice with Act-mOVA/Kb−/− splenocytes and evaluated the expansion of WT and Def6−/− CD8+ T cells, which were identified based on their distinct CD45 congenic markers, at the peak of the response (day 7). We observed a substantial clonal expansion of donor WT OT-I cells in the blood (Fig. 3A) and spleen (Fig. 3C) as evidenced by frequency and absolute number of the recipient mice. By contrast, the expansion of Def6−/− OT-I T cells was significantly diminished (Fig. 3A,C). Calculation of the ratio of WT to Def6−/− OT-I cells in the recipient mice showed that WT CD8+ cells expanded on average ~2.5 times more than the Def6−/− cells (Fig. 3B). However, the expanded Def6−/− OT-I cells displayed a frequency of SLEC and MPEC similar to that of WT OT-I cells (Fig. 3E), and a only small decrease in T-bet and Eomes expression (Fig. 3D) and in IFN-γ/TNF-α-producing cells (Fig. 3F) compared to WT cells. Furthermore, Def6−/− and WT donor OT-I T cells displayed similar cytolytic activity in vivo and ex vivo (Suppl. Figs. 1A and 1B, respectively) and CD8+ T cell degranulation determined by cell surface modulation of CD107a/LAMP-1 upon Ag encounter (Suppl Fig. 1C). Overall, these results recapitulate the data obtained in intact immunized mice (Fig. 1 and 2) and, moreover, clearly indicate that the defect in the priming and expansion of Ag-specific T cells observed in Def6−/− mice is CD8+ T cell-intrinsic.
Figure 3. SLAT regulates CD8+ T cell expansion in a T cell-intrinsic manner.
Fifty naïve WT (CD45.1+) OT-I and Def6−/− (KO; CD45.2+) OT-I CD8+ T cells were adoptively co-transferred into CD45.1/2+ B6 recipient mice that were immunized one day later with 5 × 106 Act-mOVA/Kb−/− splenocytes (A–F) or 1 × 107 cfu OVA-expressing Listeria monocytogenes Act A deficient (Lm-OVA Act A−) (G–H) followed by analysis at day 7. A, Frequency of WT and KO OT-I T cells among the CD8+ T cell population in the blood of a representative mouse (n=10). B and G, Average ratio of WT OT-I to KO OT-I CD8+ T cells (n=10). The WT/KO ratio in non-immunized animals is set at 1. C, Absolute number of WT and KO OT-I CD8+ T cells among total splenocytes from recipient mice (n=3). D, Expression of the KLRG-1 and CD127 on CD45.1+ and CD45.2+ peripheral blood CD8+ cells. E and H, Mean expression ± SEM of T-bet and Eomes by CD8+ T cells. Data are representative of at least three independent experiments. *P<0.05 (two-tailed unpaired t-test). F, Frequency of WT and KO OT-I CD8+ T cells producing IFN-γ alone (IFN-γ+TNF-α−) or both IFN-γ and TNF-α (IFN-γ+TNF-α+) among total splenocytes determined by ICS. Data are representative of five (A–F) and three (G–H) independent experiments.
The cross-priming model using Act-mOVA/Kb−/− splenocytes as immunogens has been shown to result in accelerated acquisition of memory phenotype and function, and effector CD8+ T cells cross-primed under these conditions respond vigorously to short-interval reexposure to antigen (26). This accelerated memory development is evident from the prevalence of MPECs over SLECs 7 days after antigen priming (Figs. 3B,E). We took advantage of this system to further examine the importance of SLAT in the secondary expansion of CD8+ T cells following ActA-deficient (ActA−) Lm-OVA challenge. We observed a drastically diminished (4–5-fold) accumulation of Def6−/− OT-I CD8+ T cells by comparison with WT cells 5 days after Lm-OVA infection (Suppl. Fig. 2A–C). In contrast, the phenotypic (Suppl. Fig. 2D,E) and functional (Suppl. Fig. 2F) parameters of Ag-specific CD8+ T cells were not affected by Def6 deletion (Suppl. Fig 2).
We extended this analysis to a different priming model, which does not display the accelerated memory phenotype, namely, priming of mice with Lm-Ova. When CD8+ T cell priming was analyzed 7 days after Lm-OVA immunization, we observed similar results to those obtained in mice primed with Act-mOVA/Kb−/− splenocytes, i.e., impaired expansion of the Def6−/− T cells (Fig. 3G). The delayed development of T cell memory was evident from the finding that on day 7 post-priming, the antigen-specific response as determined was dominated by SLECs (Fig. 3H). As in the case of CD8+ T cells from Act-mOVA/Kb−/− splenocyte-primed mice (Suppl. Fig. 2A–C), Def6−/− T cells also displayed an impaired secondary T cell expansion following ActA− Lm-OVA rechallenge at day 54 (Suppl. Fig. 3).
Impaired proliferation and enhanced death of Def6−/− CD8+ T cells
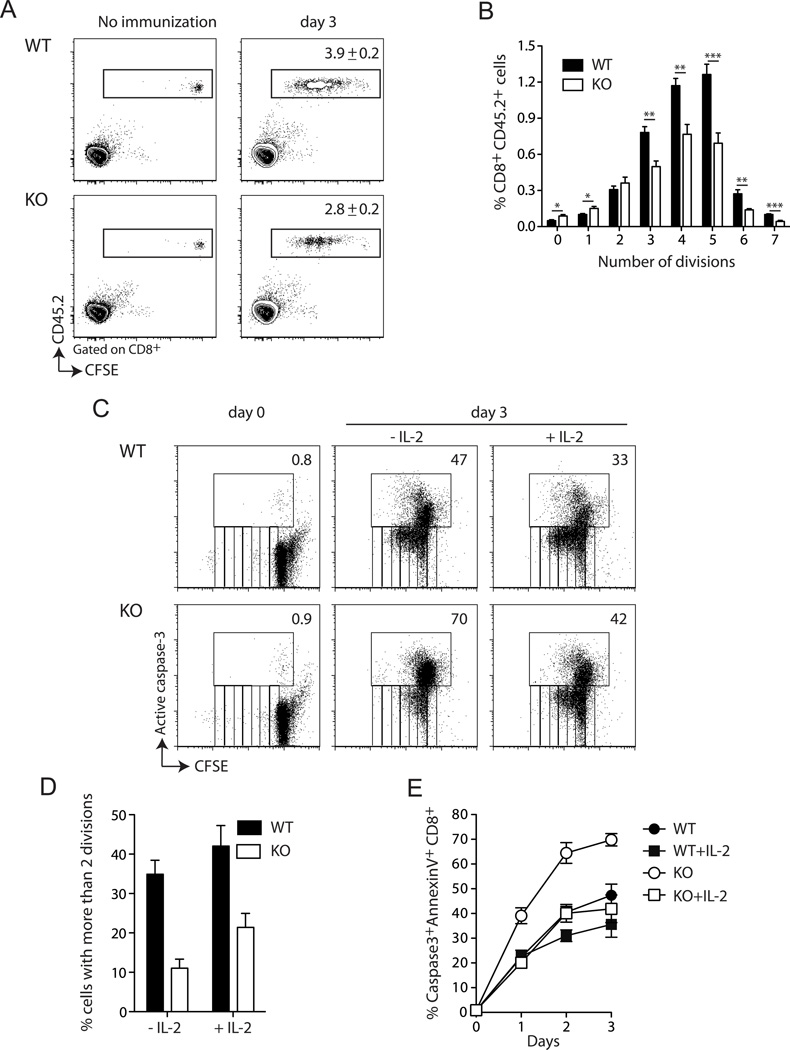
To further investigate the cause of the reduced expansion of Def6−/− CD8+ T cells, we first evaluated their proliferative capacities in vivo. We transferred CFSE-labeled WT or Def6−/− CD45.2+ OT-I CD8+ T cells into WT B6.SJL (CD45.1+) mice, immunized the recipients with Act-mOVA/Kb−/− splenocytes and analyzed the accumulation of the donor cells and their division profile by CFSE dilution in recipient spleens. Def6−/− CD8+ T cells exhibited impaired accumulation when compared to WT cells, reflecting a significantly lower number of cycling cells (Fig. 4A). Analysis of cell number recovered at each division cycle showed that Def6−/− CD8+ T cells divided at a rate similar to that of WT cells in response to OVA priming during the first two cycles, but displayed a reduced rate at subsequent division cycles (Fig. 4B). This reduced proliferation in response to anti-CD3/CD28 stimulation was confirmed by measuring [3H]thymidine incorporation (Suppl. Fig. 4A). With regard to cell survival, we could not assess the proportion of dying cells in vivo, presumably due to the rapid engulfment of apoptotic cells by phagocytes. However, we determined the frequency of dividing vs. dying CD8+ T cells in vitro by CFSE-labeling and concurrent staining with active caspase-3 and Annexin V mAbs, respectively. We confirmed that Def6−/− CD8+ T cells divide at a slower rate than WT cells in response to anti-CD3/CD28 stimulation (Fig. 4C and D). This proliferative defect was accompanied by a 2-fold reduction in the proportion of surviving Def6−/− CD8+ T cells compared to WT cells at all time points assayed (Fig. 4C and E). Consistent with their reduced proliferation along with enhanced cell death, anti-CD3/CD28-stimulated Def6−/− CD8+ T cells displayed a drastic decrease in IL-2 production (Suppl. Fig. 4B), although CD3/CD28 engagement induced intact upregulation of CD25 and the activation marker CD69 in Def6−/− CD8+ T cells (Suppl. Fig. 4C). Since IL-2 has been shown to be required for survival (but not for the initiation of CD8 T cell cycling) in order to sustain CD8 T cell primary expansion (27), we assessed whether addition of exogenous IL-2 could rescue the survival and/or the proliferation defect of Def6−/− CD8+ T cells. Addition of IL-2 restored Def6−/− CD8+ T cell survival at all times assayed (Fig. 4C,E), but did not rescue the proliferative defect (Fig. 4C,D), indicating that the impaired proliferation of Def6−/− CD8+ T cells reflects a cell-intrinsic defect, while the in vitro survival defect was a consequence of diminished IL-2 production. These data collectively suggest that in the absence of SLAT, CD8+ T cell cycling is intrinsically reduced and IL-2-dependent survival is decreased, with both of these effects combining to result in a drastic defect in primary CD8+ T cell expansion.
Figure 4. SLAT is required for proliferation and IL-2-dependent survival of CD8+ T cells.
Five × 105 WT or KO (CD45.2+) OT-I CD8+ T cells were labeled with CFSE and injected i.v. into naïve B6.SJL (CD45.1+) mice. One day later, the mice were immunized with 5 × 106 Act-mOVA/Kb−/− splenocytes. Splenocytes were harvested 3 days later, and the proliferation of OT-I cells was analyzed by FACS analysis of CFSE dilution after gating on live CD8+ T cells. A, Numbers in the right panels indicate the % of WT or KO OT-I CD8+ T cells among CD8+ splenocytes (mean ± SEM of 6–7 mice). B, Frequency of OT-I cells at each cell division cycle. *P<0.05, **P<0.005, ***P<0.0005 (two-tailed unpaired t-test). C–E, CFSE-labeled purified CD8+ T cells from WT or Def6−/− (KO) mice were stimulated with plate-coated anti-CD3 (5µg/ml) plus soluble anti-CD28 (2.5µg/ml) mAbs for 72h in the presence or absence of exogenous IL-2 (100 U/ml). Cells were then stained with Annexin V and active caspase 3 Ab and analyzed by flow cytometry. C, CFSE dilution and active caspase-3 staining allow to concurrently assess cell division and cell death of CD8+ T cells. Numbers indicate the % of active caspase-3+ cells. D, the percentage of active caspase-3−CD8+ T cells with more than 2 divisions upon 72h of stimulation is shown. E, the percentage of active caspase-3+ Annexin V+ CD8+ T cells at the indicated times is determined. Data are representative of four (A–B) and three (C–E) independent experiments.
Dependence of Ag-specific CD8+ T cell expansion on SLAT-mediated Cdc42 and NFAT1 activation
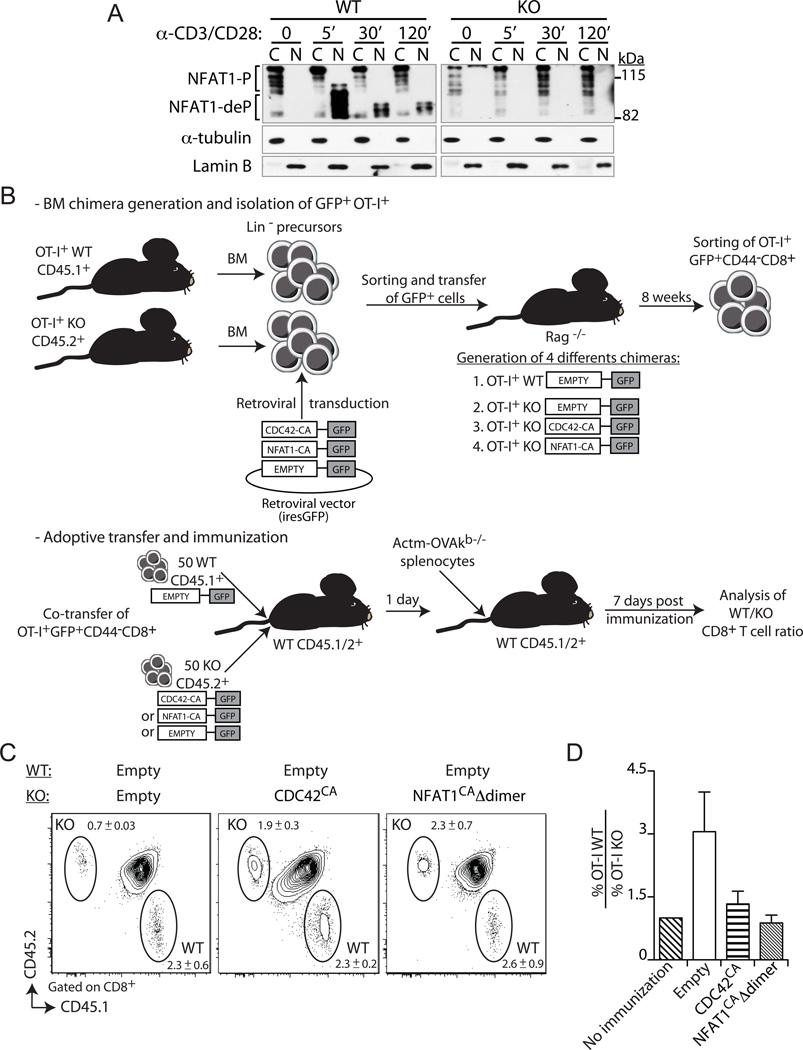
Next, we investigated the molecular mechanism underlying SLAT-mediated CD8+ T cell expansion. Since SLAT functions as a TCR-regulated GEF for the Rho GTPase, Cdc42, and this activity is required for subsequent NFAT activation and, consequently, for differentiation of CD4+ T cells into effector Th cells (6), we first determined whether NFAT activation in CD8+ T cells was also dependent on SLAT. TCR/CD28 costimulation of naïve WT CD8+ T cells resulted in the rapid (5 min) translocation of NFAT1 from the cytoplasm to the nucleus (28, 29); this translocation was still evident after 2 h (Fig. 5A). In sharp contrast, no NFAT1 nuclear translocation was observed in stimulated Def6−/− CD8+ T cells (Fig. 5A), indicating that SLAT is required for NFAT1 activation in naïve CD8+ T cells as well. This result prompted us to further assess whether constitutively active forms of Cdc42 or NFAT1 can restore the impaired expansion of Def6−/− CD8+ T cells in vivo. For this purpose, we used a retrovirus-mediated stem cell gene transfer to generate retrogenic (Rg) BM chimeric mice, which served as a source of naïve CD8+ T cell expressing constitutively active Cdc42 (Cdc42CA) or NFAT1 (NFAT1CA) (Fig. 5B). In the case of NFAT, we used an active mutant that contains two additional point mutations, which prevent NFAT homodimerization (the anergy-inducing form of NFAT), but allow NFAT:AP-1 heterodimerization leading to productive T cell activation (termed hereafter “NFAT1CAΔdimer”) (18). This mutant was able to constitutively transactivate an NFAT-responsive reporter luciferase gene in unstimulated T cells (Suppl. Fig. 2G). Sorted GFP+ (transduced) BM stem cells, isolated as lin− cells, were transferred into sublethally irradiated Rag1−/− recipients, thus allowing the development of donor-derived T lymphocytes in vivo (30). Six to 8 weeks after BM transfer, naïve (CD62Lhigh CD44low) peripheral blood OT-I CD8+ T cells were sorted and a 1:1 mixture of 50 each of mock-transduced WT cells and Def6−/− CD45.2+ cells transduced with either Cdc42CA or NFAT1CAΔdimer were adoptively transferred into WT CD45.1/2+ recipient mice (Fig. 5B). Seven days after challenge with Act-mOVA splenocytes, we found that the defective Def6−/− CD8+ T cell primary expansion was restored when the cells were transduced with Cdc42CA as well as with NFAT1CAΔdimer (Fig. 5C,D). Likewise, when re-challenged with Lm-OVA, the Def6−/− OT-I CD8+ T cells expressing Cdc42CA or NFAT1CAΔdimer, but not the mock-transduced Def6−/− cells, displayed secondary expansion very similar to that of the control, mock-transduced WT cells (Suppl. Fig. 2H,I). Together, these findings suggest that defective NFAT signaling, which depends on SLAT-mediated Cdc42 activation, is the cause of the impaired Ag-specific CD8+ T cell expansion.
Figure 5. Constitutively active Cdc42 or NFAT1 rescue primary expansion of Def6−/− CD8+ T cells.
A, Primary WT and KO CD8+ T cells were activated with anti-CD3 plus -CD28 (5 and 2.5µg/ml, respectively) mAbs for the indicated times. Cytoplasmic (C) and nuclear (N) fractions were immunoblotted with an NFAT1-specific Ab. Fractions were also immunoblotted with α-tubulin- and lamin B-specific Abs to confirm purity of the cytosolic and nuclear fractions, respectively. NFAT1-P, phosphorylated NFAT1, NFAT1-deP, dephosphorylated NFAT1. B, Experimental setup used to assess the role of SLAT-mediated NFAT1 and CDC42 activation in CD8+ T cell expansion. The top panel describes the generation of retrogenic mice, followed by sorting of retrovirally transduced (GFP+) naïve WT or KO CD8+ T cells, which are used as donor cells for co-adoptive transfers into immunized WT recipients, as described in bottom panel. C and D, WT (CD45.1+) or KO (CD45.2+) BM progenitor cells from respective OT-I TCR-Tg mice were transduced either with the control empty retrovirus (WT and KO; Mock) or with retroviruses encoding the constitutively active Cdc42 (KO; Cdc42CA) or NFAT1 (KO; NFAT1CAΔdimer). Transduced GFP+ BM cells were sorted and injected i.v. into sublethally irradiated Rag1−/− mice. Eight weeks later, WT or KO naïve OT-I CD8+ cells (tetramer+, CD62Lhigh, and CD44low) were sorted from spleen cell suspensions. WT and KO cells (50 cells each) were transferred as indicated into recipient B6 (CD45.1/2+) mice. One day later, the recipient mice were immunized with 5 × 106 Act-mOVA/Kb−/− splenocytes and CD8+ T cell expansion of the transferred cells analyzed on day 7 post-challenge. C, Frequency of WT and KO OT-I CD8+ T cells among CD8+ T cells in the blood (mean ± SEM, n=5). D, Average ratio of WT OT-I to KO OT-I CD8+ T cells (n=5). The ratio in non-immunized animals is set at 1. Data are representative of three independents experiments.
DISCUSSION
In this study, we investigated the impact of Def6 deficiency on different phases of antigen-specific CD8+ T cell responses, i.e., activation, expansion, effector function, and memory development. We report that SLAT is a critical CD8+ T cell-intrinsic positive regulator T cell expansion, but is not required for CD8+ T cell differentiation into effector or memory cells. Moreover, we provide evidence that the reduced antigen-induced expansion of CD8+ T cells in the absence of SLAT results primarily from a T cell-intrinsic defective activation and proliferation of clonal precursors, inducing consequently impaired IL-2-dependent cell survival. Lastly, our finding that the expression of constitutively active mutants of Cdc42 or NFAT1 in naïve CD8+ T cells rescued the defective Ag-specific CD8+ T cell response suggests that SLAT controls CD8+ T cell expansion through a Cdc42/NFAT pathway.
The CD8+ T cell expansion defect was observed both in a polyclonal environment, i.e., in intact immunized mice and in a TCR-transgenic adoptive transfer system. The results of the adoptive transfer experiments provide evidence that the expansion defect is intrinsic to the antigen-specific CD8+ T cells, although it does not exclude potential contribution by other cell types that also express SLAT such as DC or CD4+ T cells, which and are well-known to play a critical role in the generation of an optimal CD8+ T cell response. However, we have previously shown that Def6−/− dendritic cells did not display any defect in antigen presentation to CD4+ T cells (11).
The proliferation of naïve CD8+ T cells during a primary response follows a two-phase pattern. The first phase, occurring in the secondary lymphoid organs (SLOs), reflects an initial IL-2-independent expansion of Ag-specific T cells following interaction with APCs. The second phase, which correlates with emigration of the activated CD8+ T cells to peripheral, non-lymphoid target tissues, is associated with the acquisition of effector functions and corresponds to an IL-2-dependent prolonged proliferation of CD8+ T cells within the target organs (27, 31, 32). It is highly likely that SLAT play a prominent role in CD8+ T proliferation during the early phase of proliferation in SLOs, where the initial antigen-specific T cell-APC cell interaction occurs. This notion is supported by several findings: First, the expansion defect in Def6−/− CD8+ T cells was detected in the spleen. Second, the proliferation defect was observed as early as 3 days post-immunization, when the cells did not yet migrate to the periphery. Third, the acquisition of effector functions and CD8+ T cell survival, associated with the latter stage of the proliferative phase, were not altered in the absence of SLAT. Finally, the impaired proliferation observed in response to anti-CD3/CD28 stimulation was not rescued by addition of exogenous IL-2.
In CD4+ T cells, SLAT has previously been shown to play a key role in TCR-mediated activation and antigen-specific immunological synapse (IS) formation and stabilization at the T-cell/APC interface via its actin regulatory function, a process that is critical for optimal T cell activation in SLOs (6, 33). Furthermore, we found that the expression of constitutive active Cdc42, a target of SLAT, rescued the defective expansion of Slat−/− CD8+ T cells, demonstrating, for the first time, a role of SLAT and its effector Cdc42 in CD8+ T cell expansion in vivo. On the basis of these observations, and although a role of SLAT in IS formation in CD8+ T cells remains to be formally demonstrated, we hypothesize that altered IS formation, most likely due to abrogated Cdc42-mediated actin accumulation at the T cell-APC interphase, accounts for the impaired priming and proliferation of Def6−/− CD8+ T cells in vivo. In support of this concept, a recent study showed that SLAT accumulates at the center of the IS, where it may contribute to the activation of Cdc42, which is colocalized at the same site (34). However, several studies ascribe to CDC42 and its effector, Wiskott-Aldrich Syndrome protein (WASp), a function in later stages of CD8+ T cell differentiation, i.e., in the polarization of cytolytic effectors at the CTL-target cell interface, rather than in CD8+ T cell priming (35, 36).
Our data suggest that SLAT functions in CD8+ T cells is a manner distinct from that of other GEFs that are known to activate Cdc42, such as DOCK8 and Vav, since these other GEFs were reported to play a key role in the persistence of CD8+ T cell memory (37) and in CTL lytic activity via regulation of cytolytic effector polarization toward target cells (38, 39). In agreement with the idea that SLAT-mediated CDC42 activation plays a role in CD8+ T cell priming, we have shown that Def6 deficiency does not impair the formation of effector KLRG1hiCD127lo (SLEC) and memory KLRG1lo CD127hi (MPEC) CTL populations in response to immunization with Lm-OVA or Actm-OVA/Kb−/− splenocytes. However, the number of memory cells generated after contraction was reduced, most likely reflecting a secondary outcome of the initial expansion defect. Moreover, a more severe defect in secondary expansion upon antigen rechallenge was observed (5–8-fold reduction in the numbers of Def6−/− T cells depending on the immunization model) compared to the 2–3-fold defect in T cell expansion following antigen priming (Figs. S2 and S3). This difference suggests an additional role of SLAT in memory T cell expansion, in addition to its crucial role in primary CD8+ T cell expansion. Supporting a role of Cdc42 in the first phase of CD8+ T cell differentiation from naïve into effector cells, a gene expression profile of naïve vs. effector CD8+ T cells has shown that Cdc42 was upregulated in effector, but not in memory, CD8+ T cells by comparison with naïve CD8+ T cells (40). However, in the case of Def6 deficiency, the cytolytic activity, degranulation, and cytokine production evaluated in equivalent numbers of CTL from immunized mice was also not altered. Altogether, these observations suggest that SLAT, unlike other Cdc42 activators, may be part of a qualitatively unique TCR-induced signaling signalosome, or it may be involved in stage-specific signaling pathways that control CD8+ T cell expansion, but not other phases of the response. Of note, these findings held true in several distinct experimental settings, which differ by the strength of the antigenic stimulation and level of inflammation, i.e., the splenocyte cross-priming pathway (in the absence of overt systemic inflammation) vs. the more inflammatory Lm-OVA model. Consistent with these in vivo findings, Def6−/− CD8+ T cells also showed a clear proliferative defect in response to anti-CD3/CD28-mediated costimulation in vitro independently of the concentration of the anti-CD3 antibody (Suppl. Fig 4A). These findings suggest that SLAT is not critically involved in setting signaling thresholds but, rather, it qualitatively modulates TCR signaling.
Primary CTL expansion in vivo is the result of the integration of signals originating from a variety of different signaling cascades, which imprint differentiation of clonal precursors during initial priming into various functional subsets by orchestrating gene programs governed by master transcriptional factors such as STAT1 (41) and Bcl11b (42). Our previous studies in CD4+ T cells show that SLAT is a key component of the Ca2+/NFAT signaling pathway, thereby controlling CD4+ T cell differentiation into effector Th1, Th2 or Th17 subsets (6, 10, 11, 21). While extensively studied in the context of CD4+ T cell activation and differentiation, relatively few studies explored the role for NFAT during CD8+ T cell responses, and none of these specifically addressed its role in clonal expansion of CD8+ T cells. NFAT1 has been shown to partially regulate IFN-γ production by naïve CD8+ T cells (13). In a model of chronic viral infection, silencing of cytokine production has been linked to a selective impairment of NFAT nuclear translocation in CD8+ T cells, whereas cell-directed effector functions, such as degranulation and cytotoxicity remained intact (15). Finally, the calcineurin-NFAT pathway has been implicated in CD8+ T cell tolerance in vivo (14). Since SLAT controls NFAT activation in a CDC42-dependent manner in CD4+ T cells, and we showed here that Cdc42CA rescued the defective expansion of the Def6−/− CD8+ T cells, we propose that impaired SLAT- dependent, CDC42-mediated NFAT activation is the underlying mechanism for the impaired expansion of CD8+ T cells. Consistent with such a mechanism, our results show that NFAT1 failed to translocate to the nucleus in stimulated Def6−/− CD8+ T cells and, second, a constitutively active form of NFAT1 bypassed Def6 deficiency and restored CD8+ T cell expansion. Thus, our findings reveal a heretofore unappreciated role for NFAT1 in CD8+ T cell biology by demonstrating for the first time that SLAT controls the clonal expansion of CD8+ T cells in vivo through a CDC42/NFAT signaling pathway. Our findings differ from another study (13) reporting that Nfat1−/− CD8+ T cells show a drastic impairment in IFN-γ production after in vitro anti-CD3 stimulation, which we did not observe in the Def6−/− T cells. Thus, the absence of SLAT is likely to lead to more compound alterations in signaling compared to NFAT1 deletion since SLAT may have additional targets beyond NFAT, including some target(s) that could potentially negatively regulate IFN-γ production and antagonize positive regulation by NFAT. In this scenario, IFN-γ expression would be intact even in the absence of SLAT. Further work aimed at elucidating the molecular pathways through which SLAT links Cdc42 to NFAT activation in cycling CD8+ T, and identifying SLAT-interacting partners in its regulatory signaling complex, may unveil targets for the development of vaccination and therapeutic strategies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yun-Cai Liu for helpful comments, Ann J. Canonigo-Balancio for mouse genotyping, Cheryl Kim, Kurt Van Gunst and Anthony Jose for assistance with flow cytometry and cell sorting. This is manuscript number 1507 from La Jolla Institute for Allergy and Immunology, La Jolla, CA.
This work was supported by National Institutes of Health grants AI068320 (A.A), AI076972 (SPS), the Kurz Family Foundation (SPS) and fellowships from the Diabetes & Immune Disease National Research Institute (S.B) and Philippe Foundation (S.B).
Abbreviations used
- GEF
Guanine nucleotide exchange factor
- Lm-OVA
Listeria monocytogenes expressing OVA
- LN
lymph node
- MPEC
memory precursor effector cells
- SLAT
SWAP-70-like adapter of T cells
- SLEC
short-lived effector cells
REFERENCES
- 1.Sprent J, Judge AD, Zhang X. Cytokines and memory-phenotype CD8+ cells. Adv Exp Med Biol. 2002;512:147–153. doi: 10.1007/978-1-4615-0757-4_20. [DOI] [PubMed] [Google Scholar]
- 2.Arens R, Schoenberger SP. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol Rev. 235:190–205. doi: 10.1111/j.0105-2896.2010.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 4.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 5.Parish IA, Kaech SM. Diversity in CD8(+) T cell differentiation. Curr Opin Immunol. 2009;21:291–297. doi: 10.1016/j.coi.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becart S, Balancio AJ, Charvet C, Feau S, Sedwick CE, Altman A. Tyrosine-phosphorylation-dependent translocation of the SLAT protein to the immunological synapse is required for NFAT transcription factor activation. Immunity. 2008;29:704–719. doi: 10.1016/j.immuni.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka Y, Bi K, Kitamura R, Hong S, Altman Y, Matsumoto A, Tabata H, Lebedeva S, Bushway PJ, Altman A. SWAP-70-like adapter of T cells, an adapter protein that regulates early TCR-initiated signaling in Th2 lineage cells. Immunity. 2003;18:403–414. doi: 10.1016/s1074-7613(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Fanzo JC, Hu C, Cox D, Jang SY, Lee AE, Greenberg S, Pernis AB. T cell receptor engagement leads to the recruitment of IBP, a novel guanine nucleotide exchange factor, to the immunological synapse. J Biol Chem. 2003;278:43541–43549. doi: 10.1074/jbc.M308960200. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Lee A, Hu C, Fanzo J, Goldberg I, Cattoretti G, Pernis AB. Molecular cloning of IBP, a SWAP-70 homologous GEF, which is highly expressed in the immune system. Hum Immunol. 2003;64:389–401. doi: 10.1016/s0198-8859(03)00024-7. [DOI] [PubMed] [Google Scholar]
- 10.Becart S, Charvet C, Canonigo Balancio AJ, De Trez C, Tanaka Y, Duan W, Ware C, Croft M, Altman A. SLAT regulates Th1 and Th2 inflammatory responses by controlling Ca2+/NFAT signaling. J Clin Invest. 2007;117:2164–2175. doi: 10.1172/JCI31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canonigo-Balancio AJ, Fos C, Prod'homme T, Becart S, Altman A. SLAT/Def6 plays a critical role in the development of Th17 cell-mediated experimental autoimmune encephalomyelitis. J Immunol. 2009;183:7259–7267. doi: 10.4049/jimmunol.0902573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung-Theung-Long S, Mondor I, Guiraud M, Lamare C, Nagaleekar V, Paulet PE, Rincon M, Guerder S. Impaired NFAT transcriptional activity in antigen-stimulated CD8 T cells linked to defective phosphorylation of NFAT transactivation domain. J Immunol. 2009;182:6807–6814. doi: 10.4049/jimmunol.0803539. [DOI] [PubMed] [Google Scholar]
- 13.Teixeira LK, Fonseca BP, Vieira-de-Abreu A, Barboza BA, Robbs BK, Bozza PT, Viola JP. IFN-gamma production by CD8+ T cells depends on NFAT1 transcription factor and regulates Th differentiation. J Immunol. 2005;175:5931–5939. doi: 10.4049/jimmunol.175.9.5931. [DOI] [PubMed] [Google Scholar]
- 14.Fehr T, Lucas CL, Kurtz J, Onoe T, Zhao G, Hogan T, Vallot C, Rao A, Sykes M. A CD8 T cell-intrinsic role for the calcineurin-NFAT pathway for tolerance induction in vivo. Blood. 2007;115:1280–1287. doi: 10.1182/blood-2009-07-230680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agnellini P, Wolint P, Rehr M, Cahenzli J, Karrer U, Oxenius A. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proc Natl Acad Sci U S A. 2007;104:4565–4570. doi: 10.1073/pnas.0610335104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benedict CA, Loewendorf A, Garcia Z, Blazar BR, Janssen EM. Dendritic cell programming by cytomegalovirus stunts naive T cell responses via the PD-L1/PD-1 pathway. J Immunol. 2008;180:4836–4847. doi: 10.4049/jimmunol.180.7.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells. Nat Immunol. 2011;12:908–913. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soto-Nieves N, Puga I, Abe BT, Bandyopadhyay S, Baine I, Rao A, Macian F. Transcriptional complexes formed by NFAT dimers regulate the induction of T cell tolerance. J Exp Med. 2009;206:867–876. doi: 10.1084/jem.20082731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 20.Villalba M, Coudronniere N, Deckert M, Teixeiro E, Mas P, Altman A. A novel functional interaction between Vav and PKCtheta is required for TCR-induced T cell activation. Immunity. 2000;12:151–160. doi: 10.1016/s1074-7613(00)80168-5. [DOI] [PubMed] [Google Scholar]
- 21.Becart S, Altman A. SWAP-70-like adapter of T cells: a novel Lck-regulated guanine nucleotide exchange factor coordinating actin cytoskeleton reorganization and Ca2+ signaling in T cells. Immunol Rev. 2009;232:319–333. doi: 10.1111/j.1600-065X.2009.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 24.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 25.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pham NL, Pewe LL, Fleenor CJ, Langlois RA, Legge KL, Badovinac VP, Harty JT. Exploiting cross-priming to generate protective CD8 T-cell immunity rapidly. Proc Natl Acad Sci U S A. 2010;107:12198–12203. doi: 10.1073/pnas.1004661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 28.Ruff VA, Leach KL. Direct demonstration of NFATp dephosphorylation and nuclear localization in activated HT-2 cells using a specific NFATp polyclonal antibody. J Biol Chem. 1995;270:22602–22607. doi: 10.1074/jbc.270.38.22602. [DOI] [PubMed] [Google Scholar]
- 29.Okamura H, Aramburu J, Garcia-Rodriguez C, Viola JP, Raghavan A, Tahiliani M, Zhang X, Qin J, Hogan PG, Rao A. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol Cell. 2000;6:539–550. doi: 10.1016/s1097-2765(00)00053-8. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa R, Mason SM, Michie AM. Determining the role of specific signaling molecules during lymphocyte development in vivo: instant transgenesis. Nat Protoc. 2006;1:1185–1193. doi: 10.1038/nprot.2006.178. [DOI] [PubMed] [Google Scholar]
- 31.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 32.Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 33.Fanzo JC, Yang W, Jang SY, Gupta S, Chen Q, Siddiq A, Greenberg S, Pernis AB. Loss of IRF-4-binding protein leads to the spontaneous development of systemic autoimmunity. J Clin Invest. 2006;116:703–714. doi: 10.1172/JCI24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singleton KL, Gosh M, Dandekar RD, Au-Yeung BB, Ksionda O, Tybulewicz VL, Altman A, Fowell DJ, Wulfing C. Itk controls the spatiotemporal organization of T cell activation. Sci Signal. 2011;4:ra66. doi: 10.1126/scisignal.2001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Meester J, Calvez R, Valitutti S, Dupre L. The Wiskott-Aldrich syndrome protein regulates CTL cytotoxicity and is required for efficient killing of B cell lymphoma targets. J Leukoc Biol. 2010;88:1031–1040. doi: 10.1189/jlb.0410197. [DOI] [PubMed] [Google Scholar]
- 36.Sinai P, Nguyen C, Schatzle JD, Wulfing C. Transience in polarization of cytolytic effectors is required for efficient killing and controlled by Cdc42. Proc Natl Acad Sci U S A. 2010;107:11912–11917. doi: 10.1073/pnas.0913422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randall KL, Chan SS, Ma CS, Fung I, Mei Y, Yabas M, Tan A, Arkwright PD, Al Suwairi W, Lugo Reyes SO, Yamazaki-Nakashimada MA, Garcia-Cruz Mde L, Smart JM, Picard C, Okada S, Jouanguy E, Casanova JL, Lambe T, Cornall RJ, Russell S, Oliaro J, Tangye SG, Bertram EM, Goodnow CC. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208:2305–2320. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cella M, Fujikawa K, Tassi I, Kim S, Latinis K, Nishi S, Yokoyama W, Colonna M, Swat W. Differential requirements for Vav proteins in DAP10- and ITAM-mediated NK cell cytotoxicity. J Exp Med. 2004;200:817–823. doi: 10.1084/jem.20031847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Billadeau DD, Brumbaugh KM, Dick CJ, Schoon RA, Bustelo XR, Leibson PJ. The Vav-Rac1 pathway in cytotoxic lymphocytes regulates the generation of cell-mediated killing. J Exp Med. 1998;188:549–559. doi: 10.1084/jem.188.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 41.Quigley M, Huang X, Yang Y. STAT1 signaling in CD8 T cells is required for their clonal expansion and memory formation following viral infection in vivo. J Immunol. 2008;180:2158–2164. doi: 10.4049/jimmunol.180.4.2158. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Rozell M, Verma RK, Albu DI, Califano D, VanValkenburgh J, Merchant A, Rangel-Moreno J, Randall TD, Jenkins NA, Copeland NG, Liu P, Avram D. Antigen-specific clonal expansion and cytolytic effector function of CD8+ T lymphocytes depend on the transcription factor Bcl11b. J Exp Med. 2010;207:1687–1699. doi: 10.1084/jem.20092136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.