Abstract
Autism spectrum disorders (ASD) form a common group of neurodevelopmental disorders appearing to be under polygenic control, but also strongly influenced by multiple environmental factors. The brain mechanisms responsible for ASD are not understood and animal models paralleling related emotional and cognitive impairments may prove helpful in unraveling them. BTBR T+tf/J (BTBR) mice display behaviors consistent with the three diagnostic categories for ASD. They show impaired social interaction and communication as well as increased repetitive behaviors. This review covers much of the data available to date on BTBR behavior, neuroanatomy and physiology in search for candidate biomarkers, which could both serve as diagnostic tools and help to design effective treatments for the behavioral symptoms of ASD.
Keywords: BTBR T+tf/J mouse, autism spectrum disorder, social interaction, agenesis of corpus callosum, biomarkers
1. Introduction
Autism spectrum disorders (ASD) form a group of behaviorally defined neurodevelopmental disorders characterized by three core symptom clusters: social interaction impairment, communication deficits, and ritualistic-repetitive behaviors [1–4]. The rapid increase in diagnoses of ASD over the past years places current estimates of prevalence at about 1% of young children [5–9]. The etiology of ASD remains unclear, with multiple factors likely to contribute to an autistic phenotype. Although there is evidence for a strong influence of genetic factors, with 60–90% concordance in monozygotic twins and 4–10% in dizygotic twins [10] with a recent study suggesting rates approaching 30% for the latter [11], ASD appears to be polygenic, with hundreds of contributing loci [12,13]. ASD is more often diagnosed in boys, with the sex ratio of 4.3:1 [14] for classic autism and as high as 11:1 in Asperger Syndrome [15], suggesting that at least some of the genes involved are X-linked. Since only 10–15% of ASD cases can be associated with monogenic disorders such as Rett syndrome, Fragile X syndrome or tuberous sclerosis complex [16–18] the remaining majority of cases are described as idiopathic. Most likely the autistic phenotype is a result of the complex interaction of a number of genes with a range of environmental factors.
ASD patients have been shown to display cortical gray matter overgrowth and impaired migration of neurons during early development resulting in heterotopias, as well as thinning of the corpus callosum and malformations of the ventricular system [19–22]. The relatively low penetration of these changes in the population of ASD patients is not sufficient to consider them major causes of the behavioral phenotype of ASD. Other suggested impairments include: imbalance in excitatory and inhibitory neurotransmission, abnormal formation of dendritic spines, disrupted secondary messenger systems, extracellular matrix and synaptic protein malfunction, neuroinflamation during fetal development and later in life (for review see [23]). Approximately one-third of autistic individuals also suffer from other conditions such as epilepsy [24], anxiety disorders [25], obsessive compulsive disorder [26]or Attention Deficit Hyperactivity Disorder [26]. Gastro-intestinal and metabolic problems have also been reported in autistic patients [27,28]. But again, none of these features constitutes a strong and reliable biomarker for ASD. With the increasing costs of treatment, estimated to be $3.99 million over the lifetime of a patient born in 2000 [29], the need for early diagnosis and reliable biomarkers grows. It is therefore crucial to develop animal models with a strong autism-relevant behavioral phenotype to aid in the establishment of biomarkers. So far, the mouse model most reliably filling all three diagnostic criteria for ASD is the BTBR T+tf/J (BTBR) mouse.
2. Behavior
2.1. Social behavior
2.1.1. Three-chambered test
The three-chambered test is commonly used to assess social approach by measuring the amount of time the test mouse spends in a chamber containing a live stimulus mouse (the social chamber) compared to the amount of time spent in a chamber not containing a social stimulus (the non social chamber). This high-throughput test is often conducted as a binary assay that determines the presence or absence of a social preference, but does not compare the magnitude of social approach behavior between strains. In the three-chambered test for social approach, BTBR males failed to show a preference for the social chamber [30–38]. This is in contrast to C57BL/6J (B6) mice, often used as a comparison strain, and other strains which spent significantly more time in the social chamber when compared to the non-social chamber [30]. There are conflicting reports on the social approach behavior of BTBR females. In the three-chamber test, BTBR females have been reported as both displaying a preference for the social chamber [38] and not displaying a social preference [33,36]. These conflicting results may be due to use of different types of stimulus mice, suggesting that the social behavior of female BTBR mice is more sensitive to the partner strain.
2.1.2. Free interaction test
Free interaction tests place two animals together in an area, such as a clean housing cage, with measurement of interactive and non-interactive behaviors. In free interaction, adult BTBR mice showed reductions in sniffing and following behaviors [31,39], regardless of the stimulus animal [37]. Free interaction testing of juvenile mice showed similar results: BTBR mice showed reduced amounts of nose-to-nose sniffing, allogrooming and play behavior, while showing normal amounts of nose-to-anogenital sniffing and high amounts of self grooming [31].
2.1.3. Visible burrow system
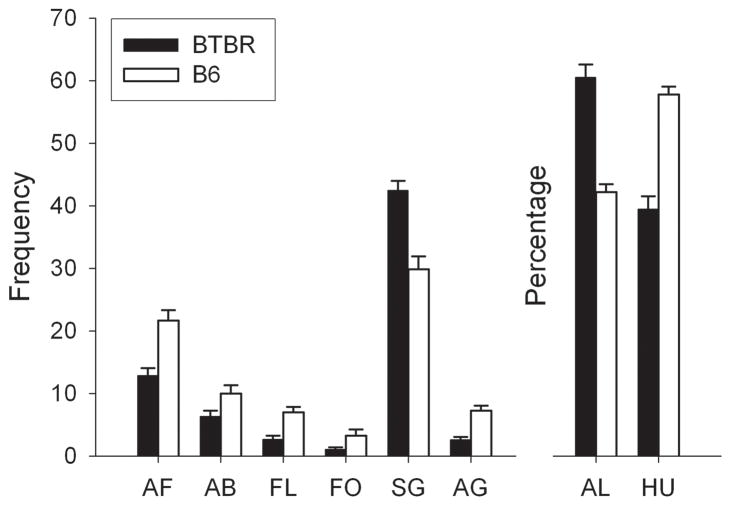
The Visible Burrow System (VBS) Test places subjects in an apparatus containing features that are present in the natural environment of the mouse [40]. An arrangement of burrows is connected to an open “surface” area by tunnels, allowing many behaviors to occur as they would in the wild. In the VBS, BTBR mice showed reductions in all interactive behaviors including approach, follow, flight, grooming another mouse, and huddling; and increases in non-dyadic behaviors such as self-grooming and being alone (Fig. 1) [32].
Figure 1.
Social interactions are reduced in BTBR mice tested in the semi-natural Visible Burrow System. All comparisons between BTBR and B6 mice are significant, p < 0.05. Approach front (AF), approach back (AB), flight (FL), follow and chase (FO), self groom (SG), and allogroom (AG) are presented as mean frequency ± SEM. Alone (AL) and huddle (HU) are presented as percentage of total time ± SEM. Modified from [32].
2.1.4. Social proximity test
The social proximity test places two animals together in a small chamber where contact with one another is virtually unavoidable. This test provides a microanalysis of behavior in a situation where the focus is not whether animals will avoid social contact, but instead concentrates on how mice will react when forced to interact. In social proximity testing [38] involving same-strain pairs, BTBR male mice showed decreased nose tip-to-nose tip, and upright behaviors; and increased nose-to-anogenital, crawl over and crawl under behaviors, in comparison to B6 mice. BTBR females also made fewer nose tip-to-nose tip and upright behaviors; and increased crawl over and crawl under behaviors. These data suggest that BTBR mice have an aversion for reciprocal frontal (face-to-face) orientations as indicated by reduced nose tip-to-nose tip behavior as well as upright behavior, in which two facing animals with contacting vibrissae move to stand on their hind legs. The increased crawl over and crawl under behaviors provide further support for this interpretation as these may function in avoidance of face-to-face encounters. Increased nose-to-anogenital behavior may serve a similar purpose in avoiding facial contact while obtaining identifying information present in pheromones.
Social proximity testing of male pairs containing one BTBR mouse and one B6 mouse produced results complementary to same-strain testing: BTBR showed increased nose-to-anogenital and crawl under behaviors and decreased nose-to-head behaviors. As nose-tip-to-nose tip requires that the two animals of a pair have an identical orientation, the BTBR and B6 pair members were not different; although this measure declined to the level seen in BTBR pairs during the same-strain tests. The dynamics of this decline could be seen in the numerous instances of approach by the B6 mouse, in which the BTBR pair member jerked its head away, resulting in the B6 mouse contacting the side of the BTBR mouse’s head. A difference in crawl over behavior was also absent in mixed strain pairings as BTBR mice would crawl under the B6 mouse and remain there, leaving the B6 few other options than to crawl over.
2.1.5. Social conditioned place preference
The social conditioned place preference test is used to determine if mice display a preference for an area that has been associated with social interaction. The multi-phase test is run in the same three-chambered apparatus used for social approach testing, with additional spatial cues added to each outer chamber (such as vertical and horizontal stripes). During the conditioning phase, subjects are placed in the outer chamber containing the stimulus mouse (the social chamber), with doors closed, and allowed to interact freely. Subjects are then immediately placed into the opposite outer chamber which does not contain a stimulus mouse (the non-social chamber), with doors closed. During the test phase, stimulus mice are not used and subjects are allowed to freely explore the apparatus with all doors open. Duration of time spent in the chamber conditioned as the social chamber, and the chamber conditioned as the non-social chamber is measured. In this test, BTBR mice conditioned for 10 days failed to show a preference for the social chamber during testing; while a preference for this chamber was displayed by B6 mice, suggesting that BTBR mice lack social motivation [41]. It is possible that reduced social interaction during conditioning may have influenced the absence of preference for the social chamber during testing; however, the authors report that BTBR mice spent a normal proportion of time near the stimulus animal.
2.1.6. Cross-fostering and cross-rearing
Studies which altered the environment of the BTBR have demonstrated the importance of juvenile sociability. Cross-fostering of BTBR pups to B6 dams [34] did not improve social or grooming behaviors of BTBR mice, suggesting that the postnatal maternal environment is not primarily involved in the manifestation of abnormal behaviors displayed by this strain. In contrast, manipulation of the BTBR rearing environment produced changes in sociability. BTBR mice housed at weaning with highly social B6 mice for 20 days showed increased social approach behavior [42]. This interesting result indicated that continuous juvenile exposure to social peers improved or prevented the characteristic social deficits of BTBR mice.
2.2. Communication
2.2.1. Ultrasonic vocalizations and scent marking
Auditory and olfactory stimuli provide mice with meaningful environmental and social information [43–45] with ultrasonic vocalizations (USVs) and urinary scent marking serving as two primary modes of communication in mice [43,46]. Rodent pups emit USVs when separated from their lactating dam, prompting the dam to retrieve the pup and return it to the nest [47]. When mouse pups were removed from the homecage and tested for maternal separation-induced vocalizations, BTBR mice emitted an unusual pattern of vocalizations. These were more frequent with high amplitude harmonics [48]. When tested as adults, BTBR mice emitted a reduced number of USVs in male-to-male, male-to-female, and female-to-female encounters [49], as well as in males presented with female urine [50]. The pattern of vocalizations in adult BTBR mice of both sexes was different from the one observed in B6 mice, with an over expression of unstructured calls in male and short calls in female BTBR mice [49]. BTBR male mice also deposited few scent marks in the presence of female urine [50,51]. The reduction in scent marking to female urine does not appear to be due to an overall olfactory deficit as BTBR mice are capable of discriminating different odors [30,37]. The authors suggested that elevated USVs in BTBR pups may provide parallels to high levels of inconsolable crying in infants that are later diagnosed with autism [48]; while, reduced USVs and scent marking in BTBR adults are relevant to communication impairments in autistic children [49–51].
2.2.2. Social transmission of food preference
While USVs and scent marking serve as the primary means of communication in mice, the transmission of food preference between animals has also been used to assess communication. The social transmission of food preference test [52] allows a “demonstrator” mouse to consume food with a strong cued flavor such as cinnamon and then allows the demonstrator mouse to interact with an “observer” mouse. The observer is later tested for food flavor preference between the cued flavor (cinnamon in this example) and an un-cued flavor (such as cocoa). In this test, BTBR mice ate less of the cued flavor introduced by the demonstrator, suggesting an impaired transmission of food preference; although it should be noted that interactions between BTBR demonstrator and BTBR observer mice were reduced, which may have influenced the result [31].
2.3. Stereotyped behaviors and resistance to change
2.3.1. Repeated and stereotyped behaviors
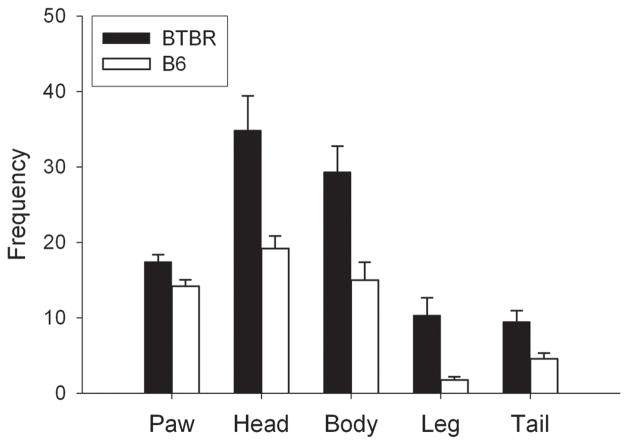
Several studies have shown that BTBR mice display elevated levels of overall self-grooming [31,33,53,54] as well as elevated levels of several grooming components: paw lick, head wash, body groom, leg lick and tail/genital groom (Fig. 2) [53]. BTBR mice are tufted mutants (hence the “tf” symbol in BTBR T+tf/J), which are characterized by repeated patterned hair loss [55]. If such a condition is associated with itching sensations, then this may account for elevated levels of grooming. Investigation into this possibility showed that the number of hairs lost during self-grooming was not correlated with any of the grooming variables measured [53], suggesting that itching does not underlie the high amounts of grooming behaviors in BTBR mice. A microanalysis of grooming structure showed that BTBR mice performed a higher percentage of area-specific rostral to caudal grooming transitions than B6 mice, indicating a more stereotyped pattern of grooming behavior [53]. BTBR mice also displayed high levels of bar biting [53], and marble burying [54], two additional measures of stereotyped motor behavior in laboratory rodents [56,57]. In a hole board test, BTBR mice displayed a preference for a particular corner hole [58]. A novel object contact test revealed similar findings. In a chamber containing four novel objects in each corner, BTBR mice showed a greater preference for individual objects manifested by significantly greater contact time to the first and second most investigated objects and reduced time with the least preferred object compared to controls. BTBR mice also displayed more episodes of visiting the objects in particular orders, even though they made no more visits than did B6 mice [53]. For example, BTBR mice might have repeatedly performed the sequence of visiting object 1, object 3, object 4, and then object 2. These results indicate that BTBR mice not only show more repeated behaviors, but they tend to do so in stereotyped patterns as well.
Figure 2.
Individual grooming components are elevated in BTBR mice. All comparisons between BTBR and B6 mice are significant, p < 0.05. Data are presented as mean frequency ± SEM. Modified from [53].
2.3.2. Cognitive inflexibility and insistence on sameness
Cognitive inflexibility, characterized by resistance to change, has been correlated with stereotypies [59]; although reports of its manifestation in autism vary [60,61]. Tests for resistance to change require mice to first learn an established routine and then to deviate from that to learn a new routine. The Morris water maze tests for spatial learning by measuring the time required to find a hidden, submerged platform first during an acquisition phase, then in a new location in a reversal phase. In this test, BTBR mice performed normally in the acquisition phase, but showed impaired performance in finding the platform [37] and did not show a preference for the quadrant containing the platform [30] in the reversal phase. Another test of acquisition and reversal allowed mice to choose between two compartments, one of which was baited with food 80% of the time, with the other bated 20% of the time for the acquisition phase [54]. During the reversal phase, the probability of baiting for each compartment was switched. In this test, BTBR mice showed normal acquisition, but deficits in reversal; however, BTBR performed normally in both phases when one arm was baited 100% of the time [30,54]. These findings indicate impaired reversal learning and cognitive inflexibility in BTBR mice, which are dependent on the type of reinforcer and the percentage of reinforcement.
2.4. Anxiety-related and stress-related behaviors
2.4.1. Elevated maze tests and light/dark exploration
Reports of anxiety behavior in BTBR mice have been inconsistent. The elevated plus maze (EPM) is a commonly used test of general anxiety that measures the number of entries and duration of time spent in an open arm compared to a closed arm, with more open arm activity generally indicating less anxiety. In the EPM, studies report that BTBR mice show normal [30], high [36], and low [62] number of entries into the open arms; as well as normal [30,36,63], and low [62] open arm durations. In the elevated zero maze, a test similar to the EPM, BTBR mice were consistent across studies in spending more time in the open arms [31,62]. The light/dark exploration test also assesses anxiety-related behaviors and is based on the aversion of mice to brightly lighted areas. In this test, the BTBR mice did not differ from B6 mice in the number of transitions between the light and dark compartments, the time spent in the dark chamber, or in the latency to enter the dark chamber.
2.4.2. Mouse defense test battery
In the mouse defense test battery (MDTB) a hand-held anesthetized rat is used to measure behaviors in response to predator exposure in various subtests/contexts [64]. In the MDTB [62], BTBR mice showed more vocalizations when contact with the predator was forced. However, BTBR mice also allowed the predator to approach within shorter distances before fleeing in an approach/avoidance setting. In addition, BTBR mice displayed increased locomotion as well as increased wall jumping and defensive upright behaviors after, but not before, the predator was introduced, suggesting increased predator-induced anxiety. In the MDTB, BTBR mice displayed complex patterns of behavior that were inconsistent with respect to generalized anxiety.
2.4.3. General reactivity and depression-like tests
The stress-reactive behaviors of BTBR mice appear to vary with the stressor. BTBR mice show normal reactivity to an acoustic startle stimulus and tail flick; along with normal prepulse inhibition and low reactivity to a hot plate stimulus [65]. When the EPM was preceded by tail suspension, BTBR showed enhanced anxiety-like behavior [63]. In tests of depression-like behaviors, BTBR mice displayed low levels of immobility in the forced swim test and the tail suspension test, suggesting a reduced tendency to depression-like behavior [65].
2.5. Behavior Summary
BTBR mice display a wide range of behaviors relevant to autism. When assessed in tests conducted in multiple independent laboratories, male BTBR mice showed overwhelmingly consistent and robust reductions in various social behaviors, impairments in two different types of communication and heightened displays of various repeated and stereotyped behaviors. While findings of the stress-related and anxiety-related behaviors of BTBR mice are inconsistent and appear to be vary with the type of stimulus, the data from these studies collectively support the notion that general anxiety does not underlie the abnormal behaviors displayed by this strain.
3. Candidate biomarkers
In addition to the strong ASD-like behavioral profile, BTBR mice have several physiological and neurological features resembling those observed in ASD diagnosed patients. Because of its consistent autism-relevant behavioral phenotype there is an increasing attention to the biology of the BTBR mouse.
3.1. Genetic background
BTBR is an inbred strain developed originally at Columbia University by crossing mice carrying the wildtype T (brachyury) gene [66] with the stock carrying tufted (tf) mutation [55]. Afterwards the strain was outcrossed to c129 mice and then maintained by inbreeding. Currently the BTBR strain is commercially available from The Jackson Laboratory (Bar Harbor, ME) and is a part of Mouse Phenome Project. Several attempts have been made to describe the genes underlying the behavior differences, including social behavior, of BTBR mice. Nadler et al. [67] performed a large scale comparison of gene expression in 10 inbred mouse strains including BTBR and B6 mice, in an attempt to correlate this expression with motor coordination on the rota-rod test. The results pointed to a correlation of motor activity with expression of several transcripts in the cerebellum, hippocampus and amygdala, but did not focus on between strain differences other than giving a general statement that cluster analysis placed BTBR mice closest to BALB/cByJ mice in terms of overall expression patterns. A genome-wide linkage analysis performed on BTBR mice backcrossed to obtain an obese phenotype (ob/ob) identified 3 major loci differentiating BTBR from B6 mice and influencing plasma glucose and/or insulin levels. One of these was located on chromosome 2 and was related to insulin sensitivity and the other two were located on chromosomes 16 and 19 and affected fasting glucose and insulin levels [68]. Analysis of 124 putative autism candidate genes revealed that there are four genes with nonsynonymous coding single nucleotide polymorphisms (SNPs) that differentiate B6 from BTBR mice. Two of them, Smo (smoothened, signaling protein regulated by Sonic hedgehog) and Pkd1 (polycystic kidney disease 1) were common for other inbred strains. Of the remaining two, Slc6a4 encoding serotonin transporter was altered in B6 mice, while Kmo encoding kynurenine 3-hydroxylase was altered in BTBR mice. The later consists of 3 different SNPs located on chromosomes 9 and 13, of which the one located in chromosome 13 is a cytosine/tymine substitution in a sequence coding a transmembrane part of the protein. This part is a conserved domain identical in mice and humans [31]. This could possibly constitute a relevant biomarker. Kynurenine 3-hydroxylase is involved in synthesis of kynurenic acid, a potent antagonist of glutamate and nicotinic receptors, involved in neuroprotection, dendritic spine formation and dopamine release [69–73]. Further analysis showed that BTBR mice also carry a Disrupted in Schizophrenia (Disc1) mutation, a 25bp deletion causing a frame shift and a premature termination of translation of the protein. It is located on chromosome 7 [http://jaxmice.jax.org/strain/002282.html]. Quantitative trait loci analyses based on offspring from BTBR and BALB/cByJ mouse crosses identified two additional regions on the X chromosome, that are possibly related to one of the neuroanatomical abnormalities observed in BTBR mice, namely agenesis of corpus callosum. The exostosin 1 (Ext1), involved in synthesis of guidance molecules crucial for the crossing of corpus callosum neuronal fibers across the midline during development (E14–E17), was found to be downregulated in BTBR mice (for further information see http://phenome.jax.org).
3.2. Neuroanatomy: interactions with genetic and molecular mechanisms
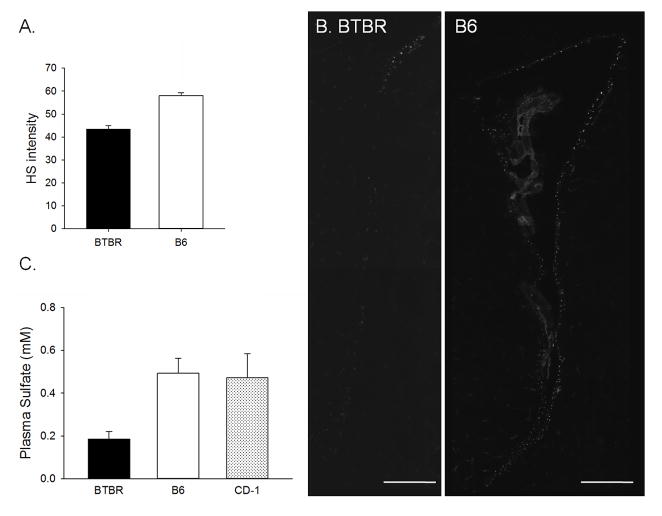
BTBR mice show severe abnormalities of the corpus callosum, often accompanied with a substantial reduction of the hippocampal commissure (HC) [74,75], a collapse of lateral ventricles (LV, [76]) with lateral displacement of the hippocampi [77]. The length of the subventricular zone (SVZ) is decreased in BTBR mice and both the concentration of laminin and heparan sulfate (HS) therein are diminished (Fig. 3a–b [76]). The latter being likely an effect of downregulation of Ext1 expression as Ext1 is involved in synthesis and elongation of HS polysaccharides [78]. HS in its properly sulfated form is considered a major guidance factor during CC development. The Ext1 KO as well as Hs6st1−/− and Hs2st−/− mice, lacking sulfotransferases responsible for sulfation of the polysaccharide HS chains, have been shown to display aCC, and altered guidance of other neuronal tracts in the brain [79,80]. Whether the intact CC is a prerequisite for development of normal social behaviors in mice is disputable, as postnatal day (PND) 7 lesion of CC in B6 mice was shown not to affect their social behavior [36]. Unfortunately it is difficult to assess the effect of earlier disruption of CC, as Ext1 KO, as well as Hs6st1−/− and Hs2st−/− mice are not postnatally viable to show any social behavior deficits. It seems more likely that it is the lack of the guidance factors influencing CC genesis, rather than the functioning of the CC itself, that disturbs the behaviors of BTBR mice. Conditional knockouts of Ext1 (Ext1CKO, CaMKII-Cre2834;Ext1flox/flox, [81]), in which Ext1 is disrupted from PND21 on, display abnormal social interactions, increased repetitive behaviors and impaired ultrasonic vocalization. The Ext1CKO animals also showed attenuated neuronal activation to social stimuli in the amygdala and impaired excitatory synaptic transmission therein, suggesting that HS is involved in correct functioning of glutamatergic synapses. Notably, Stephenson et al. [82] recently reported that BTBR mice show substantial forebrain reductions in the neurodevelopmental proteins PSA-NCAM (as well as NG2, NeuroD and DCX). PSA-NCAM interactions have effects on both HS and glutamate receptors [83], promoting NMDA and FGF2 dependent synaptogenesis. Moreover both PSA-NCAM and HS have been shown to bind to FGF-2 [83,84] this way affecting neurogenesis. Indeed, BTBR mice have recently been reported to show decreased neurogenesis in the dentate gyrus of the hippocampus [82]. This in turn has recently been linked to the autism-like behavioral phenotype by Wei and colleagues [85] showing that juvenile neurogenesis is crucial for development of normal social repertoire. Our lab has also shown that BTBR mice also have decreased general levels of plasma sulfates (Fig. 3c, [86]), which is in accordance with clinical data from ASD patients [28,87] and could indicate that abnormal sulfate metabolism or excretion may be affecting HS levels in these mice. Also, the Disc1 mutation carried by BTBR mice has been shown to affect the formation of CC [88], as well as the incorporation and migration of newly born neurons [89–91]. In addition to neurogenesis or migration patterns, upregulation of the Ras/Raf/ERK1/2 signaling pathway has been reported in BTBR, suggesting an exaggerated apoptosis occurring in the BTBR brain [92]. Altogether these data point to serious alterations of neurogenesis and neuronal death, as well as developmental migration of cells in the BTBR mouse brain, all of which are potentially mediated by alterations in the HS system and the factors it modulates.
Figure 3.
Brain heparan sulfate and plasma sulfate levels are decreased in BTBR mice. All comparisons between BTBR mice and other strains are significant, p < 0.05. A. The intensity of heparan sulfate immunolabelling (on 1–256 scale of greyness) in the fractones located around lateral ventricles. B. Photomicrograph showing the difference in the size of the lateral ventricles and the number of heparan sulfate positive fractones surrounding the ventricles in B6 and BTBR mice; scale bar represents 250um. C. Plasma sulfate levels in BTBR mice as compared with B6 and CD-1 mice. A and B modified from [76]; C modified from [86].
3.3. Physiology
3.3.1. Metabolism
BTBR metabolism was a subject of interest for many years before the discovery of their social behavior impairment. BTBR leptin ob/ob mice are prone to develop type2, insulin resistant diabetes and were studied as an animal model for this disease. Even in the absence of leptin ob/ob BTBR mice have been shown to have elevated plasma insulin levels as compared to B6 mice [93]. Male BTBR mice are prone to abdominal obesity, hypertrigliceridemia and hypercholesterolemia, with decreased insulin-stimulated uptake of glucose to white adipose tissue [94,95]. Surprisingly, in the light of a clinically proven link between gestational diabetes and autism [96], these impairments are not observed in BTBR females. Insulin resistance in BTBR males is correlated with increased expression of several genes in the adipose tissue including cytoskeletal and focal adhesion molecules (Actb, Acta2, Flna, Diap, Rhoa), proteins involved in inflammation (Pir’s, Fos, Ptgs2, Syk, Cflar, H2ab1) and phosphatases (Ptprs, Pp2r4, Pp6c) as well as leptin (for detailed description see [94].
3.3.2. Regional Brain Activation
The c-Fos protein is often used for assessment of event-related neuronal activation. Recently it has been used to assess changes in neuronal activation with social stimulation in Ext1CKO mice [81] showing reduction in activation of the basolateral and medial amygdala, as well as in the ventral orbitofrontal cortex, compared to controls. In our lab we have used c-Fos immunocytochemistry to compare neuronal activation patterns in a number of brain areas, finding wide-spread regional differences of BTBR compared to B6 mice in both the home cage and in response to social and nonsocial stimuli [Meyza et al. in prep].
3.3.3. Stress and other hormones
BTBR mice show an increased basal plasma levels of corticosterone (CORT) [63,65], suggested to affect their anxiety levels [62,63]. The link was questioned by Silverman and colleagues [65] as no concomitant increase in CRF peptide or mRNA expression was observed after exposure to stressors in BTBR mice. However, they found that BTBR mice have higher levels of glucocorticoid receptors in the CA1 (but not CA2) field of the hippocampus, which could indicate an altered negative feedback loop for the hypothalamo-pituitary-adrenal axis (HPA axis)They also suggest that elevated basal CORT might be related to insulin resistance observed in BTBR..
Surprisingly, the same study showed that BTBR mice have increased levels of plasma oxytocin as compared with B6 mice. Oxytocin (OT) signaling deficiency (observed in oxytocin knockout mice, OTKO; oxytocin receptor knockout mice, OTRKO and CD38KO mice where the CD38 enzyme regulates the Ca2+ dependent secretion of oxytocin) has been shown to produce social behavior impairments. These can be rescued (except in OTRKO mice) by intranasal supplementation of oxytocin, non-peptide agonists of OTRs or OT release promoting factors (for review see [97]). Therefore an elevated plasma OT in BTBR mice seems to be inconsistent with their asocial behavior phenotype. One possible explanation for this discrepancy is that BTBR mice display abnormalities in serotonergic neurotransmission, including reduction in SERT and 5HT1a binding in several brain areas [98]. As oxytocinergic supraoptic and paraventricular nuclei of the hypothalamus are rich in serotonergic fibers and receptors [99] and 5HT1a receptors agonists have been proved to release OT [100,101] it is possible that the 5HT deficiencies of BTBR mice are responsible for their reduced social behaviors, despite elevated levels of OT.
BTBR mice also show increased circulating progesterone and 5αpregnan-3α-ol-20-one levels with increased levels of the latter in the hypothalamus [102], suggesting that the higher basal CORT observed in these mice may result from impaired feedback of 5αpregnan-3α-ol-20-one on the HPA axis. In male mice the disruption of 5αpregnan-3α-ol-20-one synthesis results in enhanced aggression and decreased cognitive function [103]. In line with this view, BTBR males display very little or no aggression in social proximity or resident-intruder tests (unpublished observations). Similarly, both BTBR mice and their F1 offspring with B6 mice display higher levels of plasma testosterone [94], which on one hand might be responsible for their insulin resistance, as testosterone can inhibit the production of adiponectin [104], and on the other, stimulates the expression of TNF-α in macrophages [105] this way modulating the immune reactivity of BTBR mice.
3.4. Immune system
The immune responses of BTBR mice are different from those observed in B6 mice on both humoral and cellular levels. Heo and colleagues [106] showed that BTBR mice have higher basal levels of plasma IgG, IgE, anti-brain antibodies (Abs), and also of brain tissue deposited IgG and IgE. They also show higher expression of proinflammatory cytokines such as IL-33, IL-18 and IL-1b. Upon stimulation with keyhole limpet hemocyanin (KLH) BTBR mice produced 2–3 times more anti-KLH antibodies than B6 mice. In contrast to humoral immunity, their cellular response to listeriosis was much weaker than that of B6 mice even though the basal levels of CD8+T cells was higher in the BTBR mesenteric lymph nodes (but not in the spleen or blood), and CD4+T cells were elevated both in the mesenteric lymph nodes and circulating blood. The brain-blood barrier permeability index measured with Evans blue was similar in BTBR and B6 mice. In the brain the number of mast cells was much higher in BTBR mice (as compared to almost none in B6 mice), which implies an ongoing neuroinflammation. Also the microglia of BTBR differed from B6 in the number of MHC class II-expressing cells. These immune differences were found not to be related to changes in NF-κB signaling [107]. Similar results have recently been reported for ASD patients [108].
3.5. Neurotransmitters
The serotonergic system of the BTBR mouse has been an area of interest since citalopram [109], fluoxetine and buspirone were shown to decrease depression-like behavior and increase sociability [98,110]. Major differences between BTBR and B6 mice were observed in the density and binding to SERT and the binding to 5HT1a receptors but with no difference in binding to the 5HT2a receptor [59]. The latter finding is in line with studies showing that risperidone a DA/5HT2a, c antagonist/blocker had no influence on social behavior [98,110] although it did reduce marble burying [98]. In ASD patients risperidone has been used to suppress aggression, but has not been successfully used to improve social behavior [111]. Reductions in repetitive selfgrooming were observed in BTBR mice after administration of 2-methyl-6-(phenylethynyl)-pyridine (MPEP), a mGluR5 receptor antagonist [112], which could indicate that alterations in NMDA tone may be responsible for excessive selfgrooming in these mice. Moreover, GRN-529, a GluR5 antagonist reduced not only repetitive behaviors in BTBR mice but also improved some parameters of their social approach and social interactions [113]. Enhancement of GABA neurotransmission by diazepam reduces BTBR jump escape, upright and crawl under behaviors in the social proximity test [38]. As all of these behaviors have some connection to defensiveness, these changes are compatible with a view that BTBR mice do show heightened social anxiety. However, the core nose-to-nose contact measure, as well as increased nose-to-anus approaches and crawl over behaviors did not change with diazepam, indicating that social anxiety cannot account for all the social deficiencies of the BTBR mouse. We have also employed high performance liquid chromatography (HPLC) to assess concentration of norepinephrine, dopamine and serotonin and their metabolites in several brain areas [Jensen et al., in prep] finding a possible shift in levels of these between telencephalic and hindbrain sites under basal (homecage) conditions
4. Conclusions and future directions
Many of the BTBR characteristics, both behavioral and physiological, resemble those of individuals diagnosed with ASD. Agenesis of the CC and other neuroanatomic aberrations in BTBR mice, linked with studies on alterations in HS, and in conjunction with recent findings that inhibitors of mGluR5 can reduce an autism-like behavioral phenotype in BTBR mice, open a new and interesting area for further studies. Similarly, the BTBR immune system impairments, which are in line with observations made in ASD patients, pose further questions regarding the causes of these neuroinflammatory changes and the differences between cellular and humoral immune responses to challenge observed in these mice. Early fetal exposure to pathogens has been implicated in the pathogenesis of autism [114] suggesting the value of examining maternal immune activation manipulations in BTBR mice, and, in other mouse strains varying in immune compromise.
ASD encompasses a broad spectrum of abnormalities, most likely reflecting the diverse etiology of the disorder. The BTBR mouse has been proposed as an animal model for autism due to behavioral similarities to the three core symptoms of the disorder. The physiological and neuroanatomical features of BTBR mice have since been compared to human data from ASD patients, finding many similarities. However, some of the alterations found in BTBR mice are only present in a small subset of ASD patients. For example, the lack of CC observed in BTBR mice is rare among ASD patients; although, reductions in size of the CC is commonly observed in ASD [115–117]. Similarly, Disc1 mutation, carried by BTBR mice, has been associated with ASD in some [118,119], but not all [120], cases. The autistic-like phenotype of the BTBR mice may therefore model only a fraction of the ASD cases resulting from similar neuronal/physiological aberrations. Nonetheless, this strain of mice seems to be a valuable animal model, due to its strong face validity for ASD. BTBR mice may prove crucial in understanding the mechanisms underlying the autistic phenotype, leading to discovery of early detectable, reliable biomarkers for ASD, the search for which is far from over.
Highlights.
The BTBR T+tf/J mouse is an animal model of autism spectrum disorders with strong face validity
The neuroanatomy and physiology of the BTBR mouse resembles that of some individuals with ASD
Lack of corpus callosum and impaired synaptogenesis and neurogenesis may be linked with heparan sulfate reduction
The glutamatergic transmission seems crucial for restoring sociability of BTBR mice
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4. Amer Psychiatric Pub; 2000. [Google Scholar]
- 2.Baird G, Cass H, Slonims V. Diagnosis of autism. BMJ. 2003;327:488–93. doi: 10.1136/bmj.327.7413.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–55. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- 4.Lord C. Methods and measures of behavior in the diagnosis of autism and related disorders. Psychiatr Clin North Am. 1991;14:69–80. [PubMed] [Google Scholar]
- 5.Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord. 2003;33:365–82. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- 6.Maenner MJ, Durkin MS. Trends in the prevalence of autism on the basis of special education data. Pediatrics. 2010;126:e1018–1025. doi: 10.1542/peds.2010-1023. [DOI] [PubMed] [Google Scholar]
- 7.Maughan B, Iervolino AC, Collishaw S. Time trends in child and adolescent mental disorders. Curr Opin Psychiatry. 2005;18:381–5. doi: 10.1097/01.yco.0000172055.25284.f2. [DOI] [PubMed] [Google Scholar]
- 8.Rutter M. Autism research: lessons from the past and prospects for the future. J Autism Dev Disord. 2005;35:241–57. doi: 10.1007/s10803-004-2003-9. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. MMWR Morbidity and Mortality Weekly Reports. 2012. Prevalence of Autism Spectrum Disorders —Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. [PubMed] [Google Scholar]
- 10.Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- 11.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch Gen Psychiatry. 2011;68:1095–102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Betancur C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 13.Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci (Regul Ed) 2011;15:409–16. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, Mandell DS, Miller LA, Pinto-Martin J, Reaven J, Reynolds AM, Rice CE, Schendel D, Windham GC. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–58. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- 15.Gillberg C, Cederlund M, Lamberg K, Zeijlon L. Brief report: “the autism epidemic”. The registered prevalence of autism in a Swedish urban area. J Autism Dev Disord. 2006;36:429–35. doi: 10.1007/s10803-006-0081-6. [DOI] [PubMed] [Google Scholar]
- 16.Zoghbi HY. MeCP2 dysfunction in humans and mice. J Child Neurol. 2005;20:736–40. doi: 10.1177/08830738050200090701. [DOI] [PubMed] [Google Scholar]
- 17.Hagerman R, Au J, Hagerman P. FMR1 premutation and full mutation molecular mechanisms related to autism. J Neurodev Disord. 2011;3:211–24. doi: 10.1007/s11689-011-9084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chévere-Torres I, Maki JM, Santini E, Klann E. Impaired social interactions and motor learning skills in tuberous sclerosis complex model mice expressing a dominant/negative form of tuberin. Neurobiol Dis. 2012;45:156–64. doi: 10.1016/j.nbd.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 2011;1380:138–45. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidal CN, Nicolson R, Boire J-Y, Barra V, DeVito TJ, Hayashi KM, Geaga JA, Drost DJ, Williamson PC, Rajakumar N, Toga AW, Thompson PM. Three-dimensional mapping of the lateral ventricles in autism. Psychiatry Res. 2008;163:106–15. doi: 10.1016/j.pscychresns.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wegiel J, Kuchna I, Nowicki K, Imaki H, Wegiel J, Marchi E, Ma SY, Chauhan A, Chauhan V, Bobrowicz TW, de Leon M, Louis LAS, Cohen IL, London E, Brown WT, Wisniewski T. The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 2010;119:755–70. doi: 10.1007/s00401-010-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ecker C, Suckling J, Deoni SC, Lombardo MV, Bullmore ET, Baron-Cohen S, Catani M, Jezzard P, Barnes A, Bailey AJ, Williams SC, Murphy DGM. Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch Gen Psychiatry. 2012;69:195–209. doi: 10.1001/archgenpsychiatry.2011.1251. [DOI] [PubMed] [Google Scholar]
- 23.Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29:349–58. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurol. 2002;1:352–8. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 25.van Steensel FJA, Bögels SM, Perrin S. Anxiety Disorders in Children and Adolescents with Autistic Spectrum Disorders: A Meta-Analysis. Clin Child Fam Psychol Rev. 2011;14:302–17. doi: 10.1007/s10567-011-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Tager-Flusberg H, Lainhart JE. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord. 2006;36:849–61. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- 27.Maenner MJ, Arneson CL, Levy SE, Kirby RS, Nicholas JS, Durkin MS. Brief Report: Association Between Behavioral Features and Gastrointestinal Problems Among Children with Autism Spectrum Disorder. J Autism Dev Disord. 2011 doi: 10.1007/s10803-011-1379-6. [DOI] [PubMed] [Google Scholar]
- 28.Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, Gehn E, Loresto M, Mitchell J, Atwood S, Barnhouse S, Lee W. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr Metab (Lond) 2011;8:34. doi: 10.1186/1743-7075-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Federal Reserve Bank of Minneapolis. Consumer Price Index (estimate) 1800–2008 [Google Scholar]
- 30.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–63. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 32.Pobbe RLH, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214:443–9. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–89. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25:515–21. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–77. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang M, Abrams DN, Zhang JY, Weber MD, Katz AM, Clarke AM, Silverman JL, Crawley JN. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiology & Behavior. 2012 doi: 10.1016/j.physbeh.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Defensor EB, Pearson BL, Pobbe RLH, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav Brain Res. 2011;217:302–8. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–6. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arakawa H, Blanchard DC, Blanchard RJ. Colony formation of C57BL/6J mice in visible burrow system: identification of eusocial behaviors in a background strain for genetic animal models of autism. Behav Brain Res. 2007;176:27–39. doi: 10.1016/j.bbr.2006.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearson BL, Bettis JK, Meyza KZ, Yamamoto LH, Blanchard DC, Blanchard RJ. Absence of social conditioned place preference in BTBR T+tf/J mice: relevance for social motivation testing in rodent models of autism. Behavioral Brain Research. doi: 10.1016/j.bbr.2012.04.040. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2011;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–15. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheetham SA, Thom MD, Jury F, Ollier WER, Beynon RJ, Hurst JL. The genetic basis of individual-recognition signals in the mouse. Curr Biol. 2007;17:1771–7. doi: 10.1016/j.cub.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Hurst JL. Female recognition and assessment of males through scent. Behav Brain Res. 2009;200:295–303. doi: 10.1016/j.bbr.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 46.Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev. 2008;32:1236–48. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Amato FR, Scalera E, Sarli C, Moles A. Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behav. Genet. 2005;35:103–12. doi: 10.1007/s10519-004-0860-9. [DOI] [PubMed] [Google Scholar]
- 48.Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS ONE. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roullet FI, Wöhr M, Crawley JN. Female urine-induced male mice ultrasonic vocalizations, but not scent-marking, is modulated by social experience. Behav Brain Res. 2011;216:19–28. doi: 10.1016/j.bbr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryan BC, Young NB, Moy SS, Crawley JN. Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behav Brain Res. 2008;193:235–42. doi: 10.1016/j.bbr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearson BL, Pobbe RLH, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, Blanchard RJ. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:228–35. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res. 2012;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lyon MF. Hereditary Hair Loss in the Tufted Mutant of the House Mouse. J Hered. 1956;47:101–3. [Google Scholar]
- 56.Nevison C, Hurst J, Barnard C. Why do male ICR(CD-1) mice perform bar-related (stereotypic) behaviour? Behavioural Processes. 1999;47:95–111. doi: 10.1016/s0376-6357(99)00053-4. [DOI] [PubMed] [Google Scholar]
- 57.Londei T, Valentini AM, Leone VG. Investigative burying by laboratory mice may involve non-functional, compulsive, behaviour. Behav Brain Res. 1998;94:249–54. doi: 10.1016/s0166-4328(97)00162-9. [DOI] [PubMed] [Google Scholar]
- 58.Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008;188:178–94. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanimura Y, Yang MC, Lewis MH. Procedural learning and cognitive flexibility in a mouse model of restricted, repetitive behaviour. Behav Brain Res. 2008;189:250–6. doi: 10.1016/j.bbr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Zandt F, Prior M, Kyrios M. Repetitive behaviour in children with high functioning autism and obsessive compulsive disorder. J Autism Dev Disord. 2007;37:251–9. doi: 10.1007/s10803-006-0158-2. [DOI] [PubMed] [Google Scholar]
- 61.Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends Cogn Sci (Regul Ed) 2009;13:74–82. doi: 10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pobbe RLH, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+ tf/J mouse strain. Behav Brain Res. 2011;216:446–51. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: an unusual behavioral phenotype. Behav Brain Res. 2009;197:462–5. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 64.Blanchard DC, Griebel G, Blanchard RJ. The Mouse Defense Test Battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003;463:97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- 65.Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010;171:1197–208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dobrovolskaya-Zavadskaya N. L’irradiation des testicules et heredite chez la souris. Archives De Biologie. 1928;38:457–501. [Google Scholar]
- 67.Nadler JJ, Zou F, Huang H, Moy SS, Lauder J, Crawley JN, Threadgill DW, Wright FA, Magnuson TR. Large-scale gene expression differences across brain regions and inbred strains correlate with a behavioral phenotype. Genetics. 2006;174:1229–36. doi: 10.1534/genetics.106.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clee SM, Nadler ST, Attie AD. Genetic and genomic studies of the BTBR ob/ob mouse model of type 2 diabetes. Am J Ther. 2005;12:491–8. doi: 10.1097/01.mjt.0000178781.89789.25. [DOI] [PubMed] [Google Scholar]
- 69.Alkondon M, Pereira EFR, Yu P, Arruda EZ, Almeida LEF, Guidetti P, Fawcett WP, Sapko MT, Randall WR, Schwarcz R, Tagle DA, Albuquerque EX. Targeted deletion of the kynurenine aminotransferase ii gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via alpha7 nicotinic receptors in the hippocampus. J Neurosci. 2004;24:4635–48. doi: 10.1523/JNEUROSCI.5631-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–73. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sapko MT, Guidetti P, Yu P, Tagle DA, Pellicciari R, Schwarcz R. Endogenous kynurenate controls the vulnerability of striatal neurons to quinolinate: Implications for Huntington’s disease. Exp Neurol. 2006;197:31–40. doi: 10.1016/j.expneurol.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Wu H-Q, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm. 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- 73.Yu P, Di Prospero NA, Sapko MT, Cai T, Chen A, Melendez-Ferro M, Du F, Whetsell WO, Jr, Guidetti P, Schwarcz R, Tagle DA. Biochemical and phenotypic abnormalities in kynurenine aminotransferase II-deficient mice. Mol Cell Biol. 2004;24:6919–30. doi: 10.1128/MCB.24.16.6919-6930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wahlsten D, Metten P, Crabbe JC. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain Res. 2003;971:47–54. doi: 10.1016/s0006-8993(03)02354-0. [DOI] [PubMed] [Google Scholar]
- 75.Kusek GK, Wahlsten D, Herron BJ, Bolivar VJ, Flaherty L. Localization of two new X-linked quantitative trait loci controlling corpus callosum size in the mouse. Genes Brain Behav. 2007;6:359–63. doi: 10.1111/j.1601-183X.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- 76.Meyza KZ, Blanchard DC, Pearson BL, Pobbe RLH, Blanchard RJ. Fractone-associated N-sulfated heparan sulfate shows reduced quantity in BTBR T+tf/J mice: a strong model of autism. Behav Brain Res. 2012;228:247–53. doi: 10.1016/j.bbr.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mercier F, Cho Kwon Y, Kodama R. Meningeal/vascular alterations and loss of extracellular matrix in the neurogenic zone of adult BTBR T+ tf/J mice, animal model for autism. Neurosci Lett. 2011;498:173–8. doi: 10.1016/j.neulet.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 78.Busse M, Feta A, Presto J, Wilén M, Grønning M, Kjellén L, Kusche-Gullberg M. Contribution of EXT1, EXT2, and EXTL3 to heparan sulfate chain elongation. J Biol Chem. 2007;282:32802–10. doi: 10.1074/jbc.M703560200. [DOI] [PubMed] [Google Scholar]
- 79.Inatani M, Irie F, Plump AS, Tessier-Lavigne M, Yamaguchi Y. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science. 2003;302:1044–6. doi: 10.1126/science.1090497. [DOI] [PubMed] [Google Scholar]
- 80.Conway CD, Howe KM, Nettleton NK, Price DJ, Mason JO, Pratt T. Heparan sulfate sugar modifications mediate the functions of slits and other factors needed for mouse forebrain commissure development. J Neurosci. 2011;31:1955–70. doi: 10.1523/JNEUROSCI.2579-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Irie F, Badie-Mahdavi H, Yamaguchi Y. Autism-like socio-communicative deficits and stereotypies in mice lacking heparan sulfate. Proceedings of the National Academy of Sciences of the United States of America; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stephenson DT, O’Neill SM, Narayan S, Tiwari A, Arnold E, Samaroo HD, Du F, Ring RH, Campbell B, Pletcher M, Vaidya VA, Morton D. Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis. Mol Autism. 2011;2:7. doi: 10.1186/2040-2392-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Senkov O, Tikhobrazova O, Dityatev A. PSA-NCAM: Synaptic functions mediated by its interactions with proteoglycans and glutamate receptors. Int J Biochem Cell Biol. 2012;44:591–5. doi: 10.1016/j.biocel.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 84.Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–57. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- 85.Wei L, Meaney MJ, Duman RS, Kaffman A. Affiliative behavior requires juvenile, but not adult neurogenesis. J Neurosci. 2011;31:14335–45. doi: 10.1523/JNEUROSCI.1333-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corley MJ, Meyza KZ, Blanchard DC, Blanchard RJ. Reduced sulfate plasma concentrations in the BTBR T+tf/J mouse model of autism. Physiology & Behavior. doi: 10.1016/j.physbeh.2012.04.010. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waring RH, Klovrza LV. Sulphur Metabolism in Autism. Journal of Nutritional and Environmental Medicine. 2000;10:25–32. [Google Scholar]
- 88.Osbun N, Li J, O’Driscoll MC, Strominger Z, Wakahiro M, Rider E, Bukshpun P, Boland E, Spurrell CH, Schackwitz W, Pennacchio LA, Dobyns WB, Black GCM, Sherr EH. Genetic and functional analyses identify DISC1 as a novel callosal agenesis candidate gene. Am J Med Genet A. 2011;155A:1865–76. doi: 10.1002/ajmg.a.34081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Steinecke A, Gampe C, Valkova C, Kaether C, Bolz J. Disrupted-in-Schizophrenia 1 (DISC1) is necessary for the correct migration of cortical interneurons. J Neurosci. 2012;32:738–45. doi: 10.1523/JNEUROSCI.5036-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meyer KD, Morris JA. Disc1 regulates granule cell migration in the developing hippocampus. Hum Mol Genet. 2009;18:3286–97. doi: 10.1093/hmg/ddp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu X, Yang C-H, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng H-J, Ming G, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–58. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zou H, Yu Y, Sheikh AM, Malik M, Yang K, Wen G, Chadman KK, Brown WT, Li X. Association of upregulated Ras/Raf/ERK1/2 signaling with autism. Genes Brain Behav. 2011;10:615–24. doi: 10.1111/j.1601-183X.2011.00702.x. [DOI] [PubMed] [Google Scholar]
- 93.Stoehr JP, Nadler ST, Schueler KL, Rabaglia ME, Yandell BS, Metz SA, Attie AD. Genetic obesity unmasks nonlinear interactions between murine type 2 diabetes susceptibility loci. Diabetes. 2000;49:1946–54. doi: 10.2337/diabetes.49.11.1946. [DOI] [PubMed] [Google Scholar]
- 94.Flowers JB, Oler AT, Nadler ST, Choi Y, Schueler KL, Yandell BS, Kendziorski CM, Attie AD. Abdominal obesity in BTBR male mice is associated with peripheral but not hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2007;292:E936–945. doi: 10.1152/ajpendo.00370.2006. [DOI] [PubMed] [Google Scholar]
- 95.Ranheim T, Dumke C, Schueler KL, Cartee GD, Attie AD. Interaction between BTBR and C57BL/6J genomes produces an insulin resistance syndrome in (BTBR x C57BL/6J) F1 mice. Arterioscler Thromb Vasc Biol. 1997;17:3286–93. doi: 10.1161/01.atv.17.11.3286. [DOI] [PubMed] [Google Scholar]
- 96.Gardener H, Spiegelman D, Buka SL. Prenatal Risk Factors for Autism: Comprehensive Meta-Analysis. BJP. 2009;195:7–14. doi: 10.1192/bjp.bp.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Modi ME, Young LJ. The oxytocin system in drug discovery for autism: Animal models and novel therapeutic strategies. Horm Behav. 2012;61:340–50. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jørgensen H, Kjaer A, Knigge U, Møller M, Warberg J. Serotonin stimulates hypothalamic mRNA expression and local release of neurohypophysial peptides. J Neuroendocrinol. 2003;15:564–71. doi: 10.1046/j.1365-2826.2003.01032.x. [DOI] [PubMed] [Google Scholar]
- 100.Bagdy G, Kalogeras KT. Stimulation of 5-HT1A and 5-HT2/5-HT1C receptors induce oxytocin release in the male rat. Brain Res. 1993;611:330–2. doi: 10.1016/0006-8993(93)90521-n. [DOI] [PubMed] [Google Scholar]
- 101.Jørgensen H, Riis M, Knigge U, Kjaer A, Warberg J. Serotonin receptors involved in vasopressin and oxytocin secretion. J Neuroendocrinol. 2003;15:242–9. doi: 10.1046/j.1365-2826.2003.00978.x. [DOI] [PubMed] [Google Scholar]
- 102.Frye CA, Llaneza DC. Corticosteroid and neurosteroid dysregulation in an animal model of autism, BTBR mice. Physiol Behav. 2010;100:264–7. doi: 10.1016/j.physbeh.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Agís-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci US A. 2007;104:18736–41. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu A, Chan KW, Hoo RLC, Wang Y, Tan KCB, Zhang J, Chen B, Lam MC, Tse C, Cooper GJS, Lam KSL. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–80. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 105.Ashcroft GS, Mills SJ. Androgen receptor-mediated inhibition of cutaneous wound healing. J Clin Invest. 2002;110:615–24. doi: 10.1172/JCI15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heo Y, Zhang Y, Gao D, Miller VM, Lawrence DA. Aberrant immune responses in a mouse with behavioral disorders. PLoS ONE. 2011;6:e20912. doi: 10.1371/journal.pone.0020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Malik M, Tauqeer Z, Sheikh AM, Wen G, Nagori A, Yang K, Brown WT, Li X. NF-κB signaling in the brain of autistic subjects. Mediators Inflamm. 2011;2011:785265. doi: 10.1155/2011/785265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Manzardo AM, Henkhaus R, Dhillon S, Butler MG. Plasma cytokine levels in children with autistic disorder and unrelated siblings. International Journal of Developmental Neuroscience. 2012;30:121–7. doi: 10.1016/j.ijdevneu.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology (Berl) 2005;183:257–64. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- 110.Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol Biochem Behav. 2011;97:586–94. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 111.West L, Waldrop J, Brunssen S. Pharmacologic treatment for the core deficits and associated symptoms of autism in children. J Pediatr Health Care. 2009;23:75–89. doi: 10.1016/j.pedhc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 112.Burket JA, Herndon AL, Winebarger EE, Jacome LF, Deutsch SI. Complex effects of mGluR5 antagonism on sociability and stereotypic behaviors in mice: possible implications for the pharmacotherapy of autism spectrum disorders. Brain Res Bull. 2011;86:152–8. doi: 10.1016/j.brainresbull.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 113.Silverman JL, Smith DG, Rizzo SJS, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, Crawley JN. Negative Allosteric Modulation of the mGluR5 Receptor Reduces Repetitive Behaviors and Rescues Social Deficits in Mouse Models of Autism. Sci Transl Med. 2012;4:131ra51–131ra51. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr. Res. 2011;69:26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Casanova MF, El-Baz A, Elnakib A, Switala AE, Williams EL, Williams DL, Minshew NJ, Conturo TE. Quantitative analysis of the shape of the corpus callosum in patients with autism and comparison individuals. Autism. 2011;15:223–38. doi: 10.1177/1362361310386506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hardan AY, Minshew NJ, Keshavan MS. Corpus callosum size in autism. Neurology. 2000;55:1033–6. doi: 10.1212/wnl.55.7.1033. [DOI] [PubMed] [Google Scholar]
- 117.Hardan AY, Pabalan M, Gupta N, Bansal R, Melhem NM, Fedorov S, Keshavan MS, Minshew NJ. Corpus callosum volume in children with autism. Psychiatry Res. 2009;174:57–61. doi: 10.1016/j.pscychresns.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Crepel A, Breckpot J, Fryns J-P, De la Marche W, Steyaert J, Devriendt K, Peeters H. DISC1 duplication in two brothers with autism and mild mental retardation. Clin Genet. 2010;77:389–94. doi: 10.1111/j.1399-0004.2009.01318.x. [DOI] [PubMed] [Google Scholar]
- 119.Kilpinen H, Ylisaukko-Oja T, Hennah W, Palo OM, Varilo T, Vanhala R, Nieminen-von Wendt T, von Wendt L, Paunio T, Peltonen L. Association of DISC1 with autism and Asperger syndrome. Mol Psychiatry. 2008;13:187–96. doi: 10.1038/sj.mp.4002031. [DOI] [PubMed] [Google Scholar]
- 120.Lim SM, Kim H-J, Nam M, Chung J-H, Park YH. Association study of DISC1 in Korean population with autism spectrum disorders. Psychiatr Genet. 2009;19:160. doi: 10.1097/YPG.0b013e32832a9bd1. [DOI] [PubMed] [Google Scholar]
- 121.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–23. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]